Abstract
Surgery has been reported to suppress cell-mediated immunity; however, the detailed mechanisms responsible for this remain unclear. We obtained information on expressions of T-cell immunoglobulin domain and mucin domain 3 (TIM-3) and programmed cell death 1 (PD-1) on both CD4+ and CD8+ T cells postoperatively from colorectal cancer patients and evaluated by multicolor flow cytometry. The functions of CD4+ and CD8+ T cells based on TIM-3 and PD-1 expressions were determined by quantitating the concentration of interferon-γ (IFN-γ) by enzyme-linked immunosorbent assay. The study demonstrated a rapid and significant decrease in number of total lymphocytes, CD4+ T cells and CD8+ T cells. These immune cells reached the minimum on day 1 and then increased, but still significantly decreased on days 3 and 7. The frequency of PD-1+CD4+ T cells and TIM-3+CD4+ T cells significantly increased after colorectal surgery. IFN-γ secretion by PD-1+TIM-3+CD4+ T cells was significantly decreased compared to secretion by either PD-1+TIM3-CD4+ T cells or PD-1–TIM-3–CD4+ T cells. Furthermore, IFN-γ secretion by PD-1+TIM-3+CD8+ T cells was significantly decreased compared to secretion by either PD-1–TIM-3+CD8+ T cells or PD-1–TIM-3–CD8+ T cells. The expression of PD-1 and TIM-3 was closely related to impaired cell-mediated immunity that was observed after surgery for colorectal cancer. New treatment targeting TIM-3 and PD-1 on CD4+ and CD8+ T cells during the perioperative period may provide a breakthrough in the treatment of colorectal cancer patients.
Keywords: colorectal cancer, programmed cell death 1, surgical trauma, T-cell immunoglobulin domain and mucin domain 3
Colorectal cancer is one of the most prevalent solid organ cancers in both men and women. Approximately one million cases are recorded every year worldwide, and over half a million patients die of this disease yearly (Parkin et al., 2005). Surgical removal of the primary tumor provides the best chance of long-term disease-free survival for patients with colorectal cancer. Unfortunately, a significant number of colorectal cancer patients typically develop metastases, even after successful resection of the primary tumor. It is now becoming clear that surgery itself contributes to development of both local recurrences and distant metastases. Surgical trauma and the concomitant wound-healing processes induce local and systemic changes, including impairment of tissue integrity and production of inflammatory mediators and angiogenic factors. Subsequently, this can lead to immune suppression and enhanced growth or adhesion of tumor cells, all of which increase the chance of exfoliated tumor cells developing into secondary malignancies ( van der Bij et al., 2009).
Surgery has been reported to suppress cell-mediated immunity, which plays a key role in host defense against bacterial and viral infections (Gupta and Watson, 2001; Jacobi et al., 2002). T cells are a critical component of cellular immunity that are involved in the recruitment and activation of effector cells, as well as amplification of the specific immune response to pathogens and cancer cells. T-cell dysfunctions are characterized by diminished cytokine production, impaired killing and hypoproliferation. According to Wichmann et al. (2005), there is a significant decrease in cell number of lymphocyte subpopulations, such as helper T lymphocytes (Th), cytotoxic T lymphocytes and natural killer cells after surgery. After conventional cholecystectomy, T cells produce significantly less interferon-γ (IFN-γ), tumor necrosis factor-α and interleukin-2 (Brune et al., 1999). Surgical stress is known to induce a shift in the Th1/Th2 balance toward Th2, which suggests downregulation of cell-mediated immunity and upregulation of antibody-mediated immunity after surgery (Decker et al., 1996). However, the detailed mechanisms responsible for the dysfunction of T cells after surgery remain unclear.
Recent studies have demonstrated that programmed cell death 1 (PD-1) and T-cell immunoglobulin domain and mucin domain 3 (TIM-3) expression on CD4+ and CD8+ T cells is closely associated with T-cell dysfunction in human immunodeficiency virus (HIV), hepatitis C virus (HCV) and cancer patients. PD-1 (CD279) was identified as a gene that was upregulated in a T-cell hybridoma undergoing apoptotic cell death, and was thus named PD-1 (Okazaki and Honjo, 2006). PD-1 is inducibly expressed on CD4+ T cells, CD8+ T cells, natural killer-like T cells, B cells and monocytes upon activation (Greenwald et al., 2005; Okazaki and Honjo, 2006). Recent studies have shown that PD-1 is highly expressed by CD8+ T cells during chronic lymphocytic choriomeningitis virus infection, and that the PD-1–PD-1-ligand (PD-L) pathway plays a major role in regulating T-cell exhaustion during infection ( Barber et al., 2006). Furthermore, several new studies suggest a role for the PD-1–PD-L pathway in exhaustion of virus-specific CD8+ T cells during HIV infection. Several reports show that PD-1 expression is elevated on HIV-specific CD8+ T cells and that blocking the PD-1–PD-L pathway leads to increased T-cell proliferation and effector cytokine production (Day et al., 2006; Petrovas et al., 2006; Trautmann et al., 2006). A previous work has shown that PD-L is upregulated in HIV infection (Trabattoni et al., 2003). Collectively, these observations suggest that the PD-1–PD-L pathway may indeed be operating during chronic HIV infection.
TIM-3 is a transmembrane protein constitutively expressed on type 1 CD8+ cytotoxic T cells (Tc1) in mice and humans (Monney et al., 2002). Several lines of evidence support the role of TIM-3 as an inhibitory molecule that downregulates effector Th1/Tc1 cell responses. In mice, blocking the TIM-3–TIM-3 ligand (TIM-3L) pathway resulted in hyperproliferation of Th1-type cells and abrogated the induction of peripheral and transplantation tolerance (Sabatos et al., 2003; Sánchez-Fueyo et al., 2003). TIM-3 interacts with its ligand galectin-9 to induce cell death in Th1 cells (Zhu et al., 2005). In humans, TIM-3 expression is defective in CD4+ T cells producing high levels of IFN-γ, as well as those isolated from the cerebrospinal fluid of patients with multiple sclerosis (Koguchi et al., 2006). Recently, TIM-3 upregulation has been reported in HIV-specific and HCV-specific CD8+ T cells in patients with progressive HIV infection and chronic hepatitis C, respectively (Jones et al., 2008; Golden-Mason et al., 2009). TIM-3+ HIV- and HCV-specific CD8+ T cells were distinct from the PD-1+ CD8+ T cells and exhibited T-cell dysfunction. In addition to virus infection, PD-1 and TIM-3 expression on CD4+ and CD8+ T cells are also related to T-cell exhaustion in cancer patients (Fourcade et al., 2010; Sakuishi et al., 2010).
Given that PD-1 and TIM-3 expressions on CD4+ and CD8+ T cells are closely associated with T-cell dysfunction in HIV, HCV and cancer patients, it is likely that these might be related to suppression of cell-mediated immunity induced after surgery. In the present study, therefore, we investigated the expressions of PD-1 and TIM-3 on lymphocytes to determine the role of these molecules in the immune-suppression that occurs after operation for colorectal cancer.
Materials and Methods
Patients and normal donors
Thirty-six patients (20 males and 16 females), treated at the Tottori University Hospital and pathologically diagnosed with colorectal cancer, were enrolled in this study. None of the patients received radiotherapy, chemotherapy or other medical interventions before surgery. Informed consent for blood donations was obtained from all individuals. The clinicopathological findings were determined according to the Japanese Classification of Colorectal Carcinoma (Japanese Society for Cancer of the Colon and Rectum, 2009). The clinicopathological characteristics of the patients enrolled in the present study are shown in Table 1.
Table 1. Clinicopathologic characteristics in colorectal cancer patients in the present study.
| Clinicopathologic factor | n (%) | |
| Gender | Male | 20 (56) |
| Female | 16 (44) | |
| Age (yr) | 66.8 ± 3.1 | |
| Procedure | Open | 11 (30) |
| Laparoscopic | 25 (70) | |
| Cancer site | Cecum | 2 (5) |
| Ascending | 6 (16) | |
| Transverse | 5 (14) | |
| Descending | 1 (3) | |
| Sigmoid | 6 (16) | |
| Rectum | 17 (47) | |
| Depth of invasion* | M/SM/MP | 16 (44) |
| SS/SE/SI (A1/A2/AI) | 20 (56) | |
| Lymph node metastasis | Absent | 26 (72) |
| Present | 10 (28) | |
| Lymphatic invasion | Absent | 7 (19) |
| Present | 29 (81) | |
| Vascular invasion | Absent | 8 (22) |
| Present | 28 (78) | |
| Liver metastasis | Absent | 35 (97) |
| Present | 1 (3) | |
| Lung metastasis | Absent | 34 (94) |
| Present | 2 (6) | |
| Stage of disease | 0 | 2 (6) |
| I | 12 (33) | |
| II | 11 (30) | |
| III | 8 (22) | |
| IV | 3 (8) |
*M, tumor invasion of mucosa;
SM, tumor invasion of submucosa;
MP, tumor invasion of muscularis propria;
SS, tumor invasion of subserosa.
SE, tumor invasion of serosa;
SI, direct tumor invasion of other organs or structures;
A1, tumor invasion through muscularis propria into a non-peritonealized part;
A2, tumor invasion of non-peritonealized, pericolic or perirectal tissues;
AI, direct tumor invasion of other organs or structures.
Preparation of peripheral blood mononuclear cells
Approximately 30 mL of peripheral blood was drawn from the patients before surgery as well as on days 1, 3, 7 and 30 after surgery, and blood samples were prepared by centrifugation over a Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient.
Flow cytometry analysis
Fluorescence-activated cell sorting (FACS) analysis was performed using an FACS Calibur (Becton Dickinson, Franklin Lakes, NJ). The following antibodies were used to determine cell phenotype: anti-CD4-FITC, anti-CD8-FITC, anti-PD-1-PE (BD PharMingen, Franklin Lakes, NJ), anti-TIM-3-PE (R&D Systems, Minneapolis, MN) and anti-CD3-PE-Cy5 (Biolegend, San Diego, CA).
Measurement of IFN-γ concentrations
CD4+ or CD8+ T cells were sorted based on TIM-3–PD-1–, TIM-3+PD-1–, TIM–3-PD-1+ and TIM-3+PD-1+ phenotype. Each population was seeded in anti-CD3 antibody-coated 96-well plates (1 } 104 cells/well; Nunc A/S, Roskilde, Denmark). After 48 h incubation, supernatants were collected. The IFN-γ concentrations of the supernatants were measured by enzyme-linked immunosorbent assay (ELISA) using a human IFN-γ ELISA Kit (R&D Systems).
Media
Complete culture medium consisting of RPMI 1640 (Invitrogen, Carlsbad, CA), 1% L-glutamine (Invitrogen), 1% penicillin/streptomycin (Invitrogen), 2.5% HEPES buffer solution (Invitrogen), 1% sodium pyruvate (Sigma-Aldrich, St. Louis, MO), 1% essential amino acids (Sigma-Aldrich) and 20% fetal bovine serum (Sigma-Aldrich) was used.
Statistical analysis
To determine statistical differences between 2 groups, either paired or unpaired t-tests were used. The accepted level of significance was P < 0.05. The GraphPad Prism software (GraphPad Software, La Jolla, CA) was used for all statistical analyses.
Results
Absolute leukocyte and lymphocyte counts and C-reactive protein levels after colorectal cancer operation
The number of total leukocytes increased postoperatively and reached a maximum on day 1 (Fig. 1a). The number of neutrophils also increased postoperatively, reached a maximum on day 1 and then decreased, but still significantly elevated on day 3 (Fig. 1b). Further, a significant increase in C-reactive protein levels was also observed on days 1, 3 and 7 compared with preoperative values (Fig. 1c). On the other hand, a rapid and significant decrease in number of total lymphocytes (Fig. 2a), CD4+ T cells (Fig. 2b) and CD8+ T cells (Fig. 2c) was also observed. These cells reached a minimum on day 1 and then increased, but still significantly decreased on days 3 and 7.
Fig. 1.
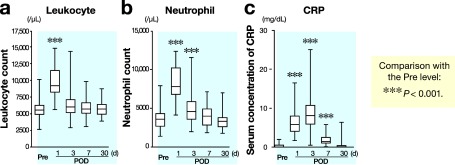
Changes in number of total leukocytes (a), neutrophil count (b) and serum concentration of C-reactive protein (c) after surgery. POD, postoperative day; Pre, before operation.
Fig. 2.
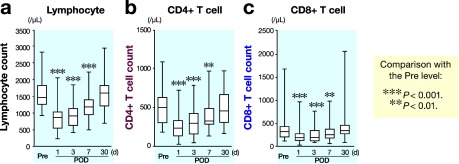
Changes in number of total lymphocytes (a), CD4+ T cells (b) and CD8+ T cells (c) after surgery. POD, postoperative day; Pre, before operation.
PD-1 and TIM-3 expressions on CD4+ and CD8+ T cells after colorectal cancer operation
Figure 3a shows representative results of PD-1 and TIM-3 expressions on CD4+ T cells in peripheral blood obtained from colorectal cancer patients on postoperative day 3 by FACS. PD-1 and TIM-3 expressions on both CD4+ and CD8+ T cells was examined and the frequency of PD-1+CD4+ T cells (Fig. 3b) and TIM-3+CD4+ T cells (Fig. 3c) significantly increased after colorectal surgery. Figure 4a shows representative results of PD-1 and TIM-3 expressions on CD8+ T cells in peripheral blood obtained from colorectal cancer patients on postoperative day 3 by FACS. Moreover, a significant increase in the frequency of PD-1+CD8+ T cells (Fig. 4b) and TIM-3+CD8+ T cells (Fig. 4c) was also observed after colorectal cancer operation.
Fig. 3.
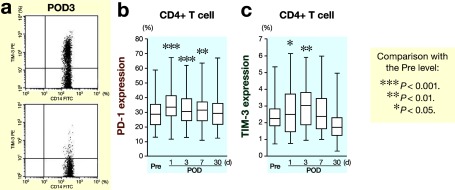
FACS analysis of leukocytes obtained from patients after surgery.
aRepresentative results of PD-1 and TIM-3 expressions on CD4+ T cells in peripheral blood obtained from colorectal cancer patients on postoperative day 3 by FACS.
bChanges in PD-1 expression on CD4+ T cells after surgery.
cChanges in TIM-3 expression on CD4+ T cells after surgery.
FACS, fluorescence-activated cell sorting; PD-1, programmed cell death 1; TIM-3, T-cell immunoglobulin domain and mucin domain 3; POD, postoperative day; Pre, before operation.
Fig. 4.
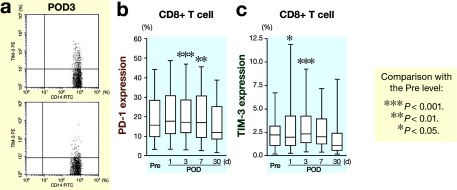
FACS analysis of leukocytes obtained after surgery.
aRepresentative results of PD-1 and TIM-3 expressions on CD8+ T cells in peripheral blood obtained from colorectal cancer patients on postoperative day 3 by FACS.
bChanges in PD-1 expression on CD8+ T cells after surgery.
cChanges in TIM-3 expression on CD8+ T cells after surgery.
FACS, fluorescence-activated cell sorting; PD-1, programmed cell death 1; TIM-3, T-cell immunoglobulin domain and mucin domain 3; POD, postoperative day; Pre, before operation.
Functional differences of CD4+ and CD8+ T cells depending on PD-1 and TIM-3 expressions
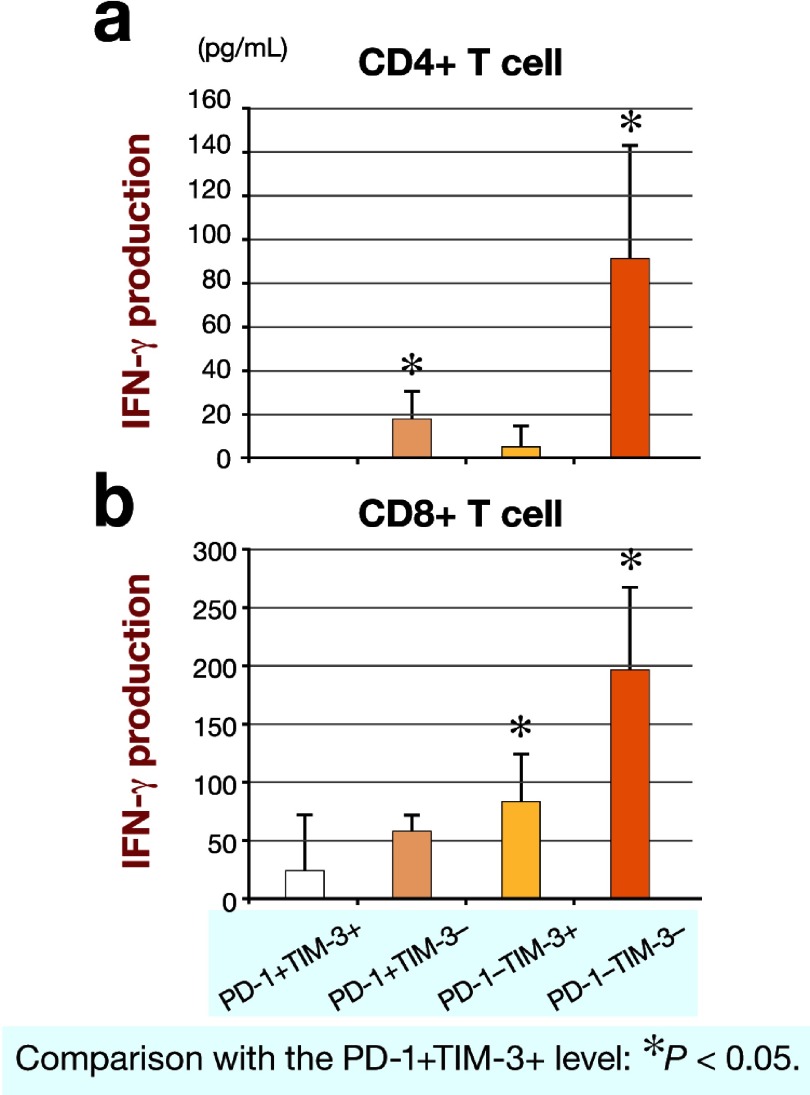
Each phenotype of CD4+ and CD8+ T cells based on PD-1 and TM-3 expressions was sorted, and the function of each phenotype was determined by measuring IFN-γ secretion. IFN-γ secretion by PD-1+TIM-3+CD4+ T cells was significantly less than that by either PD-1+TIM3–CD4+ T cells or PD-1–TIM-3–CD4+ T cells (Fig. 5a). Furthermore, IFN-γ secretion by PD-1+TIM-3+CD8+ T cells was significantly less than that by either PD-1–TIM-3+CD8+ T cells or PD-1–TIM-3–CD8+ T cells (Fig. 5b).
Fig. 5.
IFN-γ productions by CD4+ T cells (a) and CD8+ T cells (b) based on PD-1 and TIM-3 expressions. IFN-γ, interferon-γ PD-1, programmed cell death 1; TIM-3, T-cell immunoglobulin domain and mucin domain 3.
Discussion
During the perioperative and postoperative periods, a complex biologic response takes place in response to surgical stress. This response is intended to restore homeostasis as one aspect of host defenses against surgical stress. Thus, it is a very important response for the host. On the other hand, surgical stress induces suppression of cell-mediated immunity that is harmful to patients with cancer who underwent operation, since it may diminish a patient's ability to prevent postoperative infections. Of importance is that surgical stress-induced suppression of cell-mediated immunity may place a patient at higher risk for tumor recurrence. To assess postoperative cell-mediated immune function in the present study, the absolute number of circulating immunocompetent cells (CD4+ and CD8+ T cells) was measured, since the absolute number of circulating immunocompetent cells have been considered good indicators of the individual patient's cell-mediated immune function. In the present patients, the absolute number of total lymphocytes, CD4+ T cells and CD8+ T cells significantly decreased after colorectal cancer operation. These results showed evidence of impaired function of cell-mediated immunity after operation, consistently with a previous report by Wichmann et al. (2005).
Although there are numerous reports demonstrating inadequate cellular immunity after surgery, most studying the effect of surgical trauma on immune function have generally assessed differences in cell number and activity of immune cell subsets, as well as determined levels of circulating factors and mRNA expression of select genes ( Hamid et al., 1984; Hansbrough et al., 1984 ; Faist et al., 1986; Ogawa et al., 2000; Kalff et al., 2003). However, the detailed mechanisms responsible for these defects in cellular immunity remain unclear. Therefore, we determined PD-1 and TIM-3 expressions on CD4+ and CD8+ T cells to demonstrate one mechanism likely responsible for impaired cell-mediated immunity after colorectal surgery. Our data clearly demonstrated that PD-1 expression on both CD4+ and CD8+ T cells significantly increased after operation. Furthermore, TIM-3 expression on both CD4+ and CD8+ T cells also significantly increased after operation. Of importance is that IFN-γ production by CD4+ and CD8+ T cells was related to TIM-3 and PD-1 expressions on CD4+ and CD8+ T cells in the present study, indicating that TIM-3+ PD-1+ CD4+ and CD8+ T cells are highly dysfunctional compared with other CD4+ and CD8+ T cells. These data indicate that both TIM-3 and PD-1 expressions on CD4+ and CD8+ T cells is related to impaired function of these cells after operation.
Laparoscopic-assisted colectomy has gained acceptance for the treatment of colorectal cancer patients. The main advantage of laparoscopic surgery over open surgery is decreased invasiveness that results in a rapid return to preoperative activity levels and significantly shorter hospitalization. With regard to prognosis, some prospective randomized clinical trials have suggested that there were no differences between laparoscopic and open surgery (Guillou et al., 2005; Veldkamp et al., 2005). Of importance is that a randomized clinical trial demonstrated the prognosis of patients who underwent laparoscopic surgery is significantly better than those who underwent open surgery (Lacy et al., 2008). This might be due to well preserved cell-mediated immunity in patients who underwent laparoscopic surgery. In fact, our preliminary data indicated that TIM-3 expression on CD4+ and CD8+ T cells obtained from patients who underwent laparoscopic surgery was significantly lower than that obtained from patients who underwent open surgery, indicating that cell-medicated immunity is well preserved in patients who underwent laparoscopic surgery compared with those who underwent open surgery. Further investigations are required to clearly demonstrate this matter.
Surgery induces tumor cell dissemination, and handling of the tumor during resection can result in the spilling of tumor cells, as free floating tumor cells in the peritoneum, circulation and liver increase (Weitz et al., 1999; Conzelmann et al., 2005). Therefore, suppression of cell-mediated immunity after operation for colorectal cancer may place the patient at higher risk for tumor recurrence. In fact, according to several clinical studies that analyzed peri- and post-operatively obtained blood, tumor cell presence is a strong predictor of colorectal cancer recurrence (Koch et al., 2005; Allen-Mersh et al., 2007). Our data clearly demonstrated that both TIM-3 and PD-1 expressions were related to impaired function of T cells after operation for colorectal cancer. Therefore, PD-1 and TIM-3 might be good targets to restore impaired cell-mediated immunity after operation for colorectal cancer. In fact, blockade of both the TIM-3–TIM-3L and PD-1–PD-L1 pathways reverses the function of T cells, and is effective in controlling tumor growth.
In conclusion, PD-1 and TIM-3 expressions are closely related to the impaired cell-mediated immunity that is observed after surgery for colorectal cancer, and the development of specific treatments targeting TIM-3 and PD-1 on CD4+ and CD8+ T cells during the perioperative period may provide a breakthrough in the treatment of colorectal cancer patients.
References
- 1.Allen-Mersh TG, McCullough TK, Patel H, Wharton RQ, Glover C, Jonas SK.Role of circulating tumour cells in predicting recurrence after excision of primary colorectal carcinoma. Br J Surg 2007; 94: 96–105 [DOI] [PubMed] [Google Scholar]
- 2.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH.Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439: 682–687 [DOI] [PubMed] [Google Scholar]
- 3.Brune IB, Wilke W, Hensler T, Holzmann B, Siewert JR.Downregulation of T helper type 1 immune response and altered pro-inflammatory and anti-inflammatory T cell cytokine balance following conventional but not laparoscopic surgery. Am J Surg 1999; 177: 55– 60 [DOI] [PubMed] [Google Scholar]
- 4.Conzelmann M, Linnemann U, Berger MR.Detection of disseminated tumour cells in the liver of cancer patients. Eur J Surg Oncol 2005 ; 31: 977–985 [DOI] [PubMed] [Google Scholar]
- 5.Kubo M, Sasako M, Gotoda T, Ono H, Fujishiro M, Saito D.PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006; 443: 350–354 [DOI] [PubMed] [Google Scholar]
- 6.Decker D, Schondorf M, Bidlingmaier F, Hirner A, von Ruecker AA.Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery 1996; 119: 316 –325 [DOI] [PubMed] [Google Scholar]
- 7.Faist E, Kupper TS, Baker CC, Chaudry IH, Dwyer J, Baue AE. Depression of cellular immunity after major injury. Its association with posttraumatic complications and its reversal with immunomodulation. Arch Surg 1986; 121: 1000–1005 [DOI] [PubMed] [Google Scholar]
- 8.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients . J Exp Med 2010; 207: 2175 –2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM.Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005; 365: 1718– 1726 [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Watson DI.Effect of laparoscopy on immune function. Br J Surg 2001; 88: 1296–1306 [DOI] [PubMed] [Google Scholar]
- 11.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol 2009; 83: 9122–9130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol 2005; 23: 515–548 [DOI] [PubMed] [Google Scholar]
- 13.Hamid J, Bancewicz J, Brown R, Ward C, Irving MH, Ford WL. The significance of changes in blood lymphocyte populations following surgical operations. Clin Exp Immunol 1984; 56: 49–57 [PMC free article] [PubMed] [Google Scholar]
- 14.Hansbrough JF, Bender EM, Zapata-Sirvent R, Anderson J. Altered helper and suppressor lymphocyte populations in surgical patients. A measure of postoperative immunosuppression. Am J Surg 1984; 148: 303–307 [DOI] [PubMed] [Google Scholar]
- 15.Jacobi CA, Wenger F, Opitz I, Muller JM. Immunologic changes during minimally invasive surgery. Dig Surg 2002; 19: 459–463 [DOI] [PubMed] [Google Scholar]
- 16.Japanese Society for Cancer of the Colon and Rectum, the Japanese classification of colorectal carcinoma. 2nd English ed. Tokyo : Kanehara; 2009. [Google Scholar]
- 17.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med 2008; 205: 2763–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalff JC, Turler A, Schwarz NT, Schraut WH, Lee KK, Tweardy DJ. Intra-abdominal activation of a local inflammatory response within the human muscularis externa during laparotomy . Ann Surg 2003; 237 : 301–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch M, Kienle P, Hinz U, Antolovic D, Schmidt J, Herfarth C. Detection of hematogenous tumor cell dissemination predicts tumor relapse in patients undergoing surgical resection of colorectal liver metastases. Ann Surg 2005; 241: 199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koguchi K, Anderson DE, Yang L, O'Connor KC, Kuchroo VK, Hafler DA. Dysregulated T cell expression of TIM3 in multiple sclerosis. J Exp Med 2006; 203: 1413–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg 2008; 248: 1–7 [DOI] [PubMed] [Google Scholar]
- 22.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002; 415: 536–541 [DOI] [PubMed] [Google Scholar]
- 23.Ogawa K, Hirai M, Katsube T, Murayama M, Hamaguchi K, Shimakawa T. Suppression of cellular immunity by surgical stress . Surgery 2000; 127: 329–336 [DOI] [PubMed] [Google Scholar]
- 24.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance . Trends Immunol 2006; 27 : 195–201 [DOI] [PubMed] [Google Scholar]
- 25.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108 [DOI] [PubMed] [Google Scholar]
- 26.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med 2006; 203 : 2281–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabatos CA, Chakravarti S, Cha E, Schubart A, Sánchez-Fueyo A, Zheng XX. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol 2003; 4: 1102–1110 [DOI] [PubMed] [Google Scholar]
- 28.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 2010; 207: 2187–2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sánchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol 2003; 4: 1093–1101 [DOI] [PubMed] [Google Scholar]
- 30.Trabattoni D, Saresella M, Biasin M, Boasso A, Piacentini L, Ferrante P. B7-H1 is up-regulated in HIV infection and is a novel surrogate marker of disease progression. Blood 2003; 101: 2514–2520 [DOI] [PubMed] [Google Scholar]
- 31.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 2006; 12: 1198–1202 [DOI] [PubMed] [Google Scholar]
- 32.van der Bij GJ, Oosterling SJ, Beelen RH, Meijer S, Coffey JC, van Egmond M. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann Surg 2009; 249: 727–734 [DOI] [PubMed] [Google Scholar]
- 33.Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 2005; 6: 477–484 [DOI] [PubMed] [Google Scholar]
- 34.Weitz J, KienleP P, Magener A, Koch M, Schrodel A, Willeke F. Detection of disseminated colorectal cancer cells in lymph nodes, blood and bone marrow. Clin Cancer Res 1999; 5: 1830–1836 [PubMed] [Google Scholar]
- 35.Wichmann MW, Huttl TP, Winter H, Spelsberg F, Angele MK, Heiss MM. Immunological effects of laparoscopic vs open colorectal surgery: a prospective clinical study. Arch Surg 2005; 140: 692–697 [DOI] [PubMed] [Google Scholar]
- 36.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 2005; 6: 1245–1252 [DOI] [PubMed] [Google Scholar]