Abstract
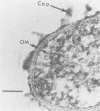
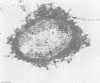
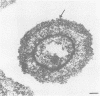
The etiological agent of the sexually transmitted genital ulcer disease chancroid was first described in 1889 by Auguste Ducrey following repeated autoinoculation of purulent ulcer material from a series of patients. The organism was isolated on artificial media a decade later but has remained difficult to isolate consistently, resulting in controversy over its characteristics and role as the causative agent of chancroid. Because of its fastidious growth requirements, including unknown components in blood, the organism was included in the original description of the genus Haemophilus. Requirement for exogenous hemin and limited phenotypic characteristics, including structural and antigenic properties, suggested that Haemophilus ducreyi was a valid member of the genus Haemophilus. Recent studies of respiratory quinones, deoxyribonucleic acid hybridization, and competition for homologous transformation of the type species, H. influenzae, suggest that H. ducreyi is unrelated to any of the present species of the family Pasteurellaceae, which includes members of the genera Haemophilus, Actinobacillus, and Pasteurella. This review summarizes the early studies with H. ducreyi and our current knowledge of the microbiology of this important human pathogen.
Full text
PDF
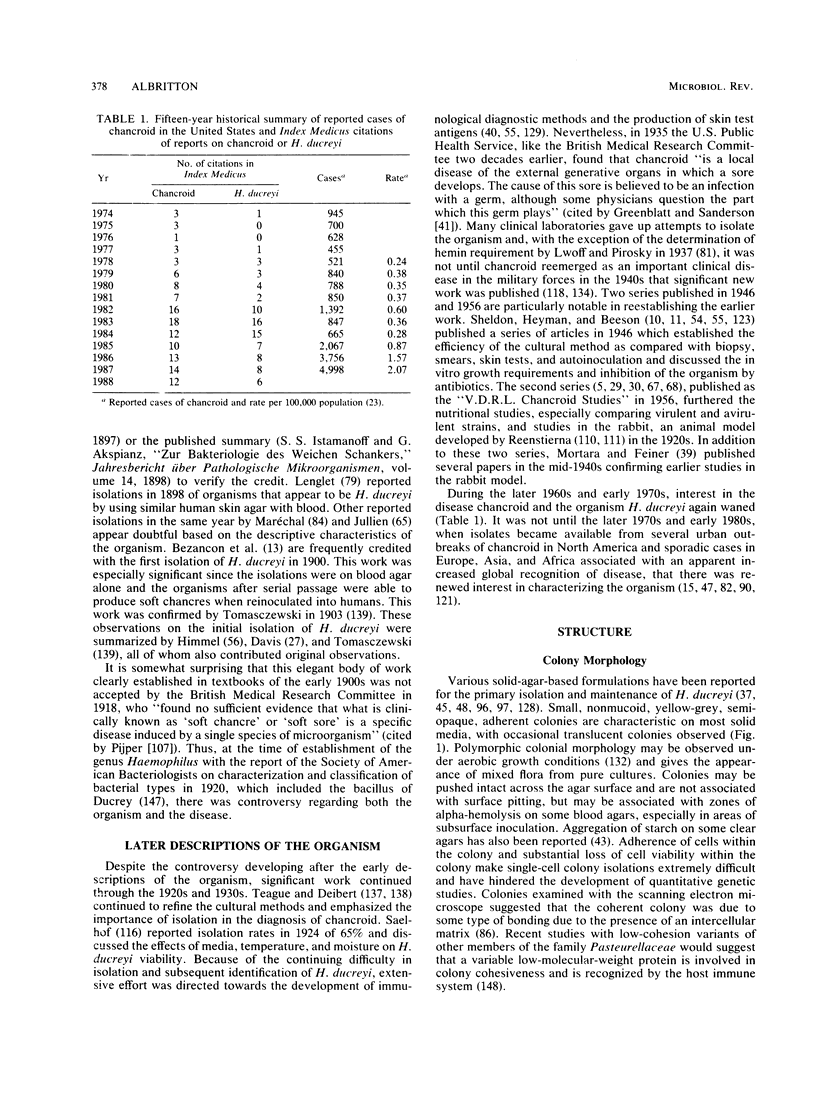





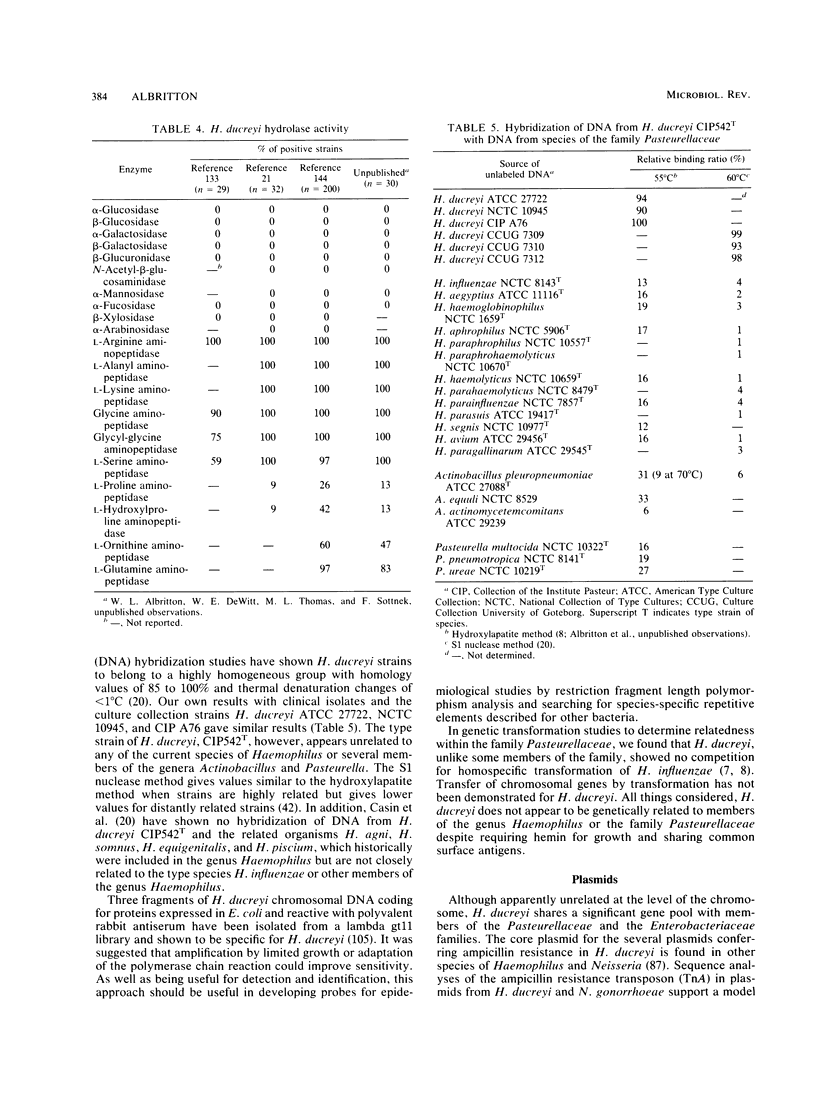





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AJELLO G. W., DEACON W. E., PAUL L., WALLS K. W. Nutritional studies of a virulent strain of Haemophilus ducreyi. J Bacteriol. 1956 Dec;72(6):802–808. doi: 10.1128/jb.72.6.802-808.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeck D., Johnson A. P., Taylor-Robinson D. Antigenic analysis of Haemophilus ducreyi by Western blotting. Epidemiol Infect. 1988 Aug;101(1):151–157. doi: 10.1017/s0950268800029319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albritton W. L., Setlow J. K., Thomas M., Sottnek F., Steigerwalt A. G. Heterospecific transformation in the genus Haemophilus. Mol Gen Genet. 1984;193(2):358–363. doi: 10.1007/BF00330693. [DOI] [PubMed] [Google Scholar]
- BUTLER L. O. A defined medium for Haemophilus influenzae and Haemophilus parainfluenzae. J Gen Microbiol. 1962 Jan;27:51–60. doi: 10.1099/00221287-27-1-51. [DOI] [PubMed] [Google Scholar]
- Blackmore C. A., Limpakarnjanarat K., Rigau-Pérez J. G., Albritton W. L., Greenwood J. R. An outbreak of chancroid in Orange County, California: descriptive epidemiology and disease-control measures. J Infect Dis. 1985 May;151(5):840–844. doi: 10.1093/infdis/151.5.840. [DOI] [PubMed] [Google Scholar]
- Borchardt K. A., Hoke A. W. Simplified laboratory technique for diagnosis of chancroid. Arch Dermatol. 1970 Aug;102(2):188–192. [PubMed] [Google Scholar]
- Cagle G. D. Fine structure and distribution of extracellular polymer surrounding selected aerobic bacteria. Can J Microbiol. 1975 Mar;21(3):395–408. doi: 10.1139/m75-055. [DOI] [PubMed] [Google Scholar]
- Casin I. M., Sanson-Le Pors M. J., Gorce M. F., Ortenberg M., Pérol Y. The enzymatic profile of Haemophilus ducreyi. Ann Microbiol (Paris) 1982 Nov-Dec;133(3):379–388. [PubMed] [Google Scholar]
- Cazarré E. A., Barreto T. M. Hemophilus ducreyi: alguns aspectos metabólicos e morfológicos pesquisados pelo emprego de precursores radioativos e microscopia electrônica. Rev Inst Med Trop Sao Paulo. 1974 Mar;16(2):95–102. [PubMed] [Google Scholar]
- Chen S. T., Clowes R. C. Nucleotide sequence comparisons of plasmids pHD131, pJB1, pFA3, and pFA7 and beta-lactamase expression in Escherichia coli, Haemophilus influenzae, and Neisseria gonorrhoeae. J Bacteriol. 1987 Jul;169(7):3124–3130. doi: 10.1128/jb.169.7.3124-3130.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary B. P., Kumari S., Bhatia R., Agarwal D. S. Bacteriological study of chancroid. Indian J Med Res. 1982 Sep;76:379–385. [PubMed] [Google Scholar]
- DEACON W. E., ALBRITTON D. C., EDMUNDSON W. F., OLANSKY S. Study of Ducrey's bacillus and recognition of a gram-positive smooth phase. Proc Soc Exp Biol Med. 1954 Jun;86(2):261–264. doi: 10.3181/00379727-86-21068. [DOI] [PubMed] [Google Scholar]
- DEACON W. E., ALBRITTON D. C., OLANSKY S., KAPLAN W. V.D.R.L. chancroid studies. I. A simple procedure for the isolation and identification of Hemophilus ducreyi. J Invest Dermatol. 1956 May;26(5):399–406. doi: 10.1038/jid.1956.51. [DOI] [PubMed] [Google Scholar]
- DEACON W. E., OLANSKY S., ALBRITTON D. C., KAPLAN W. VDRL chancroid studies. IV. Experimental chancroid, prophylaxis and treatment. Antibiotic Med Clin Ther (New York) 1956 Mar;2(3):143–147. [PubMed] [Google Scholar]
- Demarco de Hormaeche R., Thornley M. J., Glauert A. M. Demonstration by light and electron microscopy of capsules on gonococci recently grown in vivo. J Gen Microbiol. 1978 May;106(1):81–91. doi: 10.1099/00221287-106-1-81. [DOI] [PubMed] [Google Scholar]
- Denys G. A., Chapel T. A., Jeffries C. D. An indirect fluorescent antibody technique for Haemophilus ducreyi. Health Lab Sci. 1978 Jul;15(3):128–132. [PubMed] [Google Scholar]
- Diaz-Mitoma F., Benningen G., Slutchuk M., Ronald A. R., Brunham R. C. Etiology of nonvesicular genital ulcers in Winnipeg. Sex Transm Dis. 1987 Jan-Mar;14(1):33–36. doi: 10.1097/00007435-198701000-00007. [DOI] [PubMed] [Google Scholar]
- Dylewski J., Nsanze H., Maitha G., Ronald A. Laboratory diagnosis of Haemophilus ducreyi: sensitivity of culture media. Diagn Microbiol Infect Dis. 1986 Mar;4(3):241–245. doi: 10.1016/0732-8893(86)90103-3. [DOI] [PubMed] [Google Scholar]
- EDDY B. P. The Voges-Proskauer reaction and its significance: a review. J Appl Bacteriol. 1961 Apr;24:27–41. doi: 10.1111/j.1365-2672.1961.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Grimont P. A. Use of DNA reassociation in bacterial classification. Can J Microbiol. 1988 Apr;34(4):541–546. doi: 10.1139/m88-092. [DOI] [PubMed] [Google Scholar]
- Hafiz S., McEntegart M. G., Kinghorn G. R. Sheffield medium for cultivation of Haemophilus ducreyi. Br J Vener Dis. 1984 Jun;60(3):196–198. doi: 10.1136/sti.60.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Lian C. J., Wilt J. C., Albritton W. L., Ronald A. R. Determination of the hemin requirement of Haemophilus ducreyi: evaluation of the porphyrin test and media used in the satellite growth test. J Clin Microbiol. 1978 Mar;7(3):243–246. doi: 10.1128/jcm.7.3.243-246.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Lian C. J., Wilt J. C., Ronald A. R. Antimicrobial susceptibility of Haemophilus ducreyi. Antimicrob Agents Chemother. 1978 Apr;13(4):608–612. doi: 10.1128/aac.13.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Lian C. J., Wilt J. C., Ronald A. R. Comparison of specimen collection and laboratory techniques for isolation of Haemophilus ducreyi. J Clin Microbiol. 1978 Jan;7(1):39–43. doi: 10.1128/jcm.7.1.39-43.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Slutchuk M., Scatliff J., Sherman E., Wilt J. C., Ronald A. R. Epidemiologic, clinical, laboratory, and therapeutic features of an urban outbreak of chancroid in North America. Rev Infect Dis. 1980 Nov-Dec;2(6):867–879. doi: 10.1093/clinids/2.6.867. [DOI] [PubMed] [Google Scholar]
- Hannah P., Greenwood J. R. Isolation and rapid identification of Haemophilus ducreyi. J Clin Microbiol. 1982 Nov;16(5):861–864. doi: 10.1128/jcm.16.5.861-864.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Loftus T. A. Monoclonal antibodies reactive with all strains of Haemophilus ducreyi. Infect Immun. 1984 Apr;44(1):196–198. doi: 10.1128/iai.44.1.196-198.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander I. M., Lindner B., Brade H., Altmann K., Lindberg A. A., Rietschel E. T., Zähringer U. Chemical structure of the lipopolysaccharide of Haemophilus influenzae strain I-69 Rd-/b+. Description of a novel deep-rough chemotype. Eur J Biochem. 1988 Nov 15;177(3):483–492. doi: 10.1111/j.1432-1033.1988.tb14398.x. [DOI] [PubMed] [Google Scholar]
- Hendley J. W., Powell K. R., Rodewald R., Holzgrefe H. H., Lyles R. Demonstration of a capsule on Neisseria gonorrhoeae. N Engl J Med. 1977 Mar 17;296(11):608–611. doi: 10.1056/NEJM197703172961105. [DOI] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. Y., Vogt M., Modan M. Defined medium for growth of Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):513–516. doi: 10.1128/jb.101.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holländer R. Correlation of the function of demethylmenaquinone in bacterial electron transport with its redox potential. FEBS Lett. 1976 Dec 15;72(1):98–100. doi: 10.1016/0014-5793(76)80821-6. [DOI] [PubMed] [Google Scholar]
- Inzana T. J. A chemically defined medium induces resistance to lipopolysaccharide antibody in Haemophilus influenzae type b. Microb Pathog. 1986 Oct;1(5):483–489. doi: 10.1016/0882-4010(86)90010-0. [DOI] [PubMed] [Google Scholar]
- Jantzen E., Berdal B. P., Omland T. Cellular fatty acid composition of Haemophilus species, Pasteurella multocida, Actinobacillus Actinomycetemcomitans and Haemophilus vaginalis (Corynebacterium vaginale). Acta Pathol Microbiol Scand B. 1980 Apr;88(2):89–93. doi: 10.1111/j.1699-0463.1980.tb02611.x. [DOI] [PubMed] [Google Scholar]
- Jensen R. A. Biochemical pathways in prokaryotes can be traced backward through evolutionary time. Mol Biol Evol. 1985 Mar;2(2):92–108. doi: 10.1093/oxfordjournals.molbev.a040338. [DOI] [PubMed] [Google Scholar]
- Johnson A. P., Abeck D., Davies H. A. The structure, pathogenicity and genetics of Haemophilus ducreyi. J Infect. 1988 Sep;17(2):99–106. doi: 10.1016/s0163-4453(88)91487-9. [DOI] [PubMed] [Google Scholar]
- KAPLAN W., DEACON W. E., OLANSKY S., ALBRITTON D. C. V.D.R.L. chancroid studies. II. Experimental chancroid in the rabbit. J Invest Dermatol. 1956 May;26(5):407–414. doi: 10.1038/jid.1956.52. [DOI] [PubMed] [Google Scholar]
- KAPLAN W., DEACON W. E., OLANSKY S., ALBRITTON D. C. V.D.R.L. chancroid studies. III. Use of Ducrey skin test vaccines on rabbits. J Invest Dermatol. 1956 May;26(5):415–419. doi: 10.1038/jid.1956.53. [DOI] [PubMed] [Google Scholar]
- Kampmeier R. H. The recognition of Haemophilus ducreyi as the cause of soft chancre. Sex Transm Dis. 1982 Oct-Dec;9(4):212–213. doi: 10.1097/00007435-198210000-00012. [DOI] [PubMed] [Google Scholar]
- Kasper D. L. The polysaccharide capsule of Bacteroides fragilis subspecies fragilis: immunochemical and morphologic definition. J Infect Dis. 1976 Jan;133(1):79–87. doi: 10.1093/infdis/133.1.79. [DOI] [PubMed] [Google Scholar]
- Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976 Mar;93(1):9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- Kinghorn G. R., Hafiz S., McEntegart M. G. Pathogenic microbial flora of genital ulcers in Sheffield with particular reference to herpes simplex virus and Haemophilus ducreyi. Br J Vener Dis. 1982 Dec;58(6):377–380. doi: 10.1136/sti.58.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. D., Luginbuhl G. H. Simplified media for the growth of Haemophilus influenzae from clinical and normal flora sources. J Gen Microbiol. 1979 Aug;113(2):409–411. doi: 10.1099/00221287-113-2-409. [DOI] [PubMed] [Google Scholar]
- Korting H. C., Abeck D., Johnson A. P., Ballard R. C., Taylor-Robinson D., Braun-Falco O. Lectin typing of Haemophilus ducreyi. Eur J Clin Microbiol Infect Dis. 1988 Oct;7(5):678–680. doi: 10.1007/BF01964253. [DOI] [PubMed] [Google Scholar]
- Lee B. C., Bryan L. E. Penicillin-binding proteins of Haemophilus ducreyi. Antimicrob Agents Chemother. 1989 Jun;33(6):983–986. doi: 10.1128/aac.33.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Lactoperoxidase and Iodo-Gen-catalyzed iodination labels inner and outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983 Jul;155(1):443–446. doi: 10.1128/jb.155.1.443-446.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke-Olesen L., Larsen L., Pedersen T. G., Gaarslev K. Epidemic of chancroid in Greenland 1977-78. Lancet. 1979 Mar 24;1(8117):654–655. doi: 10.1016/s0140-6736(79)91091-2. [DOI] [PubMed] [Google Scholar]
- Marsch W. C., Haas N., Stüttgen G. Ultrastructural detection of Haemophilus ducreyi in biopsies of chancroid. Arch Dermatol Res. 1978 Nov 10;263(2):153–157. doi: 10.1007/BF00446436. [DOI] [PubMed] [Google Scholar]
- McEntegart M. G., Hafiz S., Kinghorn G. R. Haemophilus ducreyi infections--time for reappraisal. J Hyg (Lond) 1982 Dec;89(3):467–478. doi: 10.1017/s0022172400071035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicol P. J., Ronald A. R. The plasmids of Haemophilus ducreyi. J Antimicrob Chemother. 1984 Dec;14(6):561–564. [PubMed] [Google Scholar]
- Morse S. A. Chancroid and Haemophilus ducreyi. Clin Microbiol Rev. 1989 Apr;2(2):137–157. doi: 10.1128/cmr.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayyar K. C., Stolz E., Michel M. F. Rising incidence of chancroid in Rotterdam. Epidemiological, clinical, diagnostic, and therapeutic aspects. Br J Vener Dis. 1979 Dec;55(6):439–441. doi: 10.1136/sti.55.6.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre G. N. Identification of Haemophilus ducreyi in the clinical laboratory. J Med Microbiol. 1982 May;15(2):243–245. doi: 10.1099/00222615-15-2-243. [DOI] [PubMed] [Google Scholar]
- Nsanze H., Fast M. V., D'Costa L. J., Tukei P., Curran J., Ronald A. Genital ulcers in Kenya. Clinical and laboratory study. Br J Vener Dis. 1981 Dec;57(6):378–381. doi: 10.1136/sti.57.6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nsanze H., Plummer F. A., Maggwa A. B., Maitha G., Dylewski J., Piot P., Ronald A. R. Comparison of media for the primary isolation of Haemophilus ducreyi. Sex Transm Dis. 1984 Jan-Mar;11(1):6–9. doi: 10.1097/00007435-198401000-00002. [DOI] [PubMed] [Google Scholar]
- Oberhofer T. R., Back A. E. Isolation and cultivation of Haemophilus ducreyi. J Clin Microbiol. 1982 Apr;15(4):625–629. doi: 10.1128/jcm.15.4.625-629.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odumeru J. A., Ronald A. R., Albritton W. L. Characterization of cell proteins of Haemophilus ducreyi by polyacrylamide gel electrophoresis. J Infect Dis. 1983 Oct;148(4):710–714. doi: 10.1093/infdis/148.4.710. [DOI] [PubMed] [Google Scholar]
- Odumeru J. A., Wiseman G. M., Ronald A. R. Relationship between lipopolysaccharide composition and virulence of Haemophilus ducreyi. J Med Microbiol. 1987 Mar;23(2):155–162. doi: 10.1099/00222615-23-2-155. [DOI] [PubMed] [Google Scholar]
- Odumeru J. A., Wiseman G. M., Ronald A. R. Role of lipopolysaccharide and complement in susceptibility of Haemophilus ducreyi to human serum. Infect Immun. 1985 Nov;50(2):495–499. doi: 10.1128/iai.50.2.495-499.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odumeru J. A., Wiseman G. M., Ronald A. R. Virulence factors of Haemophilus ducreyi. Infect Immun. 1984 Feb;43(2):607–611. doi: 10.1128/iai.43.2.607-611.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov N. M., Delektorskii V. V., Tishchenko L. D., Omel'chenko O. G. Ul'trastruktura vozbuditelia miagkogo shankra. Vestn Dermatol Venerol. 1976 Nov;(11):37–38. [PubMed] [Google Scholar]
- PAGE L. A. Haemophilus infections in chickens. I. Characteristics of 12 Haemophilus isolates recovered from diseased chickens. Am J Vet Res. 1962 Jan;23:85–95. [PubMed] [Google Scholar]
- Parr T. R., Jr, Bryan L. E. Lipopolysaccharide composition of three strains of Haemophilus influenzae. Can J Microbiol. 1984 Sep;30(9):1184–1187. doi: 10.1139/m84-185. [DOI] [PubMed] [Google Scholar]
- Parsons L. M., Shayegani M., Waring A. L., Bopp L. H. DNA probes for the identification of Haemophilus ducreyi. J Clin Microbiol. 1989 Jul;27(7):1441–1445. doi: 10.1128/jcm.27.7.1441-1445.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. P., Schuffman S. S., Sell S. H. Electron microscopic studies of complexes of Haemophilus infuenzae, type b, with specific antibodies. Immunology. 1972 Jul;23(1):101–105. [PMC free article] [PubMed] [Google Scholar]
- Saunders J. M., Folds J. D. Immunoblot analysis of antigens associated with Haemophilus ducreyi using serum from immunised rabbits. Genitourin Med. 1986 Oct;62(5):321–328. doi: 10.1136/sti.62.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalla W. O., Sanders L. L., Schmid G. P., Tam M. R., Morse S. A. Use of dot-immunobinding and immunofluorescence assays to investigate clinically suspected cases of chancroid. J Infect Dis. 1986 May;153(5):879–887. doi: 10.1093/infdis/153.5.879. [DOI] [PubMed] [Google Scholar]
- Schmid G. P., Sanders L. L., Jr, Blount J. H., Alexander E. R. Chancroid in the United States. Reestablishment of an old disease. JAMA. 1987 Dec 11;258(22):3265–3268. [PubMed] [Google Scholar]
- Schryvers A. B., Wong S. S., Bryan L. E. Antigenic relationships among penicillin-binding proteins 1 from members of the families Pasteurellaceae and Enterobacteriaceae. Antimicrob Agents Chemother. 1986 Oct;30(4):559–564. doi: 10.1128/aac.30.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon W. H., Heyman A. Studies on Chancroid: I. Observations on the Histology with an Evaluation of Biopsy as a Diagnostic Procedure. Am J Pathol. 1946 Mar;22(2):415–425. [PMC free article] [PubMed] [Google Scholar]
- Slootmans L., Vanden Berghe D. A., Van Dyck E., Piot P. Susceptibility of 40 Haemophilus ducreyi strains to 34 antimicrobial products. Antimicrob Agents Chemother. 1983 Oct;24(4):564–567. doi: 10.1128/aac.24.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sng E. H., Lim A. L., Rajan V. S., Goh A. J. Characteristics of Haemophilus ducreyi. A study. Br J Vener Dis. 1982 Aug;58(4):239–242. doi: 10.1136/sti.58.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottnek F. O., Biddle J. W., Kraus S. J., Weaver R. E., Stewart J. A. Isolation and identification of Haemophilus ducreyi in a clinical study. J Clin Microbiol. 1980 Aug;12(2):170–174. doi: 10.1128/jcm.12.2.170-174.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A. W. Identification of Haemophilus ducreyi. Antonie Van Leeuwenhoek. 1981 Mar;47(1):89–90. doi: 10.1007/BF00399078. [DOI] [PubMed] [Google Scholar]
- Sturm A. W., Stolting G. J., Cormane R. H., Zanen H. C. Clinical and microbiological evaluation of 46 episodes of genital ulceration. Genitourin Med. 1987 Apr;63(2):98–101. doi: 10.1136/sti.63.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A. W., Zanen H. C. Characteristics of Haemophilus ducreyi in culture. J Clin Microbiol. 1984 May;19(5):672–674. doi: 10.1128/jcm.19.5.672-674.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A. W., Zanen H. C. Enzymic activity of Haemophilus ducreyi. J Med Microbiol. 1984 Oct;18(2):181–187. doi: 10.1099/00222615-18-2-181. [DOI] [PubMed] [Google Scholar]
- Taylor D. N., Echeverria P., Hanchalay S., Pitarangsi C., Slootmans L., Piot P. Antimicrobial susceptibility and characterization of outer membrane proteins of Haemophilus ducreyi isolated in Thailand. J Clin Microbiol. 1985 Mar;21(3):442–444. doi: 10.1128/jcm.21.3.442-444.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuyau J. E., Sims W., Williams R. A. The acid end-products of glucose metabolism of oral and other haemophili. J Gen Microbiol. 1984 Jul;130(7):1787–1793. doi: 10.1099/00221287-130-7-1787. [DOI] [PubMed] [Google Scholar]
- Van Dyck E., Piot P. Enzyme profile of Haemophilus ducreyi strains isolated on different continents. Eur J Clin Microbiol. 1987 Feb;6(1):40–43. doi: 10.1007/BF02097188. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe D. A. Selenium and the growth of Haemophilus ducreyi. J Clin Pathol. 1987 Oct;40(10):1174–1177. doi: 10.1136/jcp.40.10.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson P. J., Deneer H. G., Potter A., Albritton W. Characterization of a streptomycin-sulfonamide resistance plasmid from Actinobacillus pleuropneumoniae. Antimicrob Agents Chemother. 1989 Feb;33(2):235–238. doi: 10.1128/aac.33.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow C. E., Broadhurst J., Buchanan R. E., Krumwiede C., Rogers L. A., Smith G. H. The Families and Genera of the Bacteria: Final Report of the Committee of the Society of American Bacteriologists on Characterization and Classification of Bacterial Types. J Bacteriol. 1920 May;5(3):191–229. doi: 10.1128/jb.5.3.191-229.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow C. E., Broadhurst J., Buchanan R. E., Krumwiede C., Rogers L. A., Smith G. H. The Families and Genera of the Bacteria: Preliminary Report of the Committee of the Society of American Bacteriologists on Characterization and Classification of Bacterial Types. J Bacteriol. 1917 Sep;2(5):505–566. doi: 10.1128/jb.2.5.505-566.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss C. Selected low-cohesion variants of Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus lack distinct antigens recognized by human antibodies. Arch Microbiol. 1989;151(2):133–136. doi: 10.1007/BF00414427. [DOI] [PubMed] [Google Scholar]