Abstract
The C1 neurons are a nodal point for blood pressure control and other autonomic responses. Here we test whether these rostral ventrolateral medullary catecholaminergic (RVLM-CA) neurons use glutamate as a transmitter in the dorsal motor nucleus of the vagus (DMV). After injecting Cre-dependent adeno-associated virus (AAV2) DIO-Ef1α-channelrhodopsin2(ChR2)-mCherry (AAV2) into the RVLM of dopamine-β-hydroxylase Cre transgenic mice (DβHCre/0), mCherry was detected exclusively in RVLM-CA neurons. Within the DMV >95% mCherry-immunoreactive(ir) axonal varicosities were tyrosine hydroxylase (TH)-ir and the same proportion were vesicular glutamate transporter 2 (VGLUT2)-ir. VGLUT2-mCherry colocalization was virtually absent when AAV2 was injected into the RVLM of DβHCre/0;VGLUT2flox/flox mice, into the caudal VLM (A1 noradrenergic neuron-rich region) of DβHCre/0 mice or into the raphe of ePetCre/0 mice. Following injection of AAV2 into RVLM of TH-Cre rats, phenylethanolamine N-methyl transferase and VGLUT2 immunoreactivities were highly colocalized in DMV within EYFP-positive or EYFP-negative axonal varicosities. Ultrastructurally, mCherry terminals from RVLM-CA neurons in DβHCre/0 mice made predominantly asymmetric synapses with choline acetyl-transferase-ir DMV neurons. Photostimulation of ChR2-positive axons in DβHCre/0 mouse brain slices produced EPSCs in 71% of tested DMV preganglionic neurons (PGNs) but no IPSCs. Photostimulation (20 Hz) activated PGNs up to 8 spikes/s (current-clamp). EPSCs were eliminated by tetrodotoxin, reinstated by 4-aminopyridine, and blocked by ionotropic glutamate receptor blockers. In conclusion, VGLUT2 is expressed by RVLM-CA (C1) neurons in rats and mice regardless of the presence of AAV2, the C1 neurons activate DMV parasympathetic PGNs monosynaptically and this connection uses glutamate as an ionotropic transmitter.
Introduction
The C1 neurons are a subset of catecholaminergic neurons that reside at the rostral end of the ventrolateral medulla and, in rats, express the adrenaline-synthesizing enzyme phenylethanolamine N-methyl transferase (PNMT) (Hökfelt et al., 1974; Ross et al., 1983). Their contribution to the regulation of sympathetic vasomotor tone and blood pressure is thoroughly documented and is attributed to their direct projections to the intermediolateral cell column (Ross et al., 1983; Guyenet, 2006; Abbott et al., 2009; Marina et al., 2011). Less known is the fact that the C1 neurons also have dense axonal projections to the dorsal motor nucleus of the vagus (DMV; Hökfelt et al., 1974; Card et al., 2006). This connection suggests that the C1 neurons may also regulate the parasympathetic component of the autonomic nervous system. A major objective of this study is to test this hypothesis and to identify whether the input from the C1 cells to the DMV is monosynaptic and excitatory.
Contrary to most brainstem noradrenergic neurons, adult C1 neurons in rats contain readily detectable levels of vesicular glutamate transporter 2 (VGLUT2) mRNA (Stornetta et al., 2002) and the cognate protein is present within PNMT-immunoreactive (ir) terminals presumed to originate from these neurons (Card et al., 2006; Rosin et al., 2006). Conversely, >90% of C1 neurons lack a plasmalemmal monoamine transporter (Lorang et al., 1994; Comer et al., 1998) and there is no direct evidence yet that their axonal terminals release catecholamines (Sved, 1989). PNMT-ir varicosities, unlike those of dopaminergic neurons, typically make conventional synapses in adult rats (Milner et al., 1988, 1989; Moss et al., 2011; Bérubé-Carrière et al., 2012). These predominantly asymmetric synapses are structurally reminiscent of forebrain glutamatergic synapses. However, evidence that adult C1 cells release glutamate is based on in vivo pharmacology, therefore of an indirect nature (Morrison et al., 1989; Abbott et al., 2012). In sum, the C1 cells appear to be excitatory and could be using glutamate as one of their transmitters, but definitive evidence is lacking.
In the present study we used a combination of neuroanatomical and electrophysiological approaches to test whether adult rostral ventrolateral medullary catecholaminergic (RVLM-CA) neurons form conventional excitatory glutamatergic synapses with DMV neurons in mice. Channelrhodopsin-2 (ChR2) was selectively introduced into the RVLM-CA neurons by injecting a Cre-dependent adeno-associated virus (AAV2) into the RVLM of transgenic mice that express Cre recombinase exclusively in dopamine-β-hydroxylase (DβH)-containing neurons (Stornetta et al., 2012). We examined whether the axonal varicosities of the RVLM-CA neurons contain VGLUT2 and whether these terminals form conventional synapses with vagal preganglionic neurons (PGNs). We used tyrosine hydroxylase (TH)-Cre transgenic rats (Witten et al., 2011) to verify some of the mouse findings and to ascertain that the axonal projections from the C1 cells to the DMV possess VGLUT2-ir varicosities regardless of whether the C1 neurons had been exposed to AAV2. Finally, we recorded from vagal parasympathetic PGNs in slices of adult mice and examined the effects produced by photostimulating the ChR2-expressing nerve fibers originating from the RVLM-CA cells.
Materials and Methods
Animals
Animal use was in accordance with guidelines approved by the University of Virginia Animal Care and Use Committee. All electrophysiology experiments were done using 21 DβHCre/0 mice (9 females, 12 males). Histology experiments were done with 13 DβHCre/0 mice (seven males, six females). DβH-Cre mice were obtained from the Mutant Mouse Regional Resource Center at the University of California, Davis, CA (Tg(Dbh-cre)KH212Gsat/Mmcd; stock # 032081-UCD). The transgenic line was maintained as hemizygous (Cre/0) on a C57BL/6J background. Vglut2(flox/flox) mice (JAX stock #012898; STOCK Slc17a6tm1Lowl/J) (Tong et al., 2007) maintained as homozygous (flox/flox) were bred with DβH-Cre mice to generate DβHCre/0; VGlut2flox/0 and subsequently crossed with Vglut2flox/flox for two generations to generate DβH-Cre(Cre/0); Vglut2(flox/flox) mice in which VGLUT2 should be absent from any Cre-expressing noradrenergic or adrenergic neurons. Three of these DβHCre/0; Vglut2flox/flox mice (two females, one male) were also used for histology experiments. Histology experiments were also conducted on three ePetCre/0 mice (two males, one female) kindly provided by E.S. Deneris (Case Western Reserve University, Cleveland, OH) (Scott et al., 2005) and five THCre/0 rats (all males, rat line obtained from Ilana Witten (Princeton University, New Jersey) and Karl Deisseroth (Stanford University, California) (Witten et al., 2011) and maintained as hemizygous (Cre/0) on a Long–Evans background. All animals were at least 8 weeks old at the time of AAV2 injection.
AAV2 vectors
DIO-eF1α-ChR2-mCherry AAV serotype 2 and DIO-eF1α-EYFP AAV serotype 2 (Atasoy et al., 2008) were obtained from the University of North Carolina Vector core (Chapel Hill, NC) (AAV2 titer: 1012 viral molecules per milliliter).
Stereotaxic injections of AAV2 into the RVLM of mice or rats
DβH-Cre and DβHCre/0; Vglut2flox/flox and ePetCre/0 mice were anesthetized with a mixture of ketamine (100 mg/kg) and dexmedetomidine (0.2 mg/kg) given intraperitoneally and received three injections of undiluted DIO-eF1α-ChR2-mCherry AAV2 (360 nl total volume) into the left RVLM, the left caudal VLM (A1 catecholaminergic region), or the raphe obscurus after methods previously described (Depuy et al., 2011; Stornetta et al., 2012).
TH-Cre rats were anesthetized with a mixture of ketamine (75 mg/kg), xylazine (5 mg/kg), and acepromazine (1 mg/kg). A total of 500 nl of DIO-eF1α-EYFP AAV2 was injected in three RVLM locations aligned in the longitudinal direction using methods previously described (Abbott et al., 2012).
In vitro electrophysiology
Four to 12 weeks after AAV2 injection, DβHCre/0 mice were anesthetized with a mixture of ketamine (120 mg/kg) and xylazine (12 mg/kg) given intraperitoneally, then decapitated. The brainstem was removed and sectioned into 300 μm slices in the transverse plane in ice-cold, N-methyl-d-glucamine (NMDG)-substituted artificial CSF (ACSF) containing the following (in mm): 92 NMDG, 2.5 KCl, 1.25 NaH2PO4, 10 MgSO4, 0.5 CaCl2, 20 HEPES, 30 NaHCO3, 25 glucose, 2 thiourea, 5 Na-ascorbate, and 3 Na-pyruvate (∼300 mOsm/kg). After 10 min at 33°C, brain slices were transferred to aerated physiological extracellular ACSF containing the following (in mm): 119 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 MgSO4, 2 CaCl2, 26 NaHCO3, 12.5 glucose, 2 thiourea, 5 Na-ascorbate, and 3 Na-pyruvate and maintained at room temperature (23−25°C). All recordings were performed at room temperature in submerged slices continuously perfused with aerated physiological ACSF.
The transverse slices from which DMV neurons were recorded included the area postrema or were immediately adjacent to this level in either direction. To record postsynaptic currents (PSC), electrodes (2–6 MΩ tip resistance) were filled with an intracellular solution containing the following (in mm): 140 Cs-methanesulfonate, 10 HEPES, 10 Tris-phosphocreatine, 3 ATP-Na, 0.3 GTP-Na, and 1 EGTA, 2 MgCl2, pH 7.3. The calculated E[Cl-] was −88 mV. EPSCs were recorded at Vhold of −70 mV (−79.4 mV after junction potential correction). IPSCs were recorded at Vhold of 0 mV (−9.4 mV after junction potential correction).
For current-clamp recordings a similar pipette solution was used replacing Cs-methanesulfonate with K-gluconate (junction potential 14.8 mV). Corrected membrane potentials are reported in the text and figures. Biocytin-filled electrodes were used to label the recorded cells intracellularly in seven experiments. Whole-cell voltage-clamp and current-clamp recordings were performed on visually identified DMV neurons (68 neurons in total). Three mice received an injection of Fluoro-Gold (FG) (5 ml/kg, i.p., 1% solution in sterile water; (Fluorochrome) and 8 FG neurons were recorded in these mice. These neurons responded exactly as the DMV neurons selected on the basis of location only and the results were pooled.
Recordings were performed using a MultiClamp 700B amplifier and pClamp 10 software (Molecular Devices). Signals were lowpass filtered at 4 kHz and digitized at 10 kHz. Only cells with series resistance that remained <25 MΩ were included in the analysis.
For photostimulation of ChR2 terminals, the tip of a 200 μm diameter optical fiber coupled to a 473 nm DPSS laser (IkeCool) was placed ∼300 μm away from the DMV. Delivery of optical pulses (1 ms duration) was controlled by a digitizer (Digidata 1440A; Molecular Devices) controlled by episodic protocols run in pClamp 10 (Molecular Devices). The laser/fiber was calibrated for 5 mW steady-state output before each experiment.
Histology
Perfusions.
All animals receiving AAV2 injections and used exclusively for histology (both mice and rats) survived 4–6 weeks after injections and were anesthetized (pentobarbital overdose) and perfused transcardially first with heparinized saline and then with 4% paraformaldehyde (PFA) (mice with 10 ml saline, then 80 ml PFA and rats with 100 ml saline and then 400 ml PFA) and brains removed and postfixed in 4% PFA for 2–5 d. For ultrastructural analysis, five of the AAV2-injected DβH-Cre mice were anesthetized as above and perfused transcardially with 20 ml of heparinized saline (1000 U/ml) followed by fixative (25 ml of 2% PFA with 3.75% acrolein from Electron Microscope Sciences, followed by 30 ml of 2% PFA). Brains from these animals were removed and postfixed for at least 3 h in 2% PFA before sectioning.
Recorded brain slices.
After recording, the slices that contained biocytin-filled neurons were transferred to 4% PFA for 2–5 d at 4°C.
FG injections.
Four 4- to 6-week-old C57BL/6J mice received intraperitoneal injections of 200 μl of 1% FG. After 3–5 d, the mice were deeply anesthetized and perfused. Brains were removed and postfixed in 4% PFA for up to 5 d. Brains were sectioned and reacted for immunohistochemistry as described below.
Sectioning and immunohistochemistry.
Transverse sections (30 μm thick) were cut through the DMV with a vibrating microtome (Leica) and collected into a cryoprotectant solution and stored at −20°C before further processing. All histological procedures were done with free-floating sections. Sections were rinsed, blocked, and incubated in primary antibodies TH, choline acetyl-transferase (ChAT), FG, dsRed, PNMT, and VGLUT2, as previously described (Bochorishvili et al., 2012; Stornetta et al., 2012). Sections were then rinsed and incubated for 45–60 min in appropriate secondary antibody, then rinsed, mounted on gelatinized glass slides, dehydrated through a graded series of alcohols, and covered with DPX. Slides were examined using a Zeiss Axioskop2 and photographed with a Zeiss MRC camera (Bochorishvili et al., 2012; Stornetta et al., 2012). Ultrastructural procedures were done exactly as described by Bochorishvili et al. (2012).
Statistical analysis
All data were analyzed using the GraphPad Prism v5.04 statistical package. Statistical significance (p < 0.05) was determined using parametric ANOVA (one-way or repeated measures) with post hoc Student–Newman–Keuls, or nonparametric analysis with Kruskal–Wallis (Dunn's post hoc) or Wilcoxon signed rank test. Before statistical analysis, normality of distribution was determined with the Kolmogorov–Smirnov test and homogeneity of variance was tested with Bartlett's test. Unless stated otherwise, data are expressed as mean ± SEM.
Results
ChR2-mCherry labeled terminals originating from RVLM-CA neurons innervate the DMV and contain VGLUT2
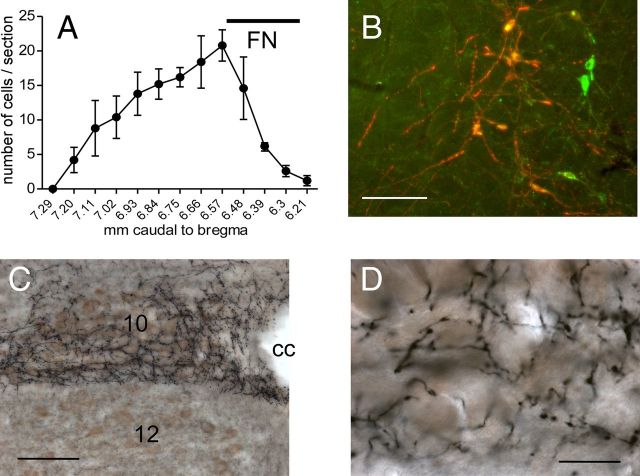
The location of the neurons that expressed ChR2-mCherry following injection of DIO-ChR2-mCherry AAV2 was examined in five mice that were also used for ultrastructural analysis of labeled terminals within the DMV. Consistent with our previous study in which we used the same electrophysiologically guided procedure to inject the vector into the RVLM (Stornetta et al., 2012), the mCherry-ir neurons were confined to the RVLM, mostly caudal to the facial motor nucleus (Fig. 1A). The mCherry-positive neurons were 97 ± 1% TH-ir, hence potentially catecholaminergic (Fig. 1B).
Figure 1.
RVLM-CA neurons innervate the DMV in DβH-Cre mice. A, Rostrocaudal distribution of mCherry + TH-ir neurons (average of 5 cases). The ChR2-expressing neurons were confined to the RVLM. FN, caudal extent of the facial motor nucleus, provided as reference. B, Dual-labeled neurons appear orange-yellow. Untransfected TH-ir neurons appear green. Photomicrograph of the RVLM 1 month after injection of AAV2-DIO-ChR2-mCherry into the same region in a DβH-Cre mice. mCherry immunoreactivity (red) is detectable exclusively in TH-ir neurons (green). Scale bar, 100 μm. C, Photomicrograph showing the dense plexus of nerve terminals arising from mCherry-ir RVLM-CA neurons. ChR2-mCherry is visualized with the nickel-DAB method resulting in a black color. ChAT-ir is visualized with DAB resulting in a brown color. Note the dense terminal field in the DMV (10) that avoids the hypoglossal nucleus (12). cc, Central canal. Scale bar, 200 μm. D, Higher power photomicrograph of the DMV innervation seen in C showing fibers and synaptic boutons arising from ChR2-expressing RVLM-CA neurons in close proximity of the DMV cholinergic neurons. Scale bar, 30 μm.
In all mice, the DMV contained a dense network of mCherry-ir terminals that made close appositions with ChAT-ir, i.e., cholinergic neurons (Fig. 1C,D).
Evidence that neurons recorded in the DMV were parasympathetic preganglionic cells
We confirmed that these DMV cholinergic neurons were parasympathetic PGNs by showing that all of these cholinergic neurons contained FG in mice that had received an intraperitoneal injection of this retrogradely transported dye. After intraperitoneal injection of FG in three animals, neurons labeled with FG within the DMV were counted and the proportion of FG-labeled DMV neurons that were immunoreactive for ChAT (three sections per mouse) was determined. The coincidence was 95.1 ± 1.5% (total of 1219 neurons counted, example in Fig. 2A1–A3). Brainstem slices were made from an additional three mice injected intraperitoneally with FG and 8 FG-positive DMV neurons cells were recorded and filled with biocytin as described previously (Fig. 2B1–B3). The extensive filling with biocytin allowed for computer-assisted reconstruction of the dendrites and axonal processes of four cells (Fig. 2C) showing a long, presumably axonal, process exiting along the expected trajectory for a parasympathetic PGN.
Figure 2.
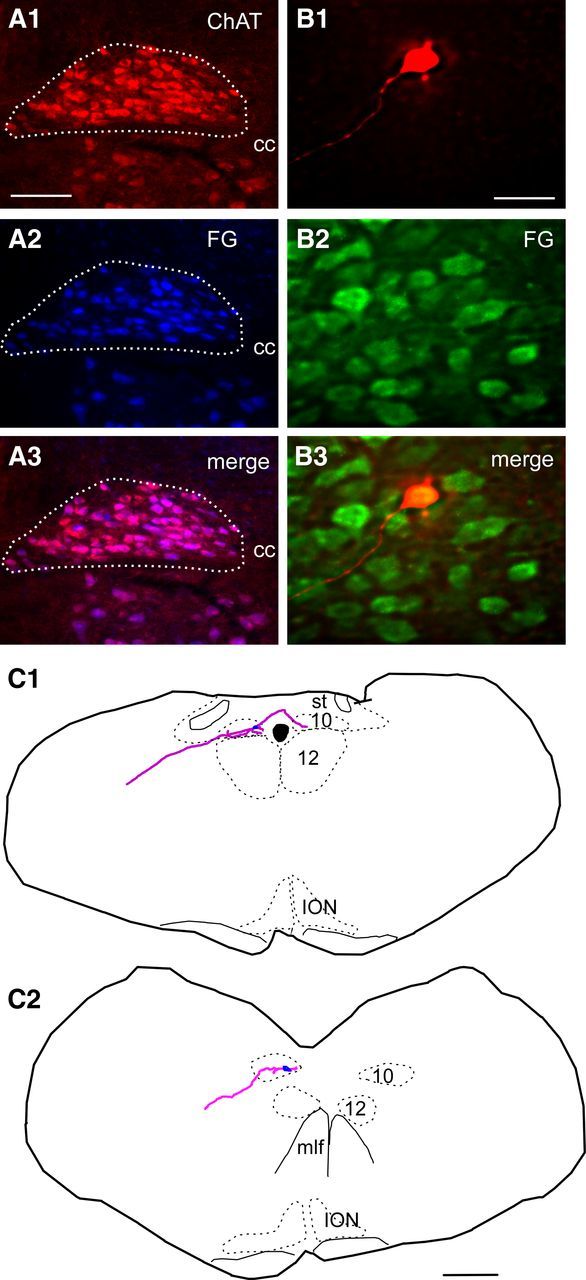
Verification that FG labels ChAT neurons in the DMV and that the recorded neurons are vagal premotor neurons. A1–A3, Photomicrograph of DMV illustrating that ChAT-ir neurons (red) in DMV(A1) are also FG positive (blue; A2). A3, Merged image of A1 and A2; double-labeled neurons appear pink. Scale bar: (in A1) A1–A3, 100 μm. B, Example of a slice reacted for immunohistochemistry to reveal the biocytin label (B1) showing the cell is also FG-ir (green; B2). B3, Merge of B1 and B2 showing the cell is within the DMV and also FG, thus projecting outside the brain. Scale bar: (in B1) B1–B3, 50 μm. C1, C2, Illustrations of biocytin-filled neurons recorded in slices and reconstructed showing the dendrites and axon projecting toward the periphery of the section. Scale bar: (in C2), C1, C2, 0.5 mm. cc, Central canal; ION, inferior olivary nucleus; st, solitary tract; mlf, medial longitudinal fasciculus.
Since almost all mCherry-expressing cells in the RVLM of DIO-ChR2-mCherry AAV2-injected animals were TH-ir, we expected that a similarly high proportion of the mCherry-ir terminals located in the DMV would also be TH-ir, but the ChR2-containing axons present in the DMV could conceivably have originated primarily from the very few TH-negative hence apparently noncatecholaminergic neurons that expressed the transgene. To exclude this possibility, in three DβHCre/0 mice injected with AAV2, coronal sections were reacted for simultaneous immunofluorescent detection of TH and mCherry and we counted singly (mCherry-ir) and dually labeled axonal varicosities (both mCherry and TH-ir) in the region of the DMV outlined in Figure 3A, which also corresponds to the region that was explored electrophysiologically. In this region, most (98.6 ± 0.2%) of the mCherry-positive axonal varicosities were TH-ir (Fig. 3 B1–B3; 1370 total terminals counted in three sections, one section per mouse). Parallel experiments were done to determine what proportion of the ChR2-expressing axonal varicosities contained VGLUT2-ir in the same region of the DMV. Most (97 ± 1%) mCherry-ir axonal varicosities were also VGLUT2-ir (Fig. 3 D1–D3; 1950 total terminals counted in three sections, one section per mouse). On statistical grounds, this evidence demonstrates that most ChR2 axonal varicosities contain both VGLUT2 and TH immunoreactivities. By performing triple-label experiments, we were able to find direct evidence of the presence of all three markers (mCherry, VGLUT2, and TH) within the same terminals in the DMV, although the degree of coincidence was not quantified (Fig. 3 C1–C3).
Figure 3.
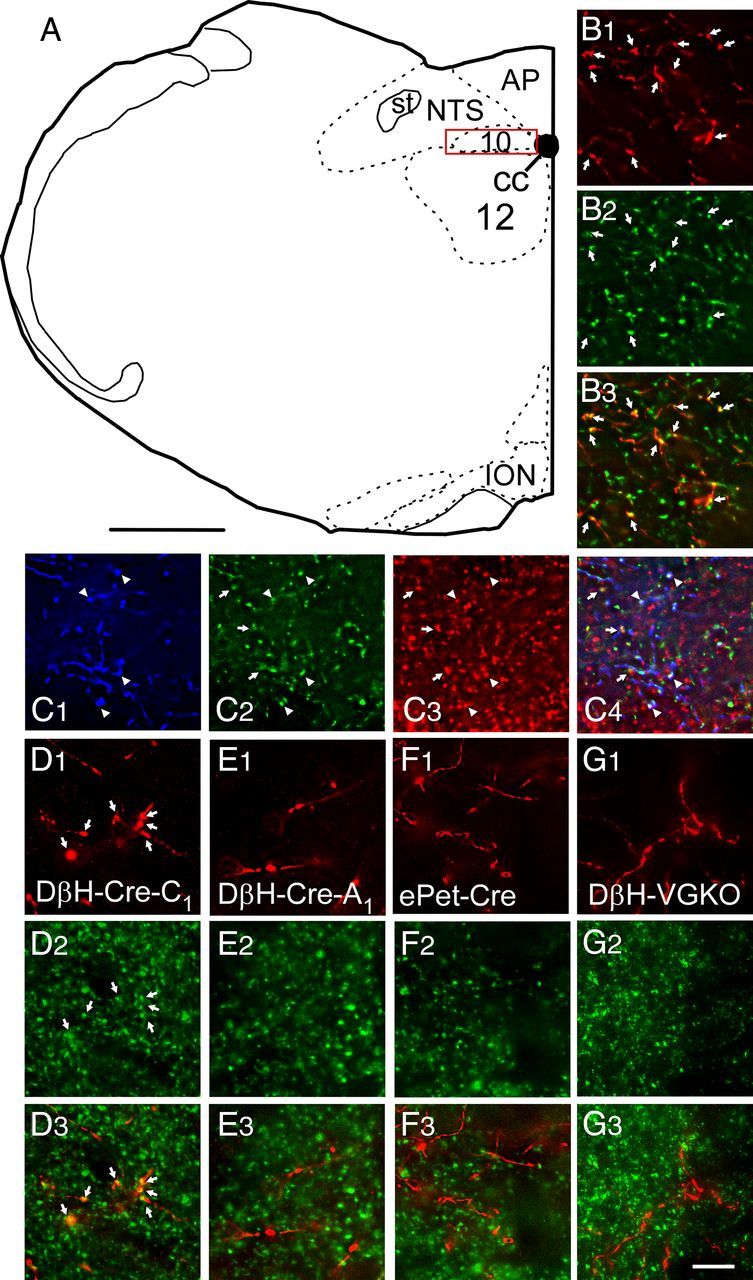
VGLUT2 is present in DMV varicosities that originate from RVLM-CA neurons but is absent from those that emanate from A1 noradrenergic and raphe serotonergic neurons. A, Drawing of mouse brain coronal hemisection at ∼7.64 mm caudal to bregma. The red box represents the region of the DMV where axonal varicosities were counted and the photomicrographs were taken. Scale bar, 500 μm. B1, Photomicrograph of mCherry-ir terminals (red) in the DMV from a DβH-Cre mouse injected in RVLM with DIO-ChR2-mCherry AAV2. B2, TH-ir varicosities within the same field. B3, Merged image (B1 + B2) showing that all the mCherry-ir varicosities are yellow, i.e., they contain TH. C, Same experiment as in B but revealing mCherry with a rabbit secondary tagged with Dylight 649 and pseudocolored blue (C1) and both TH-ir (green; C2) and VGLUT2-ir varicosities (red; C3). C4, Merged image (C1 + C2 + C3) showing triple-labeled terminals (appearing white, arrowheads) as well as terminals double labeled only for TH and VGLUT2 (appearing yellow, arrows). D1, Photomicrograph of mCherry-ir terminals (red) in the DMV from a DβH-Cre mouse injected in RVLM with DIO-ChR2-mCherry AAV2. D2, VGLUT2-ir varicosities within the same field. D3, Merged image (B1 + B2) showing that all the mCherry-ir varicosities are yellow, i.e., they contain VGLUT2. E, Similar experiment to B except the DβH-Cre mouse was injected in the A1 region (caudal VLM). E1, mCherry. E2, VGLUT2. E3, Merge of C1 + C2. Note that the mCherry-ir varicosities are red, i.e., they are not VGLUT2-ir. F, Similar experiment to B except the AAV2 was injected into the raphe obscurus of an ePet-Cre mouse. F1, mCherry. F2, VGLUT2. F3, Merge of F1 + F2. Note that the mCherry-ir varicosities are red, i.e., they are not VGLUT2-ir. G, Similar experiment to B except the AAV2 was injected into the RVLM of a DβH-Cre(Cre/0); Vglut2(flox/flox) mouse (DβH-VGKO). G1, mCherry. G2, VGLUT2. G3, Merge of G1 + G2. Note that the mCherry-ir varicosities are red, i.e., they are not VGLUT2-ir. 10, DMV; 12, hypoglossal motor nucleus; AP, area postrema; cc, central canal; ION, inferior olivary nucleus; NTS, nucleus of the solitary tract; st, solitary tract. Scale bar: (in G3) B–G, 10 μm.
Control experiments were done to verify that we correctly identified the presence of VGLUT2 in the terminals of the RVLM-CA neurons and to exclude the possibility that the presence of VGLUT2 might be an artifact caused by AAV2 transduction. In three DβHCre/0 mice, DIO-ChR2-mCherry AAV2 was injected more caudally within the VLM where the A1 group of noradrenergic neurons resides. In rats, very few A1 neurons contain VGLUT2 mRNA suggesting that they are not glutamatergic (Stornetta et al., 2002). The cell bodies of these caudal VLM catecholaminergic neurons expressed high levels of transgene (data not shown). These cells also projected to the dorsal vagal complex and adjacent nucleus of the solitary tract but very few of their axonal varicosities (8.7 ± 1.5%) were also VGLUT2-ir (Fig. 3E1–E3; 567 total terminals counted in one section each from three mice). In the second control group, we injected the same amount of DIO-ChR2-mCherry AAV2 into the raphe obscurus of three ePetCre/0 mice, a procedure that results in selective expression of the transgene by serotonergic neurons (Scott et al., 2005; Depuy et al., 2011). These serotonergic neurons also abundantly innervated the dorsal vagal complex. Most of their varicosities were not VGLUT2-ir (Fig. 3F1--F3). Only 7.4 ± 1% of the mCherry-ir terminals originating from these raphe neurons appeared VGLUT-ir (1137 terminals counted from one section each of three mice). The third control group consisted of three DβH-CreCre/0; VGLUT2flox/flox mice that received the standard injections of DIO-ChR2-mCherry AAV2 in the C1 region. The DMV of these mice contained approximately the same density of mCherry-ir axonal projections as the control (DβHCre/0) mice but only 8.2 ± 3.2% of the mCherry varicosities were also VGLUT2-ir (Fig. 3 G1–G3). In summary, within the mouse dorsal motor nucleus of the vagus, the axonal varicosities of the RVLM-CA neurons (presumptive C1 cells) typically contained both TH and VGLUT2, whereas very few terminals from A1 noradrenergic and raphe obscurus serotonergic neurons exposed to the same AAV2 contained VGLUT2. The absence of VGLUT2 in serotonergic neurons conforms to expectation since this particular vesicular transporter is not known to be expressed by these neurons in adult tissue (Gras et al., 2002). The small number of VGLUT2-positive terminals originating from A1 and raphe could conceivably be false positive results caused by the close juxtaposition of two terminals from different neurons and likely represents the “background.” Finally, we demonstrated the selectivity of the immunohistochemical detection of VGLUT2 within the varicosities emanating from RVLM-CA cells by showing that VGLUT2 immunoreactivity was present only at background level when the Cre-dependent vector was injected into DβHCre/0; VGLUT2flox/flox mice.
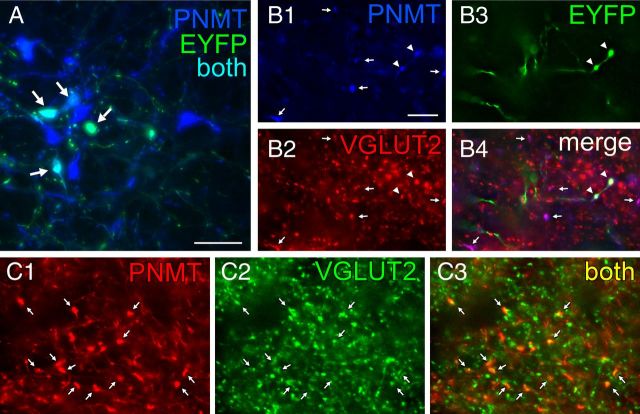
The final light microscopy histological experiments were conducted in TH-Cre rats and had three goals. One was to test whether the projection from RVLM-CA neurons to DMV also exists in rats and is also glutamatergic. The second goal was to verify that this projection emanates from the C1 (PNMT-positive) neurons. To do so we located the adrenaline-synthesizing enzyme PNMT that is diagnostic of the C1 neurons and is coexpressed with TH and DβH in these neurons (Phillips et al., 2001). The third goal was to verify, in rats, that AAV2 does not reactivate a latent glutamatergic phenotype in these catecholaminergic neurons, a possibility raised previously to explain discrepancies between electrophysiological results suggesting a contribution of glutamate to neurotransmission in adult mesolimbic dopaminergic neurons and the lack of supportive ultrastructural evidence (Stuber et al., 2010; Moss et al., 2011; Bérubé-Carrière et al., 2012). We injected DIO eF1α EYFP AAV2 into the RVLM of three TH-Cre rats, which caused a high level of expression in PNMT-ir neurons (typical injection site shown in Fig. 4A). EYFP was also detected in a smaller number of RVLM neurons devoid of PNMT immunoreactivity suggesting some degree of ectopic Cre recombinase expression (data not illustrated). Numerous triple-labeled terminals containing EYFP, VGLUT2, and PNMT immunoreactivities were observed in each rat within the DMV (Fig. 4B). Within this structure almost all PNMT-ir terminals were also VGLUT2-ir whether or not they were labeled with EYFP, suggesting that AAV2 transduction could not have explained the presence of VGLUT2 in PNMT-ir terminals. To further verify this point, we examined the DMV of two TH-Cre rats that had not been injected with AAV2. Virtually all PNMT-ir terminals identified within the DMV were also VGLUT2-ir (Fig. 4C). In summary, RVLM-CA neurons also innervate the DMV in rats. As in mice, the axonal projections of these neurons to the DMV contain VGLUT2 and the presence of VGLUT2 in these neurons is not an artifact caused by AAV2 transduction.
Figure 4.
Axonal varicosities from C1 neurons in rat DMV contain PNMT and VGLUT2. A, Photomicrograph showing that injection of AAV2-DIO-EYFP into the RVLM drives expression of EYFP in C1 (PNMT-ir) neurons in TH-Cre rats. EYFP-ir is in green and PNMT-ir is in blue. Double-labeled neurons appear aqua (arrows). Scale bar, 50 μm. B, Virtually all adrenergic varicosities (PNMT-ir; blue) within the DMV (B1, denoted by arrows or arrowheads) are also glutamatergic (VGLUT2-ir, red; B2). The EYFP terminals (green) (B3, arrowheads) originating from C1 RVLM neurons are both adrenergic (red) and glutamatergic (green). B4, Merge of B1–B3. Triple-labeled terminals (arrowheads) appear white. Double-label terminals (PNMT + VGLUT2) appear purple (arrows) when the colors are merged. C, Adrenergic terminals in DMV are also glutamatergic in naive TH-Cre rats (no AAV2 injection). Adrenergic nerve terminals (PNMT-ir; red) located in DMV (C1) are also glutamatergic (VGLUT2-ir, green; C2), arrows. These terminals appear yellow when the colors are merged in C3 (arrows). Note that practically all the PNMT-ir terminals are also VGLUT2-ir similar to the virus-injected rat shown in B. Scale bar: (in B1) B, C, 10 μm.
ChR2-mCherry-labeled terminals from RVLM-CA neurons make monosynaptic contacts with cholinergic DMV neurons
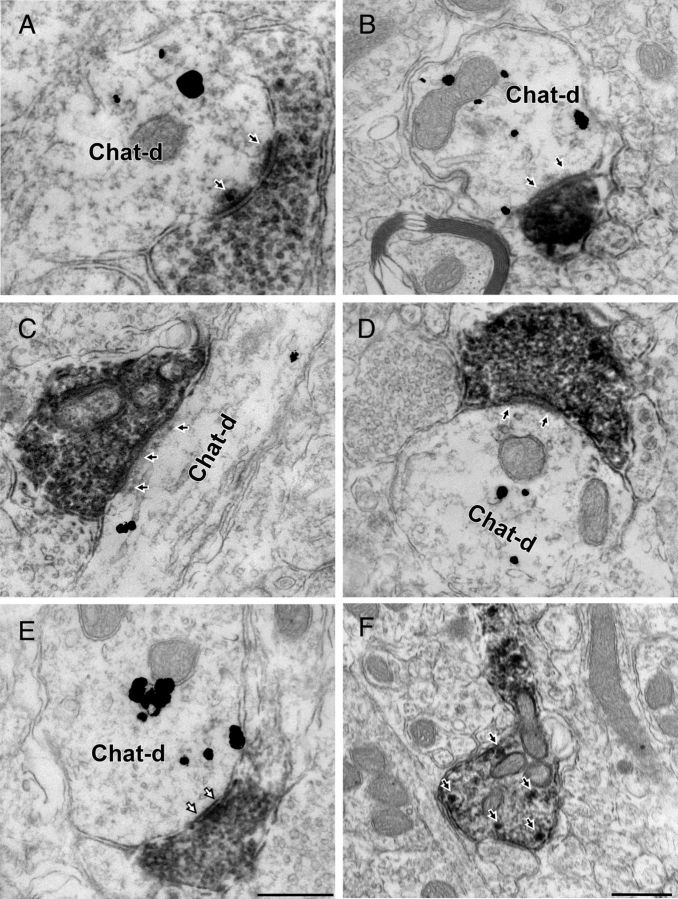
We identified 113 mCherry-ir [diaminobenzidine (DAB)-labeled] profiles within the DMV using tissue from five mice processed for electron microscopy (EM). The DMV region examined is specified in Figure 3A. These profiles consisted exclusively of unmyelinated axons and nerve terminals. Immunogold-silver labeling for ChAT was observed only within perikarya and dendrites. In tissue dually labeled for mCherry and ChAT we identified 65 synaptic contacts between mCherry-ir varicosities and ChAT-ir profiles (Fig. 5A–D; Table 1). The majority of these synapses (N = 48, 74%) were asymmetric (Fig. 5A–D), but 17 symmetric axodendritic or axosomatic synapses also were detected (26%; Fig. 5E). A small proportion of mCherry-positive boutons made synaptic contact onto dendrites that lacked detectable immunoreactivity for ChAT (n = 18; 12 asymmetric and 6 symmetric contacts). Because ChAT immunodetection was restricted to the surface of the sections, these negative results are not clearly interpretable. They could either be false negative results or denote that, within the confines of the DMV, RVLM-CA neurons also innervate neurons other than the parasympathetic PGNs.
Figure 5.
RVLM-CA neurons make synapses with DMV cholinergic neurons. A–D, EMs of the DMV from DβH-Cre mice injected with DIO-ChR2-mCherry AAV2 in RVLM. mCherry-ir axonal varicosities labeled with immunoperoxidase reaction product make synapses onto dendrites containing gold-silver labeling for ChAT. The postsynaptic density (black arrows) indicates that these synapses are asymmetric and presumably glutamatergic. E, Example of symmetric synapse (white arrows) between mCherry-ir (peroxidase) terminal and ChAT-ir (gold) dendrite (Chat-d). F, Example of mCherry-positive axon containing dense core vesicles (white arrows) reminiscent of catecholaminergic terminals. Scale bar: (in E) A–E, 0.5 μm; F, 0.5 μm.
Table 1.
Synaptic contacts formed by mCherry-ir terminals and ChAT-ir neurons in DMV
| Mouse | Primary antibody | DsRed terminals | Synapses (%) | Asymmetric (%) | Symmetric (%) |
|---|---|---|---|---|---|
| N = 5 | DsRed/rabbit ChAT/goat | 113 | 65 (59%) | 48 (74%) | 17 (26%) |
Dense core vesicles consistent with catecholamine-releasing organelles were detected within the more lightly labeled mCherry-positive varicosities (Fig. 5F) but most terminals contained a level of peroxidase reaction product that was too high to reliably observe such vesicles.
Photostimulation of RVLM-CA terminals in the DMV evokes postsynaptic inward currents in DMV neurons
DMV neurons were recorded in brain slices prepared from adult DβHCre/0 mice ∼1 month after injection of AAV2-DIO-ChR2-mCherry into RVLM. The dorsal vagal complex from AAV2-injected mice contained mCherry-positive fibers that were clearly visible (observed with epifluorescence using Zeiss filter set 20: excitation 546 nm, emission 575 nm). The slices used for recording did not contain mCherry-positive somata, confirming that few if any A1 noradrenergic neurons had been exposed to the AAV2. mCherry-positive somata were consistently observed in the expected VLM location in more rostral slices.
We believe that we recorded exclusively from DMV neurons because of their fairly obvious location in coronal slices. Moreover, in several instances when we recorded from DMV neurons visualized with FG, we obtained the same results as with DMV neurons sampled without FG guidance. Also, several intracellular fills labeled DMV neurons with an axon that extended mediolaterally toward an exiting vagal rootlet (Fig. 2) as expected for parasympathetic PGNs. Finally, the membrane potential and discharge characteristics of the DMV neurons recorded in current-clamp were similar to those reported previously (Browning et al., 1999; Martinez-Peña y Valenzuela et al., 2004).
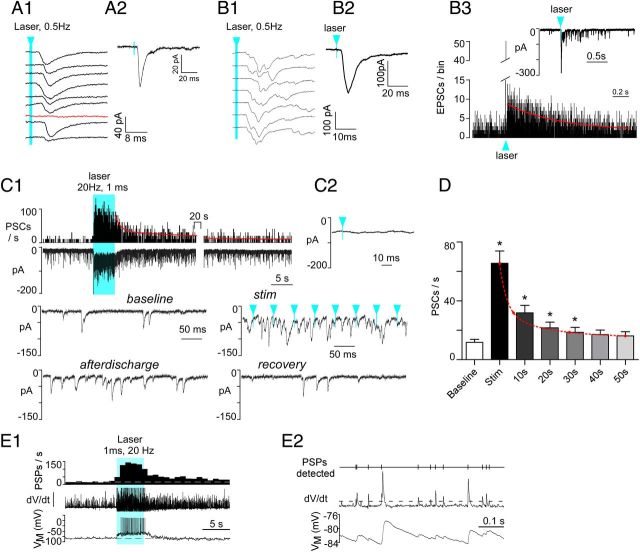
Blue light (473 nm, 5 mW, 1 ms pulses) was delivered by placing a 200-μm-thick optical fiber 300 μm above the dorsal vagal complex. The light pulses had no detectable effect in DMV neurons prepared from naive mice (seven DMV neurons clamped at −79.4 mV). In contrast, the light produced EPSCs (or EPSPs) in 71% of the DMV neurons recorded from AAV2-injected DβH-Cre mice (48 of 68 cells; 21 mice). The electrophysiological effects produced by the light were therefore ChR2 dependent. Low-frequency light pulses (1 ms, 0.5 Hz) evoked a compound, inward PSC in most DMV neurons clamped at a holding potential of −79.4 mV (Fig. 6A1,A2). PSCs were elicited with a latency of 5.5 ± 0.3 ms (23 neurons) from laser onset and had an amplitude of 71.3 ± 17.6 pA (13 neurons). When the PSC was of small amplitude (<60 pA), occasional failures were observed (Fig. 6A1). When the evoked PSC had a larger amplitude (60–120 pA; Fig. 6B1,B2), it was more prominently multiphasic and a PSC after-discharge lasting nearly a full second (decay τ = 0.6 s) was commonly observed (Fig. 6B3).
Figure 6.
Photostimulation of ChR2-labeled axons produces PSCs in DMV neurons and increases their discharge rate. A1, Compound PSC (note variable latency) evoked in a DMV neuron clamped at −79 mV by nine consecutive 1 ms light flashes (473 nm) delivered at 0.5 Hz. The red trace denotes a failure. Blue arrowhead and line indicates time of laser onset throughout figure. A2, Event-triggered average of the PSCs evoked in the cell shown in A1 (75 stimuli). B1, Compound PSC evoked in another DMV neuron (VM −79 mV) by nine consecutive 1 ms light flashes (473 nm) delivered every 2 s. This second example illustrates a larger and more multiphasic response. B2, Event-triggered average of the EPSC evoked in the cell shown in B1 (75 stimuli). B3, The inset shows the EPSC after-discharge that follows the large initial EPSC and the main graph is a peri-event histogram depicting the probability of occurrence of EPSCs following the laser flashes. The red line is a single exponential with a time constant of 0.6 s that fits the kinetics of the after-discharge with a probability of 0.9. C1, Top trace, Integrated rate histogram of the PSC frequency before, during, and after photostimulation (20 Hz, 1 ms, train duration 5 s, laser on time represented by solid blue block). Second trace from top, Original recording obtained from DMV neuron clamped at VM −79 mV. Lower traces, Higher resolution excerpts from the original recording. Note that, during high-frequency photostimulation, the evoked PSCs are asynchronous with respect to the light pulses. Solid blue block indicates period of photostimulation. C2, Event-triggered average of the PSCs evoked by photostimulation (100 light pulses). Note that the trace is flat indicating asynchrony between PSCs and light pulses. D, Group data showing the average kinetics of the after-discharge (N = 16 neurons); *p < 0.001 between group and baseline except 30 s, p < 0.05. E1, Response of a DMV neuron to photostimulation of RVLM-CA neuron input (1 ms, 20 Hz, 5 s). Bottom trace, Original current-clamp recording (−10 pA bias current injected to silence the cell). Middle trace, First differential of original trace to highlight postsynaptic potentials (PSPs). Top trace, PSP frequency (integrated rate histogram, 1 s bin). Period of photostimulation is indicated by solid blue block. E2, High-resolution excerpt illustrating (from the bottom up) the original recording, the first differential used to detect PSPs and analyze their frequency (dotted line, detection threshold) and the detected PSPs.
High-frequency photostimulation (1 ms, 20 Hz, 5 s), produced a very large increase in PSC frequency with a concurrent inward shift in the holding current (Fig. 6C1). Unlike with the single pulse paradigm, the PSCs elicited by train stimulation occurred at random relative to the laser pulses (Fig. 6C1, stim). Accordingly, during train stimulation, stimulus-triggered waveform averages were flat (Fig. 6C2). Train stimulation almost always produced a strong after-discharge of PSCs that took >30 s to return to the prestimulus (baseline) frequency (Fig. 6C1, after-discharge, D). PSCs were binned in 10 s intervals following the end of the photostimulus and between groups differences were determined by nonparametric Friedman's analysis (F = 87.4; p < 0.0001) followed by Dunn's post hoc test for multiple comparisons (Fig. 6D). The decay in PSC frequency following a train of stimuli could be modeled with two exponentials (τfast = 2.3 ± 0.4 s; τslow = 48 ± 18 s, N = 16 neurons).
We also recorded from DMV neurons in whole-cell current-clamp mode to determine whether the excitatory input from RVLM-CA neurons was strong enough to elicit action potentials. On average, DMV neurons had a resting membrane potential of −63 ± 1.5 mV (N = 7) and a baseline-firing rate of 0.8 ± 0.2 Hz. In every recorded cell (N = 8), photostimulation of RVLM-CA terminals using a 5 s train (1 ms, 20 Hz) of laser light bursts produced a massive increase in EPSP frequency and markedly increased the resting discharge rate of the recorded neuron. The DMV neuron illustrated in Figure 6, E1 and E2, was kept just below firing threshold by injection of bias current (−10 pA) and the first differential of the original voltage recording (dV/dt) is shown as a means of better distinguishing the occurrence of postsynaptic potentials. In the seven cells in which no bias current was applied, discharge rate was increased from 0.8 ± 0.3 spikes/s to 3.7 ± 0.8 spikes/s (p < 0.01, paired t test).
Photostimulation of ChR2-expressing RVLM-CA terminals elicits excitatory PSCs exclusively
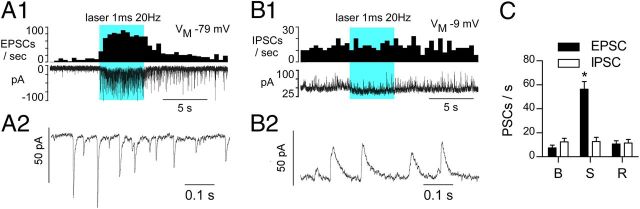
Photostimulation (5 s trains, 1 ms, 5 mW) was performed while the DMV neurons were clamped at two discrete holding potentials to differentiate excitatory, cationic currents (inward; Vm = −79.4 mV) from inhibitory, anionic currents (outward; Vm = −9.4 mV; representative examples in Fig. 7A1,B1). At Vm of −79.4 mV the photostimulus train produced a barrage of inward, short-lasting EPSCs (decay τ = 6.5 ± 0.3 ms; amplitude = 12.7 ± 1.8 pA; N = 8; =. 7A1,A2,C). When the neuron was clamped at Vm= −9.4 mV, longer lasting outward IPSCs (decay τ = 16.5 ± 1.1 ms; amplitude = 20.7 ± 1.5 pA; n = 8) were typically observed during the resting period, but the frequency of these events was unaffected by photostimulation (Fig. 7B1,B2,C). At rest the recorded DMV neurons received an approximately equal frequency of EPSCs and IPSCs (Fig. 7C). Two-way repeated-measures ANOVA followed by Bonferroni post hoc test for multiple comparisons indicated that only EPSC frequency was altered during photostimulation: influence of photostimulation (F(2,32) = 54.43, p < 0.0001), influence of PSC (EPSC vs IPSC; F(1,16) = 8.56, p < 0.01), and interaction of photostimulation and PSC (F(2,32) = 51.2, p < 0.0001).
Figure 7.
Photostimulation of ChR2-expressing axons produces exclusively EPSCs in DMV neurons. A1, DMV neuron voltage-clamped at VM −79 mV (cesium chloride filled electrode). Top trace, EPSC frequency (integrated rate histogram with 0.5 s bin); bottom trace, original recording. Solid blue block represents the period of photostimulation. A2, Excerpt of EPSCs recorded during the early part of the after discharge. B1, Same neuron voltage-clamped at VM −9 mV. Top trace illustrates the lack of change of IPSC frequency during photostimulation; bottom trace is the original recording. B2, Excerpt of the recording trace obtained during the stimulation period. C, Group data (N = 9 neurons) showing inhibitory and excitatory PSC frequency at baseline (B), during the 20 Hz stimulus (S), and after full recovery (R). p < 0.0001.
Thus, based on this sample of eight neurons, photostimulation of the axons of RVLM-CA neurons does not elicit IPSCs in DMV neurons. The absence of detectable mRNA transcripts for glycine transporter 2 or glutamic acid decarboxylase in RVLM-CA neurons in rats agrees with these findings (Stornetta and Guyenet, 1999; Stornetta et al., 2004).
The EPSCs evoked in DMV neurons by stimulating RVLM-CA terminals are glutamatergic
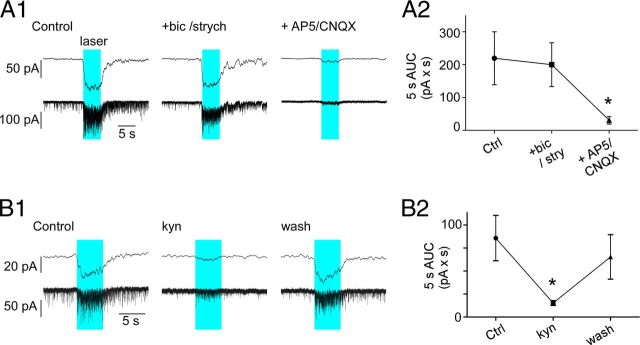
The strong increase in EPSC frequency and associated inward shift in membrane current elicited by trains of photostimuli (1 ms, 20 Hz, 5 s) in DMV neurons clamped at −79.4 mV was unchanged by the addition of bicuculline and strychnine to block GABA and glycine-mediated currents, leaving the total integrated current (area under the curve; AUC) during the stimulus period unchanged (Fig. 8A2). However, addition of the NMDA/AMPA antagonists AP5/CNQX produced near complete blockade of spontaneous EPSCs and EPSCs elicited during the photostimulation (Fig. 8A1,A2). Group data were analyzed with one-way ANOVA for repeated measures (F(2,6) = 7.7; p < 0.05) followed by Student–Newman–Keuls (SNK) post hoc test for multiple comparisons. The AP5/CNQX mixture also greatly attenuated the peak amplitude (93.1 ± 2.6%) and integrated AUC (91.1 ± 2.4%) of the averaged compound EPSC evoked by single pulse photostimulation (1 ms, 0.5 Hz; N = 4; not illustrated). The broad spectrum excitatory amino acid antagonist kynurenic acid (1 mm) reversibly reduced background EPSC activity and also markedly attenuated the inward current generated by the trains of photostimuli (1 ms 20 Hz, 5 s; 82.3 ± 3.9% attenuation; p < 0.01; Fig. 8B1,B2). Like AP5/CNQX, kynurenic acid also significantly reduced the peak amplitude and integrated AUC of the EPSCs elicited by low-frequency stimulation (respectively, 66.2 ± 4.4% and 72.7 ± 4.1% attenuation; p < 0.01; N = 9; not illustrated). Group data for effects of kynurenic acid were analyzed by one-way ANOVA for repeated measures (F(2,22) = 7.8; p < 0.01) followed by SNK post hoc test for multiple comparisons.
Figure 8.
EPSCs evoked in DMV neurons are glutamatergic. A1, Response of a DMV neuron clamped at VM −79 mV to photostimulation (1 ms, 20 Hz, 5 s; blue block represents period of photostimulation). From left to right: control response, response following application of bicuculline (bic, 10 μm), and strychnine (strych, 20 μm) and response after further addition of ionotropic glutamate receptor blockers AP5 (50 μm) and CNQX (10 μm[scap]). Bottom traces, original recordings; top traces lowpass filtered traces (5 Hz). A2, Group data (N = 4). AUC obtained from the original recording trace. Asterisk indicates statistical significance from both control and bicuculline/strychnine. B1, Response of a DMV neuron clamped at VM −79 mV to photostimulation (1 ms, 20 Hz, 5 s). From left to right: control response, response following application of kynurenate (kyn, broad spectrum ionotropic glutamate receptor antagonist, 1 mm). Bottom traces, original recordings; top traces lowpass filtered traces (5 Hz). B2, Group data (N = 7). AUC obtained from the original recording traces. *p < 0.05 indicates statistical significance from both control and recovery period.
Glutamatergic input from RVLM-CA neurons to DMV neurons is monosynaptic
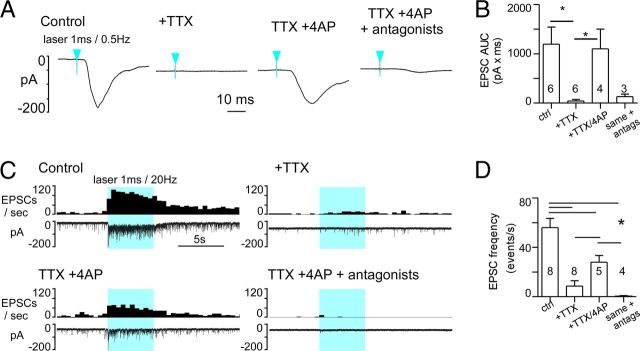
To determine whether the excitatory glutamatergic input from RVLM-CA neurons to the DMV was monosynaptic or polysynaptic, we first blocked voltage-gated sodium (Nav) channels with 1 μm tetrodotoxin (TTX), then we applied 100 μm 4-aminopyridine (4-AP) to block the Kv channels that are critical for the repolarization of the axon (Shu et al., 2007). This protocol eliminates action potential-dependent, therefore potentially polysynaptic events, but enhances the direct depolarization of ChR2-positive terminals and the local release of neurotransmitter during photostimulation (Petreanu et al., 2009). As found by the latter authors, the compound EPSC evoked by single-pulse stimulation (1 ms, 0.5 Hz) was virtually eliminated by TTX (Fig. 9A,B). After coapplication of TTX and 4-AP, a slower, ChR2-dependent EPSC that relied on glutamate transmission was reinstated (Fig. 9A,B). Group data were analyzed by the nonparametric Kruskal–Wallis test (H = 14.3, p < 0.01) followed by Dunn's post hoc test for multiple comparisons. Use of a 5 s train of stimuli (1 ms, 20 Hz) produced similar results (Fig. 9C). Application of TTX alone significantly blunted the overall response to train stimulation. The increase in evoked EPSCs was delayed and only at the very end of the 5 s stimulus period did a small increase in EPSC frequency become apparent. This suggested that TTX imposed a significant slowing of the terminal depolarization during the photostimulus, requiring seconds to reach a “threshold” for Ca2+ entry and vesicular docking. Coapplication of TTX and 4-AP restored the response toward the control value, particularly the rapid increase in EPSCs occurring early in the stimulus train (Fig. 9C). This action potential-independent transmission relied on ionotropic glutamate receptors as blockade with NMDA/AMPA antagonists eliminated the effect of photostimulation (Fig. 9C,D). Group data were analyzed by one-way ANOVA (F(3.21) = 17.8, p < 0.0001) followed by SNK post hoc test for multiple comparisons. The number of repeats per group is unequal due to loss of recorded neurons at different points along the experiments. Significance of comparisons: CNQX/AP5 versus control and TTX versus control, p < 0.001; 4-AP versus control, p < 0.01; CNQX/AP5 versus TTX/4-AP, TTX versus 4AP, p < 0.05; and CNQX/AP5 versus TTX, n.s.
Figure 9.
Photostimulation of ChR2-expressing fibers activate DMV neurons after action potential blockade. A, EPSC evoked in a DMV neuron (VM: −79 mV) by 1 ms light pulses at 0.5 Hz (average of 75 consecutive stimuli) before any drug (left trace), following TTX application (1 μm), after addition of 4-AP (TTX + 4-AP, 100 μm), and in the presence of TTX, 4-AP, and glutamatergic antagonists CNQX and AP5. Blue arrowheads indicate time of laser onset. B, Group data (N = 6 neurons). Asterisks indicate significant differences between groups joined by horizontal bars. C, Experiment illustrating the effect of the same sequence of drug application on the response of a DMV neuron to a train of high-frequency photostimulation (1 ms, 20 Hz, 5 s). Solid blue block indicates period of photostimulation. D, Group data (N = 8). Asterisks indicate significant differences between groups joined by horizontal bars.
Discussion
This study provides the first direct evidence that the C1 cells make excitatory glutamatergic synapses in the adult brain. We also show that these neurons, better known for their contribution to sympathetic tone generation, activate parasympathetic premotor neurons in the dorsal vagal complex.
Monosynaptic glutamatergic input from the C1 cells to the DMV: anatomical evidence
We show here that, within the DMV, the axonal varicosities of the catecholaminergic neurons located in the RVLM contain VGLUT2 immunoreactivity in both rats and mice. Importantly, we show that VGLUT2 immunoreactivity correctly identified the presence of VGLUT2 protein because it was no longer detectable in DβH-CreCre/0; Vglut2flox/flox mice. Few axonal varicosities originating from the presumed A1 noradrenergic cells or from raphe obscurus serotonergic neurons were detectably VGLUT2-ir consistent with the lack of VGLUT2 mRNA in serotonin neurons and relative paucity of VGLUT2 mRNA in A1 neurons (Stornetta et al., 2002). In short, as in the rat, RVLM-CA neurons have a glutamatergic phenotype. Although we were not able to identify PNMT in the RVLM-CA neurons of mice, the CNS projections of these neurons are virtually identical to those of the C1 cells in rats (Card et al., 2006; Stornetta et al., 2012).
The present ultrastructural study shows that RVLM-CA neurons establish conventional synapses with DMV neurons. The predominantly asymmetric morphology of these synapses is consistent with prior ultrastructural studies of PNMT-ir terminals in rats (Milner et al., 1988, 1989). In the forebrain, asymmetric synapses are associated with ionotropic glutamatergic transmission (Sheng and Kim, 2011). The significance of the 25% symmetric synapses between RVLM-CA neurons and DMV neurons is elusive. The postsynaptic thickening could have been missed for lack of serial sectioning. The existence of a subset of RVLM-CA neurons that do not use glutamate as a transmitter is possible.
Monosynaptic input from the C1 cells to the DMV neurons: electrophysiological evidence
Photostimulation of slices from control mice had no effect on DMV neurons and photostimulation had no effect in 30% of DMV neurons in the experimental mice. The electrophysiological effects produced by photostimulation therefore required expression of ChR2.
Although the recorded DMV neurons received on average an approximately equal frequency of spontaneous inhibitory and excitatory PSCs, photostimulation of ChR2-expressing fibers evoked EPSCs exclusively. These EPSCs were unaffected by adding glycinergic and GABAA receptor blockers but were greatly attenuated by administration of blockers of ionotropic glutamate receptors such as kynurenate or a mixture of CNQX and AP5. This evidence indicates that the PSCs evoked by photostimulating the C1 axons were mediated primarily by glutamate.
Consistent with the ultrastructural results, the glutamatergic input from the C1 cells to the DMV is monosynaptic since EPSCs could still be evoked by photostimulating the ChR2-labeled terminal field when fast sodium channels were blocked by TTX and these EPSCs reappeared after addition of 4-AP, a potassium blocker that prolongs the depolarization of the terminals and reinstates vesicular release (Petreanu et al., 2009). Finally the reinstated PSCs were blocked by the combination of CNQX and AP5 demonstrating their glutamatergic nature.
In summary, photostimulation of the axonal processes of RVLM-CA neurons elicited EPSCs in a substantial proportion of DMV PGNs. These EPSCs were monosynaptic and glutamatergic. Fast evoked IPSCs were not found, consistent with the absence of GAD or GlyT2 mRNA in RVLM-CA neurons (Stornetta and Guyenet, 1999; Stornetta et al., 2004).
Is the release of glutamate by the C1 neurons an injury response caused by the AAV?
Cultured dopaminergic neurons make glutamatergic synapses (Sulzer et al., 1998) and, based on evidence similar to ours, others (Stuber et al., 2010; Tecuapetla et al., 2010) have proposed that the dopaminergic neurons that innervate nucleus accumbens also use glutamate as an excitatory transmitter in adult mice. Consistent with this evidence, VGLUT2 mRNA is present in TH-ir neurons within the A10 region (Yamaguchi et al., 2011). Yet, careful ultrastructural studies have failed to detect the presence of VGLUT2 in the dopaminergic varicosities of adult mice (Moss et al., 2011). These negative anatomical findings could mean that glutamate and dopamine are stored and released by separate varicosities (Broussard, 2012). A more disturbing possibility would be that adeno-associated vectors somehow injure adult dopaminergic neurons and reinstate a glutamatergic phenotype that these cells only express early during development or in culture (Bérubé-Carrière et al., 2009). A similar explanation seems highly unlikely in the case of the C1 neurons for the following reasons. First, the AAV2 did not induce VGLUT2 in noradrenergic (A1) or serotonergic neurons. We did find a very low percentage of mCherry terminals that appeared to contain VGLUT2 in these control experiments in mice, but this percentage was the same regardless of whether the transgene was expressed by A1 or serotonergic cells, therefore a technical artifact is the more likely explanation. Second, in the absence of AAV2, most rat C1 cells contain VGLUT2 mRNA (Stornetta et al., 2002). Third, we did observe VGLUT2 and TH-ir doubly labeled terminals that were not transduced with mCherry in the DMV of the DBH-Cre RVLM-injected mice. Fourth, we showed that, in TH-Cre rats, the terminals of the C1 neurons, identified within the DMV by PNMT immunohistochemistry, were VGLUT2-ir regardless of whether or not they arose from C1 neurons that were AAV2 transfected. Fifth, unlike the mesencephalic dopaminergic neurons (Bérubé-Carrière et al., 2012), the C1 neurons commonly make conventional synapses with thickened postsynaptic densities reminiscent of glutamatergic excitatory synapses in rats (Milner et al., 1988, 1989) and in mice (present study). Finally, the notion that the C1 neurons use glutamate as one of their transmitters is consistent with numerous pharmacological observations in vivo (Morrison et al., 1989; Abbott et al., 2012). In sum, we found no evidence suggesting that AAV2 induces the synthesis of VGLUT2 in the C1 cells or in medullary noradrenaline or serotonergic neurons. Our conclusion is that the C1 neurons of adult rodents normally express this transporter and use glutamate as an ionotropic transmitter, at least in the DMV.
Do the C1 neurons release catecholamines?
The C1 neurons presumably also release catecholamines inclusive of adrenaline since they express all the enzymes necessary for the synthesis and vesicular storage of these bioamines (Armstrong et al., 1982; Sevigny et al., 2008), but supportive neurophysiological evidence is lacking and the present experiments were not designed to explore this aspect of C1 neuron biology. We did observe dense core vesicles in the more lightly labeled synaptic boutons originating from the C1 cells but dense core vesicles also store peptides, which are plentiful in C1 cells (Chalmers et al., 1987; Halliday et al., 1988; Stornetta et al., 1999; Farnham et al., 2008). The C1 neurons express on their soma α2-adrenergic receptors that are negatively coupled to calcium and potassium conductances (Li et al., 1995, 1998). These autoreceptors may mediate autoinhibition or autocrine regulations analogous to those found in non-neural cells such as the endothelium and macrophages (Engler et al., 2005; Sorriento et al., 2012). The catecholamines released by the C1 cells could also activate presynaptic autoreceptors like the CNS noradrenergic neurons (Trendelenburg et al., 2003). Adrenaline is a β2-adrenoceptor-selective agent that facilitates transmitter overflow in sympathetic neurons and could conceivably exert a similar effect in C1 cells (Stjärne and Brundin, 1976).
In sum, our study demonstrates the existence of a strong glutamatergic component to synaptic transmission between the C1 cells and DMV neurons. The circumstances under which C1 neurons release catecholamines and the contribution of these substances to C1 neuron signaling remain to be determined.
Physiological implications of the innervation of DMV neurons by the C1 cells
The contribution of the C1 cells to blood pressure and sympathetic tone regulation is well established. It is partly the result of the massive projections of these neurons to the intermediolateral cell column and lamina X and likely involves glutamate signaling (Ross et al., 1983, 1984; Guyenet, 2006). C1 neurons also regulate adrenaline release by the adrenal glands (Ritter et al., 1998, 2001; Li et al., 2009) and subsets of C1 neurons innervate the paraventricular nucleus of the hypothalamus and regulate the CRF-ACTH-corticosterone cascade (Sawchenko et al., 2000).
The present study indicates that the DMV is another synaptic target of the C1 neurons. DMV neurons regulate liver metabolism, gastrointestinal function including motility and secretions, immune responses, and probably cardiac function (Standish et al., 1995; Rogers et al., 1999; Olofsson et al., 2012). A broad spectrum of these parasympathetic efferents is likely to be targeted by a subset of C1 cells given that two-thirds of randomly sampled DMV neurons responded to photostimulation of their axons.
Given that C1 cells are activated by various stressors (Pirnik et al., 2004) and have been implicated in the cardiovascular response to stress (Chen et al., 2012), the C1 neurons, via their synaptic inputs to the DMV, may also be at least partially responsible for the negative digestive and immunological consequences of stress.
Footnotes
This work was supported by National Institutes of Health grants HL28785 and HL74011 (P.G.G.), and 1F32HL096280-01 (S.D.D.).
References
- Abbott SB, Stornetta RL, Socolovsky CS, West GH, Guyenet PG. Photostimulation of channelrhodopsin-2 expressing ventrolateral medullary neurons increases sympathetic nerve activity and blood pressure in rats. J Physiol. 2009;587:5613–5631. doi: 10.1113/jphysiol.2009.177535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott SB, Kanbar R, Bochorishvili G, Coates MB, Stornetta RL, Guyenet PG. C1 neurons excite locus coeruleus and A5 noradrenergic neurons along with sympathetic outflow in rats. J Physiol. 2012;590:2897–2915. doi: 10.1113/jphysiol.2012.232157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Ross CA, Pickel VM, Joh TH, Reis DJ. Distribution of dopamine-, noradrenaline-, and adrenaline- containing cell bodies in the rat medulla oblongata: demonstrated by the immunocytochemical localization of catecholamine biosynthetic enzymes. J Comp Neurol. 1982;212:173–187. doi: 10.1002/cne.902120207. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bérubé-Carrière N, Riad M, Dal BG, Lévesque D, Trudeau LE, Descarries L. The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J Comp Neurol. 2009;517:873–891. doi: 10.1002/cne.22194. [DOI] [PubMed] [Google Scholar]
- Bérubé-Carrière N, Guay G, Fortin GM, Kullander K, Olson L, Wallén-Mackenzie A, Trudeau LE, Descarries L. Ultrastructural characterization of the mesostriatal dopamine innervation in mice, including two mouse lines of conditional VGLUT2 knockout in dopamine neurons. Eur J Neurosci. 2012;35:527–538. doi: 10.1111/j.1460-9568.2012.07992.x. [DOI] [PubMed] [Google Scholar]
- Bochorishvili G, Stornetta RL, Coates MB, Guyenet PG. Pre-Botzinger complex receives glutamatergic innervation from galaninergic and other retrotrapezoid nucleus neurons. J Comp Neurol. 2012;520:1047–1061. doi: 10.1002/cne.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard JI. Co-transmission of dopamine and glutamate. J Gen Physiol. 2012;139:93–96. doi: 10.1085/jgp.201110659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J Physiol. 1999;517:521–532. doi: 10.1111/j.1469-7793.1999.0521t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: implications for the central control of cardiovascular regulation. J Comp Neurol. 2006;499:840–859. doi: 10.1002/cne.21140. [DOI] [PubMed] [Google Scholar]
- Chalmers J, Kapoor V, Mills E, Minson J, Morris M, Pilowsky P, West M. Do pressor neurons in the ventrolateral medulla release amines and neuropeptides? Can J Physiol Pharmacol. 1987;65:1598–1604. doi: 10.1139/y87-251. [DOI] [PubMed] [Google Scholar]
- Chen D, Jancovski N, Bassi JK, Nguyen-Huu TP, Choong YT, Palma-Rigo K, Davern PJ, Gurley SB, Thomas WG, Head GA, Allen AM. Angiotensin type 1A receptors in C1 neurons of the rostral ventrolateral medulla modulate the pressor response to aversive stress 21. J Neurosci. 2012;32:2051–2061. doi: 10.1523/JNEUROSCI.5360-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer AM, Qi J, Christie DL, Gibbons HM, Lipski J. Noradrenaline transporter expression in the pons and medulla oblongata of the rat: localisation to noradreneric and some C1 adrenergic neurones. Mol Brain Res. 1998;62:65–76. doi: 10.1016/s0169-328x(98)00241-1. [DOI] [PubMed] [Google Scholar]
- Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci. 2011;31:1981–1990. doi: 10.1523/JNEUROSCI.4639-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler KL, Rudd ML, Ryan JJ, Stewart JK, Fischer-Stenger K. Autocrine actions of macrophage-derived catecholamines on interleukin-1 beta 5. J Neuroimmunol. 2005;160:87–91. doi: 10.1016/j.jneuroim.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Farnham MM, Li Q, Goodchild AK, Pilowsky PM. PACAP is expressed in sympathoexcitatory bulbospinal C1 neurons of the brain stem and increases sympathetic nerve activity in vivo. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1304–R1311. doi: 10.1152/ajpregu.00753.2007. [DOI] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Li YW, Joh TH, Cotton RG, Howe PR, Geffen LB, Blessing WW. Distribution of substance P-like immunoreactive neurons in the human medulla oblongata: co-localization with monoamine- synthesizing neurons. Synapse. 1988;2:353–370. doi: 10.1002/syn.890020403. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Fuxe K, Goldstein M, Johansson O. Immunohistochemical evidence for the existence of adrenaline neurons in the rat brain. Brain Res. 1974;66:235–251. [Google Scholar]
- Li AJ, Wang Q, Dinh TT, Ritter S. Simultaneous silencing of Npy and Dbh expression in hindbrain A1/C1 catecholamine cells suppresses glucoprivic feeding. J Neurosci. 2009;29:280–287. doi: 10.1523/JNEUROSCI.4267-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YW, Bayliss DA, Guyenet PG. C1 neurons of neonatal rats: intrinsic beating properties and α2-adrenergic receptors. Am J Physiol. 1995;269:R1356–R1369. doi: 10.1152/ajpregu.1995.269.6.R1356. [DOI] [PubMed] [Google Scholar]
- Li YW, Guyenet PG, Bayliss DA. Voltage-dependent calcium currents in bulbospinal neurons of neonatal rat rostral ventrolateral medulla: modulation by alpha2-adrenergic receptors. J Neurophysiol. 1998;79:583–594. doi: 10.1152/jn.1998.79.2.583. [DOI] [PubMed] [Google Scholar]
- Lorang D, Amara SG, Simerly RB. Cell-type-specific expression of catecholamine transporters in the rat brain. J Neurosci. 1994;14:4903–4914. doi: 10.1523/JNEUROSCI.14-08-04903.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N, Abdala AP, Korsak A, Simms AE, Allen AM, Paton JF, Gourine AV. Control of sympathetic vasomotor tone by catecholaminergic C1 neurones of the rostral ventrolateral medulla oblongata. Cardiovasc Res. 2011;91:703–710. doi: 10.1093/cvr/cvr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Peña y Valenzuela I, Rogers RC, Hermann GE, Travagli RA. Norepinephrine effects on identified neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol. 2004;286:G333–G339. doi: 10.1152/ajpgi.00289.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Morrison SF, Abate C, Reis DJ. Phenylethanolamine N-methyltransferase-containing terminals synapse directly on sympathetic preganglionic neurons in the rat. Brain Res. 1988;448:205–222. doi: 10.1016/0006-8993(88)91258-9. [DOI] [PubMed] [Google Scholar]
- Milner TA, Abate C, Reis DJ, Pickel VM. Ultrastructural localization of phenylethanolamine N- methyltransferase-like immunoreactivity in the rat locus coeruleus. Brain Res. 1989;478:1–15. doi: 10.1016/0006-8993(89)91471-6. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Ernsberger P, Milner TA, Callaway J, Gong A, Reis DJ. A glutamate mechanism in the intermediolateral nucleus mediates sympathoexcitatory responses to stimulation of the rostral ventrolateral medulla. Prog Brain Res. 1989;81:159–169. doi: 10.1016/s0079-6123(08)62006-3. [DOI] [PubMed] [Google Scholar]
- Moss J, Ungless MA, Bolam JP. Dopaminergic axons in different divisions of the adult rat striatal complex do not express vesicular glutamate transporters. Eur J Neurosci. 2011;33:1205–1211. doi: 10.1111/j.1460-9568.2011.07594.x. [DOI] [PubMed] [Google Scholar]
- Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev. 2012;248:188–204. doi: 10.1111/j.1600-065X.2012.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JK, Goodchild AK, Dubey R, Sesiashvili E, Takeda M, Chalmers J, Pilowsky PM, Lipski J. Differential expression of catecholamine biosynthetic enzymes in the rat ventrolateral medulla. J Comp Neurol. 2001;432:20–34. doi: 10.1002/cne.1086. [DOI] [PubMed] [Google Scholar]
- Pirnik Z, Mravec B, Kubovcakova L, Mikkelsen JD, Kiss A. Hypertonic saline and immobilization induce Fos expression in mouse brain catecholaminergic cell groups: colocalization with tyrosine hydroxylase and neuropeptide Y. Ann N Y Acad Sci. 2004;1018:398–404. doi: 10.1196/annals.1296.049. [DOI] [PubMed] [Google Scholar]
- Ritter S, Llewellyn-Smith I, Dinh TT. Subgroups of hindbrain catecholamine neurons are selectively activated by 2-deoxy-D-glucose induced metabolic challenge. Brain Res. 1998;805:41–54. doi: 10.1016/s0006-8993(98)00655-6. [DOI] [PubMed] [Google Scholar]
- Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol. 2001;432:197–216. doi: 10.1002/cne.1097. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE, Travagli RA. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J Physiol. 1999;514:369–383. doi: 10.1111/j.1469-7793.1999.369ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Joh TH, Park DH, Reis DJ. Adrenaline synthesizing neurons in the rostral ventrolateral medulla: a possible role in tonic vasomotor control. Brain Res. 1983;273:356–361. doi: 10.1016/0006-8993(83)90862-4. [DOI] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Joh TH, Park DH, Reis DJ. Rostral ventrolateral medulla: selective projections to the thoracic autonomic cell column from the region containing C1 adrenaline neurons. J Comp Neurol. 1984;228:168–185. doi: 10.1002/cne.902280204. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci U S A. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny CP, Bassi J, Teschemacher AG, Kim KS, Williams DA, Anderson CR, Allen AM. C1 neurons in the rat rostral ventrolateral medulla differentially express vesicular monoamine transporter 2 in soma and axonal compartments. Eur J Neurosci. 2008;28:1536–1544. doi: 10.1111/j.1460-9568.2008.06445.x. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim E. The postsynaptic organization of synapses. Cold Spring Harb Perspect Biol. 2011 doi: 10.1101/cshperspect.a005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Yu Y, Yang J, McCormick DA. Selective control of cortical axonal spikes by a slowly inactivating K+ current. Proc Natl Acad Sci U S A. 2007;104:11453–11458. doi: 10.1073/pnas.0702041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorriento D, Santulli G, Del Giudice C, Anastasio A, Trimarco B, Iaccarino G. Endothelial cells are able to synthesize and release catecholamines both in vitro and in vivo. Hypertension. 2012;60:129–136. doi: 10.1161/HYPERTENSIONAHA.111.189605. [DOI] [PubMed] [Google Scholar]
- Standish A, Enquist LW, Escardo JA, Schwaber JS. Central neuronal circuit innervating the rat heart defined by transneuronal transport of pseudorabies virus. J Neurosci. 1995;15:1998–2012. doi: 10.1523/JNEUROSCI.15-03-01998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjärne L, Brundin J. Beta2-adrenoceptors facilitating noradrenaline secretion from human vasoconstrictor nerves. Acta Physiol Scand. 1976;97:88–93. doi: 10.1111/j.1748-1716.1976.tb10238.x. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Guyenet PG. Distribution of glutamic acid decarboxylase mRNA-containing neurons in rat medulla projecting to thoracic spinal cord in relation to monoaminergic brainstem neurons. J Comp Neurol. 1999;407:367–380. [PubMed] [Google Scholar]
- Stornetta RL, Akey PJ, Guyenet PG. Location and electrophysiological characterization of rostral medullary adrenergic neurons that contain neuropeptide Y mRNA in rat. J Comp Neurol. 1999;415:482–500. doi: 10.1002/(sici)1096-9861(19991227)415:4<482::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J Comp Neurol. 2002;444:191–206. doi: 10.1002/cne.10141. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, McQuiston TJ, Guyenet PG. GABAergic and glycinergic presympathetic neurons of rat medulla oblongata identified by retrograde transport of pseudorabies virus and in situ hybridization. J Comp Neurol. 2004;479:257–270. doi: 10.1002/cne.20332. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Macon CJ, Nguyen TM, Coates MB, Guyenet PG. Cholinergic neurons in the mouse rostral ventrolateral medulla target sensory afferent areas. Brain Struct Funct. 2012 doi: 10.1007/s00429-012-0408-3. Advance online publication. Retrieved January 2, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattori T, Rayport S. Dopamine neurons make glutamatergic synapses in vitro. J Neurosci. 1998;18:4588–4602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sved AF. PNMT-containing catecholaminergic neurons are not necessarily adrenergic. Brain Res. 1989;481:113–118. doi: 10.1016/0006-8993(89)90490-3. [DOI] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg AU, Philipp M, Meyer A, Klebroff W, Hein L, Starke K. All three α2-adrenoceptor types serve as autoreceptors in postganglionic sympathetic neurons. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:504–512. doi: 10.1007/s00210-003-0829-x. [DOI] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, Stuber GD, Tye KM, Janak PH, Deisseroth K. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. J Neurosci. 2011;31:8476–8490. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]