Abstract
Knowledge of the structure and function of the genes and proteins of the rotaviruses has expanded rapidly. Information obtained in the last 5 years has revealed unexpected and unique molecular properties of rotavirus proteins of general interest to virologists, biochemists, and cell biologists. Rotaviruses share some features of replication with reoviruses, yet antigenic and molecular properties of the outer capsid proteins, VP4 (a protein whose cleavage is required for infectivity, possibly by mediating fusion with the cell membrane) and VP7 (a glycoprotein), show more similarities with those of other viruses such as the orthomyxoviruses, paramyxoviruses, and alphaviruses. Rotavirus morphogenesis is a unique process, during which immature subviral particles bud through the membrane of the endoplasmic reticulum (ER). During this process, transiently enveloped particles form, the outer capsid proteins are assembled onto particles, and mature particles accumulate in the lumen of the ER. Two ER-specific viral glycoproteins are involved in virus maturation, and these glycoproteins have been shown to be useful models for studying protein targeting and retention in the ER and for studying mechanisms of virus budding. New ideas and approaches to understanding how each gene functions to replicate and assemble the segmented viral genome have emerged from knowledge of the primary structure of rotavirus genes and their proteins and from knowledge of the properties of domains on individual proteins. Localization of type-specific and cross-reactive neutralizing epitopes on the outer capsid proteins is becoming increasingly useful in dissecting the protective immune response, including evaluation of vaccine trials, with the practical possibility of enhancing the production of new, more effective vaccines. Finally, future analyses with recently characterized immunologic and gene probes and new animal models can be expected to provide a basic understanding of what regulates the primary interactions of these viruses with the gastrointestinal tract and the subsequent responses of infected hosts.
Full text
PDF







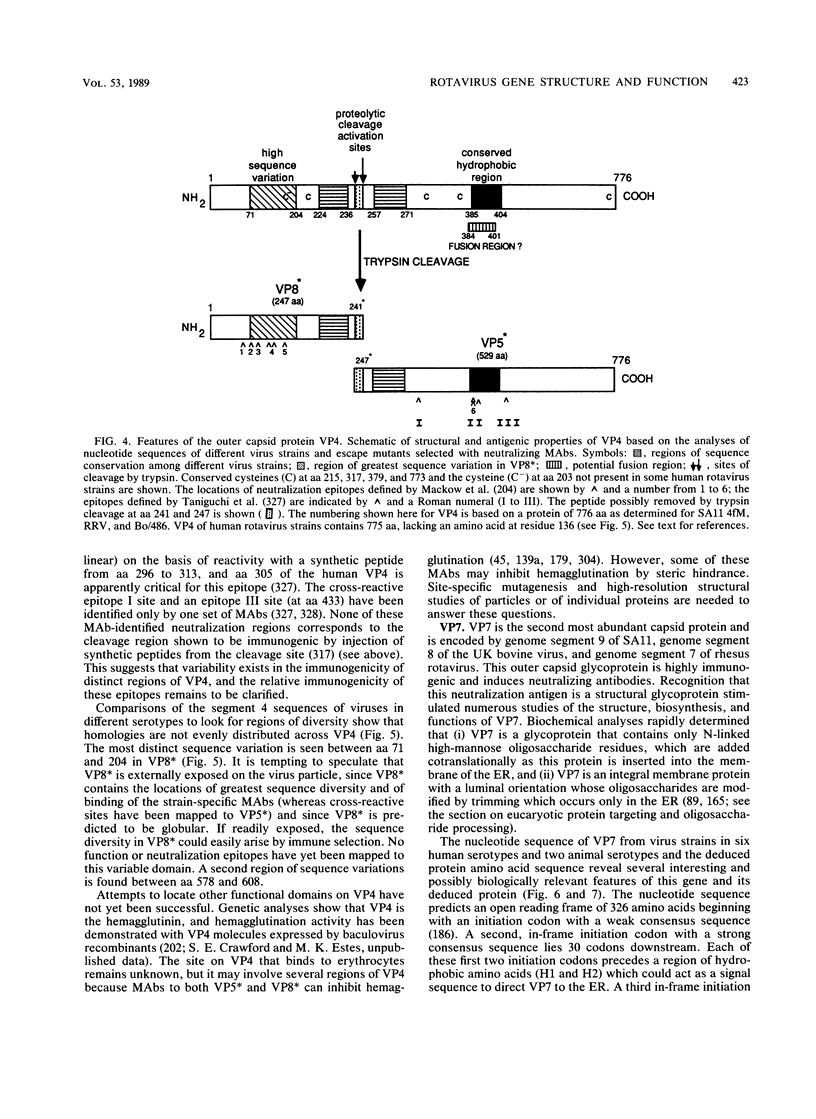
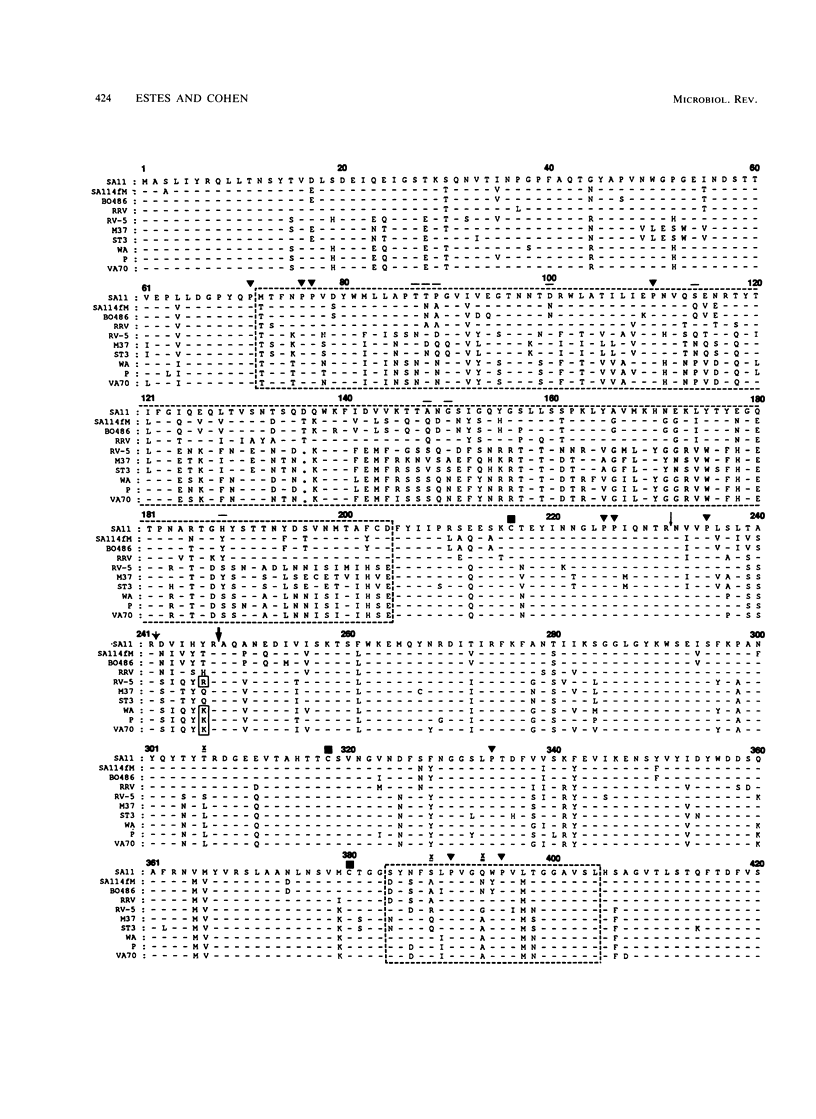
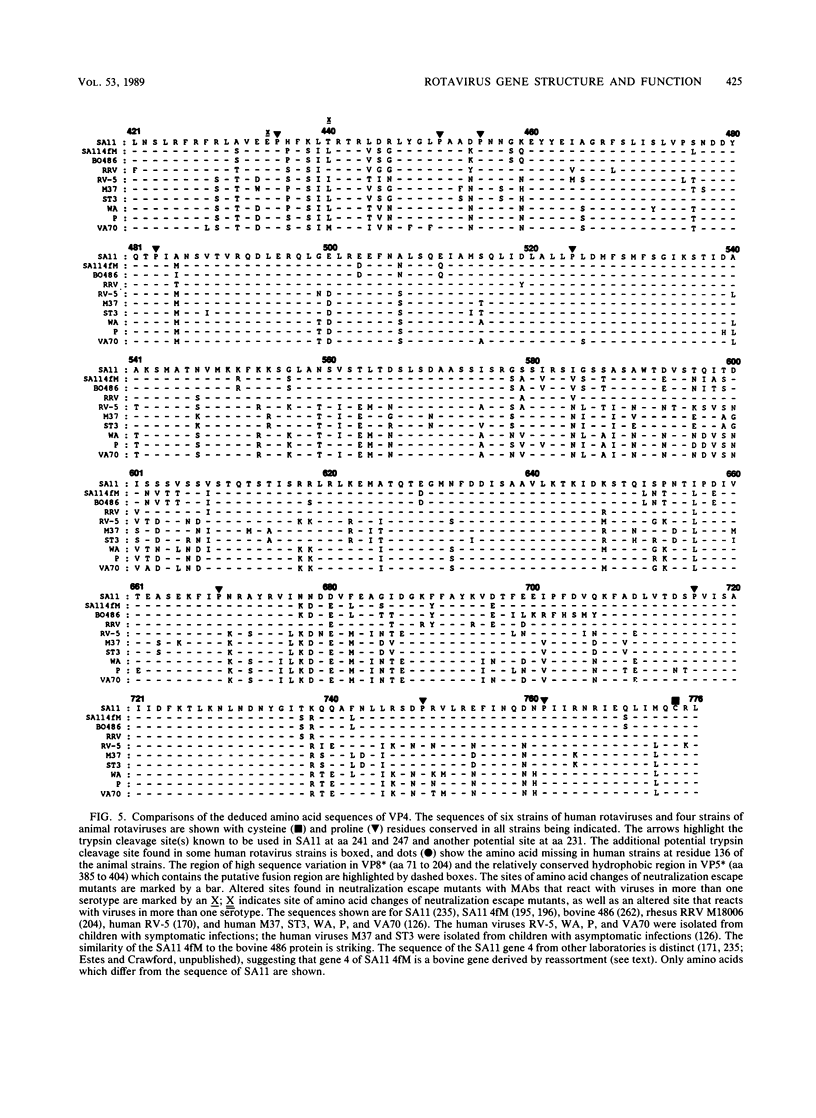

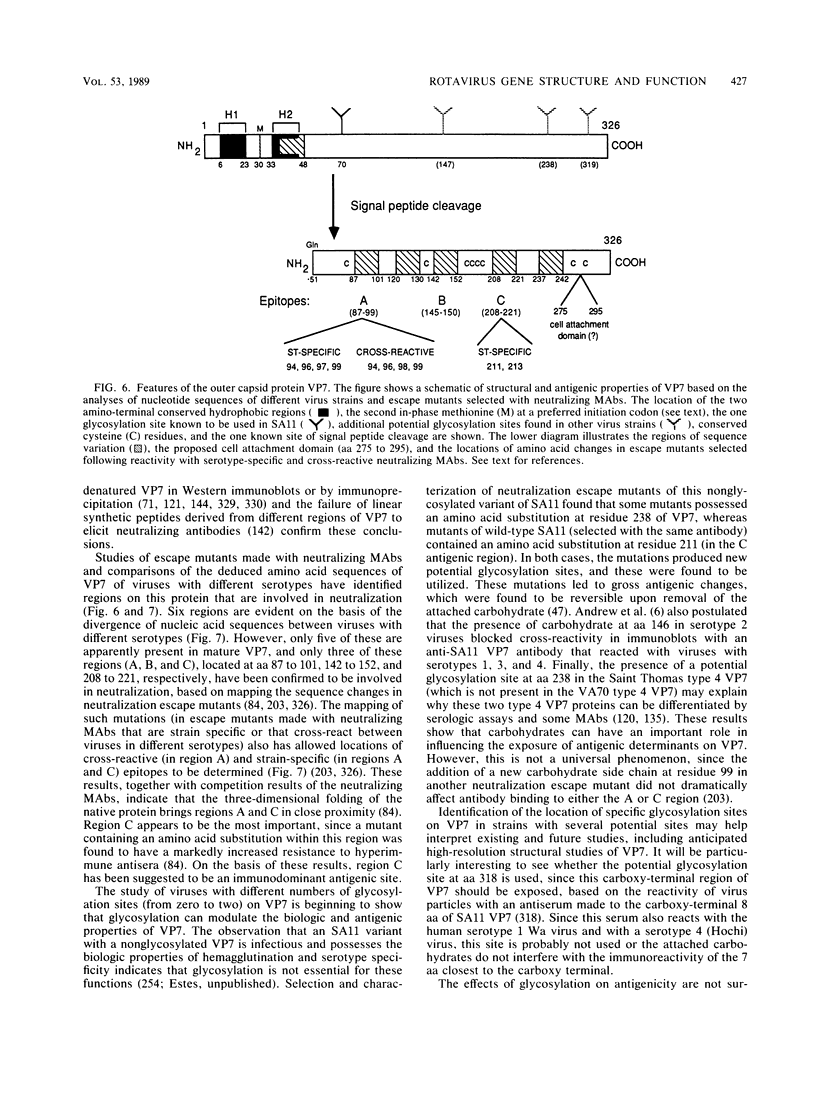



















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboudy Y., Shif I., Zilberstein I., Gotlieb-Stematsky T. Use of polyclonal and monoclonal antibodies and analysis of viral RNA in the detection of unusual group A human rotaviruses. J Med Virol. 1988 Jul;25(3):351–359. doi: 10.1002/jmv.1890250312. [DOI] [PubMed] [Google Scholar]
- Air G. M., Laver W. G., Webster R. G. Antigenic variation in influenza viruses. Contrib Microbiol Immunol. 1987;8:20–59. [PubMed] [Google Scholar]
- Albert M. J., Unicomb L. E., Tzipori S. R., Bishop R. F. Isolation and serotyping of animal rotaviruses and antigenic comparison with human rotaviruses. Brief report. Arch Virol. 1987;93(1-2):123–130. doi: 10.1007/BF01313898. [DOI] [PubMed] [Google Scholar]
- Allen A. M., Desselberger U. Reassortment of human rotaviruses carrying rearranged genomes with bovine rotavirus. J Gen Virol. 1985 Dec;66(Pt 12):2703–2714. doi: 10.1099/0022-1317-66-12-2703. [DOI] [PubMed] [Google Scholar]
- Altenburg B. C., Graham D. Y., Estes M. K. Ultrastructural study of rotavirus replication in cultured cells. J Gen Virol. 1980 Jan;46(1):75–85. doi: 10.1099/0022-1317-46-1-75. [DOI] [PubMed] [Google Scholar]
- Andrew M. E., Boyle D. B., Coupar B. E., Whitfeld P. L., Both G. W., Bellamy A. R. Vaccinia virus recombinants expressing the SA11 rotavirus VP7 glycoprotein gene induce serotype-specific neutralizing antibodies. J Virol. 1987 Apr;61(4):1054–1060. doi: 10.1128/jvi.61.4.1054-1060.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antczak J. B., Chmelo R., Pickup D. J., Joklik W. K. Sequence at both termini of the 10 genes of reovirus serotype 3 (strain Dearing). Virology. 1982 Sep;121(2):307–319. doi: 10.1016/0042-6822(82)90170-2. [DOI] [PubMed] [Google Scholar]
- Arias C. F., Ballado T., Plebañski M. Synthesis of the outer-capsid glycoprotein of the simian rotavirus SA11 in Escherichia coli. Gene. 1986;47(2-3):211–219. doi: 10.1016/0378-1119(86)90065-x. [DOI] [PubMed] [Google Scholar]
- Arias C. F., Lizano M., López S. Synthesis in Escherichia coli and immunological characterization of a polypeptide containing the cleavage sites associated with trypsin enhancement of rotavirus SA11 infectivity. J Gen Virol. 1987 Mar;68(Pt 3):633–642. doi: 10.1099/0022-1317-68-3-633. [DOI] [PubMed] [Google Scholar]
- Arias C. F., López S., Bell J. R., Strauss J. H. Primary structure of the neutralization antigen of simian rotavirus SA11 as deduced from cDNA sequence. J Virol. 1984 May;50(2):657–661. doi: 10.1128/jvi.50.2.657-661.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C. F., López S., Espejo R. T. Gene protein products of SA11 simian rotavirus genome. J Virol. 1982 Jan;41(1):42–50. doi: 10.1128/jvi.41.1.42-50.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson P. H., Lee J. T. Co-translational excision of alpha-glucose and alpha-mannose in nascent vesicular stomatitis virus G protein. J Cell Biol. 1984 Jun;98(6):2245–2249. doi: 10.1083/jcb.98.6.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au K. S., Chan W. K., Burns J. W., Estes M. K. Receptor activity of rotavirus nonstructural glycoprotein NS28. J Virol. 1989 Nov;63(11):4553–4562. doi: 10.1128/jvi.63.11.4553-4562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett A. V., 3rd, Bednarz-Prashad A. J., DuPont H. L., Pickering L. K. Rotavirus gastroenteritis. Annu Rev Med. 1987;38:399–415. doi: 10.1146/annurev.me.38.020187.002151. [DOI] [PubMed] [Google Scholar]
- Bartlett A. V., 3rd, Reves R. R., Pickering L. K. Rotavirus in infant-toddler day care centers: epidemiology relevant to disease control strategies. J Pediatr. 1988 Sep;113(3):435–441. doi: 10.1016/S0022-3476(88)80624-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassel-Duby R., Spriggs D. R., Tyler K. L., Fields B. N. Identification of attenuating mutations on the reovirus type 3 S1 double-stranded RNA segment with a rapid sequencing technique. J Virol. 1986 Oct;60(1):64–67. doi: 10.1128/jvi.60.1.64-67.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastardo J. W., Holmes I. H. Attachment of SA-11 rotavirus to erythrocyte receptors. Infect Immun. 1980 Sep;29(3):1134–1140. doi: 10.1128/iai.29.3.1134-1140.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastardo J. W., McKimm-Breschkin J. L., Sonza S., Mercer L. D., Holmes I. H. Preparation and characterization of antisera to electrophoretically purified SA11 virus polypeptides. Infect Immun. 1981 Dec;34(3):641–647. doi: 10.1128/iai.34.3.641-647.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baybutt H. N., McCrae M. A. The molecular biology of rotaviruses. VII. Detailed structural analysis of gene 10 of bovine rotavirus. Virus Res. 1984 Oct;1(7):533–541. doi: 10.1016/0168-1702(84)90011-x. [DOI] [PubMed] [Google Scholar]
- Beards G. M., Desselberger U. Determination of rotavirus serotype-specific antibodies in sera by competitive enhanced enzyme immunoassay. J Virol Methods. 1989 Apr-May;24(1-2):103–110. doi: 10.1016/0166-0934(89)90012-8. [DOI] [PubMed] [Google Scholar]
- Beards G. M. Polymorphism of genomic RNAs within rotavirus serotypes and subgroups. Arch Virol. 1982;74(1):65–70. doi: 10.1007/BF01320783. [DOI] [PubMed] [Google Scholar]
- Bellinzoni R. C., Blackhall J., Baro N., Auza N., Mattion N., Casaro A., La Torre J. L., Scodeller E. A. Efficacy of an inactivated oil-adjuvanted rotavirus vaccine in the control of calf diarrhoea in beef herds in Argentina. Vaccine. 1989 Jun;7(3):263–268. doi: 10.1016/0264-410X(89)90241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinzoni R. C., Mattion N. M., Burrone O., Gonzalez A., La Torre J. L., Scodeller E. A. Isolation of group A swine rotaviruses displaying atypical electropherotypes. J Clin Microbiol. 1987 May;25(5):952–954. doi: 10.1128/jcm.25.5.952-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinzoni R., Xi J. A., Tanaka T. N., Scodeller E., Estes M. K. Rotavirus gene detection with biotinylated single-stranded RNA probes. Mol Cell Probes. 1989 Sep;3(3):233–244. doi: 10.1016/0890-8508(89)90004-2. [DOI] [PubMed] [Google Scholar]
- Bergmann C. C., Maass D., Poruchynsky M. S., Atkinson P. H., Bellamy A. R. Topology of the non-structural rotavirus receptor glycoprotein NS28 in the rough endoplasmic reticulum. EMBO J. 1989 Jun;8(6):1695–1703. doi: 10.1002/j.1460-2075.1989.tb03561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besselaar T. G., Rosenblatt A., Kidd A. H. Atypical rotavirus from South African neonates. Brief report. Arch Virol. 1986;87(3-4):327–330. doi: 10.1007/BF01315311. [DOI] [PubMed] [Google Scholar]
- Bican P., Cohen J., Charpilienne A., Scherrer R. Purification and characterization of bovine rotavirus cores. J Virol. 1982 Sep;43(3):1113–1117. doi: 10.1128/jvi.43.3.1113-1117.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch C. J., Heath R. L., Gust I. D. Use of serotype-specific monoclonal antibodies to study the epidemiology of rotavirus infection. J Med Virol. 1988 Jan;24(1):45–53. doi: 10.1002/jmv.1890240107. [DOI] [PubMed] [Google Scholar]
- Biryahwaho B., Hundley F., Desselberger U. Bovine rotavirus with rearranged genome reassorts with human rotavirus. Brief report. Arch Virol. 1987;96(3-4):257–264. doi: 10.1007/BF01320965. [DOI] [PubMed] [Google Scholar]
- Bishop R. F., Barnes G. L., Cipriani E., Lund J. S. Clinical immunity after neonatal rotavirus infection. A prospective longitudinal study in young children. N Engl J Med. 1983 Jul 14;309(2):72–76. doi: 10.1056/NEJM198307143090203. [DOI] [PubMed] [Google Scholar]
- Boere W. A., Harmsen T., Vinjé J., Benaissa-Trouw B. J., Kraaijeveld C. A., Snippe H. Identification of distinct antigenic determinants on Semliki Forest virus by using monoclonal antibodies with different antiviral activities. J Virol. 1984 Nov;52(2):575–582. doi: 10.1128/jvi.52.2.575-582.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borras-Cuesta F., Petit-Camurdan A., Fedon Y. Engineering of immunogenic peptides by co-linear synthesis of determinants recognized by B and T cells. Eur J Immunol. 1987 Aug;17(8):1213–1215. doi: 10.1002/eji.1830170820. [DOI] [PubMed] [Google Scholar]
- Both G. W., Bellamy A. R., Siegman L. J. Nucleotide sequence of the dsRNA genomic segment 7 of Simian 11 rotavirus. Nucleic Acids Res. 1984 Feb 10;12(3):1621–1626. doi: 10.1093/nar/12.3.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Bellamy A. R., Street J. E., Siegman L. J. A general strategy for cloning double-stranded RNA: nucleotide sequence of the Simian-11 rotavirus gene 8. Nucleic Acids Res. 1982 Nov 25;10(22):7075–7088. doi: 10.1093/nar/10.22.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Jennings P. A. Gene cloning to study viral antigens: prospects for novel influenza and rotavirus vaccines. Prog Vet Microbiol Immunol. 1987;3:179–213. [PubMed] [Google Scholar]
- Both G. W., Mattick J. S., Bellamy A. R. Serotype-specific glycoprotein of simian 11 rotavirus: coding assignment and gene sequence. Proc Natl Acad Sci U S A. 1983 May;80(10):3091–3095. doi: 10.1073/pnas.80.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Siegman L. J., Bellamy A. R., Atkinson P. H. Coding assignment and nucleotide sequence of simian rotavirus SA11 gene segment 10: location of glycosylation sites suggests that the signal peptide is not cleaved. J Virol. 1983 Nov;48(2):335–339. doi: 10.1128/jvi.48.2.335-339.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Siegman L. J., Bellamy A. R., Ikegami N., Shatkin A. J., Furuichi Y. Comparative sequence analysis of rotavirus genomic segment 6--the gene specifying viral subgroups 1 and 2. J Virol. 1984 Jul;51(1):97–101. doi: 10.1128/jvi.51.1.97-101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. F., Holmes K. V. RNA-binding proteins of bovine rotavirus. J Virol. 1986 May;58(2):561–568. doi: 10.1128/jvi.58.2.561-568.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremont M., Charpilienne A., Chabanne D., Cohen J. Nucleotide sequence and expression in Escherichia coli of the gene encoding the nonstructural protein NCVP2 of bovine rotavirus. Virology. 1987 Nov;161(1):138–144. doi: 10.1016/0042-6822(87)90179-6. [DOI] [PubMed] [Google Scholar]
- Bremont M., Cohen J., McCrae M. A. Analysis of the structural polypeptides of a porcine group C rotavirus. J Virol. 1988 Jun;62(6):2183–2185. doi: 10.1128/jvi.62.6.2183-2185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger J. C. Novel rotaviruses in animals and man. Ciba Found Symp. 1987;128:5–23. doi: 10.1002/9780470513460.ch2. [DOI] [PubMed] [Google Scholar]
- Bridger J. C., Oldham G. Avirulent rotavirus infections protect calves from disease with and without inducing high levels of neutralizing antibody. J Gen Virol. 1987 Sep;68(Pt 9):2311–2317. doi: 10.1099/0022-1317-68-9-2311. [DOI] [PubMed] [Google Scholar]
- Bridger J. C., Pedley S., McCrae M. A. Group C rotaviruses in humans. J Clin Microbiol. 1986 Apr;23(4):760–763. doi: 10.1128/jcm.23.4.760-763.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. W., Beards G. M., Chen G. M., Flewett T. H. Prevalence of antibody to group B (atypical) rotavirus in humans and animals. J Clin Microbiol. 1987 Feb;25(2):316–319. doi: 10.1128/jcm.25.2.316-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. W., Mathan M. M., Mathew M., Martin R., Beards G. M., Mathan V. I. Rotavirus epidemiology in Vellore, south India: group, subgroup, serotype, and electrophoretype. J Clin Microbiol. 1988 Nov;26(11):2410–2414. doi: 10.1128/jcm.26.11.2410-2414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüssow H., Marc-Martin S., Eichhorn W., Sidoti J., Fryder V. Characterization of a second bovine rotavirus serotype. Arch Virol. 1987;94(1-2):29–41. doi: 10.1007/BF01313723. [DOI] [PubMed] [Google Scholar]
- Brüssow H., Walther I., Fryder V., Sidoti J., Bruttin A. Cross-neutralizing antibodies induced by single serotype vaccination of cows with rotavirus. J Gen Virol. 1988 Jul;69(Pt 7):1647–1658. doi: 10.1099/0022-1317-69-7-1647. [DOI] [PubMed] [Google Scholar]
- Burns J. W., Chen D., Estes M. K., Ramig R. F. Biological and immunological characterization of a simian rotavirus SA11 variant with an altered genome segment 4. Virology. 1989 Apr;169(2):427–435. doi: 10.1016/0042-6822(89)90168-2. [DOI] [PubMed] [Google Scholar]
- Burns J. W., Greenberg H. B., Shaw R. D., Estes M. K. Functional and topographical analyses of epitopes on the hemagglutinin (VP4) of the simian rotavirus SA11. J Virol. 1988 Jun;62(6):2164–2172. doi: 10.1128/jvi.62.6.2164-2172.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. W., Welch S. K., Nakata S., Estes M. K. Characterization of monoclonal antibodies to human group B rotavirus and their use in an antigen detection enzyme-linked immunosorbent assay. J Clin Microbiol. 1989 Feb;27(2):245–250. doi: 10.1128/jcm.27.2.245-250.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caust J., Dyall-Smith M. L., Lazdins I., Holmes I. H. Glycosylation, an important modifier of rotavirus antigenicity. Arch Virol. 1987;96(3-4):123–134. doi: 10.1007/BF01320955. [DOI] [PubMed] [Google Scholar]
- Chan W. K., Au K. S., Estes M. K. Topography of the simian rotavirus nonstructural glycoprotein (NS28) in the endoplasmic reticulum membrane. Virology. 1988 Jun;164(2):435–442. doi: 10.1016/0042-6822(88)90557-0. [DOI] [PubMed] [Google Scholar]
- Chan W. K., Penaranda M. E., Crawford S. E., Estes M. K. Two glycoproteins are produced from the rotavirus neutralization gene. Virology. 1986 Jun;151(2):243–252. doi: 10.1016/0042-6822(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Chanas A. C., Ellis D. S., Stamford S., Gould E. A. The interaction of monoclonal antibodies directed against envelope glycoprotein E1 of Sindbis virus with virus-infected cells. Antiviral Res. 1982 Sep;2(4):191–201. doi: 10.1016/0166-3542(82)90042-0. [DOI] [PubMed] [Google Scholar]
- Chanas A. C., Gould E. A., Clegg J. C., Varma M. G. Monoclonal antibodies to Sindbis virus glycoprotein E1 can neutralize, enhance infectivity, and independently inhibit haemagglutination or haemolysis. J Gen Virol. 1982 Jan;58(Pt 1):37–46. doi: 10.1099/0022-1317-58-1-37. [DOI] [PubMed] [Google Scholar]
- Chanock R. M., Murphy B. R., Collins P. L., Coelingh K. V., Olmsted R. A., Snyder M. H., Spriggs M. K., Prince G. A., Moss B., Flores J. Live viral vaccines for respiratory and enteric tract diseases. Vaccine. 1988 Apr;6(2):129–133. doi: 10.1016/s0264-410x(88)80014-8. [DOI] [PubMed] [Google Scholar]
- Chanock S. J., Wenske E. A., Fields B. N. Human rotaviruses and genome RNA. J Infect Dis. 1983 Jul;148(1):49–50. doi: 10.1093/infdis/148.1.49. [DOI] [PubMed] [Google Scholar]
- Chen C. M., Hung T., Bridger J. C., McCrae M. A. Chinese adult rotavirus is a group B rotavirus. Lancet. 1985 Nov 16;2(8464):1123–1124. doi: 10.1016/s0140-6736(85)90710-x. [DOI] [PubMed] [Google Scholar]
- Chen D., Burns J. W., Estes M. K., Ramig R. F. Phenotypes of rotavirus reassortants depend upon the recipient genetic background. Proc Natl Acad Sci U S A. 1989 May;86(10):3743–3747. doi: 10.1073/pnas.86.10.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarini A., Arista S., Giammanco A., Sinatra A. Rotavirus persistence in cell cultures: selection of resistant cells in the presence of foetal calf serum. J Gen Virol. 1983 May;64(Pt 5):1101–1110. doi: 10.1099/0022-1317-64-5-1101. [DOI] [PubMed] [Google Scholar]
- Chiba S., Yokoyama T., Nakata S., Morita Y., Urasawa T., Taniguchi K., Urasawa S., Nakao T. Protective effect of naturally acquired homotypic and heterotypic rotavirus antibodies. Lancet. 1986 Aug 23;2(8504):417–421. doi: 10.1016/s0140-6736(86)92133-1. [DOI] [PubMed] [Google Scholar]
- Clark B., Desselberger U. Myristylation of rotavirus proteins. J Gen Virol. 1988 Oct;69(Pt 10):2681–2686. doi: 10.1099/0022-1317-69-10-2681. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Borian F. E., Bell L. M., Modesto K., Gouvea V., Plotkin S. A. Protective effect of WC3 vaccine against rotavirus diarrhea in infants during a predominantly serotype 1 rotavirus season. J Infect Dis. 1988 Sep;158(3):570–587. doi: 10.1093/infdis/158.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. F., Dolan K. T., Horton-Slight P., Palmer J., Plotkin S. A. Diverse serologic response to rotavirus infection of infants in a single epidemic. Pediatr Infect Dis. 1985 Nov-Dec;4(6):626–631. doi: 10.1097/00006454-198511000-00006. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Hoshino Y., Bell L. M., Groff J., Hess G., Bachman P., Offit P. A. Rotavirus isolate WI61 representing a presumptive new human serotype. J Clin Microbiol. 1987 Sep;25(9):1757–1762. doi: 10.1128/jcm.25.9.1757-1762.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. M., Barnett B. B., Spendlove R. S. Production of high-titer bovine rotavirus with trypsin. J Clin Microbiol. 1979 Mar;9(3):413–417. doi: 10.1128/jcm.9.3.413-417.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. M., Roth J. R., Clark M. L., Barnett B. B., Spendlove R. S. Trypsin enhancement of rotavirus infectivity: mechanism of enhancement. J Virol. 1981 Sep;39(3):816–822. doi: 10.1128/jvi.39.3.816-822.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. M., Spendlove R. S., Barnett B. B. Role of two particle types in bovine rotavirus morphogenesis. J Virol. 1980 Apr;34(1):272–276. doi: 10.1128/jvi.34.1.272-276.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Charpilienne A., Chilmonczyk S., Estes M. K. Nucleotide sequence of bovine rotavirus gene 1 and expression of the gene product in baculovirus. Virology. 1989 Jul;171(1):131–140. doi: 10.1016/0042-6822(89)90519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Laporte J., Charpilienne A., Scherrer R. Activation of rotavirus RNA polymerase by calcium chelation. Arch Virol. 1979;60(3-4):177–186. doi: 10.1007/BF01317489. [DOI] [PubMed] [Google Scholar]
- Cohen J., Lefevre F., Estes M. K., Bremont M. Cloning of bovine rotavirus (RF strain): nucleotide sequence of the gene coding for the major capsid protein. Virology. 1984 Oct 15;138(1):178–182. doi: 10.1016/0042-6822(84)90159-4. [DOI] [PubMed] [Google Scholar]
- Cohen J. Ribonucleic acid polymerase activity associated with purified calf rotavirus. J Gen Virol. 1977 Sep;36(3):395–402. doi: 10.1099/0022-1317-36-3-395. [DOI] [PubMed] [Google Scholar]
- Conner M. E., Estes M. K., Graham D. Y. Rabbit model of rotavirus infection. J Virol. 1988 May;62(5):1625–1633. doi: 10.1128/jvi.62.5.1625-1633.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S., Fowler K. J., Bishop R. F., Cotton R. G. Neutralizing monoclonal antibodies to human rotavirus and indications of antigenic drift among strains from neonates. J Virol. 1985 Apr;54(1):14–20. doi: 10.1128/jvi.54.1.14-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S., Tursi J. M., McAdam W. J., Bishop R. F. Derivation of neutralizing monoclonal antibodies to human rotaviruses and evidence that an immunodominant neutralization site is shared between serotypes 1 and 3. Virology. 1986 Oct 30;154(2):302–312. doi: 10.1016/0042-6822(86)90456-3. [DOI] [PubMed] [Google Scholar]
- Coulson B. S., Unicomb L. E., Pitson G. A., Bishop R. F. Simple and specific enzyme immunoassay using monoclonal antibodies for serotyping human rotaviruses. J Clin Microbiol. 1987 Mar;25(3):509–515. doi: 10.1128/jcm.25.3.509-515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S. Variation in neutralization epitopes of human rotaviruses in relation to genomic RNA polymorphism. Virology. 1987 Aug;159(2):209–216. doi: 10.1016/0042-6822(87)90457-0. [DOI] [PubMed] [Google Scholar]
- Cukor G., Blacklow N. R. Human viral gastroenteritis. Microbiol Rev. 1984 Jun;48(2):157–179. doi: 10.1128/mr.48.2.157-179.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharakul T., Riepenhoff-Talty M., Albini B., Ogra P. L. Distribution of rotavirus antigen in intestinal lymphoid tissues: potential role in development of the mucosal immune response to rotavirus. Clin Exp Immunol. 1988 Oct;74(1):14–19. [PMC free article] [PubMed] [Google Scholar]
- Di Matteo A., Sarasini A., Scotta M. S., Parea M., Licardi G., Gerna G. Nosocomial outbreak of infant rotavirus diarrhea due to the appearance of a new serotype 4 strain. J Med Virol. 1989 Feb;27(2):100–104. doi: 10.1002/jmv.1890270206. [DOI] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Azad A. A., Holmes I. H. Gene mapping of rotavirus double-stranded RNA segments by northern blot hybridization: application to segments 7, 8, and 9. J Virol. 1983 Apr;46(1):317–320. doi: 10.1128/jvi.46.1.317-320.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Elleman T. C., Hoyne P. A., Holmes I. H., Azad A. A. Cloning and sequence of UK bovine rotavirus gene segment 7: marked sequence homology with simian rotavirus gene segment 8. Nucleic Acids Res. 1983 May 25;11(10):3351–3362. doi: 10.1093/nar/11.10.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Holmes I. H. Comparisons of rotavirus polypeptides by limited proteolysis: close similarity of certain polypeptides of different strains. J Virol. 1981 Dec;40(3):720–728. doi: 10.1128/jvi.40.3.720-728.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Holmes I. H. Gene-coding assignments of rotavirus double-stranded RNA segments 10 and 11. J Virol. 1981 Jun;38(3):1099–1103. doi: 10.1128/jvi.38.3.1099-1103.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Holmes I. H. Sequence homology between human and animal rotavirus serotype-specific glycoproteins. Nucleic Acids Res. 1984 May 11;12(9):3973–3982. doi: 10.1093/nar/12.9.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Lazdins I., Tregear G. W., Holmes I. H. Location of the major antigenic sites involved in rotavirus serotype-specific neutralization. Proc Natl Acad Sci U S A. 1986 May;83(10):3465–3468. doi: 10.1073/pnas.83.10.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton B. T., Gould A. R. Isolation and characterization of orbivirus genotypic variants. Virus Res. 1987 Jan;6(4):363–382. doi: 10.1016/0168-1702(87)90067-0. [DOI] [PubMed] [Google Scholar]
- Edelman R. Perspective on the development and deployment of rotavirus vaccines. Pediatr Infect Dis J. 1987 Aug;6(8):704–710. doi: 10.1097/00006454-198708000-00002. [DOI] [PubMed] [Google Scholar]
- Elleman T. C., Hoyne P. A., Dyall-Smith M. L., Holmes I. H., Azad A. A. Nucleotide sequence of the gene encoding the serotype-specific glycoprotein of UK bovine rotavirus. Nucleic Acids Res. 1983 Jul 25;11(14):4689–4701. doi: 10.1093/nar/11.14.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson B. L., Graham D. Y., Mason B. B., Estes M. K. Identification, synthesis, and modifications of simian rotavirus SA11 polypeptides in infected cells. J Virol. 1982 Jun;42(3):825–839. doi: 10.1128/jvi.42.3.825-839.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson B. L., Graham D. Y., Mason B. B., Hanssen H. H., Estes M. K. Two types of glycoprotein precursors are produced by the simian rotavirus SA11. Virology. 1983 Jun;127(2):320–332. doi: 10.1016/0042-6822(83)90147-2. [DOI] [PubMed] [Google Scholar]
- Esparza J., Gil F. A study on the ultrastructure of human rotavirus. Virology. 1978 Nov;91(1):141–150. doi: 10.1016/0042-6822(78)90362-8. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., López S., Arias C. Structural polypeptides of simian rotavirus SA11 and the effect of trypsin. J Virol. 1981 Jan;37(1):156–160. doi: 10.1128/jvi.37.1.156-160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Puerto F., Soler C., González N. Characterization of a human pararotavirus. Infect Immun. 1984 Apr;44(1):112–116. doi: 10.1128/iai.44.1.112-116.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Conner M. E., Gilger M. A., Graham D. Y. Molecular biology and immunology of rotavirus infections. Immunol Invest. 1989 Jan-May;18(1-4):571–581. doi: 10.3109/08820138909112264. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Crawford S. E., Penaranda M. E., Petrie B. L., Burns J. W., Chan W. K., Ericson B., Smith G. E., Summers M. D. Synthesis and immunogenicity of the rotavirus major capsid antigen using a baculovirus expression system. J Virol. 1987 May;61(5):1488–1494. doi: 10.1128/jvi.61.5.1488-1494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Dimitrov D. H. The molecular epidemiology of rotavirus gastroenteritis. Prog Med Virol. 1984;29:1–22. [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Mason B. B. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J Virol. 1981 Sep;39(3):879–888. doi: 10.1128/jvi.39.3.879-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y. Rotavirus antigens. Adv Exp Med Biol. 1985;185:201–214. doi: 10.1007/978-1-4684-7974-4_13. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Smith E. M., Gerba C. P. Rotavirus stability and inactivation. J Gen Virol. 1979 May;43(2):403–409. doi: 10.1099/0022-1317-43-2-403. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Mason B. B., Crawford S., Cohen J. Cloning and nucleotide sequence of the simian rotavirus gene 6 that codes for the major inner capsid protein. Nucleic Acids Res. 1984 Feb 24;12(4):1875–1887. doi: 10.1093/nar/12.4.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Palmer E. L., Obijeski J. F. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Fang Z. Y., Glass R. I., Penaranda M., Dong H., Monroe S. S., Wen L., Estes M. K., Eiden J., Yolken R. H., Saif L. Purification and characterization of adult diarrhea rotavirus: identification of viral structural proteins. J Virol. 1989 May;63(5):2191–2197. doi: 10.1128/jvi.63.5.2191-2197.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner-Valle G. P., Clayton A. V., McCrae M. A. Molecular biology of rotaviruses. III. Isolation and characterization of temperature-sensitive mutants of bovine rotavirus. J Virol. 1982 May;42(2):669–677. doi: 10.1128/jvi.42.2.669-677.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewett T. H., Bryden A. S., Davies H., Woode G. N., Bridger J. C., Derrick J. M. Relation between viruses from acute gastroenteritis of children and newborn calves. Lancet. 1974 Jul 13;2(7872):61–63. doi: 10.1016/s0140-6736(74)91631-6. [DOI] [PubMed] [Google Scholar]
- Flewett T. H., Woode G. N. The rotaviruses. Arch Virol. 1978;57(1):1–23. doi: 10.1007/BF01315633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Midthun K., Hoshino Y., Green K., Gorziglia M., Kapikian A. Z., Chanock R. M. Conservation of the fourth gene among rotaviruses recovered from asymptomatic newborn infants and its possible role in attenuation. J Virol. 1986 Dec;60(3):972–979. doi: 10.1128/jvi.60.3.972-979.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Blanco M., Vilar M., Garcia D., Perez M., Daoud N., Midthun K., Kapikian A. Z. Reactions to and antigenicity of two human-rhesus rotavirus reassortant vaccine candidates of serotypes 1 and 2 in Venezuelan infants. J Clin Microbiol. 1989 Mar;27(3):512–518. doi: 10.1128/jcm.27.3.512-518.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francavilla M., Miranda P., Di Matteo A., Sarasini A., Gerna G., Milanesi G. Expression of bovine rotavirus neutralization antigen in Escherichia coli. J Gen Virol. 1987 Nov;68(Pt 11):2975–2980. doi: 10.1099/0022-1317-68-11-2975. [DOI] [PubMed] [Google Scholar]
- Friedman M. G., Galil A., Sarov B., Margalith M., Katzir G., Midthun K., Taniguchi K., Urasawa S., Kapikian A. Z., Edelman R. Two sequential outbreaks of rotavirus gastroenteritis: evidence for symptomatic and asymptomatic reinfections. J Infect Dis. 1988 Oct;158(4):814–822. doi: 10.1093/infdis/158.4.814. [DOI] [PubMed] [Google Scholar]
- Fukudome K., Yoshie O., Konno T. Comparison of human, simian, and bovine rotaviruses for requirement of sialic acid in hemagglutination and cell adsorption. Virology. 1989 Sep;172(1):196–205. doi: 10.1016/0042-6822(89)90121-9. [DOI] [PubMed] [Google Scholar]
- Fukuhara N., Yoshie O., Kitaoka S., Konno T., Ishida N. Evidence for endocytosis-independent infection by human rotavirus. Arch Virol. 1987;97(1-2):93–99. doi: 10.1007/BF01310737. [DOI] [PubMed] [Google Scholar]
- Fukuhara N., Yoshie O., Kitaoka S., Konno T. Role of VP3 in human rotavirus internalization after target cell attachment via VP7. J Virol. 1988 Jul;62(7):2209–2218. doi: 10.1128/jvi.62.7.2209-2218.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong D. B., Nibert M. L., Fields B. N. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J Virol. 1988 Jan;62(1):246–256. doi: 10.1128/jvi.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Muthukrishnan S., Shatkin A. J. Reovirus messenger RNA contains a methylated, blocked 5'-terminal structure: m-7G(5')ppp(5')G-MpCp-. Proc Natl Acad Sci U S A. 1975 Jan;72(1):362–366. doi: 10.1073/pnas.72.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbarg-Chenon A., Bricout F., Nicolas J. C. Serological characterization of human reassortant rotaviruses. J Virol. 1986 Aug;59(2):510–513. doi: 10.1128/jvi.59.2.510-513.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Gary G. W., Jr, Black D. R., Palmer E. Monoclonal IgG to the inner capsid of human rotavirus. Arch Virol. 1982;72(3):223–227. doi: 10.1007/BF01348968. [DOI] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., di Matteo A., Parea M., Orsolini P., Battaglia M. Identification of two subtypes of serotype 4 human rotavirus by using VP7-specific neutralizing monoclonal antibodies. J Clin Microbiol. 1988 Jul;26(7):1388–1392. doi: 10.1128/jcm.26.7.1388-1392.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., di Matteo A., Passarani N., Gagliardi V., Milanesi G., Astaldi Ricotti G. C., Battaglia M. The outer capsid glycoprotein VP7 of simian rotavirus SA11 contains two distinct neutralization epitopes. J Gen Virol. 1988 Apr;69(Pt 4):937–944. doi: 10.1099/0022-1317-69-4-937. [DOI] [PubMed] [Google Scholar]
- Glass R. I., Keith J., Nakagomi O., Nakagomi T., Askaa J., Kapikian A. Z., Chanock R. M., Flores J. Nucleotide sequence of the structural glycoprotein VP7 gene of Nebraska calf diarrhea virus rotavirus: comparison with homologous genes from four strains of human and animal rotaviruses. Virology. 1985 Mar;141(2):292–298. doi: 10.1016/0042-6822(85)90260-0. [DOI] [PubMed] [Google Scholar]
- Gombold J. L., Ramig R. F. Analysis of reassortment of genome segments in mice mixedly infected with rotaviruses SA11 and RRV. J Virol. 1986 Jan;57(1):110–116. doi: 10.1128/jvi.57.1.110-116.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombold J. L., Ramig R. F. Assignment of simian rotavirus SA11 temperature-sensitive mutant groups A, C, F, and G to genome segments. Virology. 1987 Dec;161(2):463–473. doi: 10.1016/0042-6822(87)90140-1. [DOI] [PubMed] [Google Scholar]
- González S. A., Mattion N. M., Bellinzoni R., Burrone O. R. Structure of rearranged genome segment 11 in two different rotavirus strains generated by a similar mechanism. J Gen Virol. 1989 Jun;70(Pt 6):1329–1336. doi: 10.1099/0022-1317-70-6-1329. [DOI] [PubMed] [Google Scholar]
- Gorziglia M., Aguirre Y., Hoshino Y., Esparza J., Blumentals I., Askaa J., Thompson M., Glass R. I., Kapikian A. Z., Chanock R. M. VP7 serotype-specific glycoprotein of OSU porcine rotavirus: coding assignment and gene sequence. J Gen Virol. 1986 Nov;67(Pt 11):2445–2454. doi: 10.1099/0022-1317-67-11-2445. [DOI] [PubMed] [Google Scholar]
- Gorziglia M., Green K., Nishikawa K., Taniguchi K., Jones R., Kapikian A. Z., Chanock R. M. Sequence of the fourth gene of human rotaviruses recovered from asymptomatic or symptomatic infections. J Virol. 1988 Aug;62(8):2978–2984. doi: 10.1128/jvi.62.8.2978-2984.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M., Hoshino Y., Buckler-White A., Blumentals I., Glass R., Flores J., Kapikian A. Z., Chanock R. M. Conservation of amino acid sequence of VP8 and cleavage region of 84-kDa outer capsid protein among rotaviruses recovered from asymptomatic neonatal infection. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7039–7043. doi: 10.1073/pnas.83.18.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M., Hoshino Y., Nishikawa K., Maloy W. L., Jones R. W., Kapikian A. Z., Chanock R. M. Comparative sequence analysis of the genomic segment 6 of four rotaviruses each with a different subgroup specificity. J Gen Virol. 1988 Jul;69(Pt 7):1659–1669. doi: 10.1099/0022-1317-69-7-1659. [DOI] [PubMed] [Google Scholar]
- Gorziglia M., Larrea C., Liprandi F., Esparza J. Biochemical evidence for the oligomeric (possibly trimeric) structure of the major inner capsid polypeptide (45K) of rotaviruses. J Gen Virol. 1985 Sep;66(Pt 9):1889–1900. doi: 10.1099/0022-1317-66-9-1889. [DOI] [PubMed] [Google Scholar]
- Gorziglia M., Nishikawa K., Fukuhara N. Evidence of duplication and deletion in super short segment 11 of rabbit rotavirus Alabama strain. Virology. 1989 Jun;170(2):587–590. doi: 10.1016/0042-6822(89)90453-4. [DOI] [PubMed] [Google Scholar]
- Gorziglia M., Nishikawa K., Green K., Taniguchi K. Gene sequence of the VP7 serotype specific glycoprotein of Gottfried porcine rotavirus. Nucleic Acids Res. 1988 Jan 25;16(2):775–775. doi: 10.1093/nar/16.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A., Kudesia G., Allen A. M., Desselberger U. Reassortment of human rotavirus possessing genome rearrangements with bovine rotavirus: evidence for host cell selection. J Gen Virol. 1987 Jan;68(Pt 1):115–122. doi: 10.1099/0022-1317-68-1-115. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Estes M. K. Proteolytic enhancement of rotavirus infectivity: biology mechanism. Virology. 1980 Mar;101(2):432–439. doi: 10.1016/0042-6822(80)90456-0. [DOI] [PubMed] [Google Scholar]
- Green K. Y., Hoshino Y., Ikegami N. Sequence analysis of the gene encoding the serotype-specific glycoprotein (VP7) of two new human rotavirus serotypes. Virology. 1989 Feb;168(2):429–433. doi: 10.1016/0042-6822(89)90289-4. [DOI] [PubMed] [Google Scholar]
- Green K. Y., Midthun K., Gorziglia M., Hoshino Y., Kapikian A. Z., Chanock R. M., Flores J. Comparison of the amino acid sequences of the major neutralization protein of four human rotavirus serotypes. Virology. 1987 Nov;161(1):153–159. doi: 10.1016/0042-6822(87)90181-4. [DOI] [PubMed] [Google Scholar]
- Green K. Y., Sears J. F., Taniguchi K., Midthun K., Hoshino Y., Gorziglia M., Nishikawa K., Urasawa S., Kapikian A. Z., Chanock R. M. Prediction of human rotavirus serotype by nucleotide sequence analysis of the VP7 protein gene. J Virol. 1988 May;62(5):1819–1823. doi: 10.1128/jvi.62.5.1819-1823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Flores J., Kalica A. R., Wyatt R. G., Jones R. Gene coding assignments for growth restriction, neutralization and subgroup specificities of the W and DS-1 strains of human rotavirus. J Gen Virol. 1983 Feb;64(Pt 2):313–320. doi: 10.1099/0022-1317-64-2-313. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Kalica A. R., Wyatt R. G., Jones R. W., Kapikian A. Z., Chanock R. M. Rescue of noncultivatable human rotavirus by gene reassortment during mixed infection with ts mutants of a cultivatable bovine rotavirus. Proc Natl Acad Sci U S A. 1981 Jan;78(1):420–424. doi: 10.1073/pnas.78.1.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Vo P. T., Jones R. Cultivation and characterization of three strains of murine rotavirus. J Virol. 1986 Feb;57(2):585–590. doi: 10.1128/jvi.57.2.585-590.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H., McAuliffe V., Valdesuso J., Wyatt R., Flores J., Kalica A., Hoshino Y., Singh N. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect Immun. 1983 Jan;39(1):91–99. doi: 10.1128/iai.39.1.91-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood K., Lund J. C., Coulson B. S., Hudson I. L., Bishop R. F., Barnes G. L. Comparison of serum and mucosal antibody responses following severe acute rotavirus gastroenteritis in young children. J Clin Microbiol. 1988 Apr;26(4):732–738. doi: 10.1128/jcm.26.4.732-738.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn P. R., Sato F., Powell K. F., Bellamy A. R., Napier J. R., Harding D. R., Hancock W. S., Siegman L. J., Both G. W. Rotavirus neutralizing protein VP7: antigenic determinants investigated by sequence analysis and peptide synthesis. J Virol. 1985 Jun;54(3):791–797. doi: 10.1128/jvi.54.3.791-797.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsey N. A., Anderson E. L., Sears S. D., Steinhoff M., Wilson M., Belshe R. B., Midthun K., Kapikian A. Z., Chanock R. M., Samorodin R. Human-rhesus reassortant rotavirus vaccines: safety and immunogenicity in adults, infants, and children. J Infect Dis. 1988 Dec;158(6):1261–1267. doi: 10.1093/infdis/158.6.1261. [DOI] [PubMed] [Google Scholar]
- Heath R., Birch C., Gust I. Antigenic analysis of rotavirus isolates using monoclonal antibodies specific for human serotypes 1, 2, 3 and 4, and SA11. J Gen Virol. 1986 Nov;67(Pt 11):2455–2466. doi: 10.1099/0022-1317-67-11-2455. [DOI] [PubMed] [Google Scholar]
- Helmberger-Jones M., Patton J. T. Characterization of subviral particles in cells infected with simian rotavirus SA11. Virology. 1986 Dec;155(2):655–665. doi: 10.1016/0042-6822(86)90225-4. [DOI] [PubMed] [Google Scholar]
- Hilpert H., Brüssow H., Mietens C., Sidoti J., Lerner L., Werchau H. Use of bovine milk concentrate containing antibody to rotavirus to treat rotavirus gastroenteritis in infants. J Infect Dis. 1987 Jul;156(1):158–166. doi: 10.1093/infdis/156.1.158. [DOI] [PubMed] [Google Scholar]
- Ho M. S., Glass R. I., Pinsky P. F., Anderson L. J. Rotavirus as a cause of diarrheal morbidity and mortality in the United States. J Infect Dis. 1988 Nov;158(5):1112–1116. doi: 10.1093/infdis/158.5.1112. [DOI] [PubMed] [Google Scholar]
- Hofer J. M., Sato F., Street J. E., Bellamy A. R. Nucleotide sequence for gene 6 of rotavirus strain S2. Nucleic Acids Res. 1987 Sep 11;15(17):7175–7175. doi: 10.1093/nar/15.17.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Gorziglia M., Valdesuso J., Askaa J., Glass R. I., Kapikian A. Z. An equine rotavirus (FI-14 strain) which bears both subgroup I and subgroup II specificities on its VP6. Virology. 1987 Apr;157(2):488–496. doi: 10.1016/0042-6822(87)90291-1. [DOI] [PubMed] [Google Scholar]
- Hoshino Y., Saif L. J., Sereno M. M., Chanock R. M., Kapikian A. Z. Infection immunity of piglets to either VP3 or VP7 outer capsid protein confers resistance to challenge with a virulent rotavirus bearing the corresponding antigen. J Virol. 1988 Mar;62(3):744–748. doi: 10.1128/jvi.62.3.744-748.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Chanock R. M., Kapikian A. Z. Analysis by plaque reduction neutralization assay of intertypic rotaviruses suggests that gene reassortment occurs in vivo. J Clin Microbiol. 1987 Feb;25(2):290–294. doi: 10.1128/jcm.25.2.290-294.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Hrdy D. B. Epidemiology of rotaviral infection in adults. Rev Infect Dis. 1987 May-Jun;9(3):461–469. doi: 10.1093/clinids/9.3.461. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Hum C. P., Dyall-Smith M. L., Holmes I. H. The VP7 gene of a new G serotype of human rotavirus (B37) is similar to G3 proteins in the antigenic c region. Virology. 1989 May;170(1):55–61. doi: 10.1016/0042-6822(89)90351-6. [DOI] [PubMed] [Google Scholar]
- Hundley F., Biryahwaho B., Gow M., Desselberger U. Genome rearrangements of bovine rotavirus after serial passage at high multiplicity of infection. Virology. 1985 May;143(1):88–103. doi: 10.1016/0042-6822(85)90099-6. [DOI] [PubMed] [Google Scholar]
- Hundley F., McIntyre M., Clark B., Beards G., Wood D., Chrystie I., Desselberger U. Heterogeneity of genome rearrangements in rotaviruses isolated from a chronically infected immunodeficient child. J Virol. 1987 Nov;61(11):3365–3372. doi: 10.1128/jvi.61.11.3365-3372.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T., Chen G. M., Wang C. G., Yao H. L., Fang Z. Y., Chao T. X., Chou Z. Y., Ye W., Chang X. J., Den S. S. Waterborne outbreak of rotavirus diarrhoea in adults in China caused by a novel rotavirus. Lancet. 1984 May 26;1(8387):1139–1142. [PubMed] [Google Scholar]
- Imai M., Akatani K., Ikegami N., Furuichi Y. Capped and conserved terminal structures in human rotavirus genome double-stranded RNA segments. J Virol. 1983 Jul;47(1):125–136. doi: 10.1128/jvi.47.1.125-136.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M., Richardson M. A., Ikegami N., Shatkin A. J., Furuichi Y. Molecular cloning of double-stranded RNA virus genomes. Proc Natl Acad Sci U S A. 1983 Jan;80(2):373–377. doi: 10.1073/pnas.80.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. A., McCrae M. A. Molecular biology of rotaviruses. VIII. Quantitative analysis of regulation of gene expression during virus replication. J Virol. 1989 May;63(5):2048–2055. doi: 10.1128/jvi.63.5.2048-2055.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabcenell A. K., Atkinson P. H. Processing of the rough endoplasmic reticulum membrane glycoproteins of rotavirus SA11. J Cell Biol. 1985 Oct;101(4):1270–1280. doi: 10.1083/jcb.101.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabcenell A. K., Poruchynsky M. S., Bellamy A. R., Greenberg H. B., Atkinson P. H. Two forms of VP7 are involved in assembly of SA11 rotavirus in endoplasmic reticulum. J Virol. 1988 Aug;62(8):2929–2941. doi: 10.1128/jvi.62.8.2929-2941.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Flores J., Greenberg H. B. Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology. 1983 Feb;125(1):194–205. doi: 10.1016/0042-6822(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Wyatt R. G., Flores J., Sereno M. M., Kapikian A. Z., Chanock R. M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981 Jul 30;112(2):385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Kaljot K. T., Shaw R. D., Rubin D. H., Greenberg H. B. Infectious rotavirus enters cells by direct cell membrane penetration, not by endocytosis. J Virol. 1988 Apr;62(4):1136–1144. doi: 10.1128/jvi.62.4.1136-1144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantharidis P., Dyall-Smith M. L., Holmes I. H. Marked sequence variation between segment 4 genes of human RV-5 and simian SA 11 rotaviruses. Arch Virol. 1987;93(1-2):111–121. doi: 10.1007/BF01313897. [DOI] [PubMed] [Google Scholar]
- Kantharidis P., Dyall-Smith M. L., Tregear G. W., Holmes I. H. Nucleotide sequence of UK bovine rotavirus segment 4: possible host restriction of VP3 genes. Virology. 1988 Oct;166(2):308–315. doi: 10.1016/0042-6822(88)90501-6. [DOI] [PubMed] [Google Scholar]
- Kapahnke R., Rappold W., Desselberger U., Riesner D. The stiffness of dsRNA: hydrodynamic studies on fluorescence-labelled RNA segments of bovine rotavirus. Nucleic Acids Res. 1986 Apr 25;14(8):3215–3228. doi: 10.1093/nar/14.8.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A. Z., Cline W. L., Greenberg H. B., Wyatt R. G., Kalica A. R., Banks C. E., James H. D., Jr, Flores J., Chanock R. M. Antigenic characterization of human and animal rotaviruses by immune adherence hemagglutination assay (IAHA): evidence for distinctness of IAHA and neutralization antigens. Infect Immun. 1981 Aug;33(2):415–425. doi: 10.1128/iai.33.2.415-425.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A. Z., Flores J., Hoshino Y., Glass R. I., Midthun K., Gorziglia M., Chanock R. M. Rotavirus: the major etiologic agent of severe infantile diarrhea may be controllable by a "Jennerian" approach to vaccination. J Infect Dis. 1986 May;153(5):815–822. doi: 10.1093/infdis/153.5.815. [DOI] [PubMed] [Google Scholar]
- Keljo D. J., Kuhn M., Smith A. Acidification of endosomes is not important for the entry of rotavirus into the cell. J Pediatr Gastroenterol Nutr. 1988 Mar-Apr;7(2):257–263. doi: 10.1097/00005176-198803000-00016. [DOI] [PubMed] [Google Scholar]
- Keljo D. J., Smith A. K. Characterization of binding of simian rotavirus SA-11 to cultured epithelial cells. J Pediatr Gastroenterol Nutr. 1988 Mar-Apr;7(2):249–256. doi: 10.1097/00005176-198803000-00015. [DOI] [PubMed] [Google Scholar]
- Killen H. M., Dimmock N. J. Identification of a neutralization-specific antigen of a calf rotavirus. J Gen Virol. 1982 Oct;62(Pt 2):297–311. doi: 10.1099/0022-1317-62-2-297. [DOI] [PubMed] [Google Scholar]
- Kitaoka S., Fukuhara N., Tazawa F., Suzuki H., Sato T., Konno T., Ebina T., Ishida N. Characterization of monoclonal antibodies against human rotavirus hemagglutinin. J Med Virol. 1986 Aug;19(4):313–323. doi: 10.1002/jmv.1890190404. [DOI] [PubMed] [Google Scholar]
- Kitaoka S., Nakagomi T., Fukuhara N., Hoshino Y., Suzuki H., Nakagomi O., Kapikian A. Z., Ebina T., Konno T., Ishida N. Serologic characteristics of a human rotavirus isolate, AU-1, which has a "long" RNA pattern and subgroup I specificity. J Med Virol. 1987 Dec;23(4):351–357. doi: 10.1002/jmv.1890230407. [DOI] [PubMed] [Google Scholar]
- Kogasaka R., Akihara M., Horino K., Chiba S., Nakao T. A morphological study of human rotavirus. Arch Virol. 1979;61(1-2):41–48. doi: 10.1007/BF01320590. [DOI] [PubMed] [Google Scholar]
- Kohl S., Harmon M. W., Tang J. P. Cytokine-stimulated human natural killer cytotoxicity: response to rotavirus-infected cells. Pediatr Res. 1983 Nov;17(11):868–872. doi: 10.1203/00006450-198311000-00006. [DOI] [PubMed] [Google Scholar]
- Kouvelos K., Petric M., Middleton P. J. Comparison of bovine, simian and human rotavirus structural glycoproteins. J Gen Virol. 1984 Jul;65(Pt 7):1211–1214. doi: 10.1099/0022-1317-65-7-1211. [DOI] [PubMed] [Google Scholar]
- Kouvelos K., Petric M., Middleton P. J. Oligosaccharide composition of calf rotavirus. J Gen Virol. 1984 Jul;65(Pt 7):1159–1164. doi: 10.1099/0022-1317-65-7-1159. [DOI] [PubMed] [Google Scholar]
- Kozak M. Bifunctional messenger RNAs in eukaryotes. Cell. 1986 Nov 21;47(4):481–483. doi: 10.1016/0092-8674(86)90609-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Nishimura S., Smith R. E., Furuichi Y. Homologous Terminal Sequences in the Double-Stranded RNA Genome Segments of Cytoplasmic Polyhedrosis Virus of the Silkworm Bombyx mori. J Virol. 1982 Nov;44(2):538–543. doi: 10.1128/jvi.44.2.538-543.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Charpilienne A., Cohen J. Nucleotide sequence of the gene encoding for the RNA binding protein (VP2) of RF bovine rotavirus. Nucleic Acids Res. 1989 Mar 11;17(5):2126–2126. doi: 10.1093/nar/17.5.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. P., Marbehant P., Marissens D., Zissis G. Monoclonal antibodies directed against different antigenic determinants of rotavirus. J Virol. 1984 Jul;51(1):47–51. doi: 10.1128/jvi.51.1.47-51.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. P., Marissens D., Marbehant P., Zissis G. Prevalence of subgroup 1, 2, and 3 rotaviruses in Belgian children suffering from acute diarrhea (1978-1981). J Med Virol. 1983;11(1):31–38. doi: 10.1002/jmv.1890110105. [DOI] [PubMed] [Google Scholar]
- Lanata C. F., Black R. E., del Aguila R., Gil A., Verastegui H., Gerna G., Flores J., Kapikian A. Z., Andre F. E. Protection of Peruvian children against rotavirus diarrhea of specific serotypes by one, two, or three doses of the RIT 4237 attenuated bovine rotavirus vaccine. J Infect Dis. 1989 Mar;159(3):452–459. doi: 10.1093/infdis/159.3.452. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Keene J. D., Schubert M. The origins of defective interfering particles of the negative-strand RNA viruses. Cell. 1981 Oct;26(2 Pt 2):145–154. doi: 10.1016/0092-8674(81)90298-1. [DOI] [PubMed] [Google Scholar]
- Linhares A. C., Gabbay Y. B., Mascarenhas J. D., Freitas R. B., Flewett T. H., Beards G. M. Epidemiology of rotavirus subgroups and serotypes in Belem, Brazil: a three-year study. Ann Inst Pasteur Virol. 1988 Jan-Mar;139(1):89–99. doi: 10.1016/s0769-2617(88)80009-1. [DOI] [PubMed] [Google Scholar]
- Liu M., Offit P. A., Estes M. K. Identification of the simian rotavirus SA11 genome segment 3 product. Virology. 1988 Mar;163(1):26–32. doi: 10.1016/0042-6822(88)90230-9. [DOI] [PubMed] [Google Scholar]
- Losonsky G. A., Johnson J. P., Winkelstein J. A., Yolken R. H. Oral administration of human serum immunoglobulin in immunodeficient patients with viral gastroenteritis. A pharmacokinetic and functional analysis. J Clin Invest. 1985 Dec;76(6):2362–2367. doi: 10.1172/JCI112248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonsky G. A., Rennels M. B., Lim Y., Krall G., Kapikian A. Z., Levine M. M. Systemic and mucosal immune responses to rhesus rotavirus vaccine MMU 18006. Pediatr Infect Dis J. 1988 Jun;7(6):388–393. doi: 10.1097/00006454-198806000-00004. [DOI] [PubMed] [Google Scholar]
- Ludert J. E., Gil F., Liprandi F., Esparza J. The structure of the rotavirus inner capsid studied by electron microscopy of chemically disrupted particles. J Gen Virol. 1986 Aug;67(Pt 8):1721–1725. doi: 10.1099/0022-1317-67-8-1721. [DOI] [PubMed] [Google Scholar]
- Ludert J. E., Michelangeli F., Gil F., Liprandi F., Esparza J. Penetration and uncoating of rotaviruses in cultured cells. Intervirology. 1987;27(2):95–101. doi: 10.1159/000149726. [DOI] [PubMed] [Google Scholar]
- López S., Arias C. F., Bell J. R., Strauss J. H., Espejo R. T. Primary structure of the cleavage site associated with trypsin enhancement of rotavirus SA11 infectivity. Virology. 1985 Jul 15;144(1):11–19. doi: 10.1016/0042-6822(85)90300-9. [DOI] [PubMed] [Google Scholar]
- López S., Arias C. F., Méndez E., Espejo R. T. Conservation in rotaviruses of the protein region containing the two sites associated with trypsin enhancement of infectivity. Virology. 1986 Oct 15;154(1):224–227. doi: 10.1016/0042-6822(86)90445-9. [DOI] [PubMed] [Google Scholar]
- López S., Arias C. F. The nucleotide sequence of the 5' and 3' ends of rotavirus SA11 gene 4. Nucleic Acids Res. 1987 Jun 11;15(11):4691–4691. doi: 10.1093/nar/15.11.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackow E. R., Barnett J. W., Chan H., Greenberg H. B. The rhesus rotavirus outer capsid protein VP4 functions as a hemagglutinin and is antigenically conserved when expressed by a baculovirus recombinant. J Virol. 1989 Apr;63(4):1661–1668. doi: 10.1128/jvi.63.4.1661-1668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Benfield D. A., Greenberg H. B. Characterization of homotypic and heterotypic VP7 neutralization sites of rhesus rotavirus. Virology. 1988 Aug;165(2):511–517. doi: 10.1016/0042-6822(88)90595-8. [DOI] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Dang M. N., Greenberg H. B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci U S A. 1988 Feb;85(3):645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Keck J. G., Stohlman S. A., Lai M. M. High-frequency RNA recombination of murine coronaviruses. J Virol. 1986 Mar;57(3):729–737. doi: 10.1128/jvi.57.3.729-737.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. L., Palmer E. L., Middleton P. J. Ultrastructure of infantile gastroenteritis virus. Virology. 1975 Nov;68(1):146–153. doi: 10.1016/0042-6822(75)90156-7. [DOI] [PubMed] [Google Scholar]
- Mason B. B., Dheer S. K., Hsiao C. L., Zandle G., Kostek B., Rosanoff E. I., Hung P. P., Davis A. R. Sequence of the serotype-specific glycoprotein of the human rotavirus Wa strain and comparison with other human rotavirus serotypes. Virus Res. 1985 Jun;2(4):291–299. doi: 10.1016/0168-1702(85)90026-7. [DOI] [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. Biochemical mapping of the simian rotavirus SA11 genome. J Virol. 1983 May;46(2):413–423. doi: 10.1128/jvi.46.2.413-423.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. In vitro transcription and translation of simian rotavirus SA11 gene products. J Virol. 1980 Mar;33(3):1111–1121. doi: 10.1128/jvi.33.3.1111-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S. M., Mackow E. R., Greenberg H. B. Molecular determinant of rotavirus neutralization and protection. Adv Virus Res. 1989;36:181–214. doi: 10.1016/s0065-3527(08)60585-0. [DOI] [PubMed] [Google Scholar]
- Matsui S. M., Offit P. A., Vo P. T., Mackow E. R., Benfield D. A., Shaw R. D., Padilla-Noriega L., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to the heterotypic neutralization domain of VP7 and the VP8 fragment of VP4. J Clin Microbiol. 1989 Apr;27(4):780–782. doi: 10.1128/jcm.27.4.780-782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno S., Hasegawa A., Kalica A. R., Kono R. Isolation of a recombinant between simian and bovine rotaviruses. J Gen Virol. 1980 May;48(1):253–256. doi: 10.1099/0022-1317-48-1-253. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Hasegawa A., Mukoyama A., Inouye S. A candidate for a new serotype of human rotavirus. J Virol. 1985 May;54(2):623–624. doi: 10.1128/jvi.54.2.623-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno S., Inouye S. Purification of an outer capsid glycoprotein of neonatal calf diarrhea virus and preparation of its antisera. Infect Immun. 1983 Jan;39(1):155–158. doi: 10.1128/iai.39.1.155-158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno S., Mukoyama A., Hasegawa A., Taniguchi K., Inouye S. Characterization of a human rotavirus strain which is possibly a naturally-occurring reassortant virus. Virus Res. 1988 May;10(2-3):167–175. doi: 10.1016/0168-1702(88)90013-5. [DOI] [PubMed] [Google Scholar]
- Mattion N., González S. A., Burrone O., Bellinzoni R., La Torre J. L., Scodeller E. A. Rearrangement of genomic segment 11 in two swine rotavirus strains. J Gen Virol. 1988 Mar;69(Pt 3):695–698. doi: 10.1099/0022-1317-69-3-695. [DOI] [PubMed] [Google Scholar]
- McCrae M. A., Faulkner-Valle G. P. Molecular biology of rotaviruses. I. Characterization of basic growth parameters and pattern of macromolecular synthesis. J Virol. 1981 Aug;39(2):490–496. doi: 10.1128/jvi.39.2.490-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae M. A., McCorquodale J. G. Expression of a major bovine rotavirus neutralisation antigen (VP7c) in Escherichia coli. Gene. 1987;55(1):9–18. doi: 10.1016/0378-1119(87)90243-5. [DOI] [PubMed] [Google Scholar]
- McCrae M. A., McCorquodale J. G. Molecular biology of rotaviruses. V. Terminal structure of viral RNA species. Virology. 1983 Apr 15;126(1):204–212. doi: 10.1016/0042-6822(83)90472-5. [DOI] [PubMed] [Google Scholar]
- McCrae M. A., McCorquodale J. G. The molecular biology of rotaviruses. II. Identification of the protein-coding assignments of calf rotavirus genome RNA species. Virology. 1982 Mar;117(2):435–443. doi: 10.1016/0042-6822(82)90482-2. [DOI] [PubMed] [Google Scholar]
- McIntyre M., Rosenbaum V., Rappold W., Desselberger M., Wood D., Desselberger U. Biophysical characterization of rotavirus particles containing rearranged genomes. J Gen Virol. 1987 Nov;68(Pt 11):2961–2966. doi: 10.1099/0022-1317-68-11-2961. [DOI] [PubMed] [Google Scholar]
- McNulty M. S., Curran W. L., McFerran J. B. The morphogenesis of a cytopathic bovine rotavirus in Madin-Darby bovine kidney cells. J Gen Virol. 1976 Dec;33(3):503–508. doi: 10.1099/0022-1317-33-3-503. [DOI] [PubMed] [Google Scholar]
- Mertens P. P., Sangar D. V. Analysis of the terminal sequences of the genome segments of four orbiviruses. Prog Clin Biol Res. 1985;178:371–387. [PubMed] [Google Scholar]
- Meyer J. C., Bergmann C. C., Bellamy A. R. Interaction of rotavirus cores with the nonstructural glycoprotein NS28. Virology. 1989 Jul;171(1):98–107. doi: 10.1016/0042-6822(89)90515-1. [DOI] [PubMed] [Google Scholar]
- Midthun K., Greenberg H. B., Hoshino Y., Kapikian A. Z., Wyatt R. G., Chanock R. M. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J Virol. 1985 Mar;53(3):949–954. doi: 10.1128/jvi.53.3.949-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midthun K., Valdesuso J., Hoshino Y., Flores J., Kapikian A. Z., Chanock R. M. Analysis by RNA-RNA hybridization assay of intertypic rotaviruses suggests that gene reassortment occurs in vivo. J Clin Microbiol. 1987 Feb;25(2):295–300. doi: 10.1128/jcm.25.2.295-300.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. B., Both G. W. Complete nucleotide sequence of the simian rotavirus SA11 VP4 gene. Nucleic Acids Res. 1989 Mar 11;17(5):2122–2122. doi: 10.1093/nar/17.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. B., Both G. W. Simian rotavirus SA11 segment 11 contains overlapping reading frames. Nucleic Acids Res. 1988 Jul 11;16(13):6244–6244. doi: 10.1093/nar/16.13.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Musalem C., Espejo R. T. Release of progeny virus from cells infected with simian rotavirus SA11. J Gen Virol. 1985 Dec;66(Pt 12):2715–2724. doi: 10.1099/0022-1317-66-12-2715. [DOI] [PubMed] [Google Scholar]
- Nagesha H. S., Holmes I. H. New porcine rotavirus serotype antigenically related to human rotavirus serotype 3. J Clin Microbiol. 1988 Feb;26(2):171–174. doi: 10.1128/jcm.26.2.171-174.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi O., Nakagomi T., Hoshino Y., Flores J., Kapikian A. Z. Genetic analysis of a human rotavirus that belongs to subgroup I but has an RNA pattern typical of subgroup II human rotaviruses. J Clin Microbiol. 1987 Jul;25(7):1159–1164. doi: 10.1128/jcm.25.7.1159-1164.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi T., Akatani K., Ikegami N., Katsushima N., Nakagomi O. Occurrence of changes in human rotavirus serotypes with concurrent changes in genomic RNA electropherotypes. J Clin Microbiol. 1988 Dec;26(12):2586–2592. doi: 10.1128/jcm.26.12.2586-2592.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata S., Estes M. K., Graham D. Y., Loosle R., Tao H., Wang S. H., Saif L. J., Melnick J. L. Antigenic characterization and ELISA detection of adult diarrhea rotaviruses. J Infect Dis. 1986 Sep;154(3):448–455. doi: 10.1093/infdis/154.3.448. [DOI] [PubMed] [Google Scholar]
- Nakata S., Estes M. K., Graham D. Y., Wang S. S., Gary G. W., Melnick J. L. Detection of antibody to group B adult diarrhea rotaviruses in humans. J Clin Microbiol. 1987 May;25(5):812–818. doi: 10.1128/jcm.25.5.812-818.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata S., Petrie B. L., Calomeni E. P., Estes M. K. Electron microscopy procedure influences detection of rotaviruses. J Clin Microbiol. 1987 Oct;25(10):1902–1906. doi: 10.1128/jcm.25.10.1902-1906.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B. The pre-S region of hepadnavirus envelope proteins. Adv Virus Res. 1988;34:65–142. doi: 10.1016/s0065-3527(08)60516-3. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Hoshino Y., Taniguchi K., Green K. Y., Greenberg H. B., Kapikian A. Z., Chanock R. M., Gorziglia M. Rotavirus VP7 neutralization epitopes of serotype 3 strains. Virology. 1989 Aug;171(2):503–515. doi: 10.1016/0042-6822(89)90620-x. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Taniguchi K., Torres A., Hoshino Y., Green K., Kapikian A. Z., Chanock R. M., Gorziglia M. Comparative analysis of the VP3 gene of divergent strains of the rotaviruses simian SA11 and bovine Nebraska calf diarrhea virus. J Virol. 1988 Nov;62(11):4022–4026. doi: 10.1128/jvi.62.11.4022-4026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novo E., Esparza J. Composition and topography of structural polypeptides of bovine rotavirus. J Gen Virol. 1981 Oct;56(Pt 2):325–335. doi: 10.1099/0022-1317-56-2-325. [DOI] [PubMed] [Google Scholar]
- Nuttall S. D., Hum C. P., Holmes I. H., Dyall-Smith M. L. Sequences of VP9 genes from short and supershort rotavirus strains. Virology. 1989 Aug;171(2):453–457. doi: 10.1016/0042-6822(89)90614-4. [DOI] [PubMed] [Google Scholar]
- Offit P. A., Blavat G., Greenberg H. B., Clark H. F. Molecular basis of rotavirus virulence: role of gene segment 4. J Virol. 1986 Jan;57(1):46–49. doi: 10.1128/jvi.57.1.46-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Blavat G., Greenberg H. B. Reassortant rotaviruses containing structural proteins vp3 and vp7 from different parents induce antibodies protective against each parental serotype. J Virol. 1986 Nov;60(2):491–496. doi: 10.1128/jvi.60.2.491-496.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Plotkin S. A. Response of mice to rotaviruses of bovine or primate origin assessed by radioimmunoassay, radioimmunoprecipitation, and plaque reduction neutralization. Infect Immun. 1983 Oct;42(1):293–300. doi: 10.1128/iai.42.1.293-300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Taylor A. H., Hess R. G., Bachmann P. A., Plotkin S. A. Rotavirus-specific antibodies in fetal bovine serum and commercial preparations of serum albumin. J Clin Microbiol. 1984 Aug;20(2):266–270. doi: 10.1128/jcm.20.2.266-270.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Dudzik K. I. Rotavirus-specific cytotoxic T lymphocytes cross-react with target cells infected with different rotavirus serotypes. J Virol. 1988 Jan;62(1):127–131. doi: 10.1128/jvi.62.1.127-131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Shaw R. D., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J Virol. 1986 May;58(2):700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Richardson M. A., Ikegami N., Nomoto A., Furuichi Y. Nucleotide sequence of human rotavirus genome segment 10, an RNA encoding a glycosylated virus protein. J Virol. 1984 Sep;51(3):856–859. doi: 10.1128/jvi.51.3.856-859.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar A., Koblet H. Semliki Forest virus particles containing only the E1 envelope glycoprotein are infectious and can induce cell-cell fusion. Virology. 1988 Sep;166(1):17–23. doi: 10.1016/0042-6822(88)90141-9. [DOI] [PubMed] [Google Scholar]
- Patton J. T., Gallegos C. O. Structure and protein composition of the rotavirus replicase particle. Virology. 1988 Oct;166(2):358–365. doi: 10.1016/0042-6822(88)90506-5. [DOI] [PubMed] [Google Scholar]
- Patton J. T., Stacy-Phipps S. Electrophoretic separation of the plus and minus strands of rotavirus SA11 double-stranded RNAs. J Virol Methods. 1986 Jun;13(3):185–190. doi: 10.1016/0166-0934(86)90012-1. [DOI] [PubMed] [Google Scholar]
- Patton J. T. Synthesis of simian rotavirus SA11 double-stranded RNA in a cell-free system. Virus Res. 1986 Dec;6(3):217–233. doi: 10.1016/0168-1702(86)90071-7. [DOI] [PubMed] [Google Scholar]
- Pedley S., Bridger J. C., Brown J. F., McCrae M. A. Molecular characterization of rotaviruses with distinct group antigens. J Gen Virol. 1983 Oct;64(Pt 10):2093–2101. doi: 10.1099/0022-1317-64-10-2093. [DOI] [PubMed] [Google Scholar]
- Pedley S., Hundley F., Chrystie I., McCrae M. A., Desselberger U. The genomes of rotaviruses isolated from chronically infected immunodeficient children. J Gen Virol. 1984 Jul;65(Pt 7):1141–1150. doi: 10.1099/0022-1317-65-7-1141. [DOI] [PubMed] [Google Scholar]
- Persing D. H., Varmus H. E., Ganem D. A frameshift mutation in the pre-S region of the human hepatitis B virus genome allows production of surface antigen particles but eliminates binding to polymerized albumin. Proc Natl Acad Sci U S A. 1985 May;82(10):3440–3444. doi: 10.1073/pnas.82.10.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie B. L., Estes M. K., Graham D. Y. Effects of tunicamycin on rotavirus morphogenesis and infectivity. J Virol. 1983 Apr;46(1):270–274. doi: 10.1128/jvi.46.1.270-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie B. L., Graham D. Y., Estes M. K. Identification of rotavirus particle types. Intervirology. 1981;16(1):20–28. doi: 10.1159/000149243. [DOI] [PubMed] [Google Scholar]
- Petrie B. L., Graham D. Y., Hanssen H., Estes M. K. Localization of rotavirus antigens in infected cells by ultrastructural immunocytochemistry. J Gen Virol. 1982 Dec;63(2):457–467. doi: 10.1099/0022-1317-63-2-457. [DOI] [PubMed] [Google Scholar]
- Petrie B. L., Greenberg H. B., Graham D. Y., Estes M. K. Ultrastructural localization of rotavirus antigens using colloidal gold. Virus Res. 1984;1(2):133–152. doi: 10.1016/0168-1702(84)90069-8. [DOI] [PubMed] [Google Scholar]
- Pocock D. H. Isolation and characterization of two group A rotaviruses with unusual genome profiles. J Gen Virol. 1987 Mar;68(Pt 3):653–660. doi: 10.1099/0022-1317-68-3-653. [DOI] [PubMed] [Google Scholar]
- Porter A. G., Barber C., Carey N. H., Hallewell R. A., Threlfall G., Emtage J. S. Complete nucleotide sequence of an influenza virus haemagglutinin gene from cloned DNA. Nature. 1979 Nov 29;282(5738):471–477. doi: 10.1038/282471a0. [DOI] [PubMed] [Google Scholar]
- Poruchynsky M. S., Atkinson P. H. Primary sequence domains required for the retention of rotavirus VP7 in the endoplasmic reticulum. J Cell Biol. 1988 Nov;107(5):1697–1706. doi: 10.1083/jcb.107.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poruchynsky M. S., Tyndall C., Both G. W., Sato F., Bellamy A. R., Atkinson P. H. Deletions into an NH2-terminal hydrophobic domain result in secretion of rotavirus VP7, a resident endoplasmic reticulum membrane glycoprotein. J Cell Biol. 1985 Dec;101(6):2199–2209. doi: 10.1083/jcb.101.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter A. A., Cox G., Parker M., Babiuk L. A. The complete nucleotide sequence of bovine rotavirus C486 gene 4 cDNA. Nucleic Acids Res. 1987 May 26;15(10):4361–4361. doi: 10.1093/nar/15.10.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. F., Gunn P. R., Bellamy A. R. Nucleotide sequence of bovine rotavirus genomic segment 10: an RNA encoding the viral nonstructural glycoprotein. Nucleic Acids Res. 1988 Jan 25;16(2):763–763. doi: 10.1093/nar/16.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
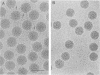
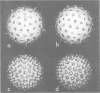
- Prasad B. V., Wang G. J., Clerx J. P., Chiu W. Three-dimensional structure of rotavirus. J Mol Biol. 1988 Jan 20;199(2):269–275. doi: 10.1016/0022-2836(88)90313-0. [DOI] [PubMed] [Google Scholar]
- Quan C. M., Doane F. W. Ultrastructural evidence for the cellular uptake of rotavirus by endocytosis. Intervirology. 1983;20(4):223–231. doi: 10.1159/000149395. [DOI] [PubMed] [Google Scholar]
- Ramig R. F. Factors that affect genetic interaction during mixed infection with temperature-sensitive mutants of simian rotavirus SA11. Virology. 1983 May;127(1):91–99. doi: 10.1016/0042-6822(83)90374-4. [DOI] [PubMed] [Google Scholar]
- Ramig R. F. Isolation and genetic characterization of temperature-sensitive mutants of simian rotavirus SA11. Virology. 1982 Jul 15;120(1):93–105. doi: 10.1016/0042-6822(82)90009-5. [DOI] [PubMed] [Google Scholar]
- Ramig R. F. Isolation and genetic characterization of temperature-sensitive mutants that define five additional recombination groups in simian rotavirus SA11. Virology. 1983 Oct 30;130(2):464–473. doi: 10.1016/0042-6822(83)90100-9. [DOI] [PubMed] [Google Scholar]
- Ramig R. F., Petrie B. L. Characterization of temperature-sensitive mutants of simian rotavirus SA11: protein synthesis and morphogenesis. J Virol. 1984 Mar;49(3):665–673. doi: 10.1128/jvi.49.3.665-673.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig R. F., Samal S. K., McConnell S. Genome RNAS of virulent and attenuated strains of bluetongue virus serotypes 10, 11, 13 and 17. Prog Clin Biol Res. 1985;178:389–396. [PubMed] [Google Scholar]
- Ramig R. F. The effects of host age, virus dose, and virus strain on heterologous rotavirus infection of suckling mice. Microb Pathog. 1988 Mar;4(3):189–202. doi: 10.1016/0882-4010(88)90069-1. [DOI] [PubMed] [Google Scholar]
- Ready K. F., Sabara M. In vitro assembly of bovine rotavirus nucleocapsid protein. Virology. 1987 Mar;157(1):189–198. doi: 10.1016/0042-6822(87)90328-x. [DOI] [PubMed] [Google Scholar]
- Richardson C. D., Choppin P. W. Oligopeptides that specifically inhibit membrane fusion by paramyxoviruses: studies on the site of action. Virology. 1983 Dec;131(2):518–532. doi: 10.1016/0042-6822(83)90517-2. [DOI] [PubMed] [Google Scholar]
- Richardson C. D., Scheid A., Choppin P. W. Specific inhibition of paramyxovirus and myxovirus replication by oligopeptides with amino acid sequences similar to those at the N-termini of the F1 or HA2 viral polypeptides. Virology. 1980 Aug;105(1):205–222. doi: 10.1016/0042-6822(80)90168-3. [DOI] [PubMed] [Google Scholar]
- Richardson M. A., Iwamoto A., Ikegami N., Nomoto A., Furuichi Y. Nucleotide sequence of the gene encoding the serotype-specific antigen of human (Wa) rotavirus: comparison with the homologous genes from simian SA11 and UK bovine rotaviruses. J Virol. 1984 Sep;51(3):860–862. doi: 10.1128/jvi.51.3.860-862.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S. C., Mercer L. E., Sonza S., Holmes I. H. Intracellular localization of rotaviral proteins. Arch Virol. 1986;88(3-4):251–264. doi: 10.1007/BF01310879. [DOI] [PubMed] [Google Scholar]
- Riepenhoff-Talty M., Dharakul T., Kowalski E., Michalak S., Ogra P. L. Persistent rotavirus infection in mice with severe combined immunodeficiency. J Virol. 1987 Oct;61(10):3345–3348. doi: 10.1128/jvi.61.10.3345-3348.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riepenhoff-Talty M., Uhnoo I., Chegas P., Ogra P. L. Effect of nutritional deprivation on mucosal viral infections. Immunol Invest. 1989 Jan-May;18(1-4):127–139. doi: 10.3109/08820138909112233. [DOI] [PubMed] [Google Scholar]
- Rixon F., Taylor P., Desselberger U. Rotavirus RNA segments sized by electron microscopy. J Gen Virol. 1984 Jan;65(Pt 1):233–239. doi: 10.1099/0022-1317-65-1-233. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Holmes I. H. Comparison of the genomes of simian, bovine, and human rotaviruses by gel electrophoresis and detection of genomic variation among bovine isolates. J Virol. 1979 Jun;30(3):839–846. doi: 10.1128/jvi.30.3.839-846.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseto A., Escaig J., Delain E., Cohen J., Scherrer R. Structure of rotaviruses as studied by the freeze-drying technique. Virology. 1979 Oct 30;98(2):471–475. doi: 10.1016/0042-6822(79)90571-3. [DOI] [PubMed] [Google Scholar]
- Roseto A., Scherrer R., Cohen J., Guillemin M. C., Charpilienne A., Feynerol C., Peries J. Isolation and characterization of anti-rotavirus immunoglobulins secreted by cloned hybridoma cell lines. J Gen Virol. 1983 Jan;64(Pt 1):237–240. doi: 10.1099/0022-1317-64-1-237. [DOI] [PubMed] [Google Scholar]
- Ruiz A. M., López I. V., López S., Espejo R. T., Arias C. F. Molecular and antigenic characterization of porcine rotavirus YM, a possible new rotavirus serotype. J Virol. 1988 Nov;62(11):4331–4336. doi: 10.1128/jvi.62.11.4331-4336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushlow K., McNab A., Olson K., Maxwell F., Maxwell I., Stiegler G. Nucleotide sequence of porcine rotavirus (OSU strain) gene segments 7, 8, and 9. Nucleic Acids Res. 1988 Jan 11;16(1):367–368. doi: 10.1093/nar/16.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabara M., Babiuk L. A., Gilchrist J., Misra V. Effect of tunicamycin on rotavirus assembly and infectivity. J Virol. 1982 Sep;43(3):1082–1090. doi: 10.1128/jvi.43.3.1082-1090.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabara M., Barrington A., Babiuk L. A. Immunogenicity of a bovine rotavirus glycoprotein fragment. J Virol. 1985 Dec;56(3):1037–1040. doi: 10.1128/jvi.56.3.1037-1040.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabara M., Gilchrist J. E., Hudson G. R., Babiuk L. A. Preliminary characterization of an epitope involved in neutralization and cell attachment that is located on the major bovine rotavirus glycoprotein. J Virol. 1985 Jan;53(1):58–66. doi: 10.1128/jvi.53.1.58-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabara M., Ready K. F., Frenchick P. J., Babiuk L. A. Biochemical evidence for the oligomeric arrangement of bovine rotavirus nucleocapsid protein and its possible significance in the immunogenicity of this protein. J Gen Virol. 1987 Jan;68(Pt 1):123–133. doi: 10.1099/0022-1317-68-1-123. [DOI] [PubMed] [Google Scholar]
- Saif L. J., Smith K. L., Landmeier B. J., Bohl E. H., Theil K. W., Todhunter D. A. Immune response of pregnant cows to bovine rotavirus immunization. Am J Vet Res. 1984 Jan;45(1):49–58. [PubMed] [Google Scholar]
- Saif L. J., Terrett L. A., Miller K. L., Cross R. F. Serial propagation of porcine group C rotavirus (pararotavirus) in a continuous cell line and characterization of the passaged virus. J Clin Microbiol. 1988 Jul;26(7):1277–1282. doi: 10.1128/jcm.26.7.1277-1282.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L. J., Weilnau P., Miller K., Stitzlein L. Isotypes of intestinal and systemic antibodies in colostrum-fed and colostrum-deprived calves challenged with rotavirus. Adv Exp Med Biol. 1987;216B:1815–1823. [PubMed] [Google Scholar]
- Sandino A. M., Jashes M., Faúndez G., Spencer E. Role of the inner protein capsid on in vitro human rotavirus transcription. J Virol. 1986 Nov;60(2):797–802. doi: 10.1128/jvi.60.2.797-802.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Suzuki H., Kitaoka S., Konno T., Ishida N. Patterns of polypeptide synthesis in human rotavirus infected cells. Arch Virol. 1986;90(1-2):29–40. doi: 10.1007/BF01314142. [DOI] [PubMed] [Google Scholar]
- Schiff L. A., Nibert M. L., Co M. S., Brown E. G., Fields B. N. Distinct binding sites for zinc and double-stranded RNA in the reovirus outer capsid protein sigma 3. Mol Cell Biol. 1988 Jan;8(1):273–283. doi: 10.1128/mcb.8.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohn A. L., Kokubun K. M., Cole G. A. Protective monoclonal antibodies define maturational and pH-dependent antigenic changes in Sindbis virus E1 glycoprotein. Virology. 1983 Oct 15;130(1):144–154. doi: 10.1016/0042-6822(83)90124-1. [DOI] [PubMed] [Google Scholar]
- Schroeder B. A., Street J. E., Kalmakoff J., Bellamy A. R. Sequence relationships between the genome segments of human and animal rotavirus strains. J Virol. 1982 Aug;43(2):379–385. doi: 10.1128/jvi.43.2.379-385.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S. K., Olive D. M., Strannegard O. O., al-Nakib W. Molecular epidemiology of human rotavirus infections based on genome segment variations in viral strains. J Med Virol. 1988 Nov;26(3):249–259. doi: 10.1002/jmv.1890260305. [DOI] [PubMed] [Google Scholar]
- Shahrabadi M. S., Babiuk L. A., Lee P. W. Further analysis of the role of calcium in rotavirus morphogenesis. Virology. 1987 May;158(1):103–111. doi: 10.1016/0042-6822(87)90242-x. [DOI] [PubMed] [Google Scholar]
- Shahrabadi M. S., Lee P. W. Bovine rotavirus maturation is a calcium-dependent process. Virology. 1986 Jul 30;152(2):298–307. doi: 10.1016/0042-6822(86)90133-9. [DOI] [PubMed] [Google Scholar]
- Shaw R. D., Fong K. J., Losonsky G. A., Levine M. M., Maldonado Y., Yolken R., Flores J., Kapikian A. Z., Vo P. T., Greenberg H. B. Epitope-specific immune responses to rotavirus vaccination. Gastroenterology. 1987 Nov;93(5):941–950. doi: 10.1016/0016-5085(87)90555-5. [DOI] [PubMed] [Google Scholar]
- Shaw R. D., Mackow E. R., Dyall-Smith M. L., Lazdins I., Holmes I. H., Greenberg H. B. Serotypic analysis of VP3 and VP7 neutralization escape mutants of rhesus rotavirus. J Virol. 1988 Sep;62(9):3509–3512. doi: 10.1128/jvi.62.9.3509-3512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. D., Stoner-Ma D. L., Estes M. K., Greenberg H. B. Specific enzyme-linked immunoassay for rotavirus serotypes 1 and 3. J Clin Microbiol. 1985 Aug;22(2):286–291. doi: 10.1128/jcm.22.2.286-291.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. D., Vo P. T., Offit P. A., Coulson B. S., Greenberg H. B. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology. 1986 Dec;155(2):434–451. doi: 10.1016/0042-6822(86)90205-9. [DOI] [PubMed] [Google Scholar]
- Singh N., Sereno M. M., Flores J., Kapikian A. Z. Monoclonal antibodies to subgroup 1 rotavirus. Infect Immun. 1983 Nov;42(2):835–837. doi: 10.1128/iai.42.2.835-837.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Stevens D. J., Daniels R. S., Douglas A. R., Knossow M., Wilson I. A., Wiley D. C. A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1779–1783. doi: 10.1073/pnas.81.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. L., Lazdins I., Holmes I. H. Coding assignments of double-stranded RNA segments of SA 11 rotavirus established by in vitro translation. J Virol. 1980 Mar;33(3):976–982. doi: 10.1128/jvi.33.3.976-982.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D. R., Fahey K. J., Wells P. W., Campbell I., Whitelaw A. Passive immunity in calf rotavirus infections: maternal vaccination increases and prolongs immunoglobulin G1 antibody secretion in milk. Infect Immun. 1980 May;28(2):344–349. doi: 10.1128/iai.28.2.344-349.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D. R., Herring A. J., Campbell I., Inglis J. M., Hargreaves F. D. Comparison of atypical rotaviruses from calves, piglets, lambs and man. J Gen Virol. 1984 May;65(Pt 5):909–914. doi: 10.1099/0022-1317-65-5-909. [DOI] [PubMed] [Google Scholar]
- Snodgrass D. R., Ojeh C. K., Campbell I., Herring A. J. Bovine rotavirus serotypes and their significance for immunization. J Clin Microbiol. 1984 Sep;20(3):342–346. doi: 10.1128/jcm.20.3.342-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler C., Musalem C., Loroño M., Espejo R. T. Association of viral particles and viral proteins with membranes in SA11-infected cells. J Virol. 1982 Dec;44(3):983–992. doi: 10.1128/jvi.44.3.983-992.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonza S., Breschkin A. M., Holmes I. H. Derivation of neutralizing monoclonal antibodies against rotavirus. J Virol. 1983 Mar;45(3):1143–1146. doi: 10.1128/jvi.45.3.1143-1146.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy-Phipps S., Patton J. T. Synthesis of plus- and minus-strand RNA in rotavirus-infected cells. J Virol. 1987 Nov;61(11):3479–3484. doi: 10.1128/jvi.61.11.3479-3484.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M. C. Rotavirus: the first five years. J Pediatr. 1980 Apr;96(4):611–622. doi: 10.1016/s0022-3476(80)80725-6. [DOI] [PubMed] [Google Scholar]
- Stirzaker S. C., Both G. W. The signal peptide of the rotavirus glycoprotein VP7 is essential for its retention in the ER as an integral membrane protein. Cell. 1989 Mar 10;56(5):741–747. doi: 10.1016/0092-8674(89)90677-6. [DOI] [PubMed] [Google Scholar]
- Stirzaker S. C., Whitfeld P. L., Christie D. L., Bellamy A. R., Both G. W. Processing of rotavirus glycoprotein VP7: implications for the retention of the protein in the endoplasmic reticulum. J Cell Biol. 1987 Dec;105(6 Pt 2):2897–2903. doi: 10.1083/jcb.105.6.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streckert H. J., Brüssow H., Werchau H. A synthetic peptide corresponding to the cleavage region of VP3 from rotavirus SA11 induces neutralizing antibodies. J Virol. 1988 Nov;62(11):4265–4269. doi: 10.1128/jvi.62.11.4265-4269.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streckert H. J., Grunert B., Werchau H. Antibodies specific for the carboxy-terminal region of the major surface glycoprotein of simian rotavirus (SA11) and human rotavirus (Wa). J Cell Biochem. 1986;30(1):41–49. doi: 10.1002/jcb.240300106. [DOI] [PubMed] [Google Scholar]
- Su C. Q., Wu Y. L., Shen H. K., Wang D. B., Chen Y. H., Wu D. M., He L. N., Yang Z. L. An outbreak of epidemic diarrhoea in adults caused by a new rotavirus in Anhui Province of China in the summer of 1983. J Med Virol. 1986 Jun;19(2):167–173. doi: 10.1002/jmv.1890190210. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Chen G. M., Hung T., Beards G. M., Brown D. W., Flewett T. H. Effects of two negative staining methods on the Chinese atypical rotavirus. Arch Virol. 1987;94(3-4):305–308. doi: 10.1007/BF01310723. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kitaoka S., Konno T., Sato T., Ishida N. Two modes of human rotavirus entry into MA 104 cells. Arch Virol. 1985;85(1-2):25–34. doi: 10.1007/BF01317003. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Sato T., Konno T., Kitaoka S., Ebina T., Ishida N. Effect of tunicamycin on human rotavirus morphogenesis and infectivity. Brief report. Arch Virol. 1984;81(3-4):363–369. doi: 10.1007/BF01310008. [DOI] [PubMed] [Google Scholar]
- Svensson L., Grahnquist L., Pettersson C. A., Grandien M., Stintzing G., Greenberg H. B. Detection of human rotaviruses which do not react with subgroup I- and II-specific monoclonal antibodies. J Clin Microbiol. 1988 Jun;26(6):1238–1240. doi: 10.1128/jcm.26.6.1238-1240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L., Sheshberadaran H., Vene S., Norrby E., Grandien M., Wadell G. Serum antibody responses to individual viral polypeptides in human rotavirus infections. J Gen Virol. 1987 Mar;68(Pt 3):643–651. doi: 10.1099/0022-1317-68-3-643. [DOI] [PubMed] [Google Scholar]
- Tanaka T. N., Conner M. E., Graham D. Y., Estes M. K. Molecular characterization of three rabbit rotavirus strains. Arch Virol. 1988;98(3-4):253–265. doi: 10.1007/BF01322173. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Hoshino Y., Nishikawa K., Green K. Y., Maloy W. L., Morita Y., Urasawa S., Kapikian A. Z., Chanock R. M., Gorziglia M. Cross-reactive and serotype-specific neutralization epitopes on VP7 of human rotavirus: nucleotide sequence analysis of antigenic mutants selected with monoclonal antibodies. J Virol. 1988 Jun;62(6):1870–1874. doi: 10.1128/jvi.62.6.1870-1874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Maloy W. L., Nishikawa K., Green K. Y., Hoshino Y., Urasawa S., Kapikian A. Z., Chanock R. M., Gorziglia M. Identification of cross-reactive and serotype 2-specific neutralization epitopes on VP3 of human rotavirus. J Virol. 1988 Jul;62(7):2421–2426. doi: 10.1128/jvi.62.7.2421-2426.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Morita Y., Urasawa T., Urasawa S. Cross-reactive neutralization epitopes on VP3 of human rotavirus: analysis with monoclonal antibodies and antigenic variants. J Virol. 1987 May;61(5):1726–1730. doi: 10.1128/jvi.61.5.1726-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa S., Urasawa T. Preparation and characterization of neutralizing monoclonal antibodies with different reactivity patterns to human rotaviruses. J Gen Virol. 1985 May;66(Pt 5):1045–1053. doi: 10.1099/0022-1317-66-5-1045. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Morita Y., Greenberg H. B., Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987 Jun;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Urasawa S. Reactivity patterns to human rotavirus strains of a monoclonal antibody against VP2, a component of the inner capsid of rotavirus. Brief report. Arch Virol. 1986;87(1-2):135–141. doi: 10.1007/BF01310550. [DOI] [PubMed] [Google Scholar]
- Tao H. Rotavirus and adult diarrhea. Adv Virus Res. 1988;35:193–218. [PubMed] [Google Scholar]
- Taylor P., Rixon F., Desselberger U. Rise per base pair in helices of double-stranded rotavirus RNA determined by electron microscopy. Virus Res. 1985 Mar;2(2):175–182. doi: 10.1016/0168-1702(85)90247-3. [DOI] [PubMed] [Google Scholar]
- Theil K. W., McCloskey C. M. Partial characterization of a bovine group A rotavirus with a short genome electropherotype. J Clin Microbiol. 1988 Jun;26(6):1094–1099. doi: 10.1128/jcm.26.6.1094-1099.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouless M. E., DiGiacomo R. F., Deeb B. J., Howard H. Pathogenicity of rotavirus in rabbits. J Clin Microbiol. 1988 May;26(5):943–947. doi: 10.1128/jcm.26.5.943-947.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouless M. E., DiGiacomo R. F., Neuman D. S. Isolation of two lapine rotaviruses: characterization of their subgroup, serotype and RNA electropherotypes. Arch Virol. 1986;89(1-4):161–170. doi: 10.1007/BF01309886. [DOI] [PubMed] [Google Scholar]
- Totterdell B. M., Banatvala J. E., Chrystie I. L., Ball G., Cubitt W. D. Systemic lymphoproliferative responses to rotavirus. J Med Virol. 1988 May;25(1):37–44. doi: 10.1002/jmv.1890250106. [DOI] [PubMed] [Google Scholar]
- Tufvesson B., Polberger S., Svanberg L., Sveger T. A prospective study of rotavirus infections in neonatal and maternity wards. Acta Paediatr Scand. 1986 Mar;75(2):211–215. doi: 10.1111/j.1651-2227.1986.tb10186.x. [DOI] [PubMed] [Google Scholar]
- Tursi J. M., Albert M. J., Bishop R. F. Production and characterization of neutralizing monoclonal antibody to a human rotavirus strain with a "super-short" RNA pattern. J Clin Microbiol. 1987 Dec;25(12):2426–2427. doi: 10.1128/jcm.25.12.2426-2427.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unicomb L. E., Coulson B. S., Bishop R. F. Experience with an enzyme immunoassay for serotyping human group A rotaviruses. J Clin Microbiol. 1989 Mar;27(3):586–588. doi: 10.1128/jcm.27.3.586-588.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Taniguchi K. Genetic reassortment between two human rotaviruses having different serotype and subgroup specificities. J Gen Virol. 1986 Aug;67(Pt 8):1551–1559. doi: 10.1099/0022-1317-67-8-1551. [DOI] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Taniguchi K., Morita Y., Sakurada N., Saeki Y., Morita O., Hasegawa S. Validity of an enzyme-linked immunosorbent assay with serotype-specific monoclonal antibodies for serotyping human rotavirus in stool specimens. Microbiol Immunol. 1988;32(7):699–708. doi: 10.1111/j.1348-0421.1988.tb01431.x. [DOI] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Taniguchi K., Wakasugi F., Kobayashi N., Chiba S., Sakurada N., Morita M., Morita O., Tokieda M. Survey of human rotavirus serotypes in different locales in Japan by enzyme-linked immunosorbent assay with monoclonal antibodies. J Infect Dis. 1989 Jul;160(1):44–51. doi: 10.1093/infdis/160.1.44. [DOI] [PubMed] [Google Scholar]
- Ushijima H., Honma H., Mukoyama A., Shinozaki T., Fujita Y., Kobayashi M., Ohseto M., Morikawa S., Kitamura T. Detection of group C rotaviruses in Tokyo. J Med Virol. 1989 Apr;27(4):299–303. doi: 10.1002/jmv.1890270408. [DOI] [PubMed] [Google Scholar]
- Vesikari T. Progress in rotavirus vaccination. Pediatr Infect Dis. 1985 Nov-Dec;4(6):612–614. doi: 10.1097/00006454-198511000-00002. [DOI] [PubMed] [Google Scholar]
- Vial P. A., Kotloff K. L., Losonsky G. A. Molecular epidemiology of rotavirus infection in a room for convalescing newborns. J Infect Dis. 1988 Apr;157(4):668–673. doi: 10.1093/infdis/157.4.668. [DOI] [PubMed] [Google Scholar]
- Ward C. W., Azad A. A., Dyall-Smith M. L. Structural homologies between RNA gene segments 10 and 11 from UK bovine, simian SA11, and human Wa rotaviruses. Virology. 1985 Jul 30;144(2):328–336. doi: 10.1016/0042-6822(85)90275-2. [DOI] [PubMed] [Google Scholar]
- Ward C. W., Elleman T. C., Azad A. A., Dyall-Smith M. L. Nucleotide sequence of gene segment 9 encoding a nonstructural protein of UK bovine rotavirus. Virology. 1984 Apr 15;134(1):249–253. doi: 10.1016/0042-6822(84)90292-7. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Knowlton D. R., Greenberg H. B. Phenotypic mixing during coinfection of cells with two strains of human rotavirus. J Virol. 1988 Nov;62(11):4358–4361. doi: 10.1128/jvi.62.11.4358-4361.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L., Knowlton D. R., Hurst P. F. Reassortant formation and selection following coinfection of cultured cells with subgroup 2 human rotaviruses. J Gen Virol. 1988 Jan;69(Pt 1):149–162. doi: 10.1099/0022-1317-69-1-149. [DOI] [PubMed] [Google Scholar]
- Welch S. K., Crawford S. E., Estes M. K. Rotavirus SA11 genome segment 11 protein is a nonstructural phosphoprotein. J Virol. 1989 Sep;63(9):3974–3982. doi: 10.1128/jvi.63.9.3974-3982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfeld P. L., Tyndall C., Stirzaker S. C., Bellamy A. R., Both G. W. Location of sequences within rotavirus SA11 glycoprotein VP7 which direct it to the endoplasmic reticulum. Mol Cell Biol. 1987 Jul;7(7):2491–2497. doi: 10.1128/mcb.7.7.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Bridger J., Hall G. A., Jones J. M., Jackson G. The isolation of reovirus-like agents (rota-viruses) from acute gastroenteritis of piglets. J Med Microbiol. 1976 May;9(2):203–209. doi: 10.1099/00222615-9-2-203. [DOI] [PubMed] [Google Scholar]
- Woode G. N., Kelso N. E., Simpson T. F., Gaul S. K., Evans L. E., Babiuk L. Antigenic relationships among some bovine rotaviruses: serum neutralization and cross-protection in gnotobiotic calves. J Clin Microbiol. 1983 Aug;18(2):358–364. doi: 10.1128/jcm.18.2.358-364.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Zheng S. L., Rosen B. I., Knight N., Gourley N. E., Ramig R. F. Protection between different serotypes of bovine rotavirus in gnotobiotic calves: specificity of serum antibody and coproantibody responses. J Clin Microbiol. 1987 Jun;25(6):1052–1058. doi: 10.1128/jcm.25.6.1052-1058.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Willoughby R., Wee S. B., Miskuff R., Vonderfecht S. Sialic acid glycoproteins inhibit in vitro and in vivo replication of rotaviruses. J Clin Invest. 1987 Jan;79(1):148–154. doi: 10.1172/JCI112775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R., Wee S. B., Eiden J., Kinney J., Vonderfecht S. Identification of a group-reactive epitope of group B rotaviruses recognized by monoclonal antibody and application to the development of a sensitive immunoassay for viral characterization. J Clin Microbiol. 1988 Sep;26(9):1853–1858. doi: 10.1128/jcm.26.9.1853-1858.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhaori G., Sun M., Ogra P. L. Characteristics of the immune response to poliovirus virion polypeptides after immunization with live or inactivated polio vaccines. J Infect Dis. 1988 Jul;158(1):160–165. doi: 10.1093/infdis/158.1.160. [DOI] [PubMed] [Google Scholar]