Abstract
Phrenic nerve is the main nerve drive to the diaphragm and its injury is a well-known complication following cardiac surgeries. It results in diaphragmatic dysfunction with reduction in lung volumes and capacities. This study aimed to evaluate the objectivity of lung volumes and capacities as an outcome measure for the prognosis of phrenic nerve recovery after cardiac surgeries. In this prospective experimental study, patients were recruited from Cardio-Thoracic Surgery Department, Educational-Hospital of College of Medicine, Cairo University. They were 11 patients with right phrenic nerve injury and 14 patients with left injury. On the basis of receiving low-level laser irradiation, they were divided into irradiated group and non-irradiated group. Measures of phrenic nerve latency, lung volumes and capacities were taken pre and post-operative and at 3-months follow up. After 3 months of low-level laser therapy, the irradiated group showed marked improvement in the phrenic nerve recovery. On the other hand, vital capacity and forced expiratory volume in the first second were the only lung capacity and volume that showed improvement consequent with the recovery of right phrenic nerve (P value <0.001 for both). Furthermore, forced vital capacity was the single lung capacity that showed significant statistical improvement in patients with recovered left phrenic nerve injury (P value <0.001). Study concluded that lung volumes and capacities cannot be used as an objective outcome measure for recovery of phrenic nerve injury after cardiac surgeries.
Keywords: Lung volumes and capacities, Phrenic nerve injury, Diaphragmatic dysfunction, Laser therapy
1. Introduction
Phrenic nerve is the main nerve supply of the essential inspiratory muscle, the diaphragm (Simansky et al., 2002; Laghi and Tobin, 2003). Phrenic nerve injury [PNI] is a well-known clinical condition following cardiac surgery (Canbaz et al., 2004; Merino-Ramirez et al., 2006; Grocott et al., 2004; Dimopoulou et al., 1998). It was reported as an incidence, estimated by electrophysiological or radiological techniques, varies from 10% to 85% (Dimopoulou et al., 1998; Cohen et al., 1997; Mazzoni et al., 1996; Chroni et al., 1995). Following the first report of phrenic nerve paresis after cardiac operation in 1963, both clinical and animal studies have shown that the application of iced saline slush to the pericardial well results in hypothermic injury of the phrenic nerves (O’Brien et al., 1991). Usage of ice slush and cold cardioplegia were among the guilty risk factors caused PNI with 45.3% incidence after cardiac surgery in a study done by El-Sobkey (2006).
Although many studies have shown that most cases of PNI following cardiac surgery are transient and of no clinical significance (Simansky et al., 2002) specially if it is unilateral (Laghi and Tobin, 2003), other studies indicated that PNI may be associated with atelectasis, pneumonia, pulmonary effusion (Devita et al., 1993) and difficult weaning in the first 2–3 days after surgery (Laghi and Tobin, 2003). In addition PNI showed to increase post-operative pulmonary complications (Deng et al., 2006). It causes diaphragmatic dysfunction (Diehl et al., 1994) and reduction of lung volumes and capacities (Simansky et al., 2002; Laghi and Tobin, 2003).
Low-level laser therapy [LLLT] has been used in treatment of peripheral nerve injury (Shin et al., 2003). A wavelength ranged from 635 to 670 nm, and power of 5 mW, has been shown to be effective in enhancing recovery of PNI after cardiac surgeries (El-Sobkey, 2006). LLLT has been described as having a stimulation effect on human tissue at local cellular level or general systemic level or both by acceleration of the photo biological or photochemical process (Sabbour, 1996; Abdou, 1995).
For PNI diagnostic and prognostic purposes, nerve conduction study [NCS] was found to be the golden standard. The disadvantages are a painful or discomfort procedure, expensive, and it is not commonly available in Cardio-Thoracic Surgery Department. On the other hand, spirometry is an easy, comfortable, safe, non-invasive and inexpensive modality to assess the lung volume and capacities (Handojo et al., 2006).
This study was conducted to assess objectivity of lung volumes and capacities as an outcome measure of phrenic nerve recovery after cardiac surgery as an alternative economical outcome measure to the golden standard NCS.
2. Methodology
This prospective experimental study was carried out in the Educational-Hospital of College of Medicine, Cairo University. Adult patients enrolled in Cardio-Thoracic Surgeries Department for open-heart surgeries were approached as the target population. Patients who met study inclusion criteria and agreed to voluntarily participate in the study were given consent form to sign. Eighty patients were enrolled as basic study group. It was clarified to the participants that, each would have the right to withdraw at any time, by notifying the researcher.
For this basic study group, 1 or 2 days before the surgery, pre-operative phrenic nerve conduction study tests, right and left phrenic nerve latencies, were measured by neurophysiologist in the clinical neurophysiologic unit. Nihon Kohden MEB-5304K device was used to transcutaneously stimulate the Phrenic nerve with square wave stimuli of 0.1 ms duration and frequency of 1 Hz. Stimulating electrodes were placed at the posterior border of the sternocleidomastoid muscle at the level of the upper margin of the thyroid cartilage, with the active electrode proximally. Recording electrodes were placed as follows; the active electrode applied in the 7th intercostal space in the mid-clavicular line and the reference electrode applied at 3.5–5 cm from the active electrode in the 9th intercostal in the anterior axillary line. Ground electrodes were placed between the stimulating and recording electrodes around the chest (Fig. 1).
Figure 1.

Sites for electrode placements for phrenic nerve transcutaneous stimulation.
Discovery portable computerized spirometer unit was used to measure the following pre-operative parameters of lung volumes and capacities; vital capacity [VC], forced vital capacity [FVC], forced expiratory volume in the first second [FEV1], FEV1 to VC ratio [FEV1/VC], FEV1 to FVC ratio [FEV1/FVC], and maximum voluntary ventilation (MVV). Spirometry was conducted from the standing position following the standards.
Post-operatively, 3–5 days, when the patient’s condition allows, as decided by the surgeon, measurement of the phrenic nerve latencies and lung volumes and capacities were repeated.
Phrenic NCS tests were used as diagnostic measure for PNI. Patients were diagnosed as phrenic nerve injured if values of Phrenic NCS exceeded 10.0 ms (Devita et al., 1993) or post-operative latencies values were more than the pre-operative values with one or more millisecond. Patients diagnosed with PNII were 28 and 52 were non-injured. Among the PNI group; 12 with right PNI and 16 with left PNI are shown in Scheme 1. Each injured group was randomly divided, by coin allocation, into two equal groups, intervention (irradiated) and placebo (non-irradiated). Patients who continued the 3-month follow-up were 25 (study group), 11 patients with right PNI (5 irradiated and 6 non-irradiated) and 14 patients with left PNI (6 irradiated and 8 non-irradiated).
Scheme 1.

Distribution of the basic study group.
Subject inclusion criteria were: (1) adult volunteer patient, signed a consent form; (2) non-smoker patient; (3) patient performing primary elective cardiac surgery; (4) patient performed coronary artery bypass graft [CABG], valvular or adult congenital deficits closure surgery; (5) patient able to understand verbally or by demonstration the instructions needed to perform spirometry test.
Subject exclusion criteria were: (1) patient with diabetes, neurological disorders or respiratory diseases; (2) patient re-do cardiac surgery; (3) patient with any learning barriers restrict spirometry test; (4) patient contraindicated for electrical stimulation as patient with pace maker.
Irradiated group received low-level laser therapy of wavelength ranged from 635 to 670 nm, and power of 5 mW for 12 successive sessions at rate of 2 sessions per week. The non-irradiated group received sham treatment with applying the laser pointer device with power switched off.
Both phrenic NCS tests and spirometry were measured pre and post-operatively and at 3 months follow-up arranged with the surgeon’s follow-up dates.
Data were collected on special forms then varied and coded. After checking normality, all data were expressed as mean and standard deviation for all continuous data. Paired t-test was used to compare phrenic nerve latency and lung volumes and capacities before and after surgery for irradiated group as well as non-irradiated group. Repeated measure ANOVA was used to compare between the progression of the irradiated group and the non-irradiated group through the 3 months follow up. Confidence interval 95% was assigned and P value <0.05 was considered.
3. Results and discussion
Incidence of PNI in the current study was 35% as 28 patients out of 80, the basic study group, were injured. This incidence was within the average of many previous studies. Siafakas et al. (1999) reported that the incidence of PNI after cardiac surgery has been in the range of 25–73% (Siafakas et al., 1999). Cohen et al. (1997) found that patients undergoing CABG have an incidence of PNI that ranges from 10% to 60% (Cohen et al., 1997). It could be said that phrenic nerve injury following cardiac surgery is variable in its incidence depending on the diligence with which it is sought (Tripp and Bolton, 1998).
Three patients were dropped because they could not keep commitment to the 12 successive LLLT sessions. Within 25 phrenic nerve injured patients (aged 44.5 ± 8.1 years) who were homogenously assigned into irradiated and non-irradiated groups, there was no significant difference between the two groups regarding the pre-operative values and 3–5 days post-operative values for phrenic nerve latencies, lung volumes and capacities (Table 1).
Table 1.
Pre and post-operative values of phrenic nerve latencies and lung volumes and capacities in irradiated and non-irradiated groups in patients with right and left phrenic nerve injury after cardiac surgeries. Pre = 1 day before the operation; post = 3–5 days after the operation.
| Stage | Outcome | Irradiated |
Non-irradiated |
P | ||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |||
| Pre-operative | Right phrenic nerve latency (ms) | 5 | 6.4 | 0.19 | 6 | 6.3 | 0.25 | >0.05 |
| Left phrenic nerve latency (ms) | 6 | 6.9 | 0.1 | 8 | 7 | 0.29 | >0.05 | |
| Right phrenic nerve injured patients | ||||||||
| VC (% of predicted values) | 5 | 54.4 | 1.13 | 6 | 52 | 2 | >0.05 | |
| FVC (% of predicted values) | 5 | 52.6 | 23.1 | 6 | 41.8 | 19.9 | >0.05 | |
| FEV1 (% of predicted values) | 5 | 59.2 | 1.63 | 6 | 58 | 1.41 | >0.05 | |
| FEV1/VC (ratio) | 5 | 114.8 | 38.6 | 6 | 104.5 | 21.7 | >0.05 | |
| FEV1/FVC (ratio) | 5 | 111.8 | 4.1 | 6 | 117.3 | 3.9 | >0.05 | |
| MVV (% of predicted values) | 5 | 43.2 | 16 | 6 | 39.4 | 18 | >0.05 | |
| Left phrenic nerve injured patients | ||||||||
| VC (% of predicted values) | 6 | 45.1 | 16.2 | 8 | 53.6 | 14.7 | >0.05 | |
| FVC (% of predicted values) | 6 | 45.1 | 3 | 8 | 47 | 3.79 | >0.05 | |
| FEV1 (% of predicted values) | 6 | 52.3 | 13.3 | 8 | 60.3 | 9.5 | >0.05 | |
| FEV1/VC (ratio) | 6 | 122 | 23.8 | 8 | 118.8 | 26 | >0.05 | |
| FEV1/FVC (ratio) | 6 | 110.5 | 3.8 | 8 | 110.3 | 9.3 | >0.05 | |
| MVV (% of predicted values) | 6 | 31 | 11.8 | 8 | 38.2 | 15.4 | >0.05 | |
| Post-operative | Right phrenic nerve latency (ms) | 5 | 7.7 | 0.08 | 6 | 7.5 | 0.15 | >0.05 |
| Left phrenic nerve latency (ms) | 6 | 8.6 | 0.33 | 8 | 8.2 | 0.26 | >0.05 | |
| Right phrenic nerve injured patients | ||||||||
| VC (% of predicted values) | 5 | 28.4 | 0.56 | 6 | 29.4 | 1.45 | >0.05 | |
| FVC (% of predicted values) | 5 | 28 | 5.5 | 6 | 33.3 | 12.5 | >0.05 | |
| FEV1 (% of predicted values) | 5 | 32.4 | 1 | 6 | 31.3 | 1.21 | >0.05 | |
| FEV1/VC (ratio) | 5 | 119 | 9.9 | 6 | 106.2 | 18.6 | >0.05 | |
| FEV1/FVC (ratio) | 5 | 115.6 | 1.9 | 6 | 101 | 32.5 | >0.05 | |
| MVV (% of predicted values) | 5 | 27.4 | 7.5 | 6 | 24.6 | 6.5 | >0.05 | |
| Left phrenic nerve injured patients | ||||||||
| VC (% of predicted values) | 6 | 26.3 | 6.2 | 8 | 33.1 | 7.5 | >0.05 | |
| FVC (% of predicted values) | 6 | 30 | 2.53 | 8 | 33.1 | 2.87 | >0.05 | |
| FEV1 (% of predicted values) | 6 | 28.8 | 7.6 | 8 | 37 | 8.8 | >0.05 | |
| FEV1/VC (ratio) | 6 | 110 | 20.2 | 8 | 109.7 | 10.9 | >0.05 | |
| FEV1/FVC (ratio) | 6 | 117 | 2.5 | 8 | 109.6 | 8.8 | >0.05 | |
| MVV (% of predicted values) | 6 | 24.8 | 10.9 | 8 | 27.8 | 8.5 | >0.05 | |
VC: vital capacity.
FVC: forced vital capacity.
FEV1: forced expiratory volume in the first second.
FEV1/VC: ratio between forced expiratory volume in the first second and vital capacity.
FEV1/FVC: ratio between forced expiratory volume in the first second and forced vital capacity.
MVV: maximum voluntary ventilation.
At 3–5 days post-operative, the 25 patients had increased right and left phrenic nerves’ latencies values more than their pre-operative values indicating PNI as a surgical complication. It is often that cardiac surgery is followed with respiratory complication even if the patient saved from PNI simply because of the mechanical corruption occurs by chest incision besides the other intraoperative factors as using the cardiopulmonary bypass machine. So, the respiratory complication after cardiac surgery is multifactor phenomenon that is why NCS tests, right and left phrenic nerve latencies, were used for diagnosis of PNI. Transcutaneous phrenic nerve stimulation is considered as “gold standard” in assessing phrenic nerve functions and is applicable even in patients who are mechanically ventilated (Dimopoulou et al., 1998). Verin et al. (2002) added that measuring electrical stimulation phrenic nerve conduction time, latency, is necessary to make the diagnosis of many types of PNI and to record their follow-up besides giving access to a non-volitional measure of the diaphragm contractile efficiency (Verin et al., 2002).
Injury to phrenic nerve may be caused by clod-induced injury with ice slush, direct trauma, or deprives the phrenic nerve from its blood supply when the internal mammary artery is used as a conduit in CABG (Canbaz et al., 2004; Shin et al., 2003; Deng et al., 2003). Cardiac surgery is the preferable operation for many patients suffering from valvular, ischemic or congenital heart problems. As it saves many patients’ lives as well as improves the quality of their lives. Despite these benefits, cardiac surgeries have respiratory complication specifically, PNI.
At 3-month follow-up, for the 11 patients with right PNI, the values of nerve latencies showed progressive decline in the five irradiated group more than the six non-irradiated group. The differences between the two groups were significant in the first, second as well as the third months. (P < 0.05, P < 0.001, P < 0.001, respectively) (Table 2) and it indicates improvement in the irradiated group.
Table 2.
Progression of right and left phrenic nerve latencies in patients with phrenic nerve injury after cardiac surgeries in irradiated and non-irradiated groups.
| Outcome | Stage | Irradiated |
Non-irradiated |
P | ||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |||
| Right phrenic nerve latency (ms) | 1st Month | 5 | 6.8 | 0.08 | 6 | 7.2 | 0.22 | <0.05 |
| 2nd Month | 5 | 6.5 | 0.1 | 6 | 7.7 | 0.06 | <0.001 | |
| 3rd Month | 5 | 6.1 | 0.24 | 6 | 7.2 | 0.24 | <0.001 | |
| Left phrenic nerve latency (ms) | 1st Month | 6 | 7.9 | 0.23 | 8 | 8.5 | 0.38 | <0.01 |
| 2nd Month | 6 | 6.9 | 0.1 | 8 | 8.8 | 0.12 | <0.001 | |
| 3rd Month | 6 | 5.8 | 0.2 | 8 | 8.4 | 0.25 | <0.001 | |
At 3-month follow-up for the 14 patients with left PNI, the values of nerve latencies showed progressive decline in the six irradiated group more than the eight non-irradiated group. The differences between the two groups were significant in the first, second and third months (P < 0.01, P < 0.001, P < 0.001, respectively) (Table 2).
The consequences of PNI are variable and depend to a large extent on the underlying condition of the patient, particularly with regard to pulmonary function. The response of the patient may range from an asymptomatic radiographic abnormality to severe pulmonary dysfunction requiring prolonged mechanical ventilation and other associated morbidities and even mortality (Tripp and Bolton, 1998). Diaphragmatic dysfunction is one of the major consequences to PNI (Diehl et al., 1994; Shin et al., 2003; Sabbour, 1996; Abdou, 1995; Handojo et al., 2006; Siafakas et al., 1999; Tripp and Bolton, 1998; Verin et al., 2002; Deng et al., 2003; Efthimiou et al., 1991; Robicsek et al., 1990). Qureshi (2009) stated that the diaphragm is the chief inspiratory muscle and that its dysfunction can lead to dyspnea and can affect ventilatory function (Qureshi, 2009). The current study results confirmed Qureshi’s statement. In the 25 phrenic nerve injured patients, 3–5 days post-operative, lung volumes and capacities showed marked reduction (Table 1).
LLLT is proved to enhance recovery of injured phrenic nerve. This was proved with decrease in nerve latency (El-Sobkey, 2006). Injured patients received LLLT during 3 months follow-up as many studies reported that recovery of PNI vary from 30 days to 2 years (Dimopoulou et al., 1998; Cohen et al., 1997; Siafakas et al., 1999; Martinez et al., 2000; Katz et al., 1998).
In the second month of laser therapy, the five right PNI irradiated patients had nerve latency values close to that of the pre-operative values. Furthermore, at the third month, they had nerve latency values even less than that of the pre-operative values by 0.22 ms. In addition, at the third month, they showed lower latency values than the six non-irradiated patients by 1.12 ms (Fig. 1).
Left phrenic nerve recovery was more obvious in the second month as nerve latency values of the six irradiated patients returned to their pre-operative values. Moreover, the values, in the third month, were less than the pre-operative values by 1.1 ms. In addition, at the third month, nerve latency values of the irradiated patients were less than that of the eight non-irradiated patients by 2.6 ms (Fig. 2). Recovery of right and left phrenic nerve latencies to the pre-operative values or even less indicates that the 3 months follow-up was acceptable period (Fig. 3).
Figure 2.
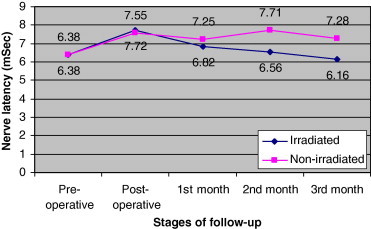
Progression of right phrenic nerve latency in patients with right phrenic nerve injury after cardiac surgeries in irradiated and non-irradiated groups.
Figure 3.
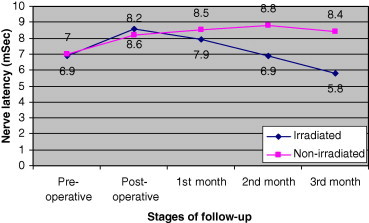
Progression of left phrenic nerve latency in patients with left phrenic nerve injury after cardiac surgeries in irradiated and non-irradiated groups.
During laser irradiation therapy, recovery of phrenic nerve needed to be objectively monitored. Nerve conduction study is somehow expensive and uncomforting to patients. Resman-Gaspersc and Pondar (2008) supported that as they mentioned that in clinical work it had been occasional to encounter difficulties in performing phrenic NCS (Resman-Gaspersc and Pondar, 2008). Measuring the electrical activity of the diaphragm itself was an alternative to directly measure the diaphragmatic compound muscle action potential. However, inability to do so was one of the limitations faced with this study as this would require the use of needle electromyography that is not feasible with cardiac surgery patients because of the severe pain it would cause especially post-operatively. That is why the present study aimed to find out another alternative objective outcome measure to monitor the prognosis of the injured phrenic nerve during its recovery.
In the present study, researchers hypothesized that as long the phrenic nerve is the main nerve drive to diaphragm, the primary inspiratory muscle, and injury of phrenic nerve results in diaphragmatic and ventilatory dysfunction, it would make sense to assume that recovery of injured phrenic nerve would improve diaphragmatic function and consequently ventilatory functions represented by lung volumes and capacities.
Measuring lung volumes and capacities is more comfortable to patients and has economical advantage on NCS. These were the reasons embarked the researchers to evaluate them as an objective outcome measure for phrenic nerve recovery to replace the NCS.
Results in this study disagreed with that hypothesis. During the 3 months of follow-up, although there was marked improvement in right and left phrenic nerve values of the 11 irradiated group (5 right and 6 left) compared with the 14 (6 right and 8 left) non-irradiated group, improvement in lung volumes and capacities was poor. It could be said that recovery on lung volumes and capacities did not show improvement consistent with recovery of the phrenic nerve latency. Instead of the small sample size of irradiated and non-irradiated patients for right PNI group and that of left PNI group, which considered one of this study limitation, compelling the radiated patients of right and left PNI in one group (11) and the non-irradiated patients (14) in another group, make their comparison more statistically acceptable. The homogeny of irradiated and non irradiated groups regarding the nerve latencies both pre-operatively and 3–5 days post-operatively, as shown in Table 1, is another statistical factor which would guarantee the recovery effect of LLLT on the injured phrenic nerves.
Results showed that in patients with right PNI, among the five measured parameters of lung volumes and capacities only the VC and FEV1 parameters showed significant improvement in the five irradiated group compared to the six non-irradiated group (P < 0.001 in the 3 months follow-up for both VC and FEV1) (Table 3).
Table 3.
Progression of vital capacity (VC), forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) in patients with phrenic nerve injury after cardiac surgeries in irradiated and non-irradiated groups.
| Outcome | Stage | Irradiated |
Non-irradiated |
P | ||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |||
| VC | 1st Month | 5 | 42.4 | 1.6 | 6 | 31.5 | 1.04 | <0.001 |
| 2nd Month | 5 | 55.2 | 0.57 | 6 | 30.6 | 1.04 | <0.001 | |
| 3rd Month | 5 | 58.2 | 0.82 | 6 | 35.1 | 1.66 | <0.001 | |
| FEV1 | 1st Month | 5 | 46.4 | 1.31 | 6 | 33.3 | 0.54 | <0.001 |
| 2nd Month | 5 | 57.4 | 0.48 | 6 | 32.3 | 1.34 | <0.001 | |
| 3rd Month | 5 | 58 | 0.76 | 6 | 35.1 | 1.66 | <0.001 | |
| FVC | 1st Month | 6 | 42.1 | 3.55 | 8 | 33.5 | 0.55 | <0.001 |
| 2nd Month | 6 | 42.3 | 3.19 | 8 | 36.1 | 3.28 | <0.05 | |
| 3rd Month | 6 | 49.1 | 1.9 | 8 | 38.3 | 4.66 | <0.001 | |
On the other hand, in patients with left PNI, no lung volumes and capacities showed improvement in the six irradiated group compared with the eight non-irradiated group except the FVC parameter. (P < 0.001, P < 0.05, and P < 0.001 in the first, second and third months of follow-up, respectively) (Table 3).
The point here is that, only one parameter (FVC in patients with left PNI) or two parameters (VC and FEV1 in patients with right PNI) of the five measured parameters of lung volumes and capacities accompanied the recovery of nerve latency. If all parameters showed improvement with the recovery of nerve latency, lung volumes and capacities would be strongly recommended as objective outcome measure for the prognosis of phrenic nerve. Nevertheless, this is not the case. Another weak point with lung volumes and capacities as an outcome measure for the prognosis of phrenic nerve is that, lung volumes and capacities showed inconsistence pattern. This is because; in patients with right PNI, VC and FEV1 were the parameters, showed significant improvement with the nerve recovery while another parameter, FVC, showed improvement with the left phrenic nerve recovery. In other words, not the same parameter improved with the nerve recovery, no consistency. If the same parameter showed improvement with right and left phrenic nerves recovery, it would be considered the selected parameter of lung volumes and capacities that can be used as mirror for value of nerve latency and it could be said that this parameter is a sensitive measure to the change on the nerve latency.
The previous phenomenon was explained by the study of De Palo and McCool (2009) as they mentioned that VC is a global rather than a specific measure of respiratory muscle function because it relies on the integrated function of the respiratory pump (muscles and nerves) with that of the chest wall bellows (airways, lung parenchyma, rib cage, and abdomen). Moreover, they stated that MVV is providing another means of evaluating the integrative function of the inspiratory pump and chest wall bellows as well (De Palo and McCool, 2009).
Another explanation is that, more time was required to translate the improvement in nerve latency into improvement in lung volumes and capacities, more than the 3 months follow-up. This explanation is supported by the study carried out by Gayan-Ramirez et al. (2008); they recorded the compound motor action potential of the diaphragm and latency of the phrenic nerve in addition to the forced vital capacity at onset of the diaphragmatic dysfunction and 12 months and 24 months after. They found that functional respiratory recovery, increase in forced vital capacity >400 ml, occurred in 43% of patients after 12 months and in 52% after 24 months. That is why they concluded that compound motor action potential did not predict functional respiratory recovery. Moreover, they stated that functional respiratory recovery is difficult to predict and may occur years after the onset of dysfunction (Gayan-Ramirez et al., 2008). Remembering the multifactor phenomenon of respiratory complication after cardiac surgery could be more reliable explanation. Patients with PNI had two main causes to their respiratory complications; firstly the nerve injury itself which been improved by LLLT and nerve recovery was proved by NCS tests, secondly other intraoperative causes or even after surgery causes as patient’s fear from breathing fully to avoid incisional chest pain. This may interfere with the ventilatory function of the respiratory system and negatively influence lung volumes and capacities and burden their improvement.
Current study, therefore, confirms that lung volumes and capacities cannot be used as an objective outcome measure and cannot be used as an alternative to NCS. In addition it could be assured that NCS, despite its disadvantages, it is still, without competitor, the only golden standard detector for peripheral nerve’s function and recovery, including the phrenic nerve, or as mentioned by Merino-Ramirez et al. (2006), it is the reference method. Zemans and Lee-Chiong, (2009) support the results as they stated that diaphragmatic dysfunction might be suggested by radiographic findings or the results of spirometry tests, but the diagnosis must be confirmed by electromyography and nerve conduction studies (Zemans and Lee-Chiong, 2009). Qureshi (2009) also stated that the diagnosis is usually suspected on chest X-ray and clinical exam and confirmed with sniff test or phrenic nerve stimulation/diaphragm electromyography (Qureshi, 2009). Canbaz et al. (2004) supported this opinion as they mentioned that when phrenic nerve injury is suspected, based on low-sensitive methods, such as chest roentgenogram, spirometry, and abnormal diaphragm movements, the diagnosis might be confirmed by a simple electrophysiologic study (Canbaz et al., 2004).
4. Conclusion
Lung volumes and capacities cannot be used as an outcome measure for recovery of phrenic nerve handled with low-level laser therapy after been injured secondary to cardiac surgeries. Nerve conduction study is the golden objective prognostic measure for phrenic nerve recovery.
Acknowledgements
We are grateful to Prof. Magdy Gomaa who helped us to have approval to access the cardiothoracic department, Cairo University Educational Hospital. We are also grateful to Prof. Ann Abdulkader for her approval for the use of the clinical neurophysiologic unit and Dr. Mai Mohamed who conducted the phrenic nerve conduction study.
References
- Abdou, E.Y., 1995. The use of laser in diabetic foot ulcers: comparative study. M.Sc. Thesis.
- Canbaz S., Turgut N., Halici U., Balci K., Ege T., Duran E. Electrophysiological evaluation of phrenic nerve injury during cardiac surgery – a prospective, controlled, clinical study. BMC Surg. 2004;4(2):1–5. doi: 10.1186/1471-2482-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chroni E., Patel R.L., Taub N., Venn G.E., Howard R.S., Panayiotopoulos C.P. A comprehensive electrophysiological evaluation of phrenic nerve injury related to open-heart surgery. Acta Neurol. Scand. 1995;91:255–259. doi: 10.1111/j.1600-0404.1995.tb07000.x. [DOI] [PubMed] [Google Scholar]
- Cohen A.J., Katz M.G., Katz R., Feld D.M., Hauptman E., Schachner A. Phrenic nerve injury after coronary artery grafting: is it always benign? Ann. Thorac. Surg. 1997;64:148–153. doi: 10.1016/s0003-4975(97)00288-9. [DOI] [PubMed] [Google Scholar]
- De Palo, V.A., McCool, F.D., 2009. Respiratory evaluation of patient with neuromuscular disease: Global Assessment of Respiratory Muscle Function. <http://www.medscape.com/viewarticle/439041_3> (accessed December 2009).
- Deng Y., Byth K., Paterson H.S. Phrenic nerve injury associated with high free right internal mammary artery harvesting. Ann. Thorac. Surg. 2003;76(2):459–463. doi: 10.1016/s0003-4975(03)00511-3. [DOI] [PubMed] [Google Scholar]
- Deng Y., Sun Z., Ma J., Paterson H.S. Semi-skeletonised internal mammary grafts and phrenic nerve injury: cause-and-effect analysis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2006;26(4):455–459. doi: 10.1007/s11596-006-0420-z. [DOI] [PubMed] [Google Scholar]
- Devita M.A., Robinson L.R., Rehder J., Hatter B., Cohen C. Incidence and natural history of phrenic neuropathy occurring during open heart surgery. Chest. 1993;103:850–856. doi: 10.1378/chest.103.3.850. [DOI] [PubMed] [Google Scholar]
- Diehl J.L., Lofaso F., Deleuze P., Similowski T., Lemaire F., Brochard L. Clinically relevant diaphragmatic dysfunction after cardiac operations. J. Thorac. Cardiovasc. Surg. 1994;107:487–498. [PubMed] [Google Scholar]
- Dimopoulou I., Daganou M., Dafni U., Karakatsani A., Khoury M., Geroulanos S. Phrenic nerve dysfunction after cardiac operations: electrophysiological evaluation of risk factors. Chest. 1998;113:8–14. doi: 10.1378/chest.113.1.8. [DOI] [PubMed] [Google Scholar]
- Efthimiou J., Butter J., Woodham C., Benson M.K., Swestaby S. Diaphragm paralysis following cardiac surgery: role of phrenic nerve cold injury. Ann. Thorac. Surg. 1991;52:1005–1008. doi: 10.1016/0003-4975(91)91268-z. [DOI] [PubMed] [Google Scholar]
- El-Sobkey, S.B., 2006. Effect of low level laser irradiation on phrenic nerve palsy after open-heart surgery. Ph.D. Thesis.
- Gayan-Ramirez G., Gosselin N., Troosters T., Bruyninckx F., Gosselink R., Decramer M. Functional recovery of diaphragm paralysis: a long-term follow-up study. Respir. Med. 2008;102(5):690–698. doi: 10.1016/j.rmed.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Grocott H.P., Clark J.A., Homi H.M., Sharma A. Other neurologic complications after cardiac surgery. Semin. Cardiothorac. Vasc. Anesth. 2004;8(3):213–226. doi: 10.1177/108925320400800304. [DOI] [PubMed] [Google Scholar]
- Handojo T., Anstey N., Kelly P., Pain M., Kenagalem E., Tjitra E. Normal spirometry, gas transfer and lung volume values in Paupua, Indonesia. Southeast Asian J. Trop. Med. Public Health. 2006;37(3) [PubMed] [Google Scholar]
- Katz M.G., Katz R., Schachner A., Cohen A.J. Phrenic nerve injury after coronary artery grafting: will it go away? Ann. Thorac. Surg. 1998;65:32–35. doi: 10.1016/s0003-4975(97)00915-6. [DOI] [PubMed] [Google Scholar]
- Laghi F., Tobin M.J. Disorders of the respiratory muscles. Am. J. Respir. Crit. Care Med. 2003;168:10–48. doi: 10.1164/rccm.2206020. [DOI] [PubMed] [Google Scholar]
- Laghi F., Tobin M.J. Disorders of the respiratory muscles. Am. J. Respir. Crit. Care Med. 2003;168:10–48. doi: 10.1164/rccm.2206020. [DOI] [PubMed] [Google Scholar]
- Martinez C.A., Armijo A., Fermoso A., Moraleda S., Mate I., Marin M. Phrenic nerve conduction study in demyelinating neuropathies and open-heart surgery. Clin. Neurophysiol. 2000;111(5):821–825. doi: 10.1016/s1388-2457(00)00250-9. [DOI] [PubMed] [Google Scholar]
- Mazzoni M., Solinas C., Sisillo E., Bortone F., Susini G. Intra-operative phrenic nerve monitoring in cardiac surgery. Chest. 1996;109(6):1455–1460. doi: 10.1378/chest.109.6.1455. [DOI] [PubMed] [Google Scholar]
- Merino-Ramirez M.A., Juan G., Ramon M., Cortijio J., Rubio E., Montero A. Electro physiologic evaluation of phrenic nerve and diaphragm function after coronary bypass surgery: prospective study of diabetes and other risk factors. J. Thoracic Cardiovasc. Surg. 2006;132(3):530–536. doi: 10.1016/j.jtcvs.2006.05.011. [DOI] [PubMed] [Google Scholar]
- O’Brien J.W., Johnson S.H., Vansteyn S.J., Craig D.M., Sharpe R.E., Mauney M.C. Effects of internal mammary artery dissection on phrenic nerve perfusion and function. Ann. Thorac. Surg. 1991;51:182–188. doi: 10.1016/0003-4975(91)91334-r. [DOI] [PubMed] [Google Scholar]
- Qureshi A. Diaphragm paralysis. Semin. Respir. Crit. Care Med. 2009;30(3):315–320. doi: 10.1055/s-0029-1222445. [DOI] [PubMed] [Google Scholar]
- Resman-Gaspersc A., Pondar S. Phrenic nerve conduction studies: technical aspect and normative data. Muscle Nerve. 2008;37(1):36–41. doi: 10.1002/mus.20887. [DOI] [PubMed] [Google Scholar]
- Robicsek F., Ducan D.G., Hawes A.C., Rice H.E., Harrill S., Robicsek S.A. Biological thresholds of cold induced phrenic nerve injury. J. Thorac. Cardiovasc. Surg. 1990;99:167–170. [PubMed] [Google Scholar]
- Sabbour, A.H., 1996. Low level laser therapy in relation to primary dysmenorrhoea. M.Sc. Thesis.
- Shin D.H., Lee E., Hyun J.K., Lee S.J., Chang Y.P., Kim J.W. Growth-associated protein-43 is elevated in injured rat sciatic nerve after low power laser irradiation. Neurosci. Lett. 2003;344(2):71–74. doi: 10.1016/s0304-3940(03)00354-9. [DOI] [PubMed] [Google Scholar]
- Siafakas N.M., Mitrouska I., Bouros D., Georgopoulos D. Surgery and respiratory muscles. Thorax. 1999;54:458–465. doi: 10.1136/thx.54.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simansky D.A., Paley M., Refaely Y., Yellin A. Diaphragm plication following phrenic nerve injury: a comparison of paediatric and adult patients. Thorax. 2002;57:613–616. doi: 10.1136/thorax.57.7.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp H.F., Bolton R. Phrenic nerve injury following cardiac surgery: a review. J. Cardiac Surg. 1998;13(3):218–223. doi: 10.1111/j.1540-8191.1998.tb01265.x. [DOI] [PubMed] [Google Scholar]
- Verin E., Straus C., Demoule A., Mialon P., Derenne J.P., Similowski T. Validation of improved recording site to measure phrenic conduction from surface electrodes in humans. J. Appl. Physiol. 2002;92:967–974. doi: 10.1152/japplphysiol.00652.2001. [DOI] [PubMed] [Google Scholar]
- Zemans, R.L., Lee-Chiong, T., 2009. Diaphragm Paresis and Paralysis. PCCU, vol. 19, lesson 21. <http://www.chestnet.org/education/online/pccu/vol19/lessons21_22/print21.php> (accessed December 2009).


