Abstract
Background and Purpose
After removal of the Foley catheter after robot-assisted radical prostatectomy (RARP), recovery of continence can take days to months. We sought to identify a simple means to predict time to recovery of postoperative continence.
Patients and Methods
Preoperative characteristics on 172 men who were undergoing RARP were entered into an electronic database. All men were queried via telephone and/or returned a 7-day log of pad use. Men without need for pads were excluded (n=41). At 4 to 7 days, responses were grouped as: one pad (n=55), two pads (n=35), or three or more pads (n=41). Patients returned self-addressed postcards noting the date of 0-pad urinary status. Univariate and multivariate analysis of variables were assessed for ability to predict time to continence.
Results
No preoperative factors, such as age, International Index of Erectile Function-5, prostate-specific antigen level, American Urological Association symptom score, body mass index, uroflowmetry, nerve-sparing status, estimated blood loss, or prostate weight, were found to predict time to continence. Pad use at 4 to 7 days, however, was highly correlated with median time to continence. The median time to continence for men using one pad was 35 days, two pads was 42 days, and for three or more pads was 73 days (P=0.0001).
Conclusions
As has been previously reported, we found no reliable baseline factors that predicted postoperative time to 0-pad continence. We did find that determining pad usage at 4 to 7 days after catheter removal strongly predicted time to pad-free continence. This method is simpler then pad weights, predicts high- and low-risk men for delayed continence, and can be used for counseling/intervention.
Introduction
Although there is strong evidence to support the use of radical prostatectomy for prostate cancer from an oncologic viewpoint, patients and physicians alike recognize that incontinence is a problem retarding widespread acceptance of surgery. Even though continence rates have been reported to be as high as 85% to 90%, when contemplating surgery, most men worry not only about incontinence but, appropriately, how long the incontinence will last. Techniques such as the posterior and anterior suspension stitches have been published without clear evidence of definitive improvement. For example, the technique described by Rocco and colleagues1 has been evaluated in randomized (controlled and noncontrolled) trials without benefit2–4; however, Nguyen and associates5 and Tewari and coworkers6 noted improvement in consecutive series. Although extensively studied, there are no reliable preoperative or baseline factors that predict time to continence.
Since 2000, a number of authors have reported that the level of incontinence immediately after catheter removal can reasonably classify the length of incontinence into slower vs faster groups.7–9 These reports examined relative incontinence, either immediately after catheter removal7 or using pad weights over the first 24 hours after catheter removal.8,9 This study's objective was to develop an outpatient method that avoids pad weighing for predicting times to recovery of postoperative continence for surgeons and their patients.
Patients and Methods
Two hundred and seven consecutive patients who were undergoing robot-assisted radical prostatectomy by a single surgeon were considered for the study; however, 35 were excluded for lack of complete follow-up (17) or competing protocol (18). Baseline characteristics (n=172), such as age, height, weight, clinical T-stage and Gleason score, prostate-specific antigen (PSA) level, International Index of Erectile Function (IIEF)-5, and pertinent medical history, were collected and entered prospectively into a dedicated electronic database. Continence was defined as the use of no pads. Patients self-reported the day that they stopped using any pads by returning subject-coded, preprinted postcards. Urinary and sexual outcomes were also obtained by validated self-administered questionnaires at 3-month intervals, including selected questions from the Expanded Prostate Cancer Index Composite-24 questionnaire, the seven-item American Urological Association (AUA) symptom score, and the IIEF-5. Institutional Review Board approval had been granted for this study. Nonclinical research associates collected all follow-up information.
Simple uroflowmetry was obtained preoperatively (Dantec, Laborie) for total voided volume (VV) and peak flow rate (PFR). A postvoid residual (PVR) detrmination was performed by ultrasound. All men were asked to report to the clinic with a full bladder. Our surgical technique has been previously described, which includes a running Van Velthoven urethrovesical anastomosis, a cautery-free technique for the prostatic vascular pedicle and neurovascular bundle, Rocco reconstruction, and local endorectal hypothermia.1,4,10,11 The indwelling catheter was removed on day 7, and cystography was not performed. All men were counseled on how to do routine Kegel exercises.
All men (100%) were contacted by telephone by the operating surgeon to formally review the pathology report, at which point they were queried (3–5 days after catheter removal) about how many pads they were using. In addition to the telephone query, patients were sent home with a self-assessed 7-day daily continence log (Appendix 1). The log reports number of pads used, pad size, and percentage of pad saturation. Patients were asked to fax the log on day 7. If we did not receive a fax, patients were contacted by one of the research associates. Men were followed until pad free or until 12 months.
Men with no need for pads in the first week were not included (n=41). Time to pad-free urinary continence was determined by postcards returned with the date of achieving pad-free status; patients were also contacted monthly if they had not returned their 0-pad postcard. Groups were compared for baseline parameters using one-way analysis of variance. Univariate analysis of time to zero pads was performed using the Kaplan-Meier method. The analysis software used was Statistical Analysis Systems (SAS Institute, Cary, NC), with statistical significance considered P<0.05.
Results
Baseline characteristics
Table 1 presents preoperative baseline characteristics for the different pad-use categories. We did not find any baseline factor(s) that distinguished between the groups or that was helpful in predicting length of incontinence. Interestingly, noninvasive uroflowmetry likewise did not demonstrate any difference in any of the standard parameters, such as VV, PVR, or PFR. Nor were there any predictive findings with perioperative factors, such as nerve-sparing status (or blood loss or length of stay—data not shown).
Table 1.
Baseline Characteristics for 1, 2, and ≥3 Pad Groups
| 1 Pad | SD | 2 pads | SD | 3+ pads | SD | P value | 0 pads | SD | 1+ pads | SD | P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 55 | 35 | 41 | 41 | 131 | |||||||
| Demographics | ||||||||||||
| Age | 63.0 | 8.7 | 61.0 | 6.6 | 61.0 | 6.2 | 0.39 | 58.0 | 8.3 | 62.0 | 7.4 | 0.015 |
| AUA | 6.0 | 6.7 | 7.0 | 5.4 | 8.0 | 6.3 | 0.81 | 8.0 | 5.5 | 7.0 | 6.2 | 0.80 |
| Bother | 2.0 | 1.4 | 2.0 | 1.5 | 1.0 | 1.4 | 0.18 | 1.0 | 1.4 | 2.0 | 1.4 | 0.95 |
| Pre-PSA | 5.0 | 5.8 | 5.9 | 5.3 | 5.0 | 2.8 | 0.51 | 5.0 | 5.4 | 5.1 | 4.9 | 0.43 |
| Prostate Weight (g) | 52.6 | 17.0 | 48.0 | 17.5 | 50.8 | 19.4 | 0.76 | 47.0 | 15.0 | 51.4 | 17.8 | 0.10 |
| BMI | 27.1 | 3.3 | 26.1 | 4.0 | 26.9 | 3.5 | 0.94 | 26.7 | 4.2 | 26.7 | 3.5 | 0.38 |
| Gleason score | 7.0 | 0.7 | 7.0 | 1.4 | 7.0 | 0.7 | 0.71 | 7.0 | 0.8 | 7.0 | 0.9 | 0.55 |
| IIEF-5 | 22.5 | 6.2 | 24.0 | 7.5 | 22.0 | 8.3 | 0.28 | 24.0 | 5.4 | 23.0 | 7.3 | 0.073 |
| Uroflowmetrics | ||||||||||||
| Volume voided (mL) | 324.0 | 210.0 | 333.0 | 207.0 | 388.0 | 197.0 | 0.75 | 362.0 | 185.0 | 342.0 | 204.0 | 0.97 |
| PVR (mL) | 69.0 | 97.6 | 68.0 | 190.0 | 94.0 | 89.7 | 0.52 | 74.0 | 82.4 | 82.5 | 127.0 | 0.53 |
| PFR (mL/s) | 16.5 | 9.0 | 16.0 | 11.7 | 15.0 | 10.9 | 0.95 | 17.0 | 8.3 | 16.0 | 10.4 | 0.93 |
| Nerve sparing | 0.33 | |||||||||||
| Bilateral | 83.6% | 80.0% | 70.7% | 87.8% | 78.6% | |||||||
| Unilateral | 16.4% | 20.0% | 26.8% | 9.8% | 20.6% | |||||||
| Non | 0% | 0% | 2.50% | 2.4% | 0.8% | |||||||
| Positive surgical margin | 7% | 17.1% | 5% | 14.6% | 9.2% | 0.32 | ||||||
| Time to zero pads (d) | 35 | 58.8 | 42 | 49.9 | 73 | 124 | 0.0002 | 3 | 23.3 | 50.0 | 88.4 | 0.0001 |
| Pad free 1 Month | 47.3% | 0.50 | 34.3% | 0.48 | 7.3% | 0.26 | <0.0001 | 90.2% | 0.30 | 31.3% | 0.47 | <0.0001 |
| Pad free 3 Months | 83.6% | 0.36 | 71.4% | 0.49 | 52.5% | 0.51 | 0.0016 | 97.6% | 0.16 | 70.8% | 0.45 | 0.0005 |
| Pad free 9 Months | 98.1% | 0.14 | 94% | 0.17 | 85.3% | 0.36 | 0.031 | 100.0% | 0 | 93.4% | 0.23 | 0.12 |
| Pad free 12 Months | 100% | 0 | 100% | 0 | 88% | 0.33 | 0.0048 | 100.0% | 0 | 96.7% | 0.18 | 0.24 |
Demographics table stratified by pad use groups and analyzed by analysis of variance. Table compares the excluded 0-pad use group with the pad use group, analyzed by t test.
P values (analysis of variance) presented are for intergroup comparisons. A simple comparison is also made between men pad free within the first 7 days after catheter removal (0 pads) with all men using pads (1+ pads) using t tests.
SD=standard deviation; AUA=American Urological Association; PSA=prostate-specific antigen; BMI=body mass index; IIEF=International Index of Erectile Function; PVR=postvoid residual; PFR=peak flow rate.
Pad use vs time to pad-free status
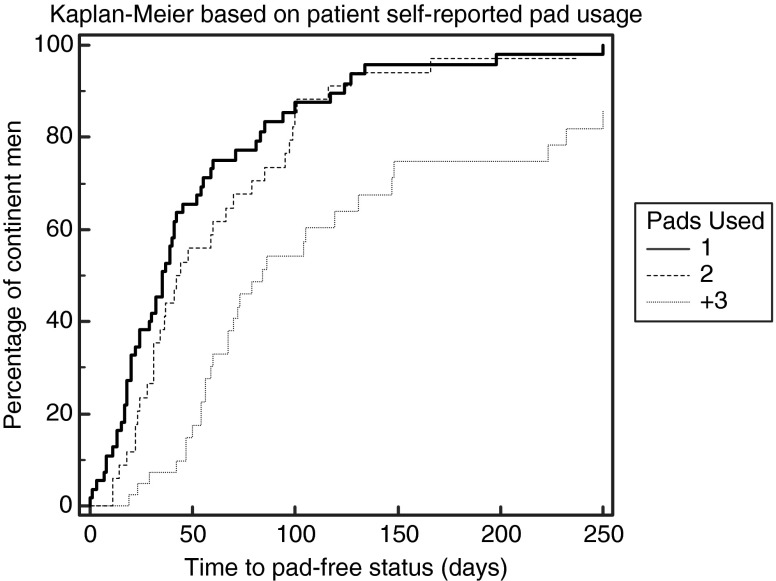
We did find a very strong correlation and predictive power of time to pad-free status simply by tracking the number of pads used and wetness (days 4 through 7). In Table 1, we present a comparison of 1 pad through 3+ pads; separately, we also present data for men with early no pad use (0 pads) compared with men using 1 or more pads (1+). Last, in addition to showing timed events, Figure 1 presents a Kaplan-Meier graph for the three self-reported pad groups (P=0.0001).
FIG. 1.
Kaplan-Meier time to pad-free continence presented as one, two, or three or more pads (P<0.0001).
Pad slope
The daily urinary log allowed us to examine pad use over the first week after catheter removal. Over the first 4 days after catheter removal in the three or more group, 34.3% decreased the quantity and/or the size of the pads used (heavy, medium, and thin sizes were available); 22.9% dropped from three or more to two pads, and 11.4% dropped to one pad. We found that any of the days from 4 to 7 equally predicted time to continence.
Discussion
The prediction of how much time it will need for any given patient to achieve pad-free status is a strategic clinical goal. Patient age is the one demographic factor most commonly linked to continence and time to continence; however, the predictive strength of age and all other baseline factors, such as body mass index, prostate weight, AUA symptom score, is weak at best.12–14 Our study confirms these previous findings as presented in Table 1. In addition, we found preoperative uroflowmetry (VV, PVR, PFR) had no predictive findings.
Because preoperative factors have not demonstrated benefit, a number of authors have focused on postoperative factors. In 2000, Twiss and associates7 were the first group we identified specifically addressing postoperative factors that helped stratify time to continence. At the time of catheter removal, they described a 5-item index to estimate the likelihood of continence at 3 months. In short, on the day of catheter removal, the bladder was filled to leak point and the bladder volume recorded. The patients were then assessed for leakage when supine, changing to sitting, changing to standing, and the ability to start and stop the urine stream. The maximum score was 18; three groups were identified (18, 17–15, and 14 or less). At 3 months, approximately 75% of men who scored a perfect 18 were pad free, 53% with scores 17 to 15 were pad free, and 34% were pad free if they scored 14 or less.
More recently, two groups in Europe have published postoperative protocols to predict time to continence. Ates and colleagues8 described a urine loss ratio (ULR): weight of pads/24 voided urine volume) measured on the day of catheter removal or approximately 2.3 days later. They defined three groups of continence, early (3M), midterm (3–12M), and late (12–24M). They found reasonable correlation of being pad free at 3 months when the ULR was 0-0.05: 89.4%; 0.05–0.15: 73.5%; and >0.15: 42.5%. They commented that there was better correlation if they measured 2.3 days vs 1 day after catheter removal. Recently, Van Kampen and coworkers9 also described a pad-weight method on the day of catheter removal.
Our study improves on these earlier studies in that it does not need pad weight and is also performed between 4 and 7 days postcatheter removal. We found that simply filling out a postcatheter log of daily pad use (Appendix A) strongly predicted prolonged urinary incontinence: For men using three or more pads, the median time to pad-free status was 73 days; two pads, the median time was reduced nearly in half, 42 days; and one pad, the median was 35 days. The pad weight methods described by Ates and associates8 and Von Kampen and colleagues9 have very similar estimates as our data does. For example in the study by Ates and associates,8 the 0–0.05 ULR correlates well with one pad estimates for continence at 3 months, the 0.05–0.15 with two pads, and >0.15 with three or more pads (89.4% vs 83.6%: 73.5% vs 71.4%: 42.5% vs 52.3%).
Another important finding was that the first 24 hours was not the optimal day for predicting time to continence. In fact, 34.3% of men using three or more pads on day 1 postcatheter removal were using two or fewer pads on day 4. All three studies use either the day of catheter removal or 1 to 2 days later. Another finding was stable pad use days 4 to 7; any of these days had equal predictability of time to continence. These findings put together introduce a fairly simple means to estimate how long the incontinence may persist.
We agree with all three previous publications that the information can be used both to counsel patients and to direct them to earlier intervention. Early noninvasive intervention techniques, such as biofeedback, muscle strengthening, etc., should be investigated as well as earlier surgical interventions could be considered.
A weakness of the present study is that we did not compare head to head the pad weight method to the daily pad log. This occurred primarily because of the expense and inconvenience of supplying patients with scales and/or keeping them hospitalized longer. Based on the large variation in time to continence in all of the proposed methods, the number of subjects needed for an appropriately powered study is prohibitive. The data from studies correlating objective pad weights indicate, however, that these large variations in time to return of continence suggest that subjective pad usage appears to be a very good surrogate. Another criticism is pad size, and % pad fullness is open to wide reporting variation; however, we found even with this wide variation, we could fit men reasonably into one of the three groups.
Conclusions
This self-reported log of daily pad use that can be fax-returned or communicated by phone query is simple, nearly cost free, and easily reproduced. The estimation of time to continence achieved on days 4 to 7 identifies for counseling and/or early intervention men who are at risk for prolonged incontinence.
Abbreviations Used
- AUA
American Urological Association
- IIEF
International Index of Erectile Function
- PFR
peak flow rate
- PSA
prostate-specific antigen
- PVR
postvoid residual
- ULR
urine loss ratio
- VV
voided volume
Appendix 1.
Daily Urinary Pad Log
| Daily Urinary Pad Log | ||
|---|---|---|
| Case #: __________ | Initials: ___________ | Surgery Date: ____/____/_____ |
| Please Fax at Highlighted Times. Thank You | ||
| Before your surgery, did you wear urinary pads for incontinence problems? □Yes, # daily? ____ □ No | ||
|
Week 1 | |||||||
|---|---|---|---|---|---|---|---|
| |
|
|
|
Check Type of Urinary Pad Used |
|
||
| Day | Date | # of Pads Used | How soaked are your pads? (0-100%) | L Light (Thin liner) | M Medium (Standard pad) | H Heavy (Disposable brief) | Comments (Use additional sheets as needed.) |
| 0 | Catheter Removed | ||||||
| 1 | |||||||
| 2 | |||||||
| 3 | |||||||
| 4 | |||||||
| 5 | |||||||
| 6 | |||||||
| 7 | Please Fax to | ||||||
| Are you currently taking medication for incontinence? □ Yes, Name:______________ Date Started: _________ □ No | |||||||
Disclosure Statement
Dr. Ahlering is a consultant for Phillips Healthcare and Astellas Pharmaceuticals. For the other authors, no competing financial interests exist.
References
- 1.Rocco F. Carmignani L. Acquati P, et al. Early continence recovery after open radical prostatectomy with restoration of the posterior aspect of the rhabdosphincter. Eur Urol. 2007;52:376–383. doi: 10.1016/j.eururo.2007.01.109. [DOI] [PubMed] [Google Scholar]
- 2.Menon M. Muhlethaler F. Campos M. Peabody JO. Assessment of early continence after reconstruction of the periprostatic tissues in patients undergoing computer assisted (robotic) prostatectomy: Results of a 2 group parallel randomized controlled trial. J Urol. 2008;180:1018–1023. doi: 10.1016/j.juro.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 3.Sammon JD. Muhletaler F. Peabody JO, et al. M Long-term functional urinary outcomes comparing single- vs double-layer urethrovesical anastomosis: Two-year follow-up of a two-group parallel randomized controlled trial. Urology. 2010;76:1102–1107. doi: 10.1016/j.urology.2010.05.052. [DOI] [PubMed] [Google Scholar]
- 4.Finley D. Osann K. Skarecky DW. Ahlering TE. Hypothermic nerve-sparing radical prostatectomy: Rationale, feasibility, and effect on early continence. Urology. 2009;73:691–696. doi: 10.1016/j.urology.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen MN. Kamoi K. Stein RJ, et al. Early continence outcomes of posterior musculofascial plate reconstruction during robotic and laparoscopic prostatectomy. BJU Int. 2008;101:1135–1139. doi: 10.1111/j.1464-410X.2007.07425.x. [DOI] [PubMed] [Google Scholar]
- 6.Tewari A. Bigelow K. Rao S, et al. Anatomic restoration technique of continence mechanism and preservation of puboprostatic collar: A novel modification to achieve early urinary continence in men undergoing robotic prostatectomy. Urology. 2007;69:726–731. doi: 10.1016/j.urology.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Twiss C. Martin S. Shore R. Lepor H. A continence index predicts the early return of urinary continence after radical retropubic prostatectomy. J Urol. 2000;164:1241–1247. [PubMed] [Google Scholar]
- 8.Ates M. Teber D. Gozen AS, et al. A new postoperative predictor of time to urinary continence after laparoscopic radical prostatectomy: The urine loss ratio. Eur Urol. 2007;52:178–185. doi: 10.1016/j.eururo.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Van Kampen M. Geraerts I. De Weerdt W. Van Poppel H. An easy prediction of urinary incontinence duration after retropubic radical prostatectomy based on urine loss the first day after catheter withdrawal. J Urol. 2009;181:2641–2646. doi: 10.1016/j.juro.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Van Velthoven RF. Ahlering TE. Peltier A, et al. Technique for laparoscopic running urethrovesical anastomosis: The single knot method. Urology. 2003;61:699–702. doi: 10.1016/s0090-4295(02)02543-8. [DOI] [PubMed] [Google Scholar]
- 11.Ahlering TE. Eichel L. Chou D. Skarecky DW. Feasibility study for robotic radical prostatectomy cautery-free neurovascular bundle preservation. Urology. 2005;65:994–997. doi: 10.1016/j.urology.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Loughlin KR. Prasad MM. Post-prostatectomy urinary incontinence: A confluence of 3 factors. J Urol. 2010;183:871–877. doi: 10.1016/j.juro.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Sandhu JS. Eastham JA. Factors predicting early return of continence after radical prostatectomy. Curr Urol Rep. 2010;11:191–197. doi: 10.1007/s11934-010-0108-6. [DOI] [PubMed] [Google Scholar]
- 14.Pick D. Osann K. Skarecky D, et al. The impact of cavernosal nerve preservation on continence after robotic radical prostatectomy. BJU Int. 2011 Jan 18; doi: 10.1111/j.1464-410X.2010.10015.x. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]