Abstract
Background
Balloon pulmonary valvuloplasty (BPV) represents the standard of management for all patients with severe pulmonary stenosis (PS) irrespective of their age. Nevertheless neonates and infants with critical PS represent a high-risk group that needs to be studied.
Methods
The study population included 72 infants with severe congenital valvular PS and four infants with imperforate pulmonary valve (PV) who were subjected to detailed history taking, full clinical examination, resting 12-lead ECG, Chest roentgenogram and transthoracic echocardiography. BPV was attempted in all infants with a peak-to-peak gradient across the PV of 50 mmHg or greater at catheterization-laboratory. Full echocardiographic evaluation was done 24 hours after the procedure as well as 3 and 6 months later.
Results
Seventy-six infants with severe PS or imperforate PV with a mean age of 5.63 ± 2.99 months were subjected to BPV with or without wire perforation. Immediately after the procedure patients had a significant reduction of the right ventricular systolic pressure (RVSP) (104.69 ± 24.98 mm Hg Vs 43.6 ± 13 mm Hg, p < 0.001) and RV-PA systolic pressure gradient (PG) (82.5 ± 23.76 mm Hg Vs 17.35 ± 8.96 mm Hg, p < 0.001). The immediate success rate defined as the drop in the RVSP to less than or equal to 50% of the baseline measurement was achieved in 85% of the cases. There was a progressive drop in the PG across the PV by Doppler echocardiogram throughout a follow-up period of six months from a mean of 93.3 ± 28.2 mm Hg to a mean of 17.4 ± 10.42 mm Hg (p < 0.001). There was a significant increase of the mean PV annulus diameter after balloon dilatation (p < 0.001). There was also a highly significant inverse correlation between the growth of the pulmonary annulus and the annular size at the baseline before dilatation (r = −0.74, p value <0.001). The incidence of PR significantly increased immediately after BPV to 64% followed by a progressive decline over a 6 months period of follow-up to 20%. There was a significant decrease in the incidence of tricuspid regurgitation (TR) over the same period of follow-up (from 55.6% at baseline to less than 20% at follow-up).
Conclusion
BPV is safe and effective to relieve critical PS in infants during the first year of life. The balloon promotes advantageous changes in both, pulmonary annulus and PG across the RVOT. In addition, the Doppler gradient observations during the follow-up support the expectation that BPV is a “curative” therapy.
Keywords: Balloon pulmonary valvuloplasty, Infants, Critical pulmonary stenosis
Abbreviations: ASD, atrial septal defect; BPV, balloon pulmonary valvuloplasty; BSA, body surface area; ECG, electrocardiogram; PADP, pulmonary artery diastolic pressure; PASP, pulmonary artery systolic pressure; PDA, patent ductus arteriosus; PFO, patent foramen ovale; PG, pressure gradient; PR, pulmonary regurgitation; PS, pulmonary stenosis; PV, pulmonary valve; RV, right ventricle; RVDP, right ventricular diastolic pressure; RVOT, right ventricular outflow tract; RVSP, right ventricular systolic pressure; TR, tricuspid regurgitation; TTE, transthoracic echocardiography; VSD, ventricualr septal defect
1. Introduction
The incidence of valvular pulmonary stenosis (PS) has been reported at 0.6 to 0.8 per 1000 live births, and when associated with other congenital cardiac lesions, it may occur in as many as 50% of all patients with congenital heart disease (Botto et al., 2001; Hoffman and Kaplan, 2002). Dysplastic valves consisting of thickened, irregular, immobile tissue, often with hypoplasia of the valve annulus and a small short main pulmonary artery are much less common (Becu et al., 1976).
The traditional method of treatment for this obstructive lesion was surgical valvotomy up to 1982, when Kan et al. (1982) introduced the technique of percutaneous balloon pulmonary valvuloplasty (BPV). The short- and mid-term results of BPV have been so good that nowadays it has become the preferred method of therapy for moderate and severe PS both in children as well as adults. It is also safe and effective for relief of PS in neonates (Ray et al., 1993; Shrivastava et al., 1993).
Neonates and infants with critical PS with right ventricular (RV) pressure equal to or greater than systemic pressure can be categorized into two distinct groups based on their clinical presentation: (1) critically ill infants with cyanosis whose pulmonary blood flow may depend on ductal patency and require urgent intervention and (2) asymptomatic infants. The anatomic and hemodynamic factors that determine the clinical presentation of infants with critical PS are not well understood (Nugent et al., 1977).
The success rates of BPV in infants with critical PS have been reported to be 55–94%; however, restenosis has been reported in 17–36% of these patients. The pulmonary valve (PV) annulus and right heart structures have been characterized as hypoplastic in some patients with critical PS, and growth of these structures relative to somatic growth after balloon pulmonary valvuloplasty has been a subject of controversy (Caspi et al., 1990; Gournay et al., 1995; Talsma et al., 1993; Zeevi et al., 1988).
2. Methods
This study was approved by our institutional review board and informed consent was obtained from the parents of all the children enrolled in the study.
2.1. Study population
The study included all infants under the age of 1 year who were referred for elective BPV in the Pediatric Cardiology Unit, Cardiology Department, Ain Shams University Hospital because of severe valvular PS or imperforate PV over 2 years time ending in December 2009.
2.2. Inclusion criteria
Patients who fulfilled the following criteria were selected for balloon dilatation of the PV: Age from birth to one year, congenital valvular PS with peak-to-peak systolic pressure gradient (PG) across the PV of >50 mm Hg regardless of symptoms, valve morphology or associated congenital anomalies such as small atrial septal defect (ASD), patent foramen ovale (PFO), small ventricular septal defect (VSD), or patent ductus arteriosus (PDA).
2.3. Exclusion criteria
-
•
Patients with associated large VSD or ASD or any other associated anomaly in which the dilatation of the PV would lead to increased risk of development of pulmonary hypertension in the future
-
•
Patient with multiple levels of right ventricular outflow tract (RVOT) obstruction who would not benefit from BPV.
2.4. Methods
2.4.1. Chest roentgenography and electrocardiography
Chest roentgenographs and electrocardiograms (ECG) were analyzed for chamber enlargement and lung vasculature.
2.4.2. Complete laboratory evaluation
Complete laboratory evaluation was carried out before catheterization.
2.4.3. Transthoracic Echocardiography (TTE)
TTE examinations were performed using a GE ViVid 5 echocardiography machine with phased array transducers. Standard echocardiographic views (parasternal long and short axis, apical 4, 2 and 5-chamber, subcostal and suprasternal views) in addition to color Doppler and continuous-wave Doppler were taken to study RV dimensions (Frommelt and Frommelt, 1999), PV morphology and flow, PV annulus and its Z-value and tricuspid valve annulus and its Z-value (Z-value was used to standardize pulmonary and tricuspid valves dimensions with body size). Z-scores <2 were taken to represent a small valve annulus while Z scores <3 marked a hypoplastic valve annulus (Gildein et al., 1996). Associated lesions such as PFO, ASD, PDA, VSD, post-stenotic dilatation and infundibular stenosis were noted. The maximum peak instantaneous systolic PG across the PV was quantified by the modified Bernoulli equation (Hatle, 1982). The presence and intensity of pulmonary regurgitation (PR) were assessed by color flow mapping. 2D Doppler echocardiographic examinations were performed immediately before and one day after BPV as well as 3 and 6 months later.
2.4.4. Balloon pulmonary valvuloplasty
Premedication with a sedative and analgesic was given to all cases, followed by local anesthesia. Continuous ECG monitoring and blood pressure measurement were carried out as well as arterial oxygen saturation by means of a pulse oximeter.
The femoral vein was accessed using Seldinger’s technique and a 5–7 F sheath was introduced into the vein. Alternative venous access sites were needed in selected cases. An angiographic catheter was placed in the RVOT and straight lateral angiograms were performed (1 cc/kg over one second) to measure the PV annulus and assess the RV and valve anatomy. The angiographic catheter was replaced with an end-hole catheter which was manipulated into the RV with the tip directed toward the RVOT. The PV was crossed and the catheter positioned in the lower lobe branch of either the left or the right pulmonary arteries. An exchange guide wire was placed in the peripheral pulmonary artery and the catheter was replaced with a dilatation balloon. The balloon was centered across the valve annulus in the lateral view and rapidly inflated by hand with diluted contrast material until the disappearance of the waist (Fig. 1). The deflated balloon was then removed while keeping the guide wire in the pulmonary artery. The guide wire permitted repositioning of an end-hole catheter into the distal pulmonary artery for pressure measurements (Moore and Lock, 2000).
Figure 1.
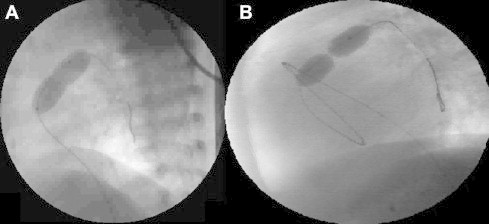
Lateral angiograms showing inflated balloons across the pulmonary valve. (A) Successful inflation of the balloon with near disappearance of the waist in an infant with severe PS, (B) sausage-like balloon with very tight waist in a case of imperforate pulmonary valve who underwent balloon dilatation after successful perforation of the pulmonary valve.
The following pressure and hemodynamic measurements were recorded before and immediately after BPV: Right ventricular systolic pressure (RVSP), right ventricular diastolic pressure (RVDP), pulmonary artery systolic pressure (PASP), Pulmonary artery diastolic pressure (PADP), RV to PA systolic PG.
The following data were also reported: Number of balloons used, type and size of each balloon used, number of inflations for each balloon, the maximum balloon annulus ratio used, the presence or absence of infundibular reaction and PG across the infundibulum.
All procedural or post-procedural complications such as respiratory or cardiac arrest, pericardial effusion or any vascular complication at the access site were recorded as well.
2.5. Statistical analysis
Continuous variables were expressed as mean ± standard deviation. Qualitative variables were expressed as percentages. A paired Student t test was used for the comparison of different variables before versus immediately after BPV as well as during the follow-up period. A P-value <0.05 was considered statistically significant. Linear correlation was applied to determine the relation between the baseline tricuspid and pulmonary valves annuli and their measurements after dilatation.
3. Results
The current study included 76 infants below 1 year of age representing 27.1% of the total number of patients referred for BPV in our institute during the given period of the study. Of these infants, 72 had severe valvular PS while four patients presented with membranous pulmonary atresia with intact inter ventricular septum.
Age of the patients ranged from 1 week to 12 months with a mean age of 5.63 ± 2.99 months. There were 48 males (63%) and 28 females (37%). Weight of the patients ranged from 3 to 9.5 kg with a mean of 6.39 ± 1.57 kg. Calculated body surface area (BSA) ranged from 0.22 to 0.56 m2. Table 1 illustrates the clinical and demographic characteristics of the study group.
Table 1.
Clinical and demographic characteristics of the patients.
| Characteristics | Findings |
|---|---|
| Gender (M/F) | 48/28 |
| Mean age (range) in months | 5.63 (0.25–12) |
| Mean BSA (range) in m2 | 0.39 (0.22–0.56) |
| Mean weight (range) in kg | 6.39 (3–9.5) |
| Cyanosis at rest | 31.5% |
| Dysplastic pulmonary valve | 13.15% |
| PFO | 59.2% |
| Associated anomalies | |
| Small VSD | 3.94%(n = 3) |
| Small ASD | 19.7%(n = 15) |
| Small PDA | 6.6%(n = 5) |
| TV disease | 5.26%(n = 4) |
| Isolated PS | 64.5%(n = 49) |
BSA = body surface area, VSD = ventricular septal defect, ASD = atrial septal defect, PDA = patent ductus arteriosus, TV = tricuspid valve, PS = pulmonary stenosis.
Table 2 shows the baseline echocardiographic data measured before BPV. Four patients with membranous pulmonary atresia underwent wire perforation of the pulmonary valve; one of these patients underwent PDA stenting as well. The remaining patients underwent BPV as previously described in the literature (Moore and Lock, 2000).
Table 2.
Baseline echocardiographic data.
| Range | SD ± Mean | |
|---|---|---|
| Peak PG | 60–165 | 28.2 ± 93.3 |
| RVSP | 80–195 | 115.6 ± 33.8 |
| Pulmonary annulus | 4–12 | 8.14 ± 1.45 |
| Pulmonary annulus Z score | −3.1–0.4 | −1.567 ± 0.895 |
| PR | 0% | |
| TR | 55.6% | |
| RVH | 72.3% |
PG = pressure gradient, RVSP = right ventricular systolic pressure, PR = pulmonary regurgitation, TR = tricuspid regurgitation, RVH = right ventricular hypertrophy.
The PV annulus measured from the right lateral angiogram ranged between 5 and 12 mm with a mean of 8.92 ± 1.44. The balloon sizes used ranged from 2.5 to 16 mm with a balloon/annulus ratio ranging from 0.9 to 1.6. Most of the patients had two consecutive balloon inflations (56%). The transvalvular PG measured during the procedure dropped from a mean of 82.5 ± 23.76 mm Hg before performing the BPV to a mean of 17.35 ± 8.96 after the procedure. There was a significant drop in the RVSP from a mean value of 104.69 ± 24.98 mm Hg to a mean value of 43.6 ± 13 mm Hg (P value <0.001). The immediate success rate defined as the drop in the RVSP to less than or equal to 50% of the baseline measurement was achieved in 85% of the cases.
There was a progressive drop in the PG across the PV by Doppler echocardiogram throughout a follow-up period of six months from a mean of 93.3 ± 28.2 mm Hg to a mean of 17.4 ± 10.42 mm Hg (Fig. 2). There was a highly significant drop from a mean of 93.3 ± 28.2 mm Hg to a mean of 24.93 ± 13.26 mm Hg 24 h after BPV (P value <0.001), this drop in the PG was maintained during the follow-up at 3 months with a mean of 19.13 ± 9.91 mm Hg and at 6 months with a mean value of 17.4 ± 10.42 mm Hg.
Figure 2.
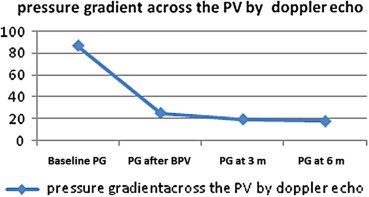
A linear curve showing the progressive drop in the pressure gradient (PG) across the pulmonary valve throughout a follow-up period of six months.
There was a progressive increase in the mean diameter of the PV annulus over the same period of follow-up from a mean value of 8.14 ± 1.45 mm to a mean value of 10.1 ± 1.64 mm. (Fig. 3). A highly significant change has been found in the pulmonary annulus Z-score with an increase from a mean of −1.567 ± 0.895 to a 6 months post BPV mean of −0.750 ± 0.995. There was a highly significant inverse correlation between the growth of the pulmonary annulus and the annular size at the baseline before dilatation (r = −0.74, P value <0.001) (Fig. 4). The incidence of PR significantly increased immediately after the BPV. The severity of PR correlated with the increase in balloon/annulus ratio as seen in Table 3. However, there was a progressive decline in PR over a 6 months period of follow-up from 64% to 20% of the patients denoting the transient nature of PR in the majority of the patients (Fig. 5).
Figure 3.
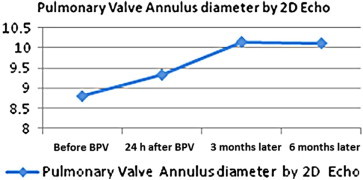
A linear curve showing the progressive increase in the mean diameter of the pulmonary valve annulus throughout a follow-up period of three months.
Figure 4.
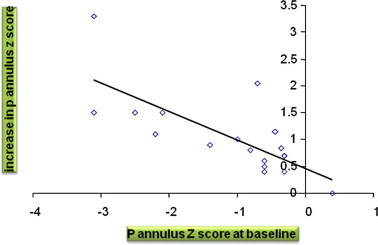
Correlation between the growth of the pulmonary annulus and the baseline annular size before dilatation.
Table 3.
Relation of the degree of PR and the balloon annulus ratio.
| Degree of PR | None | Mild | Moderate | Severe |
|---|---|---|---|---|
| Balloon/annulus ratio | 1.12 ± 0.02 | 1.29 ± 0.05 | 1.42 ± 0.09 | 1.5 ± 0.12 |
Figure 5.
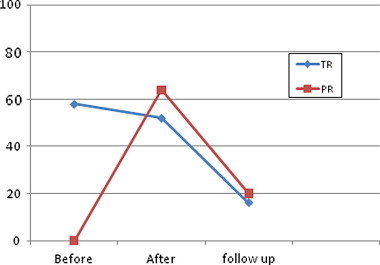
A linear curve showing the change in the incidence of both pulmonary regurgitation (PR) and tricuspid regurgitation (TR) before, immediately after and at 6 month period after balloon pulmonary valvuloplasty.
There was a decrease in the mean diameter of the tricuspid valve annulus from a mean of 17.1 ± 2.65 mm before the procedure to a mean of 16.24 ± 2.29 mm immediately after the procedure with less marked decrease during the following 6 months. There was no significant change in the tricuspid annulus Z-score with decrease from a mean of 0.4233 ± 1.214 to a 6 months post BPV mean of −0.1033 ± 1.279 (P value 0.064). However, there was a significant decrease in the incidence of tricuspid regurgitation (TR) over the same period of follow-up with a drop in the patients who had TR from 55.6% before the procedure to less than 20% at follow-up. (Figs 5 and 6).
Figure 6.
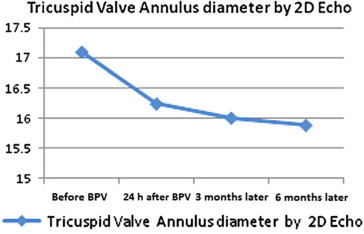
A linear curve shows a progressive decrease in the mean diameter of the tricuspid valve annulus throughout the follow-up of 6 months.
No significant change has been found in the right ventricular dimensions immediately after the BPV (19.51 ± 3.49 Vs 19.85 ± 2.28 mm) (P value = 0.51).
Regarding complication during BPV in this age group there was transient apnea in 46% of the patients, sinus bradycardia in 27.7% of the patients, self limiting perforations in 3.8% of the patients and transient loss of femoral pulse in 6.08% of the patients. The intra-procedure mortality rate was 4.1% (n = 3). One patient died due to pulmonary annulus rupture while two patients died due to persistent cardiopulmonary arrest not responding to resuscitation.
4. Discussion
BPV for congenital valvular PS is effective, safe and currently considered the therapeutic modality of choice for the treatment of PS in all age groups regardless of valve morphology (Jarrar et al., 1999). In the present study successful immediate outcome of BPV was reported in 85% of the cases. This rate is in close agreement with that of Moura and coworkers who reported a success rate of 78% in a similar study group (Moura et al., 2004).
The significant immediate reduction of both the RVSP and the PG across the RVOT in the current study was consistent with the most recent data published by Karagoz et al. (2009). There was also a progressive decrease in the PG across the RVOT throughout the follow-up period from a mean of 24.93 ± 13.26 immediately after BPV to a mean of 17.4 ± 10.42 after 6 months. This was consistent with the data published in other age groups like that of Sharieff et al. (2003) who reported a decrease in the PG from 33.5 ± 9.7 mm Hg immediately after BPV to 18.6 ± 3.4 mm Hg at follow-up. This finding might be explained by the gradual regression of infundibular hypertrophy.
Immediately after BPV we documented a sharp increase in the incidence of PR that reached 64%. This data was consistent with the data published by Poon and Menahem (2003) who reported a 61% incidence of PR the day after the procedure. Werynski et al. (2009) reported PR in 39.5% of children with critical PS immediately after BPV. However, unlike most of the data published in the literature we report a gradual decline in the incidence of PR to about 20% at 6 months follow-up (Poon and Menahem, 2003; Rao, 2007).
The current study showed a significant increase in the pulmonary annulus Z-score from a mean of −1.567 ± 0.895 to a 6 months post BPV mean of −0.750 ± 0.995. Tabatabaei et al. (1996) showed an increase in the pulmonary annulus diameter Z score from −3 ± 1.0 to 0 ± 0.1. Cazzaniga et al. (2000) also reported that the RV-PA junction Z-value grew from −1.25 ± 0.9 before valvuloplasty to −0.51 ± 0.7 at follow-up. This increase in the pulmonary valve annulus toward the normal values was also consistent with the most recent data published by Karagoz et al. (2009).
In the current study there was a significant decrease in the number of patients with TR at 6 months compared to the baseline. This data was consistent with the data in older age groups like that of Fawzy et al. (2007) who showed that severe TR in seven patients either regressed or totally disappeared at follow-up. This was also in accordance with Mechmeche et al. (2000) who found complete resolution of tricuspid insufficiency within 5 years of BPV in patients with severe PS and RV dysfunction. Similarly, Weber (2002) also demonstrated the regression of tricuspid valve insufficiency and elimination of right to left shunting at the atrial level in a similar subset of patients.
There was no significant change in the tricuspid annulus Z-score in the current study with decrease from a mean of 0.4233 ± 1.214 to a 6 months post BPV mean of −0.1033 ± 1.279. This result was similar to that obtained by Cazzaniga et al. (2000) who stated that since the tricuspid annulus is almost normal in most of the patients studied at baseline, configuration does not change substantially in the follow-up post BPV.
Regarding the mortality rate; 4.1% of our patients died compared to 8% of the patients as reported by Tabatabaei et al. (1996) and 14% of the patients as reported by Karagoz et al. (2009). This marked difference is due to the older age group of our patients. It has to be noted that BPV in even older age groups than our patients is reported with almost no mortality (Rao, 2007).
5. Study limitations
Some limitations exist in the present study. These include the relatively short period of follow-up and the single center source of data. Multicenter studies with longer follow-up periods are needed to analyze the important predictors of successful BPV in infants with severe PS and to study the outcome of this procedure in patients with critical PS and dysplastic pulmonary valves.
6. Conclusion
BPV is safe and effective to relieve critical PS in infants during the first year of life. The balloon promotes advantageous changes in both, pulmonary annulus and PG across the RVOT. In addition, the Doppler gradient observations during the follow-up support the expectation that BPV is a “curative” therapy.
Footnotes
The data submitted in this manuscript was presented by Prof. Maiy Hamdy El Sayed in the Saudi Heart Association 21st scientific session in February 2010 in a presentation titled “Valvular heart disease in pediatric age group”.
References
- Becu L., Somerville J., Gallo A. “Isolated” pulmonary valve stenosis as part of more widespread cardiovascular disease. Br. Heart J. 1976;38:472. doi: 10.1136/hrt.38.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto L.D., Correa A., Erickson J.D. Racial and temporal variations in the prevalence of heart defect. Pediatrics. 2001;107(3):1. doi: 10.1542/peds.107.3.e32. [DOI] [PubMed] [Google Scholar]
- Caspi J., Coles J.G., Benson L., Freedom R.M., Burrows E.M., Smallhorn J.F., Trusler G.A., Williams W.G. Management of neonatal critical pulmonic stenosis in the balloon valvotomy era. Ann. Thorac. Surg. 1990;49:273–278. doi: 10.1016/0003-4975(90)90149-z. [DOI] [PubMed] [Google Scholar]
- Cazzaniga M., Quero Jimenez C., Fernandez Pineda L., Daghero F., Herraiz I., Bermúdez Cañete R., Díez Balda J.I., Rico Gómez F., Maître M.J. Balloon pulmonary valvuloplasty in the neonatal period. The clinical and echocardiographic effects. Rev. Esp. Cardiol. 2000;53(3):327–336. doi: 10.1016/s0300-8932(00)75100-1. [DOI] [PubMed] [Google Scholar]
- Fawzy M.E., Hassan W., Fadel B.M., Sergani H., El Shaer F., El Widaa H., Al Sanei A. Long term results (up to 17 years) of pulmonary balloon valvuloplasty in adults and its effects on concomitant severe infundibular stenosis and tricuspid regurgitation. Am. Heart. J. 2007;153(3):433–438. doi: 10.1016/j.ahj.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Frommelt M.A., Frommelt P.C. Advances in echocardiographic diagnostic modalities for the pediatrician. Pediatr. Clin. North Am. 1999;46(2):427–439. doi: 10.1016/s0031-3955(05)70127-9. [DOI] [PubMed] [Google Scholar]
- Gildein H.P., Kleinert S., Goh T.H., Wilkinson J.L. Treatment of critical pulmonary valve stenosis by balloon dilatation in the neonate. Am. Heart J. 1996;131(5):1007–1011. doi: 10.1016/s0002-8703(96)90187-8. [DOI] [PubMed] [Google Scholar]
- Gournay V., Piechaud J., Delogu A., Sidi D., Kachaner J. Balloon valvotomy for critical stenosis or atresia of pulmonary valve in newborns. J. Am. Coll. Cardiol. 1995;26:1725–1731. doi: 10.1016/0735-1097(95)00369-X. [DOI] [PubMed] [Google Scholar]
- Hatle, L. (Ed.), 1982. Doppler Ultrasound in Cardiology: Physical Principles and Clinical Applications, first ed. Lea and Febiger, Philadelphia. pp. 76–170.
- Hoffman I.E., Kaplan S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- Jarrar M., Betbout F., Farhat M.B., Maatouk F., Gamra H., Addad F., Hammami S., Hamda K.B. Long-term invasive and noninvasive results of percutaneous balloon pulmonary valvuloplasty in children, adolescents, and adults. Am. Heart. J. 1999;138(5 Pt 1):950–954. doi: 10.1016/s0002-8703(99)70022-0. [DOI] [PubMed] [Google Scholar]
- Kan J.S., White R.I., Jr., Mitchell S.E., Gardner T.J. Percutaneous balloon valvuloplasty: a new method for treating congenital pulmonary-valve stenosis. N. Engl. J. Med. 1982;307(9):540–542. doi: 10.1056/NEJM198208263070907. [DOI] [PubMed] [Google Scholar]
- Karagoz T., Asoh K., Hickey E., Chaturvedi R., Lee K.J., Nykanen D., Benson L. Balloon dilatation of pulmonary valve stenosis in infants less than 3 Kg: a 20–year experience. Catheter Cardiovasc. Interv. 2009;74(5):753–761. doi: 10.1002/ccd.22064. [DOI] [PubMed] [Google Scholar]
- Mechmeche R., Hadrich M., Boussaada R., Marsit N., Cherif A., Farhati A., Ben Fredj S., Ezzar T. Evolution after 5 years of percutaneous pulmonary valvulotomy: report of 47 cases. Tunis. Med. 2000;78(1):30–36. [PubMed] [Google Scholar]
- Moore P., Lock J.E. Catheter intervention, balloon angioplasty, experimental studies, technology and methodology. In: Lock J.E., Keane J.F., Perry S.B., editors. Diagnostic and Interventional Catheterization in Congenital Heart Disease. 2nd ed. Kluwer Academic Publishers; Boston, USA: 2000. pp. 140–142. [Google Scholar]
- Moura C., Carrico A., Baptista M.J., Vieira A., Silva J.C., Moreira J., Areias J.C. Balloon pulmonary valvotomy performed in the first year of life. Rev. Port. Cardiol. 2004;23(1):55–63. [PubMed] [Google Scholar]
- Nugent E.W., Freedom R.M., Nora J.J. Clinical course in pulmonary stenosis. Report from the joint study of the natural history of congenital heart defects. Circulation. 1977;56(1):138. [Google Scholar]
- Poon L.K., Menahem S. Pulmonary regurgitation after percutaneous balloon valvoplasty for isolated pulmonary valvar stenosis in childhood. Cardiol. Young. 2003;13(5):444–450. [PubMed] [Google Scholar]
- Rao P.S. Percutaneous balloon pulmonary valvuloplasty: state of the art. Catheter Cardiovasc. Interv. 2007;69(5):747–763. doi: 10.1002/ccd.20982. [DOI] [PubMed] [Google Scholar]
- Ray D.G., Subramanyan R., Titus T., Tharakan J., Joy J., Venkitachalam C.G., Kumar A., Balakrishnan K.G. Balloon pulmonary valvoplasty: factors determining short- and long-term results. Int. J. Cardiol. 1993;40(1):17–25. doi: 10.1016/0167-5273(93)90226-7. [DOI] [PubMed] [Google Scholar]
- Sharieff S., Shah-e-Zaman K., Faruqui A.M. Short and intermediate term follow up results of percutaneous transluminal balloon valvuloplasty in adolescents and young adults with congenital pulmonary valve stenosis. J. Invasive Cardiol. 2003;15(9):484–487. [PubMed] [Google Scholar]
- Shrivastava S., Kumar R.K., Dev V., Saxena A., Das G. Determinants of immediate and follow-up results of pulmonary balloon valvuloplasty. Clin. Cardiol. 1993;16(6):497–502. doi: 10.1002/clc.4960160608. [DOI] [PubMed] [Google Scholar]
- Tabatabaei H., Boutin C., Nykanen D.G., Freedom R.M., Benson L.N. Morphologic and hemodynamic consequences after percutaneous balloon valvotomy for neonatal pulmonary stenosis: medium term follow up. J. Am. Coll. Cardiol. 1996;27(2):473–478. doi: 10.1016/0735-1097(95)00477-7. [DOI] [PubMed] [Google Scholar]
- Talsma M., Witsenburg M., Rohmer J., Hess J. Determinants for outcome of balloon valvuloplasty for severe pulmonary stenosis in neonates and infants up to six months of age. Am. J. Cardiol. 1993;71:1246–1248. doi: 10.1016/0002-9149(93)90662-v. [DOI] [PubMed] [Google Scholar]
- Weber H.S. Initial and late results after catheter intervention for neonatal critical pulmonary valve stenosis and atresia with intact ventricular septum: a technique in continual evolution. Catheter Cardiovasc. Interv. 2002;56(3):394–399. doi: 10.1002/ccd.10234. [DOI] [PubMed] [Google Scholar]
- Werynski P., Rudzinski A., Krol-Jawien W., Kuźma J. Percutaneous balloon valvuloplasty for the treatment of pulmonary valve stenosis in children–a single center experience. Kardiol. Pol. 2009;67(4):369–375. [PubMed] [Google Scholar]
- Zeevi B., Keane J.F., Fellows K.E., Lock J.E. Balloon dilation of critical pulmonary stenosis in the first week of life. J. Am. Coll. Cardiol. 1988;11:821–824. doi: 10.1016/0735-1097(88)90217-3. [DOI] [PubMed] [Google Scholar]


