Abstract
Neonatal fungal endocarditis is a rare but serious infection, which does not have a well-accepted management method. This is the second report of this condition in Saudi Arabia. A preterm, very low birth weight, female neonate presented with fever and shortness of breath. An echocardiogram showed moderate pericardial effusion and two masses in the heart, one in the right ventricle and the other in the inferior portion of the posterior mitral valve of the left ventricle. Blood and pericardial fluid cultures revealed an infection with Candida albicans. The patient received a 60 days course of intravenous fluconazole and amphotericin B lipid complex. At the conclusion of treatment, she was discharged in good condition with no echocardiographic evidence of pericardial effusion or fungal vegetations. Thus, a successful outcome to a serious case of fungal endocarditis was achieved through aggressive antifungal therapy with intravenous fluconazole and amphotericin B lipid complex.
Abbreviations: FE, fungal endocarditis; IE, infective endocarditis; NICU, neonatal intensive care unit; PICU, pediatric intensive care unit; C. albicans, Candida albicans; CRP, C-reactive protein
Keywords: Fungal endocarditis, Infective endocarditis, Candidemia, Candida albicans, Amphotericin B, Fluconazole
Case report
A 14-day-old female patient presented to the King Abdulaziz University Hospital Pediatric Emergency facility with a high-grade fever, progressive shortness of breath, and a dry cough. The patient also had associated decreases in oral intake and activity levels. The premature infant was born at a gestational age of 32 weeks and delivered by emergency cesarean section to a primigravida mother who had been diagnosed with severe pre-eclampsia. Born with a very low birth weight of 1400 g, the infant was admitted to the neonatal intensive care unit (NICU) for 10 days, at a different hospital. During this period, she did not require assisted ventilation, but did receive intravenous ampicillin and gentamicin for 5 days. The patient was discharged, against medical advice, due to the family’s financial situation. The infant was asymptomatic for 2 days prior to presentation.
Upon admission to our hospital, the patient’s initial vital scores revealed a core temperature of 38.1 °C (100.6 °F), a heart rate of 168 beats per minute, a respiratory rate of 76 per minute, blood pressure of 90/55 mmHg, a mean arterial pressure of 66 mmHg, and an oxygen saturation of 94%, on room air. She was irritable and in moderate respiratory distress, the first and second heart sounds were normal, and a cardiac murmur was not detected. A thorough physical examination did not reveal any other abnormalities.
The patient was admitted to the pediatric medical ward with a provisional diagnosis of neonatal sepsis. Intravenous fluids and broad-spectrum intravenous antibiotics, ampicillin and cefotaxime, were initiated following a full septic screen. Investigations revealed a total leukocytic count of 37 × 103 leukocytes/mm3, a hemoglobin level of 12.9 g/dL, and a platelet count of 215 × 103 platelets/mm3. Urea, electrolytes, coagulation profile, liver function tests, and blood gases were within normal ranges. The patient’s C-reactive protein (CRP) level was high, at 202 mg/L. A lumbar puncture was performed and cerebrospinal fluid biochemistry and cell counts were unremarkable. Cerebrospinal fluid and urine were cultured on test media, but microbiological growth was not detected. Chest X-rays showed significant enlargement of the cardiac shadow with clear lung fields. Two masses were revealed by echocardiogram, one was located in the left ventricle attached to the posterior mitral valve, measuring 15 × 8 mm, and the other was in the trabeculations of the right ventricle, measuring 10 × 6 mm (Fig. 1). There was trivial mitral regurgitation and moderate pericardial effusion with evidence of a thickened pericardium.
Figure 1.
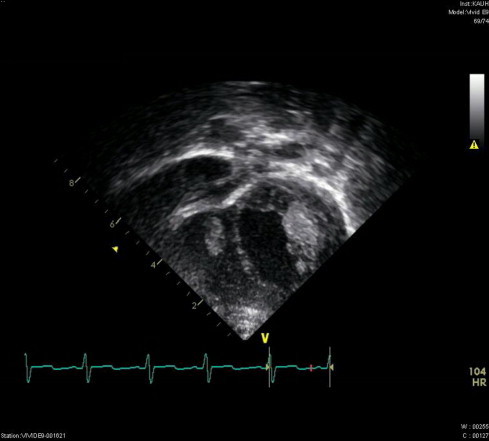
Vegetative masses in the inferior portion of the left and right ventricles. The moderate size of the pericardial effusion is also evident.
The patient was shifted to the pediatric intensive care unit (PICU) and furosemide and amphotericin B were administered. Pericardiocentesis, under echocardiographic guidance, was performed, withdrawing 15 cc of clear fluid. Pericardial fluid culture revealed multi-sensitive Candida albicans. The pericardial cell count revealed a white blood cell count of 18 × 103/μL and a red blood cell count of 6150/μL. The patient’s blood culture was also positive for C. albicans. Ampicillin and cefotaxime were discontinued and were replaced with intravenous fluconazole (6 mg/kg/day) and amphotericin B lipid complex (10 mg/kg/day). Computed tomography of the brain and ultrasonography of the kidneys excluded any seeding in those organs. After 4 days in the PICU, a follow-up echocardiogram was performed, which showed a decrease in the pericardial effusion and in the size of the masses.
The patient was moved back to the pediatric medical ward. Three additional follow-up echocardiograms were undertaken, approximately 10 days apart. These examinations all showed the absence of pericardial effusion and decreasing sizes of the vegetations. An echocardiogram, conducted 42 days after the diagnosis, was devoid of any abnormalities (Fig. 2), repeated blood cultures were negative for C. albicans, the CRP level had decreased to 3.41 mg/L, and leukocytic count decreased to 9 × 103 leukocytes/mm3. Antifungal therapy occurred over a total of 8 weeks.
Figure 2.
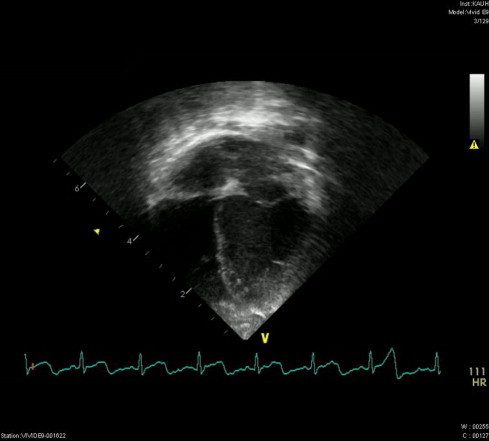
Resolution of the vegetative masses following 8 weeks of treatment.
The patient was discharged, after improving clinically, and has had three follow-up echocardiograms, performed at 2, 12, and 22 weeks after discharge. Over the course of these visits, there has not been any clinical or echocardiographic evidence of a recurrence; the discharge plan included frequent follow-up visits to the clinic for a minimum of 1 year after discharge.
The patient’ mother provided written informed consent for the publication of this case report and the accompanying images.
Discussion
Infective endocarditis (IE) is a serious, but relatively rare infection that is associated with a high degree of morbidity and mortality. The disease has an incidence of 0.8–3.3 per 1000 hospital pediatric admissions [1]. Fungal endocarditis (FE) is reported less frequently than bacterial endocarditis, with an incidence, in children, of 0–12% of the total pediatric IE admissions. Over the past few years, there has been an increasing number of documented reports of IE and, more particularly, FE [1–2]. The increased incidence of FE is more evident among infants and is diagnosed in up to 20% of the IE cases in this population [2–3]; over 60% of FE cases occur in infants less than 1-year-old [1]. This is only the second reported case in Saudi Arabia, which may suggest that the disease is either under-reported or misdiagnosed.
In neonates, FE rarely affects the native heart valves and occurs most frequently as part of a disseminated fungal infection. Risk factors for developing FE include a prolonged stay in the NICU, prolonged use of intravenous antibiotics, cardiac surgery, and the presence of an indwelling central venous catheter. The most frequent complications associated with FE are myocarditis, valve perforation, and sepsis with involvement of the brain and kidneys. In neonates, both incidence and outcome are negatively correlated to the gestational age and the birth weight [4].
Candida species are the most common causative agents of FE in infants (87%), with 42% of cases resulting from C. albicans infection. In premature infants, Candida spp. account for 64% of cases and Aspergillus spp. account for 21% [1]. FE is often difficult to diagnose, particularly in neonates, because the symptoms may be nonspecific, as seen in this case report. The disease typically occurs in otherwise critically ill patients and is often part of a confusing clinical picture, with most patients having difficulty meeting the Duke criteria for IE [5].
In the present case, the diagnosis was made within 2 days of the patient’s admission as a result, in part, of the availability of a pediatric cardiology division within the facility.
Although a consensus approach for the appropriate management of FE in neonates is lacking, a combined medical and surgical approach has been used for treating infants who have ultimately survived candidal endocarditis. Several factors should be considered in choosing the treatment modality; these include the perioperative mortality (if surgical intervention is being considered), the severity of the neonate’s illness, the side effects of the antifungal, the hemodynamic status of the patient, and the presence of any blood flow obstructions [3,6]. In the present case, surgical intervention to remove the vegetative masses was decided against because the vegetations were not causing blood flow obstructions or hemodynamic instability, and the risk of perioperative mortality was high. In addition, the likelihood of successful antifungal chemotherapy was expected to be high since the isolated species of fungus was sensitive to multiple antifungal chemotherapeutics.
Numerous antifungal agents have been used in open-label trials in neonates and children with candidemia or invasive candidiasis. The most frequently used treatment end-point in these trials has been microbiologic clearance, which provided another level of confidence in the decision to treat the present case of FE with the chosen chemotherapeutic agents. Among the antifungal agents used to treat FE, amphotericin B and its lipid preparations, [7–8], azoles [9] and caspofungin [10] have all been shown to have varying degrees of effectiveness in neonates with candidal infections. Clearance rates of 83–100% have been reported for the lipid preparations of amphotericin B, 72–97% for fluconazole, 81% for itraconazole, 85–100% for caspofungin, and 72% for micafungin [10–11].
In the present case, recognition of the two cardiac masses, which were more likely fungal balls than rhabdomyomas (based on the patient’s clinical condition), amphotericin B was started immediately, before the culture results were available. After blood and pericardial fluid cultures revealed C. albicans, amphotericin B was shifted to its lipid form and fluconazole was added to the treatment combination and administered for 8 weeks. Repeated blood cultures showed no evidence of fungal infection 6 weeks after the initiation of the antifungals. Amphotericin B lipid complex and fluconazole were continued for an additional 2 weeks, as recommended by the Clinical Practice Guidelines for the Management of Candidiasis [12].
Conclusions
FE in neonates is a rare, serious disease that has a poor prognosis, if not treated properly. Interestingly, this disease is encountered with increasing frequency in today’s highly advanced medical practices, globally. Recognizing FE early is challenging due to its nonspecific symptoms, but with a high index of suspicion and understanding of the predisposing factors, an accurate diagnosis can be made. Management with antifungal medications can be an adequate choice, even in critically ill patients.
Disclosure
The author does not have any competing interests.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Millar B.C., Jugo J., Moore J.E. Fungal endocarditis in neonates and children. Pediatr Cardiol. 2005;26:517–536. doi: 10.1007/s00246-004-0831-1. [DOI] [PubMed] [Google Scholar]
- 2.Tissières P., Jaeggi E.T., Beghetti M., Gervaix A. Increase of fungal endocarditis in children. Infection. 2005;33:267–272. doi: 10.1007/s15010-005-4122-4. [DOI] [PubMed] [Google Scholar]
- 3.Varghese G.M., Sobel J.D. Fungal endocarditis. Curr Infect Dis Rep. 2008;10:275–279. doi: 10.1007/s11908-008-0045-4. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin D.K.J., DeLong E.R., Steinbach W.J., Cotton C.M., Walsh T.J., Clark R.H. Empirical therapy for neonatal candidemia in very low birth weight infants. Pediatrics. 2003;112:543–547. doi: 10.1542/peds.112.3.543. [DOI] [PubMed] [Google Scholar]
- 5.Li J.S., Sextorn D.J., Mick N. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 6.Citak M., Rees A., Mavroudis C. Surgical management of infective endocarditis in children. Ann Thorac Surg. 1992;54:755–760. doi: 10.1016/0003-4975(92)91023-3. [DOI] [PubMed] [Google Scholar]
- 7.Cetin H., Yalaz M., Akisu M., Hilmioglu S., Metin D., Kultursay N. The efficacy of two different lipid-based amphotericin B in neonatal Candida septicemia. Pediatr Int. 2005;47:676–680. doi: 10.1111/j.1442-200x.2005.02135.x. [DOI] [PubMed] [Google Scholar]
- 8.Linder N., Klinger G., Shalit I., Levy I., Ashkenazi S., Haski G. Treatment of candidaemia in premature infants: comparison of three amphotericin B preparations. J Antimicrob Chemother. 2003;52:663–667. doi: 10.1093/jac/dkg419. [DOI] [PubMed] [Google Scholar]
- 9.Schwarze R., Penk A., Pittrow L. Treatment of candidal infections with fluconazole in neonates and infants. Eur J Med Res. 2000;5:203–208. [PubMed] [Google Scholar]
- 10.Odio C.M., Araya R., Pinto L.E., Castro C.E., Vasquez S., Alfaro B. Caspofungin therapy of neonates with invasive candidiasis. Pediatr Infect Dis J. 2004;23:1093–1097. [PubMed] [Google Scholar]
- 11.Wiley J.M., Seibel N.L., Walsh T.J. Efficacy and safety of amphotericin B lipid complex in 548 children and adolescents with invasive fungal infections. Pediatr Infect Dis J. 2005;24:167–174. doi: 10.1097/01.inf.0000153183.51258.b8. [DOI] [PubMed] [Google Scholar]
- 12.Pappas P.G., Kauffman C.A., Andes D., Benjamin D.K., Jr, Calandra T.F., Edwards J.E., Jr Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]



