Abstract
Both the therapeutic effects of regional hypothermia (RH) and somatosensory-evoked potentials (SSEP) have been intensively studied; however, the in vivo relationship between the two remains unknown. The primary focus of the current study was to investigate the impact of RH on SSEP in uninjured rats, as well as the neural safety of RH on neuronal health. An epidural perfusion model was used to keep local temperature steady by adjusting perfusion speed at 30°C, 26°C, 22°C, and 18°C for 30 min, respectively. Total hypothermic duration lasted up to 3 h. Neural signals were recorded at the end of each hypothermic period, as well as before cooling and after spontaneous rewarming. In addition, the Basso, Beattie, and Bresnahan (BBB) Locomotor Rating Scale was used to evaluate the effects of RH pre- and post-operative, combined with hematoxylin and eosin (H&E) and Fluoro-Jade C (FJC) staining. The results showed a marked declining trend in SSEP amplitude, as well as a significant prolongation in latency only during profound hypothermia (18°C). The BBB scale remained consistent at 21 throughout the entire process, signifying that no motor function injury was caused by RH. In addition, H&E and FJC staining did not show obvious histological injury. These findings firmly support the conclusion that RH, specifically profound RH, inhibits spinal cord SSEP in both amplitude and latency without neural damage in uninjured rats.
Key words: RH, spinal cord, SSEP, uninjured rat
Introduction
Hypothermia, which has been extensively studied for many years, is widely used in thoracoabdominal and descending thoracic aneurysm repair surgery for the prevention of ischemic injury to the spinal cord.1,2 Also it is considered to be a neuroprotective strategy for treating central nervous system (CNS) injuries such as traumatic brain injury, stroke, and traumatic spinal cord injury (TSCI).3–9 However, it still remains unknown whether RH can cause long-term detrimental consequences or other neurological injuries. A report by Craenen and colleagues indicated that cooling at a profound level would trigger the elevation of excitatory amino acids, resulting in neuronal death.10
One of the difficulties in the development of therapeutic hypothermia management is the absence of a reliable and sensitive measurement in clinical practice. Neuroelectrophysiological measures, including somatosensory-evoked potentials (SSEP), motor-evoked potentials (MEP), electroencephalography, and electromyography, have been used to evaluate the severity of CNS injuries, as well as therapeutic outcomes. However, these measures could easily be affected by many factors such as hypothermia. Even though the neural responses of the median nerve and uninjured brain to mild hypothermia have previously been reported, no consistent results have been found.11,12 There is still not enough published information available to demonstrate the electrophysiological effects of regional hypothermia (RH).
In the present study, a rat laminectomy model was used to determine the neuroelectrophysiological effects of RH on the spinal cord in uninjured rats. Motor function assessment and immunochemistry were also performed to determine the safety of RH.
Methods
Subjects
All experimental procedures were in accordance with the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health, and were approved by the Animal Care and Use Committee of Secondary Military Medical University. A total of 18 adult male Sprague–Dawley rats weighting 200–220g were used in this study. All rats were housed in standard conditions and given adlibitum access to food and water.
Surgical procedure
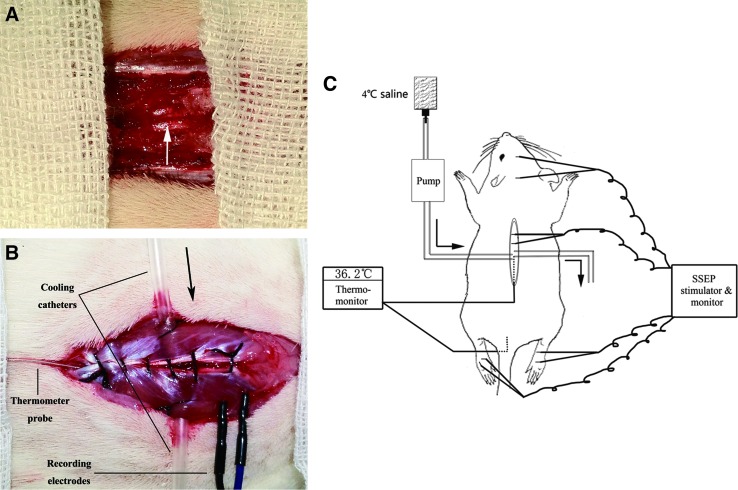
On the day of the experiment, a laminectomy was performed. Briefly, the rats were anesthetized with 10% chloral hydrate (0.35mL/100g) through intraperitoneal injection, followed by half the quantity (0.17 mL/100 g) per hour for continuous anesthesia throughout the entire experiment. Rats were placed in the prone position on a warming pad. The back region was shaved and aseptically prepared with iodine tincture twice, followed by 75% alcohol three times. The animal was given a midline longitudinal incision to expose the region of interest (T7–T12). The paravertebral muscles of the same level were retracted. Then, laminectomy was performed at T10 level using an operating microscope (M500-N, LEICA, Heerbrugg, Switzerland). The spinal dura mater surface was exposed and left intact (Fig. 1A).
FIG. 1.
The location of cooling catheters, somatosensory-evoked potentials (SSEPs) recording electrodes, and thermo-probes. A representative graph of the regional hypothermic model, thermo-monitor, and SSEP stimulator and monitor are shown. (A) The white arrow shows that the vertebral plate has been removed; complete dura mater and uninjured spinal cord are exposed under the microscope. Posterior spinal vessels are clearly seen (B), and an enlarged post-sutured view is shown as well. Black arrow indicates the direction of perfusion. (C) The thermometer probe is inserted at level T12 approximately, adjacent to the spinous process, and reaching level T10. Color image is available online at www.liebertpub.com/neu
Two self-designed epidural cooling catheters were employed to penetrate bilaterally paravertebral muscles at T10 level and stabilized using a 4-0 suture. A microdigital thermometer probe (Xinlian Equipment Co., Ltd., Nanjing, China) was placed at the same level just next to the dura mater, but without compressing the dura and the spinal cord in order to monitor temperature changes continuously. The muscles were closed using a 4-0 suture (Fig. 1B). Another temperature probe was placed in the rectum for continuous body temperature monitoring. Room temperature was maintained by an air conditioner at ∼20°C throughout the entire surgery. All the procedures were performed identically on all animals by an operator.
Hypothermia and SSEP recording
Before the induction of hypothermia, a pair of recording needle electrodes (Friendship Medical Electronics Co., Ltd. Xi'an, China) was carefully inserted into the T7–T8 and T8–T9 interspinous ligament (Fig. 1B). Another pair of subcutaneous electrodes was placed at the midpoint of the eyes at the FPZ point and at the midpoint of the ears at the CZ point. Two pairs of stimulating electrodes were used to stimulate tibial nerves of both hindlimbs without touching the nerve bundle directly. A Cadwell cascade 16 Ch system equipped with an ES-5 stimulator box (Cadwell Laboratories, Inc., WA) was used for signal recording and data analysis. Stimulation intensity was adminstered at 3.5 mA and the pulse width was 0.2 ms at a frequency of 4 Hz. Each curve was acquired after the average of 1,000 repetitions to improve the signal to noise ratio (SNR).
After the electrodes were implanted and the stimulator and software were set, the SSEP was recorded before the epidural cooling as the normal baseline. Then, hypothermia was initiated by infusing 4°C of cold saline into the epidural area through the inflow catheter, which was connected to an infusion pump (Fig. 1C). A catheter on the other side assured sufficient outflow. By adjusting the speed of perfusion, the regional temperature could be easily maintained at the levels that were used in this study: 30°C, 26°C, 22°C, and 18°C. The temperature was allowed to stabilize at the target temperature for 30 min and SSEP signals were then recorded. Finally, the perfusion was stopped and the rats were allowed to rewarm spontaneously. The temperature during rewarming was recorded every 2 min until it remained constant for a 6 min period. Neural signals were recorded again upon sufficient rewarming.
Post-operative care
All experimental equipment was removed after recording and the skin was closed with a 3-0 suture. All rats were allowed to recover in a warmed cage, and food and water was easily accessible. Saline (2 mL) was given through subcutaneous injection immediately after the operation. The antibiotic gentamicin (5 mg/kg, intramuscular) was also administered daily for the following 7 days or until animals were euthanized.
Motor function assessment
Motor function of the rat was assessed using the Basso, Beattie, and Bresnahan (BBB) Locomotor Rating Scale before the operation, after the rats recovered from anesthesia completely, and at days 1, 7, and 14 post-operative. The BBB score ranges from 0 (no hindlimb movement) to 21 (normal motor function).13 Two blinded observers evaluated the score individually, and the average value of the 2 observers' scores was used.
Histology preparation
At days 1, 7, and 14 post-operative, 6 rats were randomly selected, anesthetized, and transcardially perfused with pre-cooled saline and 4% paraformaldehyde fixation. The spinal cord was carefully removed from the vertebrae column and carefully divided into 3 parts: the experimental hypothermic segment (T10), the upper segment (T9), and the lower segment (T11). All specimens were post-fixed in 4% cold paraformaldehyde for at least 24 h, and then were transferred into 20% and 30% sucrose solution for 24 h, respectively, until the tissue sank to bottom of the container. The tissues were kept at −80°C in a refrigerator for serial sectioning.
Spinal cord tissue was cut into 5μm and 25μm thick sections by cryostat (Leica CM3050s, Germany) for hematoxylin and eosin (H&E) staining and Fluoro-Jade C (FJC) staining.
H&E staining
For histomorphological observation, H&E staining was performed using 5 μm sections. The sections were washed briefly in 0.1M phosphate buffered saline, rinsed in distilled water, and then immersed in hematoxylin solution for 5 min, followed by additional rinses in distilled water. They were differentiated in 0.1% acid alcohol (1% HCl in 70% ethanol) and blue in bluing solution 5–10 s. After washing in distilled water, the sections were immersed in eosin for 1 min and dehydrated through 80%, 95%, and 100% ethanol and xylene rinses. The sections were then mounted and observed under a microscope (Nikon E100, Japan).
FJC staining
FJC is considered to be more effective in staining degenerative neurons and has a higher affinity than Fluoro-Jade A and Fluoro-Jade B.14 The 25 μm sections were immersed into 80% alcohol solution containing 1% NaOH for 5 min, and then transferred into 70% ethanol and distilled water for 2 min, respectively. Sections were incubated in 0.06% potassium permanganate solution for 10 min. Following a water rinse, the sections were transferred to FJC dye liquor (Chemicon Europe, Hampshire, UK) for another 20 min. The sections were then washed in distilled water 3 times, air-dried in the oven until, and covered with mounting medium for further cell counting. The entire procedure was done under light-proof conditions.
Cell counting
From each segment, 1 out of every 6 serial slice sections was selected, and altogether 6 slices were collected and observed under a fluorescence microscope (Carl Zeiss Axiovert 40C CFL equipped with an Axiocam MRc, Germany). In order to obtain normal, healthy glial cells and neurons, cell counting was performed in 3 non-overlapping high-power fields from both gray (one random field for anterior horn, posterior horn, and the middle part, respectively) and white matter (randomly chosen), using a fluorescence microscope in each section. All FJC-positive cells from 36 counting areas per section were summed as the final number for statistical analysis. The number of cells was quantified by using AxioVision (Ver.4.8, Germany) in a blinded manner.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics, version 19.0.0. All data in the text are expressed as mean±SD rounded to 2 decimal places (or as noted). Paired t-tests, 1-way ANOVAs with Tamhane's T2 or least significant difference (LSD) post-tests (based on Bartlett's test), and repeated measures ANOVAs were used in data analysis; p values < 0.05 were considered significant.
Results
Temperature management and duration
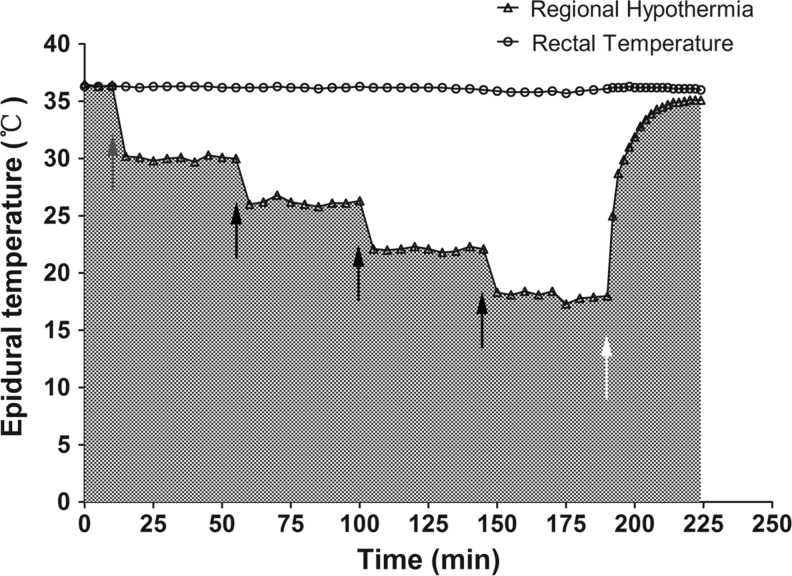
Prior to the induction of hypothermia, the initial epidural temperature was measured (35.89±0.36°C). During hypothermia, target temperatures were achieved by altering perfusion speed modulated by an infusion pump within 5 min. The regional temperature was maintained at the required level within a small fluctuation rate (within 0.5°C) for 30 min. SSEP recordings were accomplished within 10 min at each level, followed by another initiation of cooling (Fig. 2). The focal temperature after rewarming was 35.33±0.35°C, and the rectal temperature was maintained at 36±0.5°C using a warming pad during the experiment. The entire hypothermic duration was also measured from the beginning to the end of perfusion (187.11±8.30 min), with 37.22±3.23 min of rewarming.
FIG. 2.
A representative real-time recording of epidural and rectal temperature. After a 10-minute stabilization, the baseline somatosensory-evoked potentials (SSEPs) were recorded, and cold saline perfusion was initiated. Black arrows indicate the initiation of increased perfusion for the next thermo level. Rewarming occurred after all SSEP recordings were complete (white arrow). The rectal temperature remained within the normal range throughout the entire experiment.
Variation of SSEP
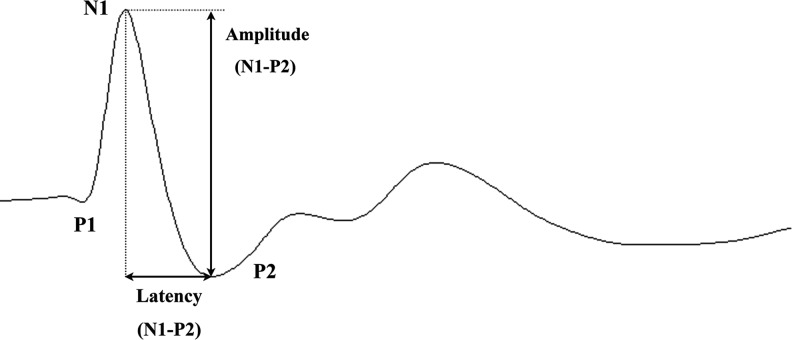
Typically, rat SSEP waveform is characterized by 3 major components: a positive peak (P1), followed by a negative peak (N1), and another positive peak (P2). In this study, the N1-P2 amplitude and latency were measured for analysis (Fig. 3).
FIG. 3.
A representative somatosensory-evoked potential (SSEP) waveform of a rat is shown, which mainly consists of three components. As labeled in this figure, N1-P2 amplitude and latency were both measured and used in the analysis.
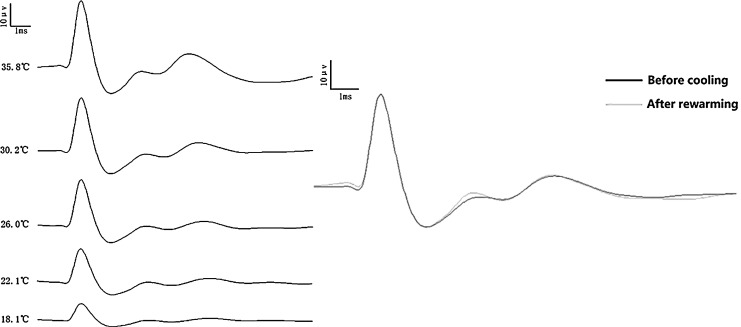
A representative group of SSEP traces at different hypothermic levels in 1 rat is shown in Figure 4 left. A marked declining trend in N1-P2 amplitude was shown in response to hypothermic changes. Additionally, a sample of two curves recorded in 1 rat before cooling (black) and after rewarming shows a great correlation (Fig. 4 right).
FIG. 4.
A sample group of somatosensory-evoked potential (SSEP) traces in a rat is shown. A large decline in amplitude is shown in one serial alteration (left). A perfect correlation between the two curves demonstrating recordings before cooling and after rewarming is also shown (right).
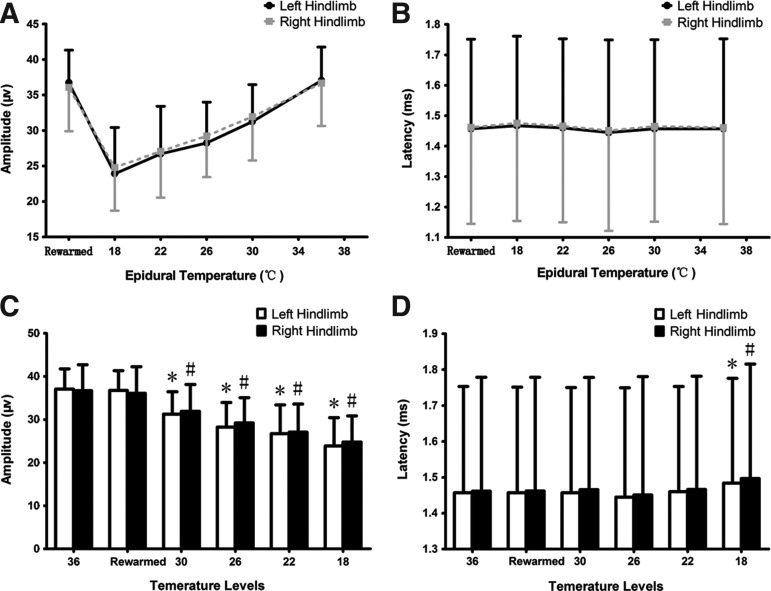
In order to verify the stability and reliability of the SSEP recording system, we compared the data on left hindlimb versus the right hindlimb at each thermo level; we found no significant differences between both regions (p>0.05; Table 1).
Table 1.
Data of SSEP Amplitude and Latency
| Left hindlimb (mean [SD]) | Right hindlimb (mean [SD]) | p value (intra-level)a | p value (inter-level)b (left/right) | |
|---|---|---|---|---|
| Amplitude (μV) | ||||
| Level 36°C | 37.04 (4.69) | 36.67 (6.05) | 0.64 | |
| Post-rewarm | 36.73 (4.60) | 36.09 (6.19) | 0.51 | 0.99/0.70 |
| Level 30°C | 31.25 (5.20) | 31.94 (6.16) | 0.33 | <0.01 for both |
| Level 26°C | 28.23 (5.75) | 29.23 (5.80) | 0.21 | <0.01 for both |
| Level 22°C | 26.71 (6.70) | 27.06 (6.53) | 0.60 | <0.01 for both |
| Level 18°C | 23.90 (6.53) | 24.78 (6.08) | 0.19 | <0.01 for both |
| Latency (ms) | ||||
| Level 36°C | 1.4572 (0.2957) | 1.4611 (0.3174) | 0.93 | |
| Post-rewarm | 1.4572 (0.2944) | 1.4617 (0.3173) | 0.92 | >0.05 for both |
| Level 30°C | 1.4567 (0.2934) | 1.4650 (0.3132) | 0.85 | >0.05 for both |
| Level 26°C | 1.4444 (0.3047) | 1.4511 (0.3295) | 0.88 | >0.05 for both |
| Level 22°C | 1.4600 (0.2932) | 1.4661 (0.3161) | 0.89 | >0.05 for both |
| Level 18°C | 1.4833 (0.2924) | 1.4967 (0.3188) | 0.76 | <0.01 for both |
Intra-level: compare left hindlimb and right hindlimb in the same thermo level.
Inter-level: compare the corresponding group with level 36°C on both hindlimbs, respectively.
Paired t-test is used for intra-level and repeated measures ANOVA for inter-level comparisons, respectively.
Data of SSEP latency are not rounded to two decimal places for precise comparison.
SSEP, somatosensory-evoked potential.
In amplitude observation, SSEPs demonstrated a significant degradation (Fig. 5A). The amplitude at the normothermic level was 37.04±4.69μV (left) and 36.67±6.05 μV (right), followed with a 5–10% decrease per level during hypothermia until it reached 23.90±6.53 μV (left) and 24.78±6.08 μV (right) at 18°C. All four hypothermic groups showed significant differences (p<0.01) compared to the 36°C group (Fig. 5C).
FIG. 5.
Analysis of somatosensory-evoked potential (SSEP) amplitude and latency. The curve shows a declining trend in amplitude in response to epidural temperature changes in the thermo-span of 36°C–18°C, and a quick recovery after rewarming on both hindlimbs (A). There is a significant difference between hypothermic groups versus the 36°C– group (p<0.01 on both hindlimbs), whereas the rewarmed group is statistically the same as the 36°C– group (p>0.05 on both hindlimbs; C). The curve of latency is much flatter, only with a significant difference at the 18°C group when compared with the 36°C– group (p<0.01 on both hindlimbs; B and D). Also, latency recovers to normal range upon rewarming (p>0.05 on both hindlimbs).
* and # refer to a significant difference (p<0.05) when comparing the corresponding group with the 36°C group on both left and right hindlimbs, respectively. (A paired t-test was used for left–right comparisons and a repeated measures ANOVA was used for intergroup comparison.)
Regarding N1-P2 latency observations, the difference is not as strong as the difference in amplitude, and is more moderate on both hindlimbs (Fig. 5B). The latency recorded during 30°C–22°C showed no statistical difference when compared with the 36°C group (Table 1). However, the latency change of both hindlimbs is significant when the epidural temperature dropped to 18°C, with a prolongation of 1.79% on the left hindlimb (1.4833±0.2924 ms) and 2.44% on right hindlimb (1.4967±0.3188 ms; p<0.01 for both hindlimbs; Fig. 5D). Both amplitude and latency recovered to a normal range upon spontaneous rewarming (p>0.05 for both hindlimbs).
Neurofunctional outcome
The motor function of all rats was normal (BBB Scale=21) pre-operative. Hindlimb movements with extensive joint movements, consistent plantar stepping, and toe clearance with tails consistently up were observed in all animals at days 1, 7, and 14 post-operative.
H&E and FJC staining
A representative image of H&E staining in the hypothermic segment is shown in Fig. 6. No obvious hemorrhage or extensive neutrophilic infiltrates were found. The neuron cell structure is regular, the nuclei are round and big with clear staining, and the dendrites are clearly visible.
FIG. 6.
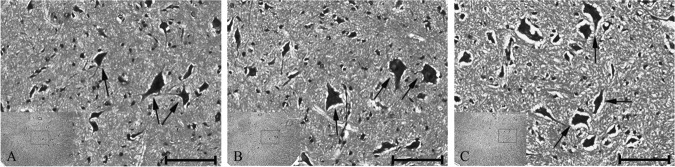
Representative hematoxylin and eosin (H&E) staining in the ventral horn of the hypothermic segment. (A) The spinal cord tissue on day 1. (B) The spinal cord tissue on day 7. (C) The spinal cord tissue on day 14. All black arrows indicate neuron cells with regular structure. Scale bar is 100μm.
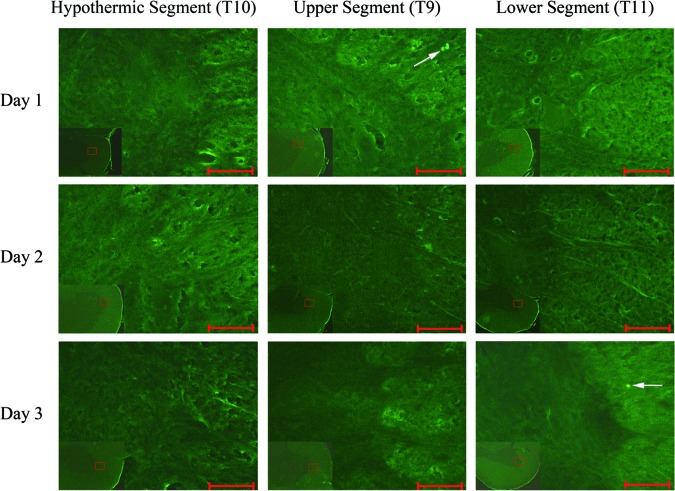
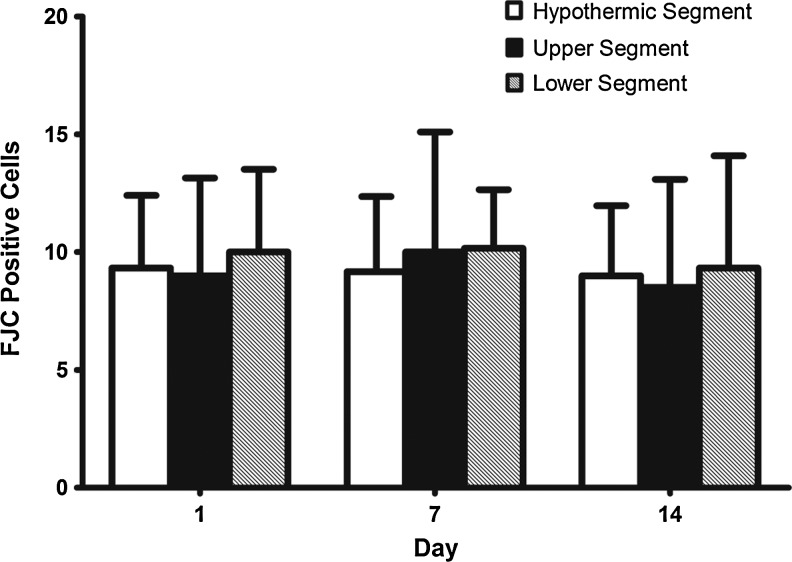
FJC-positive cells showed high green fluorescence as labeled by the white arrow (Fig. 7). The staining background was not altered in order to demonstrate a clear comparison between white and gray matter. Sporadic positive cells were visible under high-power field. No significant differences were found among the 3 segments or among the 3 time groups (p>0.05; Fig. 8).
FIG. 7.
Fluoro-Jade C staining of the spinal cord. A high-power view (10×40) is magnified in the border zone of the ventral horn and white matter. Degenerating cells are stained light green and can clearly be seen under high-power (white arrow), whereas non-degenerating cells were not stained and are therefore not visible. Scale bar is 50μm. Color image is available online at www.liebertpub.com/neu
FIG. 8.
Summary of Fluoro-Jade C staining data. The data is represented in mean±SD. No differences were observed (all p>0.05). (A 1-way ANOVA with LSD post-hoc test was used.)
Discussion
In this study, the primary focus was to investigate the electrophysiological effects of RH induced by epidural perfusion on the spinal cord without 5 different thermo levels, ranging from normothermia to profound hypothermia, in order to evaluate the neural safety of RH. The results showed that SSEP N1-P2 amplitude was quite sensitive to hypothermia, and is clearly inhibited during RH; however, the inter-peak latency was only elongated at profound hypothermia. The neural safety of RH was demonstrated by assessing BBB motor function and immunochemistry staining.
Background
CNS injury, especially TSCI, results in devastating neural dysfunction, with a high rate of deformity.15 A large percentage of the patients are left with paralysis, and irreversible neurological functional deficits, including pain, motor or sensory dysfunction, the loss of bladder control, and disturbances of visceral functions.16 Hypothermia, which has been studied since the 1960s, has been shown to be a neuroprotective procedure that reduces oxygen consumption and metabolic rate, stabilizes local blood flow, inhibits the release of excitatory neurotransmitters, and ultimately reduces neuron apoptosis.17,18 Although systemic hypothermia has been used for TSCI treatment as reported by Cappuccino,19 its large-scale application is limited because of whole-body complications, such as shivering, increased susceptibility of infection caused by immunosuppression, electrolyte disorders, insulin resistance, impaired drug clearance, and mild coagulopathy.20–24 RH, which is localized, is considered to be effective and much safer than systemic cooling.2,8,9 Therefore, it has been used to prevent ischemic SCI in thoracoabdominal aneurysm repair.25
RH has been used infrequently over the past 20 years in treating TSCI. One of the possible reasons why RH is not commonly used is that there is a lack of understanding as to the basic mechanism underlying this treatment, which is preventing translational research to move basic findings to the clinical setting. In addition, another possible reason why RH is not commonly used is the absence of efficient assessment criteria.
SSEP is believed to detect the injury not only to the posterior columns but also to the entire spinal cord.26 As a result, it is widely used in evaluating the function of the spinal cord during surgery or after SCI.27 However, SSEP could be easily impacted by many factors, including hypothermia, which would lead to the inaccurate measures in clinical practice. Although some trials have been performed, there has been no conclusion reached as to the efficacy of the measurement.
We believe it is important to clarify the relationship between RH and SSEPs, and that defining this relationship would provide clinically relevant results that might help define the factors that contribute to hypothermia protection, such as therapeutic windows.
Experimental strategy
Using the hypothermic method, we established the RH model using cold saline perfusion, which has been proven efficient and safe in epidural cooling.28,29 We found this model to be highly reliable, operable, and simple for achieving and maintaining the epidural hypothermia constant at target levels. In addition, our pilot study demonstrated a small impact on the core temperature and the regional temperature at adjacent segments when using a warming pad, which is why we chose the T9 and T11 segments as self-control groups for the immunochemistry analysis. In order to simulate representative thermo levels, we used 36°C, 30°C, 26°C, 22°C, and 18°C, which engaged normothermia, as well as mild to severe levels, which as commonly used in current laboratory experiments and clinical trials.
Based on this clinically relevant model, we believe that the results presented here may help to advance the translation of this experimental therapy to extended clinical utilization in the future. The hypothermic perfusion was produced for 30 min at each level and lasted up to 3 h after TSCI, which is thought to be a relatively short period of time to cause significant effects. However, for TSCI, there are several factors that remain unknown, including hypothermic duration, which can vary from 30 min to 6 days.30 The extent of hypothermia and severity of SCI may act as important factors as well.
In the current study, we used a non-injury model, and the thermo levels used were much lower than those used in previous studies. For these reasons, we believe that this model may potentially be used to set up a foundation for future investigation of RH. During the rewarming period, the regional temperature started rising spontaneously within the normal range immediately after the perfusion stopped. No artificial intervention was induced in order to reduce any interference, which may affect the SSEPs recording somehow after rewarming. Interestingly, the rewarmed epidural temperature did not return to the exact level as that before cooling, which is also seen in the study by Wei and colleagues.31 This result may partly be due to the fact that rewarming took place in a spontaneous way without manual interventions, except for the use of a warming pad. Additionally, it is possible that the total hypothermic duration is 3 h and may be temporarily beyond the rat's regional self-regulation.
SSEP alteration during hypothermia, and its possible mechanisms
In the present SSEP examination, the amplitude degraded as the epidural temperature decreased, and a 1/3 decrease is observed at a severe level of hypothermia. However, the effect of hypothermia on SSEP amplitude is much more complicated and has not yet clearly been studied. One experiment demonstrated that a surface hypothermia of 32°C did not change SSEP amplitude,32 whereas some recent studies found that an increase in amplitude is observed under mild and systemic hypothermia (∼32°C).11,12 Our experiment produced a deeper hypothermia (18.07±0.21°C) within the regional area, and diametrically showed a marked decrease in amplitude during the entire process of hypothermia, which is in agreement with the findings of Porkkala and coworkers, and Mark- and and colleagues.33,34 Although we are unable to explain the exact underlying mechanisms, we believe that hypothermia is likely to inhibit the activity of ion channels and the excitability of neurons, leading to a reduction of the quantity of neurotransmitter release. This hypo-excitable state most likely results in a weaker response to the stimuli, leading to a measurable decrease in amplitude.35
Unlike amplitude, it has been well established that SSEP latency can be easily prolonged during systemic hypothermia.12,32,36 Sohmer and colleagues demonstrated that the prolongation in latency was caused by disturbances both in axonal propagation and synaptic transmission caused by hypothermia.37 In accordance with this, we would expect a significant prolongation in latency during hypothermia; however, the thermo-sensitivity of latency is not as strong as that of amplitude. At first, we attributed it to the implantation of the recording electrodes in interspinous ligament caused by the restrictions on technology and equipment, and we thought it might influence the conduction of the stimuli in an unknown way. However, Jou reported the prolongation in SSEP latency during systemic hypothermia of 30°C, using the same method that we employed.38 Then we noticed that previous conclusions were mostly drawn from whole-body cooling techniques, which might potentially invoke other side effects that would impact SSEP signals. Since the effects of hypothermia on the axon, neuron, and synapse are additive and serial, it is reasonable to assume that the larger the area hypothermia impacts, the more complicated the situation might be. Therefore, we believe that RH may not cause the alteration in latency until a severe level is achieved. These findings highlight the potential benefits of using severe RH in a clinical setting. Furthermore, it is more important to note how rapidly both amplitude and latency return to a normal level upon rewarming. This result strongly suggests that the alterations in SSEP amplitude and latency are reversible and transient.
Neural safety of RH
Cold is known to cause injury to the peripheral nerves in experimental animals.39 Histological observation also shows nerve damage under exposure to < 8°C.40 Therefore, it is reasonable to assume that therapeutic RH would cause non-freezing cold nerve injury. Additionally, it is stated by Qian and colleagues that the amount of cold damage reaches its peak 24 h post-injury, including the degeneration of neurons and spontaneous hemorrhage, for which the morphological examination was performed at days 1, 7, and 14 for observing both acute and chronic injury in this study.41 The H&E staining results showed that the neuron structure was regular, and no obvious hemorrhage was seen. Furthermore, although some degenerative cells were observed in FJC fluorescent staining (which may exist in normal tissue as well), there was no difference between post-hypothermic tissues and normal tissues. Also, in addition to SSEP monitoring, a sensitive and subjective scale was used to assess the interior motor columns injury. No decline in BBB score in our study was observed, signifying that there was no locomotor injury caused by RH.
Therefore, these results strongly suggest that RH induced at the lowest temperature of 18°C does not cause nerve injury.
Conclusion
The present study demonstrates that neuroelectrophysiological signals are temporarily and reversibly inhibited by regional hypothermia, when induced at a severe level, in the spinal cord of an uninjured rat model without producing neurological injuries. However, it is critical to state that the underlying mechanisms are still unclear and remain to be clarified. Therefore, given the incomplete understanding of RH's effect on the injured spinal cord and its potential future implementation in a clinical setting, further investigation is necessary.
Acknowledgments
This work was supported by grants from Medical Science and Technology Innovation Subject of Nanjing Military Command (09MA084). The authors are grateful for support from Mengliang Zhou, Lin Zhu, Wei Li, and their colleagues in the Department of Neurosurgery, Jinling Hospital.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hsu C.C. Kwan G.N. van Driel M.L. Rophael J.A. Distal aortic perfusion during thoracoabdominal aneurysm repair for prevention of paraplegia. Cochrane Database Syst. Rev. 2012;3:D8197. doi: 10.1002/14651858.CD008197.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabayashi K. Saiki Y. Kokubo H. Takahashi G. Akasaka J. Yoshida S. Hata M. Niibori K. Miura M. Konnai T. Protection from postischemic spinal cord injury by perfusion cooling of the epidural space during most or all of a descending thoracic or thoracoabdominal aneurysm repair. Gen. Thorac. Cardiovasc. Surg. 2010;58:228–234. doi: 10.1007/s11748-009-0495-0. [DOI] [PubMed] [Google Scholar]
- 3.Faridar A. Bershad E.M. Emiru T. Iaizzo P.A. Suarez J.I. Divani A.A. Therapeutic hypothermia in stroke and traumatic brain injury. Front. Neurol. 2011;2:80. doi: 10.3389/fneur.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang J.Y. Clinical study of mild hypothermia treatment for severe traumatic brain injury. J. Neurotrauma. 2009;26:399–406. doi: 10.1089/neu.2008.0525. [DOI] [PubMed] [Google Scholar]
- 5.Ha K.Y. Kim Y.H. Neuroprotective effect of moderate epidural hypothermia after spinal cord injury in rats. Spine (Phila Pa 1976) 2008;33:2059–2065. doi: 10.1097/BRS.0b013e31818018f6. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich W.D. Cappuccino A. Cappuccino H. Systemic hypothermia for the treatment of acute cervical spinal cord injury in sports. Curr. Sports Med. Rep. 2011;10:50–54. doi: 10.1249/JSR.0b013e318205e0b3. [DOI] [PubMed] [Google Scholar]
- 7.Iumashev G.S. Cherkashina Z.A. Use of local hypothermia in complicated spinal injuries (clinical and experimental studies) Vestn. Ross. Akad. Med. Nauk. 1995;10:24–29. [PubMed] [Google Scholar]
- 8.Conrad M.F. Crawford R.S. Davison J.K. Cambria R.P. Thoracoabdominal aneurysm repair: a 20-year perspective. Ann. Thorac. Surg. 2007;83:S856–S861. doi: 10.1016/j.athoracsur.2006.10.096. S890–S892. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L. Cheng H. Shi J. Chen J. Focal epidural cooling reduces the infarction volume of permanent middle cerebral artery occlusion in swine. Surg. Neurol. 2007;67:117–121. doi: 10.1016/j.surneu.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 10.Craenen G. Jeftinija S. Grants I. Lucas J.H. The role of excitatory amino acids in hypothermic injury to mammalian spinal cord neurons. J. Neurotrauma. 1996;13:809–818. doi: 10.1089/neu.1996.13.809. [DOI] [PubMed] [Google Scholar]
- 11.Lang M. Welte M. Syben R. Hansen D. Effects of hypothermia on median nerve somatosensory evoked potentials during spontaneous circulation. J. Neurosurg. Anesthesiol. 2002;14:141–145. doi: 10.1097/00008506-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Madhok J. Wu D. Xiong W. Geocadin R.G. Jia X. Hypothermia amplifies somatosensory-evoked potentials in uninjured rats. J. Neurosurg. Anesthesiol. 2012;24:197–202. doi: 10.1097/ANA.0b013e31824ac36c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 14.Eyupoglu I.Y. Savaskan N.E. Brauer A.U. Nitsch R. Heimrich B. Identification of neuronal cell death in a model of degeneration in the hippocampus. Brain Res. Brain Res. Protoc. 2003;11:1–8. doi: 10.1016/s1385-299x(02)00186-1. [DOI] [PubMed] [Google Scholar]
- 15.Lenehan B. Street J. Kwon B.K. Noonan V. Zhang H. Fisher C.G. Dvorak M.F. The epidemiology of traumatic spinal cord injury in British Columbia, Canada. Spine (Phila Pa 1976) 2012;37:321–329. doi: 10.1097/BRS.0b013e31822e5ff8. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich W.R. Therapeutic hypothermia for spinal cord injury. Crit. Care Med. 2009;37(Suppl.7):S238–S242. doi: 10.1097/CCM.0b013e3181aa5d85. [DOI] [PubMed] [Google Scholar]
- 17.Azmoon S. Demarest C. Pucillo A.L. Hjemdahl-Monsen C. Kay R. Ahmadi N. Aronow W.S. Frishman W.H. Neurologic and cardiac benefits of therapeutic hypothermia. Cardiol. Rev. 2011;19:108–114. doi: 10.1097/CRD.0b013e31820828af. [DOI] [PubMed] [Google Scholar]
- 18.Yenari M.A. Han H.S. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat. Rev. Neurosci. 2012;13:267–278. doi: 10.1038/nrn3174. [DOI] [PubMed] [Google Scholar]
- 19.Cappuccino A. Bisson L.J. Carpenter B. Marzo J. Dietrich W.D., 3rd Cappuccino H. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine (Phila Pa 1976) 2010;35:E57–E62. doi: 10.1097/BRS.0b013e3181b9dc28. [DOI] [PubMed] [Google Scholar]
- 20.Inamasu J. Suga S. Sato S. Horiguchi T. Akaji K. Mayanagi K. Kawase T. Post-ischemic hypothermia delayed neutrophil accumulation and microglial activation following transient focal ischemia in rats. J. Neuroimmunol. 2000;109:66–74. doi: 10.1016/s0165-5728(00)00211-3. [DOI] [PubMed] [Google Scholar]
- 21.Lenhardt R. The effect of anesthesia on body temperature control. Front. Biosci. (Schol. Ed.) 2010;2:1145–1154. doi: 10.2741/s123. [DOI] [PubMed] [Google Scholar]
- 22.Melhuish T. Linking hypothermia and hyperglycemia. Nurs. Manage. 2009;40:42–45. doi: 10.1097/01.NUMA.0000365472.26379.be. [DOI] [PubMed] [Google Scholar]
- 23.Polderman K.H. Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit. Care Med. 2009;37:1101–1120. doi: 10.1097/CCM.0b013e3181962ad5. [DOI] [PubMed] [Google Scholar]
- 24.Soliman H.M. Mercan D. Lobo S.S. Mélot C. Vincent J.L. Development of ionized hypomagnesemia is associated with higher mortality rates. Crit. Care Med. 2003;31:1082–1087. doi: 10.1097/01.CCM.0000060867.17556.A0. [DOI] [PubMed] [Google Scholar]
- 25.Motoyoshi N. Takahashi G. Sakurai M. Tabayashi K. Safety and efficacy of epidural cooling for regional spinal cord hypothermia during thoracoabdominal aneurysm repair. Eur. J. Cardiothorac. Surg. 2004;25:139–141. doi: 10.1016/s1010-7940(03)00677-8. [DOI] [PubMed] [Google Scholar]
- 26.Peeling L. Hentschel S. Fox R. Hall H. Fourney D.R. Intraoperative spinal cord and nerve root monitoring: a survey of Canadian spine surgeons. Can. J. Surg. 2010;53:324–328. [PMC free article] [PubMed] [Google Scholar]
- 27.Magit D.P. Hilibrand A.S. Kirk J. Rechtine G. Albert T.J. Vaccaro A.R. Simpson A.K. Grauer J.N. Questionnaire study of neuromonitoring availability and usage for spine surgery. J. Spinal Disord. Tech. 2007;20:282–289. doi: 10.1097/01.bsd.0000211286.98895.ea. [DOI] [PubMed] [Google Scholar]
- 28.Casas C.E. Herrera L.P. Prusmack C. Ruenes G. Marcillo A. Guest J.D. Effects of epidural hypothermic saline infusion on locomotor outcome and tissue preservation after moderate thoracic spinal cord contusion in rats. J. Neurosurg. Spine. 2005;2:308–318. doi: 10.3171/spi.2005.2.3.0308. [DOI] [PubMed] [Google Scholar]
- 29.Ishikawa A. Mori A. Kabei N. Yoshitake A. Suzuki T. Katori N. Morisaki H. Yozu R. Takeda J. Epidural cooling minimizes spinal cord injury after aortic cross-clamping through induction of nitric oxide synthase. Anesthesiology. 2009;111:818–825. doi: 10.1097/ALN.0b013e3181b764f6. [DOI] [PubMed] [Google Scholar]
- 30.Kwon B.K. Mann C. Sohn H.M. Hilibrand A.S. Phillips F.M. Wang J.C. Fehlings M.G. NASS Section on Biologics. Hypothermia for spinal cord injury. Spine J. 2008;8:859–874. doi: 10.1016/j.spinee.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Wei G. Lu X.-C.M. Shear D.A. Yang X. Tortella F.C. Neuroprotection of selective brain cooling after penetrating ballistic-like brain injury in rats. Ther. Hypothermia Temp. Manage. 2011;1:33–41. doi: 10.1089/ther.2010.0007. [DOI] [PubMed] [Google Scholar]
- 32.Kottenberg–Assenmacher E. Armbruster W. Bornfeld N. Peters J. Hypothermia does not alter somatosensory evoked potential amplitude and global cerebral oxygen extraction during marked sodium nitroprusside-induced arterial hypotension. Anesthesiology. 2003;98:1112–1118. doi: 10.1097/00000542-200305000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Porkkala T. Kaukinen S. Hakkinen V. Jäntti V. Effects of hypothermia and sternal retractors on median nerve somatosensory evoked potentials. Acta Anaesthesiol. Scand. 1997;41:843–848. doi: 10.1111/j.1399-6576.1997.tb04798.x. [DOI] [PubMed] [Google Scholar]
- 34.Markand O.N. Warren C. Mallik G.S. King R.D. Brown J.W. Mahomed Y. Effects of hypothermia on short latency somatosensory evoked potentials in humans. Electroencephalogr. Clin. Neurophysiol. 1990;77:416–424. doi: 10.1016/0168-5597(90)90002-u. [DOI] [PubMed] [Google Scholar]
- 35.Weight F.F. Erulkar S.D. Synaptic transmission and effects of temperature at the squid giant synapse. Nature. 1976;261:720–722. doi: 10.1038/261720a0. [DOI] [PubMed] [Google Scholar]
- 36.Maybhate A. Hu C. Bazley F.A. Yu Q. Thakor N.V. Kerr C.L. All A.H. Potential long-term benefits of acute hypothermia after spinal cord injury: assessments with somatosensory-evoked potentials. Crit. Care Med. 2012;40:573–579. doi: 10.1097/CCM.0b013e318232d97e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sohmer H. Gold S. Cahani M. Attias J. Effects of hypothermia on auditory brain-stem and somatosensory evoked responses. A model of a synaptic and axonal lesion. Electroencephalogr. Clin. Neurophysiol. 1989;74:50–57. doi: 10.1016/0168-5597(89)90051-8. [DOI] [PubMed] [Google Scholar]
- 38.Jou I.M. Effects of core body temperature on changes in spinal somatosensory-evoked potential in acute spinal cord compression injury: an experimental study in the rat. Spine (Phila Pa 1976) 2000;25:1878–1885. doi: 10.1097/00007632-200008010-00004. [DOI] [PubMed] [Google Scholar]
- 39.Schaumburg H. Byck R. Herman R. Rosengart C. Peripheral nerve damage by cold. Arch. Neurol. 1967;16:103–109. doi: 10.1001/archneur.1967.00470190107013. [DOI] [PubMed] [Google Scholar]
- 40.Denny-Brown D. Adams R. Brenner C. Doherty M.M. The pathology of injury to nerve induced by cold. J. Neuropathol. Exp. Neurol. 1945;4:305–323. doi: 10.1097/00005072-194504040-00001. [DOI] [PubMed] [Google Scholar]
- 41.Qian X.D. Wei E.Q. Zhang L. Sheng W.W. Wang M.L. Zhang W.P. Chen Z. Pranlukast, a cysteinyl leukotriene receptor 1 antagonist, protects mice against brain cold injury. Eur. J. Pharmacol. 2006;549:35–40. doi: 10.1016/j.ejphar.2006.07.056. [DOI] [PubMed] [Google Scholar]