Abstract
The possession of specialized iron transport systems may be crucial for bacteria to override the iron limitation imposed by the host or the environment. One of the most commonly found strategies evolved by microorganisms is the production of siderophores, low-molecular-weight iron chelators that have very high constants of association for their complexes with iron. Thus, siderophores act as extracellular solubilizing agents for iron from minerals or organic compounds, such as transferrin and lactoferrin in the host vertebrate, under conditions of iron limitation. Transport of iron into the cell cytosol is mediated by specific membrane receptor and transport systems which recognize the iron-siderophore complexes. In this review I have analyzed in detail three siderophore-mediated iron uptake systems: the plasmid-encoded anguibactin system of Vibrio anguillarum, the aerobactin-mediated iron assimilation system present in the pColV-K30 plasmid and in the chromosomes of many enteric bacteria, and the chromosomally encoded enterobactin iron uptake system, found in Escherichia coli, Shigella spp., Salmonella spp., and other members of the family Enterobacteriaceae. The siderophore systems encoded by Pseudomonas aeruginosa, namely, pyochelin and pyoverdin, as well as the siderophore amonabactin, specified by Aeromonas hydrophila, are also discussed. The potential role of siderophore-mediated systems as virulence determinants in the specific host-bacteria interaction leading to disease is also analyzed with respect to the influence of these systems in the expression of other factors, such as toxins, in the bacterial virulence repertoire.
Full text
PDF

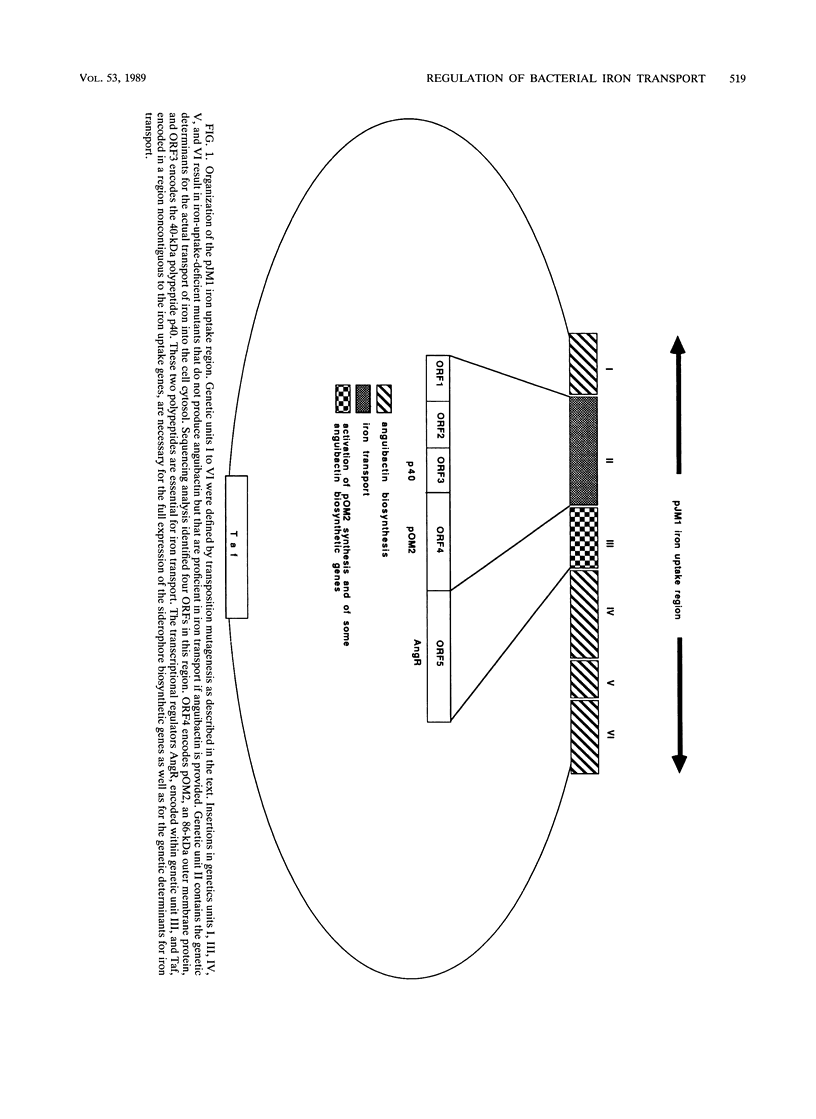
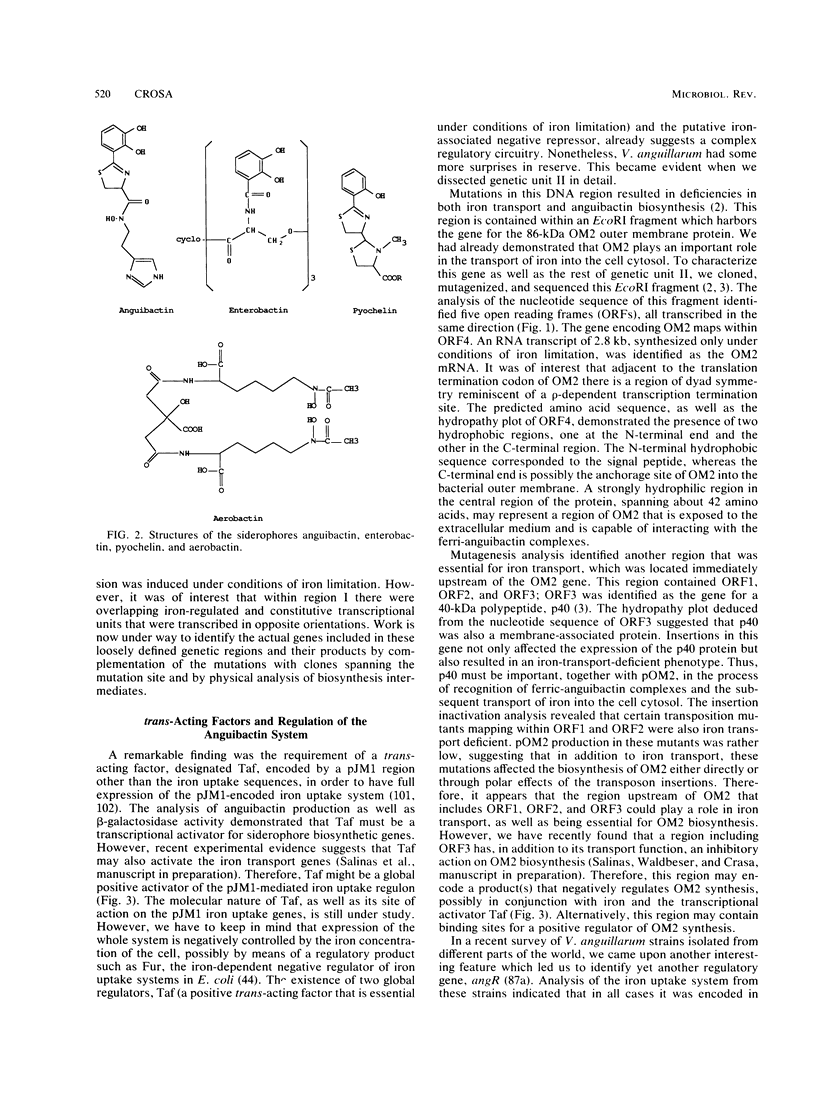
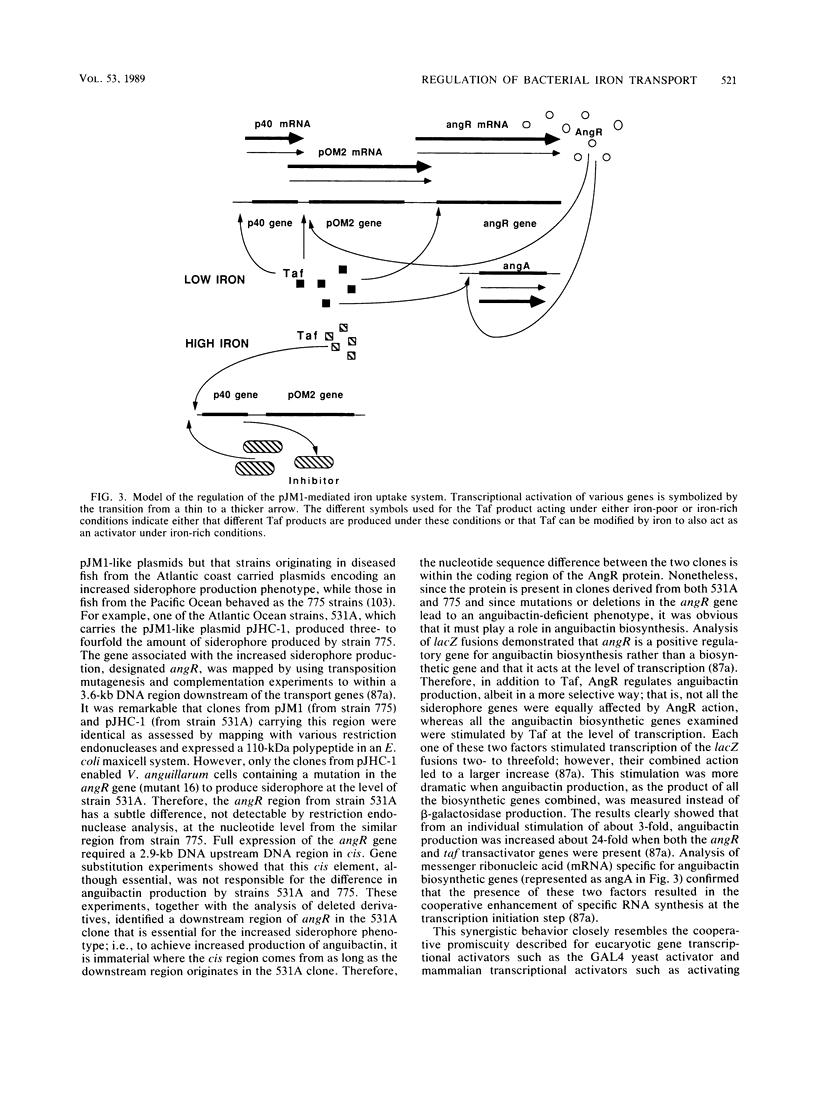

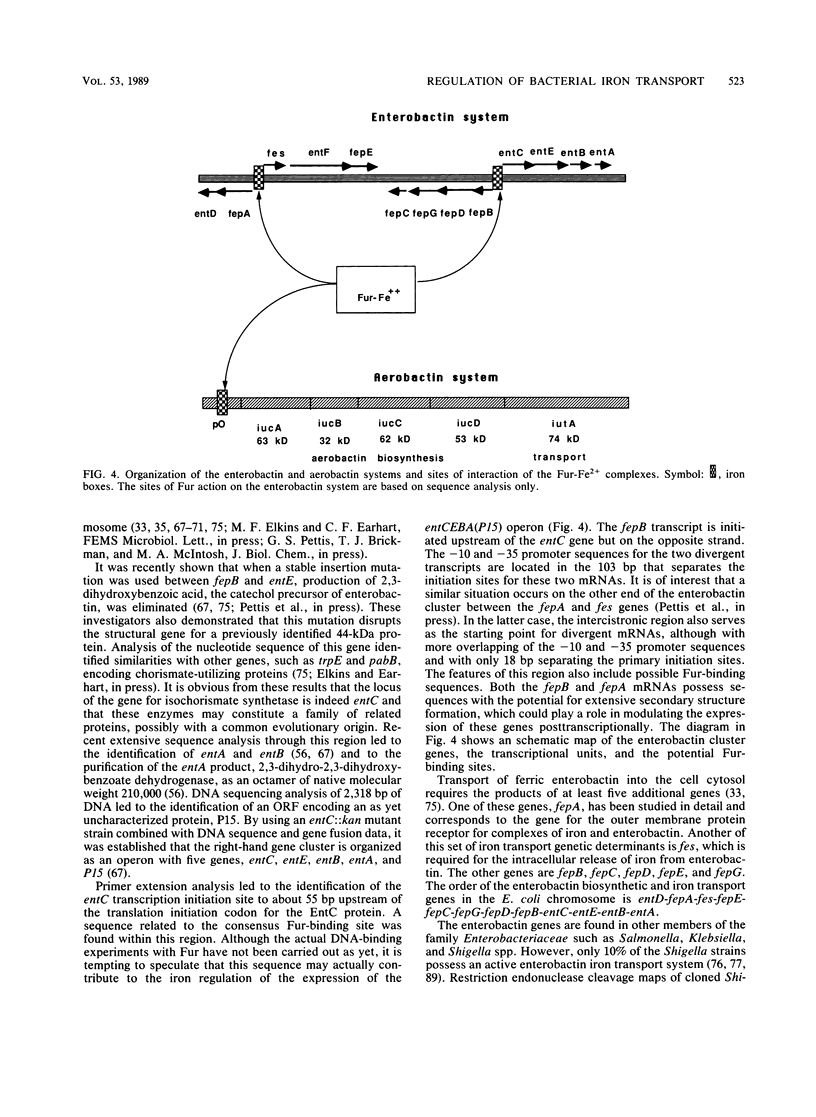







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Actis L. A., Fish W., Crosa J. H., Kellerman K., Ellenberger S. R., Hauser F. M., Sanders-Loehr J. Characterization of anguibactin, a novel siderophore from Vibrio anguillarum 775(pJM1). J Bacteriol. 1986 Jul;167(1):57–65. doi: 10.1128/jb.167.1.57-65.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Actis L. A., Potter S. A., Crosa J. H. Iron-regulated outer membrane protein OM2 of Vibrio anguillarum is encoded by virulence plasmid pJM1. J Bacteriol. 1985 Feb;161(2):736–742. doi: 10.1128/jb.161.2.736-742.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Actis L. A., Tolmasky M. E., Farrell D. H., Crosa J. H. Genetic and molecular characterization of essential components of the Vibrio anguillarum plasmid-mediated iron-transport system. J Biol Chem. 1988 Feb 25;263(6):2853–2860. [PubMed] [Google Scholar]
- Ames G. F. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- Ankenbauer R. G., Toyokuni T., Staley A., Rinehart K. L., Jr, Cox C. D. Synthesis and biological activity of pyochelin, a siderophore of Pseudomonas aeruginosa. J Bacteriol. 1988 Nov;170(11):5344–5351. doi: 10.1128/jb.170.11.5344-5351.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankenbauer R., Sriyosachati S., Cox C. D. Effects of siderophores on the growth of Pseudomonas aeruginosa in human serum and transferrin. Infect Immun. 1985 Jul;49(1):132–140. doi: 10.1128/iai.49.1.132-140.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R. R., Cole M. F., McGhee J. R. A bactericidal effect for human lactoferrin. Science. 1977 Jul 15;197(4300):263–265. doi: 10.1126/science.327545. [DOI] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987 Aug 25;26(17):5471–5477. doi: 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barghouthi S., Young R., Olson M. O., Arceneaux J. E., Clem L. W., Byers B. R. Amonabactin, a novel tryptophan- or phenylalanine-containing phenolate siderophore in Aeromonas hydrophila. J Bacteriol. 1989 Apr;171(4):1811–1816. doi: 10.1128/jb.171.4.1811-1816.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorn M. J., Iglewski B. H., Ives S. K., Sadoff J. C., Vasil M. L. Effect of iron on yields of exotoxin A in cultures of Pseudomonas aeruginosa PA-103. Infect Immun. 1978 Mar;19(3):785–791. doi: 10.1128/iai.19.3.785-791.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorn M. J., Sokol P. A., Iglewski B. H. Influence of iron on yields of extracellular products in Pseudomonas aeruginosa cultures. J Bacteriol. 1979 Apr;138(1):193–200. doi: 10.1128/jb.138.1.193-200.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Lewin J. E. The bacteriostatic effect of serum on Pasteurella septica and its abolition by iron compounds. Immunology. 1971 Mar;20(3):391–406. [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- Burkhardt R., Braun V. Nucleotide sequence of the fhuC and fhuD genes involved in iron (III) hydroxamate transport: domains in FhuC homologous to ATP-binding proteins. Mol Gen Genet. 1987 Aug;209(1):49–55. doi: 10.1007/BF00329835. [DOI] [PubMed] [Google Scholar]
- Carlioz A., Ludwig M. L., Stallings W. C., Fee J. A., Steinman H. M., Touati D. Iron superoxide dismutase. Nucleotide sequence of the gene from Escherichia coli K12 and correlations with crystal structures. J Biol Chem. 1988 Jan 25;263(3):1555–1562. [PubMed] [Google Scholar]
- Casey J. L., Hentze M. W., Koeller D. M., Caughman S. W., Rouault T. A., Klausner R. D., Harford J. B. Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science. 1988 May 13;240(4854):924–928. doi: 10.1126/science.2452485. [DOI] [PubMed] [Google Scholar]
- Cox C. D. Effect of pyochelin on the virulence of Pseudomonas aeruginosa. Infect Immun. 1982 Apr;36(1):17–23. doi: 10.1128/iai.36.1.17-23.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J Bacteriol. 1980 May;142(2):581–587. doi: 10.1128/jb.142.2.581-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Rinehart K. L., Jr, Moore M. L., Cook J. C., Jr Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4256–4260. doi: 10.1073/pnas.78.7.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton R. R., Charloteaux-Wauters M. Iron transport and storage. Eur J Biochem. 1987 May 4;164(3):485–506. doi: 10.1111/j.1432-1033.1987.tb11155.x. [DOI] [PubMed] [Google Scholar]
- Crosa J. H. A plasmid associated with virulence in the marine fish pathogen Vibrio anguillarum specifies an iron-sequestering system. Nature. 1980 Apr 10;284(5756):566–568. doi: 10.1038/284566a0. [DOI] [PubMed] [Google Scholar]
- Crosa J. H., Hodges L. L. Outer membrane proteins induced under conditions of iron limitation in the marine fish pathogen Vibrio anguillarum 775. Infect Immun. 1981 Jan;31(1):223–227. doi: 10.1128/iai.31.1.223-227.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Hodges L. L., Schiewe M. H. Curing of a plasmid is correlated with an attenuation of virulence in the marine fish pathogen Vibrio anguillarum. Infect Immun. 1980 Mar;27(3):897–902. doi: 10.1128/iai.27.3.897-902.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H. The relationship of plasmid-mediated iron transport and bacterial virulence. Annu Rev Microbiol. 1984;38:69–89. doi: 10.1146/annurev.mi.38.100184.000441. [DOI] [PubMed] [Google Scholar]
- Crosa L. M., Wolf M. K., Actis L. A., Sanders-Loehr J., Crosa J. H. New aerobactin-mediated iron uptake system in a septicemia-causing strain of Enterobacter cloacae. J Bacteriol. 1988 Dec;170(12):5539–5544. doi: 10.1128/jb.170.12.5539-5544.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Eisenstadt R. L., East S. J., Cornford R. J., Walker L. A., White A. J. Iron-regulated outer membrane proteins of Escherichia coli K-12 and mechanism of action of catechol-substituted cephalosporins. Antimicrob Agents Chemother. 1988 Dec;32(12):1879–1886. doi: 10.1128/aac.32.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo V., Herrero M., Giovannini F., Neilands J. B. Fur (ferric uptake regulation) protein and CAP (catabolite-activator protein) modulate transcription of fur gene in Escherichia coli. Eur J Biochem. 1988 May 2;173(3):537–546. doi: 10.1111/j.1432-1033.1988.tb14032.x. [DOI] [PubMed] [Google Scholar]
- Eick-Helmerich K., Hantke K., Braun V. Cloning and expression of the exbB gene of Escherichia coli K-12. Mol Gen Genet. 1987 Feb;206(2):246–251. doi: 10.1007/BF00333580. [DOI] [PubMed] [Google Scholar]
- Elish M. E., Pierce J. R., Earhart C. F. Biochemical analysis of spontaneous fepA mutants of Escherichia coli. J Gen Microbiol. 1988 May;134(5):1355–1364. doi: 10.1099/00221287-134-5-1355. [DOI] [PubMed] [Google Scholar]
- Ernst J. F., Bennett R. L., Rothfield L. I. Constitutive expression of the iron-enterochelin and ferrichrome uptake systems in a mutant strain of Salmonella typhimurium. J Bacteriol. 1978 Sep;135(3):928–934. doi: 10.1128/jb.135.3.928-934.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecker L., Braun V. Cloning and expression of the fhu genes involved in iron(III)-hydroxamate uptake by Escherichia coli. J Bacteriol. 1983 Dec;156(3):1301–1314. doi: 10.1128/jb.156.3.1301-1314.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARIBALDI J. A., NEILANDS J. B. Formation of iron-binding compounds by micro-organisms. Nature. 1956 Mar 17;177(4507):526–527. doi: 10.1038/177526a0. [DOI] [PubMed] [Google Scholar]
- Gibson F., Magrath D. I. The isolation and characterization of a hydroxamic acid (aerobactin) formed by Aerobacter aerogenes 62-I. Biochim Biophys Acta. 1969 Nov 18;192(2):175–184. doi: 10.1016/0304-4165(69)90353-5. [DOI] [PubMed] [Google Scholar]
- Griggs D. W., Konisky J. Mechanism for iron-regulated transcription of the Escherichia coli cir gene: metal-dependent binding of fur protein to the promoters. J Bacteriol. 1989 Feb;171(2):1048–1054. doi: 10.1128/jb.171.2.1048-1054.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R., Engelbrecht F., Braun V. Identification of the genes and their polypeptide products responsible for aerobactin synthesis by pColV plasmids. Mol Gen Genet. 1985;201(2):204–212. doi: 10.1007/BF00425661. [DOI] [PubMed] [Google Scholar]
- Guterman S. K. Colicin B: mode of action and inhibition by enterochelin. J Bacteriol. 1973 Jun;114(3):1217–1224. doi: 10.1128/jb.114.3.1217-1224.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Functional interaction of the tonA/tonB receptor system in Escherichia coli. J Bacteriol. 1978 Jul;135(1):190–197. doi: 10.1128/jb.135.1.190-197.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Hartmann A., Braun V. Iron transport in Escherichia coli: uptake and modification of ferrichrome. J Bacteriol. 1980 Jul;143(1):246–255. doi: 10.1128/jb.143.1.246-255.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Casey J. L., Koeller D. M., Rouault T. A., Harford J. B., Klausner R. D. A model for the structure and functions of iron-responsive elements. Gene. 1988 Dec 10;72(1-2):201–208. doi: 10.1016/0378-1119(88)90145-x. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Sparling P. F. Haemophilus influenzae can use human transferrin as a sole source for required iron. Infect Immun. 1985 Apr;48(1):248–251. doi: 10.1128/iai.48.1.248-251.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos M. L., Salinas P., Toranzo A. E., Barja J. L., Crosa J. H. Chromosome-mediated iron uptake system in pathogenic strains of Vibrio anguillarum. J Bacteriol. 1988 Apr;170(4):1920–1925. doi: 10.1128/jb.170.4.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Duncan K., Walsh C. T. Nucleotide sequence of a cluster of Escherichia coli enterobactin biosynthesis genes: identification of entA and purification of its product 2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase. J Bacteriol. 1989 Feb;171(2):791–798. doi: 10.1128/jb.171.2.791-798.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Pollack J. R., Wayne R., Ames B. N., Neilands J. B. Iron uptake in Salmonella typhimurium: utilization of exogenous siderochromes as iron carriers. J Bacteriol. 1972 Sep;111(3):731–738. doi: 10.1128/jb.111.3.731-738.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazin M. D., Moores J. C., Leong J. Cloning of the gene coding for the outer membrane receptor protein for ferric pseudobactin, a siderophore from a plant growth-promoting Pseudomonas strain. J Biol Chem. 1986 Jan 15;261(2):795–799. [PubMed] [Google Scholar]
- Marolda C. L., Valvano M. A., Lawlor K. M., Payne S. M., Crosa J. H. Flanking and internal regions of chromosomal genes mediating aerobactin iron uptake systems in enteroinvasive Escherichia coli and Shigella flexneri. J Gen Microbiol. 1987 Aug;133(8):2269–2278. doi: 10.1099/00221287-133-8-2269. [DOI] [PubMed] [Google Scholar]
- Marugg J. D., Nielander H. B., Horrevoets A. J., van Megen I., van Genderen I., Weisbeek P. J. Genetic organization and transcriptional analysis of a major gene cluster involved in siderophore biosynthesis in Pseudomonas putida WCS358. J Bacteriol. 1988 Apr;170(4):1812–1819. doi: 10.1128/jb.170.4.1812-1819.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marugg J. D., van Spanje M., Hoekstra W. P., Schippers B., Weisbeek P. J. Isolation and analysis of genes involved in siderophore biosynthesis in plant-growth-stimulating Pseudomonas putida WCS358. J Bacteriol. 1985 Nov;164(2):563–570. doi: 10.1128/jb.164.2.563-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzanke B. F., Müller G. I., Raymond K. N. Hydroxamate siderophore mediated iron uptake in E. coli: stereospecific recognition of ferric rhodotorulic acid. Biochem Biophys Res Commun. 1984 Jun 29;121(3):922–930. doi: 10.1016/0006-291x(84)90765-4. [DOI] [PubMed] [Google Scholar]
- Mickelsen P. A., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981 Aug;33(2):555–564. doi: 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles A. A., Khimji P. L. Enterobacterial chelators of iron: their occurrence, detection, and relation to pathogenicity. J Med Microbiol. 1975 Nov;8(4):477–490. doi: 10.1099/00222615-8-4-477. [DOI] [PubMed] [Google Scholar]
- Moores J. C., Magazin M., Ditta G. S., Leong J. Cloning of genes involved in the biosynthesis of pseudobactin, a high-affinity iron transport agent of a plant growth-promoting Pseudomonas strain. J Bacteriol. 1984 Jan;157(1):53–58. doi: 10.1128/jb.157.1.53-58.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahlik M. S., Brickman T. J., Ozenberger B. A., McIntosh M. A. Nucleotide sequence and transcriptional organization of the Escherichia coli enterobactin biosynthesis cistrons entB and entA. J Bacteriol. 1989 Feb;171(2):784–790. doi: 10.1128/jb.171.2.784-790.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Iron absorption and transport in microorganisms. Annu Rev Nutr. 1981;1:27–46. doi: 10.1146/annurev.nu.01.070181.000331. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- O'Brien I. G., Cox G. B., Gibson F. Enterochelin hydrolysis and iron metabolism in Escherichia coli. Biochim Biophys Acta. 1971 Jun 22;237(3):537–549. doi: 10.1016/0304-4165(71)90274-1. [DOI] [PubMed] [Google Scholar]
- Ozenberger B. A., Brickman T. J., McIntosh M. A. Nucleotide sequence of Escherichia coli isochorismate synthetase gene entC and evolutionary relationship of isochorismate synthetase and other chorismate-utilizing enzymes. J Bacteriol. 1989 Feb;171(2):775–783. doi: 10.1128/jb.171.2.775-783.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M. Synthesis and utilization of siderophores by Shigella flexneri. J Bacteriol. 1980 Sep;143(3):1420–1424. doi: 10.1128/jb.143.3.1420-1424.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Casal J. F., Crosa J. H. Aerobactin iron uptake sequences in plasmid ColV-K30 are flanked by inverted IS1-like elements and replication regions. J Bacteriol. 1984 Oct;160(1):256–265. doi: 10.1128/jb.160.1.256-265.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack J. R., Ames B. N., Neilands J. B. Iron transport in Salmonella typhimurium: mutants blocked in the biosynthesis of enterobactin. J Bacteriol. 1970 Nov;104(2):635–639. doi: 10.1128/jb.104.2.635-639.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle K., Good R. F. DNA sequence of the Escherichia coli tonB gene. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5235–5239. doi: 10.1073/pnas.80.17.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler U., Staudenmaier H., Zimmermann L., Braun V. Genetics of the iron dicitrate transport system of Escherichia coli. J Bacteriol. 1988 Jun;170(6):2716–2724. doi: 10.1128/jb.170.6.2716-2724.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Rao K., Harford J. B., Rouault T., McClelland A., Ruddle F. H., Klausner R. D. Transcriptional regulation by iron of the gene for the transferrin receptor. Mol Cell Biol. 1986 Jan;6(1):236–240. doi: 10.1128/mcb.6.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. M., Cryz S. J., Jr, Holmes R. K. Genetic and biochemical evidence for a siderophore-dependent iron transport system in Corynebacterium diphtheriae. Infect Immun. 1984 Jul;45(1):143–149. doi: 10.1128/iai.45.1.143-149.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. M., Holmes R. K. Initial characterization of the ferric iron transport system of Corynebacterium diphtheriae. J Bacteriol. 1983 Sep;155(3):1439–1442. doi: 10.1128/jb.155.3.1439-1442.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas P. C., Tolmasky M. E., Crosa J. H. Regulation of the iron uptake system in Vibrio anguillarum: evidence for a cooperative effect between two transcriptional activators. Proc Natl Acad Sci U S A. 1989 May;86(10):3529–3533. doi: 10.1073/pnas.86.10.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. P., Payne S. M. Genetics and regulation of enterobactin genes in Shigella flexneri. J Bacteriol. 1988 Dec;170(12):5579–5587. doi: 10.1128/jb.170.12.5579-5587.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Cox C. D., Iglewski B. H. Pseudomonas aeruginosa mutants altered in their sensitivity to the effect of iron on toxin A or elastase yields. J Bacteriol. 1982 Aug;151(2):783–787. doi: 10.1128/jb.151.2.783-787.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A. Surface expression of ferripyochelin-binding protein is required for virulence of Pseudomonas aeruginosa. Infect Immun. 1987 Sep;55(9):2021–2025. doi: 10.1128/iai.55.9.2021-2025.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Woods D. E. Demonstration of an iron-siderophore-binding protein in the outer membrane of Pseudomonas aeruginosa. Infect Immun. 1983 May;40(2):665–669. doi: 10.1128/iai.40.2.665-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriyosachati S., Cox C. D. Siderophore-mediated iron acquisition from transferrin by Pseudomonas aeruginosa. Infect Immun. 1986 Jun;52(3):885–891. doi: 10.1128/iai.52.3.885-891.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoebner J. A., Payne S. M. Iron-regulated hemolysin production and utilization of heme and hemoglobin by Vibrio cholerae. Infect Immun. 1988 Nov;56(11):2891–2895. doi: 10.1128/iai.56.11.2891-2895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart S. J., Greenwood K. T., Luke R. K. Hydroxamate-mediated transport of iron controlled by ColV plasmids. J Bacteriol. 1980 Jul;143(1):35–42. doi: 10.1128/jb.143.1.35-42.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai S. P., Holmes R. K. Iron regulation of the cloned diphtheria toxin promoter in Escherichia coli. Infect Immun. 1988 Sep;56(9):2430–2436. doi: 10.1128/iai.56.9.2430-2436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Avila H. Structure and gene expression of the E. coli Mn-superoxide dismutase gene. Nucleic Acids Res. 1986 Jun 11;14(11):4577–4589. doi: 10.1093/nar/14.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teintze M., Hossain M. B., Barnes C. L., Leong J., van der Helm D. Structure of ferric pseudobactin, a siderophore from a plant growth promoting Pseudomonas. Biochemistry. 1981 Oct 27;20(22):6446–6457. doi: 10.1021/bi00525a025. [DOI] [PubMed] [Google Scholar]
- Tolmasky M. E., Actis L. A., Crosa J. H. Genetic analysis of the iron uptake region of the Vibrio anguillarum plasmid pJM1: molecular cloning of genetic determinants encoding a novel trans activator of siderophore biosynthesis. J Bacteriol. 1988 Apr;170(4):1913–1919. doi: 10.1128/jb.170.4.1913-1919.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmasky M. E., Crosa J. H. Molecular cloning and expression of genetic determinants for the iron uptake system mediated by the Vibrio anguillarum plasmid pJM1. J Bacteriol. 1984 Dec;160(3):860–866. doi: 10.1128/jb.160.3.860-866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmasky M. E., Salinas P. C., Actis L. A., Crosa J. H. Increased production of the siderophore anguibactin mediated by pJM1-like plasmids in Vibrio anguillarum. Infect Immun. 1988 Jun;56(6):1608–1614. doi: 10.1128/iai.56.6.1608-1614.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toranzo A. E., Barja J. L., Potter S. A., Colwell R. R., Hetrick F. M., Crosa J. H. Molecular factors associated with virulence of marine vibrios isolated from striped bass in Chesapeake Bay. Infect Immun. 1983 Mar;39(3):1220–1227. doi: 10.1128/iai.39.3.1220-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvano M. A., Crosa J. H. Aerobactin iron transport genes commonly encoded by certain ColV plasmids occur in the chromosome of a human invasive strain of Escherichia coli K1. Infect Immun. 1984 Oct;46(1):159–167. doi: 10.1128/iai.46.1.159-167.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvano M. A., Crosa J. H. Molecular cloning, expression, and regulation in Escherichia coli K-12 of a chromosome-mediated aerobactin iron transport system from a human invasive isolate of E. coli K1. J Bacteriol. 1988 Dec;170(12):5529–5538. doi: 10.1128/jb.170.12.5529-5538.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvano M. A., Silver R. P., Crosa J. H. Occurrence of chromosome- or plasmid-mediated aerobactin iron transport systems and hemolysin production among clonal groups of human invasive strains of Escherichia coli K1. Infect Immun. 1986 Apr;52(1):192–199. doi: 10.1128/iai.52.1.192-199.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M. A., Potter S. A., Crosa J. H. Iron uptake system medicated by Vibrio anguillarum plasmid pJM1. J Bacteriol. 1983 Nov;156(2):880–887. doi: 10.1128/jb.156.2.880-887.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner P. J., Williams P. H., Bindereif A., Neilands J. B. ColV plasmid-specific aerobactin synthesis by invasive strains of Escherichia coli. Infect Immun. 1981 Aug;33(2):540–545. doi: 10.1128/iai.33.2.540-545.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters V. L., Crosa J. H. DNA environment of the aerobactin iron uptake system genes in prototypic ColV plasmids. J Bacteriol. 1986 Aug;167(2):647–654. doi: 10.1128/jb.167.2.647-654.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters V. L., Crosa J. H. Divergence of the aerobactin iron uptake systems encoded by plasmids pColV-K30 in Escherichia coli K-12 and pSMN1 in Aerobacter aerogenes 62-1. J Bacteriol. 1988 Nov;170(11):5153–5160. doi: 10.1128/jb.170.11.5153-5160.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne R., Neilands J. B. Evidence for common binding sites for ferrichrome compounds and bacteriophage phi 80 in the cell envelope of Escherichia coli. J Bacteriol. 1975 Feb;121(2):497–503. doi: 10.1128/jb.121.2.497-503.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979 Dec;26(3):925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. K., Crosa J. H. Evidence for the role of a siderophore in promoting Vibrio anguillarum infections. J Gen Microbiol. 1986 Oct;132(10):2949–2952. doi: 10.1099/00221287-132-10-2949. [DOI] [PubMed] [Google Scholar]
- Zimmermann L., Angerer A., Braun V. Mechanistically novel iron(III) transport system in Serratia marcescens. J Bacteriol. 1989 Jan;171(1):238–243. doi: 10.1128/jb.171.1.238-243.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V., Wee S., Herrero M., Neilands J. B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987 Jun;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


