Abstract
Introduction
Hepatitis C disease burden is substantially increasing in Egyptian community, it is estimated that prevalence of Hepatitis C virus (HCV) in Egyptian community reach 22% of total population. Recently there is a global alert of HCV cardiovascular complications.
Objective
To evaluate LV diastolic functions of HCV patients using tissue Doppler Imaging and NTPBNP.
Methods
30 HCV patients of 30 years, sex & BMI matched controls were evaluated by PCR, ECG, Echocardiography “conventional Doppler, pulsed wave tissue Doppler (PW-TD), strain rate imaging” & NTPBNP to assess LV diastolic functions. Mean age was 32.8 years ± 5.1 in HCV group, 29.8 years ± 6.6 in control group. Cardiovascular anomalies and predisposing factors were excluded.
Results
HCV group has shown significant increase in QTc interval, significant statistical increase in A wave, deceleration time; (p < 0.05), highly significant decrease in tissue Doppler Ea (p < 0.001), highly significant decrease in Aa (p < 0.001), highly significant increased E/Ea ratio (p value < 0.001), significant decrease in Ea/Aa ratio and significant increase in SRa (p < 0.05).
NTPBNP levels showed highly significant increase with mean value 222 pg/ml ± 283 in HCV group and 32.7 pg/ml ± 21.2 in control group (p value < 0.001). The best cut-off value of NTPBNP to detect diastolic dysfunction in HCV group was 213 pg/ml.
No statistical differences in SRe/SRa and E/SRe ratios were observed, however they had significant correlation with NTPBNP level and tissue Doppler parameters. The best cut-off value of E/SRe ratio to detect diastolic dysfunction in HCV group was 0.91, with 75% sensitivity and 100% specificity.
Conclusion and recommendation
This data show the first direct evidence that HCV infection causes diastolic dysfunction without any other predisposing factors, probably due to chronic inflammatory reaction with mild fibrosis in the heart. Previous studies did not follow strict inclusion and exclusion criteria that confirm the independent role of HCV to cause diastolic dysfunction. Tissue Doppler was more sensitive to diagnose diastolic dysfunction than conventional Doppler.
Keywords: Hepatitis C virus, Cardiomyopathy, Diastolic dysfunction, Tissue Doppler imaging, Strain rate imaging, NTproBNP
1. Introduction
Hepatitis C virus (HCV) is the cause of many different forms of heart diseases worldwide. Up till now, few cardiologists are aware of (HCV) as an etiology of heart disease and its treatment. HCV infection is seen globally, and is often undetected and therefore untreated. The burden of HCV-derived heart diseases is global, with higher prevalence in Asia, Africa, and low- and middle-income countries. Egypt has the highest prevalence of HCV in the world, apparently due to previous mass parenteral anti-schistosomal therapy (Deffic-Burban et al., 2006). HCV derived heart diseases are chronic, persistent, and devastating diseases (Matsumori et al., 2006).
The myocardium may be the target of several types of viral infections. Recently, the importance of hepatitis C virus (HCV) infection has been noted in patients with hypertrophic cardiomyopathy, dilated cardiomyopathy, myocarditis and left ventricular (LV) diastolic dysfunction (Raedle-Hurst et al., 2008; Henriksen and Møller, 2009).
Because of its high reproducibility, feasibility, and relatively preload-independence, tissue Doppler recording of the early diastolic mitral annular velocity (Ea) in conjunction with the mitral inflow velocity (E) has become the first line of diastolic evaluation. Myocardial relaxation is impaired in almost all patients with diastolic dysfunction, which is best assessed by the Ea velocity of the mitral annulus using tissue Doppler imaging (TDI). While early diastolic trans-mitral velocity (E) increases progressively as LV filling pressure increases, the mitral annular Ea velocity remains decreased at all stages of diastolic dysfunction (Thomas and Marwick, 2007).
Other recent tool is demonstrated to assess LV diastolic function which is strain and strain rate imaging, Strain, in daily language means, “stretching”. In scientific usage, the definition is extended to mean “deformation” (Asbjørn Støylen, 2005).
Extending the utility of BNP as a diagnostic marker to screen for asymptomatic or preclinical ventricular dysfunction according to the ACC/AHA guidelines (Hunt et al., 2005) in the general population has not proved cost-effective as the prevalence of heart failure is low. However, if the test is used in patients stratified for risk using clinical criteria, BNP has proved useful to “rule out” ventricular dysfunction, thus eliminating the need for other more expensive diagnostic tests in this group (Yamamoto et al., 2000).
Of all investigated neurohormones and natriuretic peptides, B type natriuretic peptide and N-terminal proBNP are the best markers for ruling out left ventricular dysfunction and to detect the degree of severity (Hammerer-Lercher et al., 2004; Park et al., 2010).
2. Aim of work
The aim of this study is to evaluate the cardiac involvement of HCV using tissue Doppler imaging “TDI” combined with NTproBNP. Any factor that can lead to cardiac insult will be excluded in order to clearly identify HCV cardiotropism.
3. Methods
3.1. Participants
This study included 60 adult patients. Patients were enrolled in two groups with body mass index, age and sex matched.
Group one included thirty patients below 40 years old infected with hepatitis C virus (HCV) while group two included 30 patients below 40 years old without HCV infection.
HCV group included 18 male patients and 12 female patients control group included 14 males and 16 females.
Patients with systolic dysfunction “Ejection Fraction (EF) less than 50%”, congenital heart disease or valvular heart disease, restrictive cardiomyopathy, hypertrophic cardiomyopathy, pericardial constriction or myocarditis were excluded.
Patients with diabetes mellitus, hypertensive, smokers or those over 40 years of age, BMI below 18.5 kg/m2 or over 30 kg/m2 and patients with history of treatment with interferon were also excluded.
Plasma sample was taken from every patient to analyze the level of N-Terminal pro Brain Natruretic Peptide (NTproBNP). Blood sample was obtained and 1 ml refrigerated EDTA plasma (lavender-top tube); was gone through immuno (electro) chemiluminescence assay (ICMA) in Roche Elecsys 2010 bench top analyzer.
3.2. Echocardiographic acquisition and analysis
Each patient had a complete two-dimensional echocardiography study using a commercially available ultrasound scanner (Vivid 7, General Electric Medical Health) with a 2.5-MHz phased-array transducer. Standard echocardiographic views, including apical four- and two-chamber views, with the patient in the left lateral decubitus position, Mitral inflow velocities were obtained by pulsed wave Doppler in the apical four-chamber view with a 1- to 2-mm sample volume placed at the tips of the mitral valve leaflets during quiet respiration. Mitral E and A wave velocities (cm/s) and deceleration time (milliseconds) were measured offline and averaged over two or three cardiac cycles. The E/A ratios were calculated.
3.3. Doppler tissue imaging
Pulsed wave Doppler tissue imaging velocities were obtained from the apical four-chamber view during quiet respiration by placing a sample volume in the lateral mitral annulus. Cursor line was more aligned with examined wall (15–20°) to decrease incidence of angle error. The peak early diastolic mitral annular velocities (Ea, cm/s) and peak late diastolic mitral annular velocities (Aa, cm/s) were measured from the time-myocardial velocity waveforms of 2–3 consecutive cardiac cycles and an average were recorded. The lateral E/Ea ratio, a measure of LV filling pressure, was calculated.
3.4. Strain rate imaging
Doppler tissue imaging recordings were taken at 134 ± 22 frames/s in the apical four-chamber view. Analysis was independently and separately performed on a commercially available analysis computer (Echopac, GE Systems) by two cardiologists blinded to the categorization of participants. Peak early diastolic strain rate (SRe) and peak late diastolic strain rate (SRa) measurements for three consecutive cardiac cycles were averaged for each participant. For longitudinal measurements, samples were placed at the basal, mid, and apical septal and lateral walls in the apical four-chamber view then Strain rate data were averaged. Participants were excluded from the study if the axis of the septum deviated from the line of the Doppler beam by more than 15–20°.
Echocardiograms of 20 participants were randomly selected and repeated measurements of E, A, and Ea, Aa, SRe and SRa were blindly performed after initial evaluation to assess intra-observer variability.
3.5. Statistical analysis
Data were analyzed using Statistical Package for Special Science (SPSS) software computer program version 15. Data were described as mean ± standard deviation (SD) for quantitative (numerical) variables and as frequency & percentage for qualitative (categorical) variables. Independent student t test was used for comparison of quantitative variables among two independent groups. Chi-square test (or Fisher’s exact test when appropriate) was used for comparison of distribution of qualitative variables among different groups. Correlation between continuous variables was performed using person correlation coefficient. Significance level (P) value:∗P > 0.05 is insignificant. ∗P ⩽ 0.05 is significant. ∗P < 0.001 is highly significant.
4. Results
A significant statistical increase was observed in QT corrected interval of resting ECG in HCV patients, compared to normal control group, p value < 0.05 “Fig. 1”. Incidence of prolonged QTc interval was 10% in HCV group. There was a highly significant statistical increase in plasma levels of NTproBNP in HCV group, p value <0.001.There was no statistical difference in HCV patients and normal control group concerning systolic function as assessed by EF measured from M-mode, Simpson’s method, p = 0.097 or tissue Doppler “Sa” wave where it was, p = 0.63 (Table 1).
Figure 1.
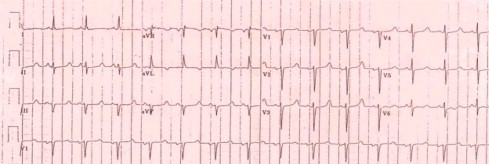
ECG of HCV infected patient showing long QTc interval.
Table 1.
Baseline and Echocardiographic characteristics of HCV group and Control group.
| Group | Mean value | Std. deviation ± | P value | |
|---|---|---|---|---|
| Age | HCV group | 32.8 | 5.1 | 0.06 |
| Control group | 29.8 | 6.6 | ||
| Heart rate | HCV group | 81.20 | 10.390 | 0.6762 |
| Control group | 79.50 | 8.935 | ||
| Arterial blood pressure “systolic” | HCV group | 121.68 | 9.013 | 0.8576 |
| Control group | 120.55 | 10.736 | ||
| Arterial blood pressure “diastolic” | HCV group | 76.00 | 9.0676 | 0.7025 |
| Control group | 77.50 | 8.8976 | ||
| Body mass index | HCV group | 25.8 | 1.85 | 0.619 |
| Control group | 25.6 | 1.86 | ||
| NTproBNP | HCV group | 222.252 | 283.444 | >0.001 |
| Control group | 32.688 | 21.234 | ||
| QTc | HCV group | 373.571 | 49.156 | 0.048 |
| Control group | 350.666 | 36.759 | ||
| EF | HCV group | 62.38 | 8.25 | 0.097 |
| Control group | 65.15 | 7.67 | ||
| Doppler E wave | HCV group | 0.852 | 0.207 | 0.512 |
| Control group | 0.821 | 0.152 | ||
| Doppler A wave | HCV group | 0.726 | 0.228 | 0.009 |
| Control group | 0.598 | 0.121 | ||
| E/A ratio | HCV group | 1.278 | 0.450 | 0.220 |
| Control group | 1.390 | 0.198 | ||
| Deceleration time | HCV group | 217.733 | 45.521 | 0.002 |
| Control group | 184.633 | 31.469 | ||
| Vp | HCV group | 73.466 | 32.722 | 0.145 |
| Control group | 83.933 | 20.844 | ||
| Sa wave | HCV group | 9.17 | 1.79 | 0.63 |
| Control group | 9.39 | 1.48 | ||
| Ea wave | HCV group | 0.106 | 0.040 | >0.001 |
| Control group | 0.260 | 0.117 | ||
| Aa wave | HCV group | 0.108 | 0.039 | >0.001 |
| Control group | 0.240 | 0.130 | ||
| Ea/Aa ratio | HCV group | 1.153 | 0.557 | 0.002 |
| Control group | 1.512 | 0.238 | ||
| E/Ea ratio | HCV group | 9.108 | 3.517 | >0.001 |
| Control group | 3.353 | 2.174 | ||
| SRe wave | HCV group | 2.066 | 1.347 | 0.6 |
| Control group | 1.927 | 0.506 | ||
| SRa wave | HCV group | 1.360 | 0.621 | 0.035 |
| Control group | 1.079 | 0.346 | ||
| SRe/SRa ratio | HCV group | 1.694 | 1.134 | 0.435 |
| Control group | 1.885 | 0.690 | ||
| E/SRe ratio | HCV group | 0.614 | 0.455 | 0.098 |
| Control group | 0.463 | 0.175 |
Table of mean values, std. deviation and p values of research parameters between HCV patients and control group. NTproBNP: N-Terminal pro Brain Natriuretic Peptide, QTc: corrected QT interval, EF: Ejection Fraction, Vp: flow propogation velocity, Sa: tissue Doppler mitral systolic velocity, Ea: tissue Doppler mitral early diastolic velocity, Aa: tissue Doppler mitral late diastolic velocity, SRe: early LV diastolic rate of deformation and SRa: late diastolic rate of deformation.
There was a significant statistical increase in A wave velocity in HCV patients and significant increase in deceleration time “DT”, p value <0.05. A highly significant decrease in Ea wave was observed in HCV patients, p value <0.001 “Fig. 2”. There was a highly significant increase in Aa wave in HCV patients, p value <0.001 “Fig. 2”. Pulsed wave tissue Doppler Ea/Aa ratio showed significant decrease of Imaging in HCV patients, p value <0.05. Incidence of reversed Ea/Aa ratio was 26.7% in HCV group. There was a highly significant statistical increase in E/Ea ratio in HCV patients, p value <0.001. Those tissue Doppler parameters “Ea, Aa, Ea/Aa and E/Ea” clearly indicate diastolic dysfunction in HCV patients (Table 1).
Figure 2.
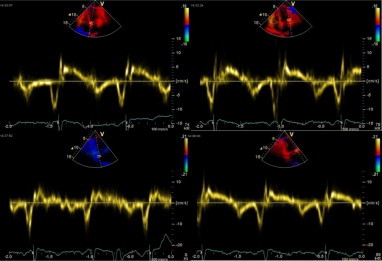
Pulsed wave tissue Doppler of a patient from HCV group, figure shows reversed Ea/Aa ratio.
There was no statistical difference between HCV patients and normal control group concerning SRe “early diastolic rate of deformation wave of strain rate imaging” or SRe/SRa ratio. However, there was a significant increase in SRa “late diastolic rate of deformation wave of strain rate imaging”, p value <0.05 “Fig. 3”.
Figure 3.
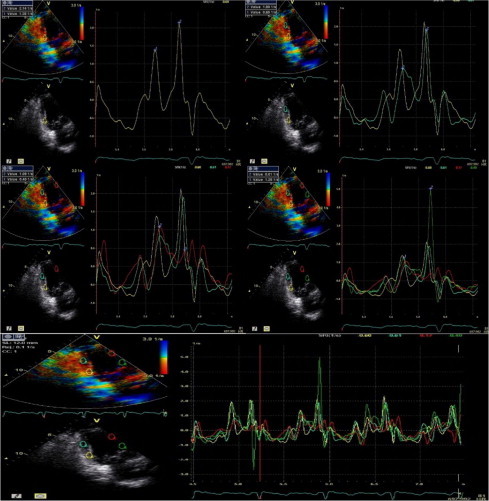
Strain rate imaging data of one of HCV patients, reversed SRe/SRa ratio can be observed.
Levels of NTproBNP had shown the following correlations: “Figs. 4–6”.
Figure 4.
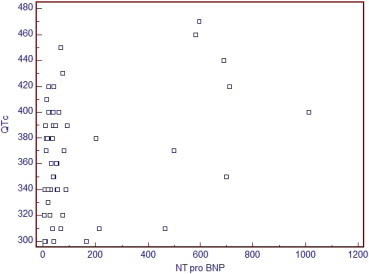
Direct relationship between NTPBNP and corrected QT interval where r = 0.334 and p value = 0.009.
Figure 5.
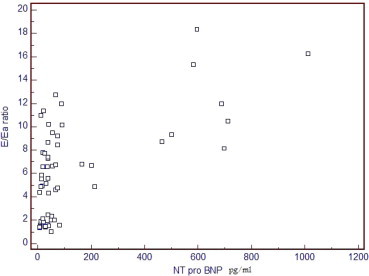
Direct relationship between NTPBNP and E/Ea ratio, r = 0.619 and p value <0.001.
Figure 6.
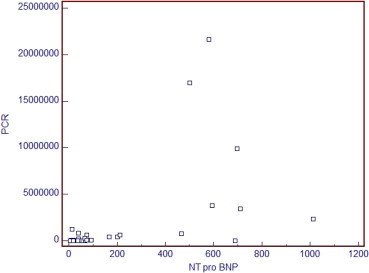
Direct relationship between NTPBNP and quantitative PCR, r = 0.515 and p = 0.004.
Strong inverse correlation with Ea/Aa ratio, r-value = “−0.777”, p value <0.001, average direct correlation with E/Ea ratio, r-value = “0.619”, p value <0.001, average direct correlation with PCR, r-value = “0.515”, p value =0.004, average direct correlation with deceleration time, r-value = “0.541”, p value <0.001 and had weak direct correlation with QTc interval, r-value = “0.334”, p value = 0.009. Howerer, no correlation was found between NTproBNP level with SRa.
5. Discussion
Our results came compatible with Dimerdal study in which the number of subjects with diastolic dysfunction in the study group (n = 18) were significantly higher than the control group (n = 4) (p < 0.05). This agrees with the results of our study, although none of our control group has shown diastolic dysfunction. That difference might be due to including older age group in his study (Demirdal et al., 2007).
Raedle-Hurst et al. has evaluated HCV patients using tissue Doppler and NTPBNP, a cutoff value of greater than 290 pg/ml was highly predictive of advanced left ventricular diastolic dysfunction in this study with significant difference in tissue Doppler indices between HCV group and normal control. Ea wave was significantly decreased while Aa wave was significantly increased. Ea/Aa ratio was significantly decreased. This study agrees with our study tissue Doppler parameters and increased levels of NTPBNP (Raedle-Hurst et al., 2008).
A previous Egyptian study was made to assess the cardiovascular effect of HCV on Egyptian patients and had found significant decrease in Doppler E wave, significant increase in A wave, significant decrease in E/A ratio, significant increase in LV wall thickness “septal & posterior” and significant prolongation of corrected QT interval. This study agrees with our results in finding significant prolongation in QTc interval and in significance of increased Doppler A wave velocity. While it contradict our results where we found no statistical difference between HCV group and normal control group concerning Doppler E wave velocity, E/A ratio and LV septal & posterior wall thickness (Haykal and Negm, 2009).
Another Japanese study aimed to determine the prevalence of hepatitis C virus (HCV) infection and myocardial injury among patients enrolled in the Myocarditis Treatment Trial. Anti-HCV antibodies were identifiable in sera and were more prevalent in patients with myocarditis and HF than in the general population. The study concluded that NTPBNP is a more sensitive marker of myocardial injury than cardiac troponins in patients with heart failure from HCV myocarditis. This agrees with our study where we found high significant increase in NTPBNP levels in HCV group compared to normal control group (Matsumori et al., 2006).
Antonelli et al. evaluated serum levels of NTproBNP in a large series of patients with HCV chronic infection (HCV+). Serum NTproBNP was assayed in 50 patients HCV+ and in 50 sex- and age-matched controls. HCV+ patients showed significantly higher mean NTproBNP level than controls (P = 0.001). By defining high NTproBNP level as a value higher than 125 pg/mL (the single cut-off point for patients under 75 years of age), 34% HCV+ and 6% controls had high NTproBNP (Fisher exact test; P < 0.001). With a cut-off point of 300 pg/mL (used to rule out chronic heart failure in patients under 75 years of age) 10% HCV+ and 0 controls had high NTproBNP (Fisher exact test; P = 0.056). With a cut-off point of 900 pg/mL (used for ruling in chronic heart failure in patients with age 50–75) 8% HCV+ patients and 0 controls had high NTproBNP (Fisher exact test; P = 0.12). The study demonstrates high levels of circulating NTproBNP in HCV+ patients compared to healthy controls. The increase of NTproBNP may indicate the presence of a sub-clinical cardiac dysfunction (Antonelli et al., 2010).
A study by Dimitroulas et al. evaluated 52 patients with Systemic Sclerosis “SSc” (mean age 55.7 +/− 10.1 years, 51 women), with conventional and tissue Doppler echocardiography. Plasma NTproBNP levels were measured in all patients. Data were compared with those obtained from 25 healthy controls comparable for age and sex. Patients with SSc had impaired left ventricular and right ventricular diastolic function expressed by inverted ratio of peak early to peak late transmittal (E/A) ratio and trans-tricuspid velocity and increased left atrial diameter compared with controls. Peak systolic mitral lateral annular motion velocity and peak early diastolic mitral lateral annular motion velocity (LV Ea) were lower, while LV E/Ea ratio was higher, in patients with SSc compared to controls. NTproBNP was associated with E, E/A ratio and mitral deceleration time. NTproBNP was significantly correlated with echocardiographic abnormalities, providing a potent link for cardiac function and neuroendocrine derangement in patients with SSc who have cardiac disease (Dimitroulas et al., 2010).
6. Study limitations
Limitations of this study were either linked to the usual limitations of tissue Doppler imaging or linked to evaluation of HCV patients. Limitations related to TDI included its inability to discriminate between active and passive motion due to tethering or overall cardiac rotational/translational motion. It is also influenced by breathing, patient motion, heart rate variations and it is angle-dependent. Limitations linked to evaluation of HCV patients were including relatively small number of patients, evaluating HCV genotype 4 which is found in Egypt and Africa while sparing the other genotypes which are more prevalent in Asia and South America. We did not link the length of infection to the abnormalities of cardiac condition due to the accidental nature of discovering the infection of HCV in Egypt (Table 1).
7. Conclusion
-
1.
Tissue Doppler was more sensitive to diagnose diastolic dysfunction than conventional Doppler.
-
2.
NTproBNP is a strong indicator of diastolic dysfunction in HCV patients and is directly related to echocardiographic parameters in addition to the level of viraemia hence we recommend its routine use as a follow up tool in HCV patients.
References
- Antonelli A. High levels of circulating N-terminal pro-brain natriuretic peptide in patients with hepatitis C. J. Viral Hepat. 2010;17(12):851–853. doi: 10.1111/j.1365-2893.2009.01237.x. [DOI] [PubMed] [Google Scholar]
- Asbjørn Støylen, 2005. Strain rate imaging. Cardiac deformation imaging by ultrasound/echocardiography-tissue Doppler and Speckle tracking. Retrieved online from <http://folk.ntnu.no/stoylen/strainrate/>.
- Deffic-Burban S. Expected increase in hepatitis C-related mortality in Egypt due to pre-2000 infections. J. Hepatol. 2006;44(3):455–461. doi: 10.1016/j.jhep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Demirdal T. Cardiomyopathy in Patients with Chronic Hepatitis C Virus. ICC Munich; Germany: 2007. (17th European Congress of Clinical Microbiology and Infectious Diseases). [Google Scholar]
- Dimitroulas T. Early detection of cardiac involvement in systemic sclerosis assessed by tissue-Doppler echocardiography: relationship with neurohormonal activation and endothelial dysfunction. J. Rheumatol. 2010;37(5):993–999. doi: 10.3899/jrheum.090931. [DOI] [PubMed] [Google Scholar]
- Hammerer-Lercher A. Natriuretic peptides as markers of mild forms of left ventricular dysfunction: effects of assays on diagnostic performance of markers. Clin. Chem. 2004;50:1174–1183. doi: 10.1373/clinchem.2003.028316. [DOI] [PubMed] [Google Scholar]
- Haykal, M., Negm, H., 2009. Cardiovasular effects of HCV in Egyptian population. CVD Prevention and Control J 4 (suppl.1).
- Henriksen JH., Møller S. Cardiac and systemic haemodynamic complications of liver cirrhosis. Scand. Cardiovasc. J. 2009;14:1–8. doi: 10.1080/14017430802691528. [DOI] [PubMed] [Google Scholar]
- Hunt S.A. ACC/AHA guideline update for the diagnosis and management of chronic heart failure in the adult A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to Update the 2001 guidelines for the evaluation and management of heart failure) J. Am. Coll. Cardiol. 2005;46:1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Matsumori A. Myocarditis and heart failure associated with hepatitis C virus infection. J. Cardiovasc. Fail. 2006;12(4):293–298. doi: 10.1016/j.cardfail.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Park H.J. Direct comparison of B-type natriuretic peptide and N-terminal pro-BNP for assessment of cardiac function in a large population of symptomatic patients. Int. J. Cardiol. 2010;140(3):336–343. doi: 10.1016/j.ijcard.2008.11.107. [DOI] [PubMed] [Google Scholar]
- Raedle-Hurst Validity of N-terminal propeptide of the brain natriuretic peptide in predicting left ventricular diastolic dysfunction diagnosed by tissue Doppler imaging in patients with chronic liver disease. Eur. J. Gastroenterol. Hepatol. 2008;20(9):865–873. doi: 10.1097/MEG.0b013e3282fb7cd0. [DOI] [PubMed] [Google Scholar]
- Thomas, H., Marwick et al., 2007. Myocardial Imaging: Textbook on Tissue Doppler and Speckle Tracking. Blackwell. ISBN: 978-1-4051-6113-8.
- Yamamoto K. Clinical criteria and biochemical markers for the detection of systolic dysfunction. J. Cardiovasc. Fail. 2000;6:194–200. doi: 10.1054/jcaf.2000.9676. [DOI] [PubMed] [Google Scholar]


