Abstract
Bacterial adhesion in natural and artificial systems has been critically reviewed to investigate the influences exerted by the presence of interfaces. Numerous investigations have demonstrated that, in the presence of a solid phase, the activity of bacterial cultures is changed. Reviewing relevant literature, two problems were encountered. One is of an experimental nature. Due to lack of similarity in experimental conditions, disparate experiments often cannot be compared; their results may even appear conflicting. The other problem is of an interpretational nature: several hypothetical theories exist which try to explain the effect of surfaces on microbial activity. These theories often confuse changes in the medium and limitations in mass transfer which are due to the presence of solid surfaces (indirect influences) with changes in cell properties (direct influences). Whenever a surface is reported to influence the metabolism of bacteria, the action is found almost exclusively to be due to changes in the medium or environment and is therefore indirect. Based on data reported in the literature, and by using thermodynamic and kinetic considerations, it is concluded that so far neither experimental nor theoretical evidence exists for a direct influence of interfaces on microbial activity.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitton G., Henis Y., Lahav N. Effect of several clay minerals and humic acid on the survival of Klebsiella aerogenes exposed to ultraviolet irradiation. Appl Microbiol. 1972 May;23(5):870–874. doi: 10.1128/am.23.5.870-874.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright J. J., Fletcher M. Amino Acid assimilation and electron transport system activity in attached and free-living marine bacteria. Appl Environ Microbiol. 1983 Mar;45(3):818–825. doi: 10.1128/aem.45.3.818-825.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busscher H. J., Weerkamp A. H., van der Mei H. C., van Pelt A. W., de Jong H. P., Arends J. Measurement of the surface free energy of bacterial cell surfaces and its relevance for adhesion. Appl Environ Microbiol. 1984 Nov;48(5):980–983. doi: 10.1128/aem.48.5.980-983.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C., Keevil C. W., Marsh P. D., Brown C. M., Wardell J. N. Surface-associated growth. Philos Trans R Soc Lond B Biol Sci. 1982 Jun 11;297(1088):517–532. doi: 10.1098/rstb.1982.0058. [DOI] [PubMed] [Google Scholar]
- Fletcher M. Attachment of Pseudomonas fluorescens to glass and influence of electrolytes on bacterium-substratum separation distance. J Bacteriol. 1988 May;170(5):2027–2030. doi: 10.1128/jb.170.5.2027-2030.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M. Measurement of Glucose Utilization by Pseudomonas fluorescens That Are Free-Living and That Are Attached to Surfaces. Appl Environ Microbiol. 1986 Oct;52(4):672–676. doi: 10.1128/aem.52.4.672-676.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. S., Gerchakov S. M., Millero F. J. Effects of inorganic particles on metabolism by a periphytic marine bacterium. Appl Environ Microbiol. 1983 Feb;45(2):411–417. doi: 10.1128/aem.45.2.411-417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. W., Young L. Y. Enumeration of particle-bound and unattached respiring bacteria in the salt marsh environment. Appl Environ Microbiol. 1980 Jul;40(1):156–160. doi: 10.1128/aem.40.1.156-160.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J. H., Pirt S. J. Quantitative aspects of growth of the methane oxidizing bacterium Methylococcus capsulatus on methane in shake flask and continuous chemostat culture. J Appl Bacteriol. 1972 Dec;35(4):597–607. doi: 10.1111/j.1365-2672.1972.tb03741.x. [DOI] [PubMed] [Google Scholar]
- Hendricks C. W. Sorption of heterotrophic and enteric bacteria to glass surfaces in the continuous culture of river water. Appl Microbiol. 1974 Oct;28(4):572–578. doi: 10.1128/am.28.4.572-578.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herson D. S., McGonigle B., Payer M. A., Baker K. H. Attachment as a factor in the protection of Enterobacter cloacae from chlorination. Appl Environ Microbiol. 1987 May;53(5):1178–1180. doi: 10.1128/aem.53.5.1178-1180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
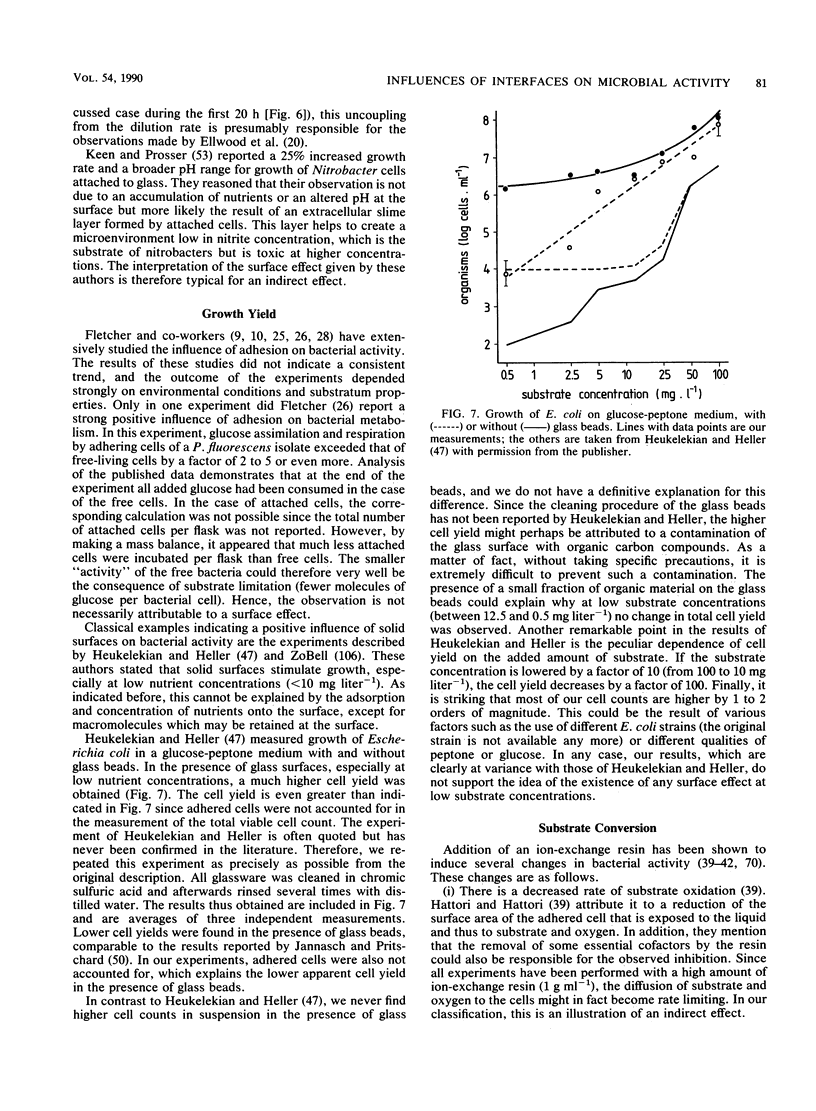
- Heukelekian H., Heller A. Relation between Food Concentration and Surface for Bacterial Growth. J Bacteriol. 1940 Oct;40(4):547–558. doi: 10.1128/jb.40.4.547-558.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey B. A., Marshall K. C. The triggering effect of surfaces and surfactants on heat output, oxygen consumption and size reduction of a starving marine Vibrio. Arch Microbiol. 1984 Dec;140(2-3):166–170. doi: 10.1007/BF00454920. [DOI] [PubMed] [Google Scholar]
- Jeffrey W. H., Paul J. H. Activity measurements of planktonic microbial and microfouling communities in a eutrophic estuary. Appl Environ Microbiol. 1986 Jan;51(1):157–162. doi: 10.1128/aem.51.1.157-162.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Mitchell R. Contribution of particle-bound bacteria to total microheterotrophic activity in five ponds and two marshes. Appl Environ Microbiol. 1982 Jan;43(1):200–209. doi: 10.1128/aem.43.1.200-209.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARSEN D. H., DIMMICK R. L. ATTACHMENT AND GROWTH OF BACTERIA ON SURFACES OF CONTINUOUS-CULTURE VESSELS. J Bacteriol. 1964 Nov;88:1380–1387. doi: 10.1128/jb.88.5.1380-1387.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeChevallier M. W., Hassenauer T. S., Camper A. K., McFeters G. A. Disinfection of bacteria attached to granular activated carbon. Appl Environ Microbiol. 1984 Nov;48(5):918–923. doi: 10.1128/aem.48.5.918-923.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz M. G., Aardema B. W., Wackernagel W. Highly efficient genetic transformation of Bacillus subtilis attached to sand grains. J Gen Microbiol. 1988 Jan;134(1):107–112. doi: 10.1099/00221287-134-1-107. [DOI] [PubMed] [Google Scholar]
- Lorenz M. G., Wackernagel W. Adsorption of DNA to sand and variable degradation rates of adsorbed DNA. Appl Environ Microbiol. 1987 Dec;53(12):2948–2952. doi: 10.1128/aem.53.12.2948-2952.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCalla T. M. Physico-Chemical Behavior of Soil Bacteria in Relation to the Soil Colloid. J Bacteriol. 1940 Jul;40(1):33–43. doi: 10.1128/jb.40.1.33-43.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngian K. F., Lin S. H., Martin W. R. Effect of mass transfer resistance on the Lineweaver-Burk plots for flocculating microorganisms. Biotechnol Bioeng. 1977 Dec;19(12):1773–1784. doi: 10.1002/bit.260191204. [DOI] [PubMed] [Google Scholar]
- Novitsky J. A. Degradation of dead microbial biomass in a marine sediment. Appl Environ Microbiol. 1986 Sep;52(3):504–509. doi: 10.1128/aem.52.3.504-509.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogram A. V., Jessup R. E., Ou L. T., Rao P. S. Effects of sorption on biological degradation rates of (2,4-dichlorophenoxy) acetic acid in soils. Appl Environ Microbiol. 1985 Mar;49(3):582–587. doi: 10.1128/aem.49.3.582-587.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikov N. S., Aseeva I. V., Chistiakova I. K. Kinetika nepreryvnogo rosta drozhzhevoi kul'tury Debaryomyces formicarius v khemostate i protochnykh kolonkakh s tverdoi fazoi. Mikrobiologiia. 1980 Sep-Oct;49(5):794–803. [PubMed] [Google Scholar]
- SUTHERLAND I. W., WILKINSON J. F. A new growth medium for virulent Bordetella pertussis. J Pathol Bacteriol. 1961 Oct;82:431–438. doi: 10.1002/path.1700820220. [DOI] [PubMed] [Google Scholar]
- Sims R. C., Little L. W. Enhanced nitrification by addition of clinoptilolite to tertiary activated sludge units. Environ Lett. 1973;4(1):27–34. doi: 10.1080/00139307309435480. [DOI] [PubMed] [Google Scholar]
- Stotzky G. Activity, ecology, and population dynamics of microorganisms in soil. CRC Crit Rev Microbiol. 1972 Nov;2(1):59–137. doi: 10.3109/10408417209108383. [DOI] [PubMed] [Google Scholar]
- Stotzky G. Influence of clay minerals on microorganisms. 3. Effect of particle size, cation exchange capacity, and surface area on bacteria. Can J Microbiol. 1966 Dec;12(6):1235–1246. doi: 10.1139/m66-165. [DOI] [PubMed] [Google Scholar]
- Stotzky G. Influence of clay minerals on microorganisms. II. Effect of various clay species, homoionic clays, and other particles on bacteria. Can J Microbiol. 1966 Aug;12(4):831–848. doi: 10.1139/m66-111. [DOI] [PubMed] [Google Scholar]
- Stotzky G., Rem L. T. Influence of clay minerals on microorganisms. I. Montmorillonite and kaolinite on bacteria. Can J Microbiol. 1966 Jun;12(3):547–563. doi: 10.1139/m66-078. [DOI] [PubMed] [Google Scholar]
- Stucki G., Alexander M. Role of dissolution rate and solubility in biodegradation of aromatic compounds. Appl Environ Microbiol. 1987 Feb;53(2):292–297. doi: 10.1128/aem.53.2.292-297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba-Rao R. V., Alexander M. Effect of sorption on mineralization of low concentrations of aromatic compounds in lake water samples. Appl Environ Microbiol. 1982 Sep;44(3):659–668. doi: 10.1128/aem.44.3.659-668.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. M., Yordy J. R., Amador J. A., Alexander M. Rates of dissolution and biodegradation of water-insoluble organic compounds. Appl Environ Microbiol. 1986 Aug;52(2):290–296. doi: 10.1128/aem.52.2.290-296.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobell C. E. The Effect of Solid Surfaces upon Bacterial Activity. J Bacteriol. 1943 Jul;46(1):39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Schraa G., Zehnder A. J. Electrophoretic mobility and hydrophobicity as a measured to predict the initial steps of bacterial adhesion. Appl Environ Microbiol. 1987 Aug;53(8):1898–1901. doi: 10.1128/aem.53.8.1898-1901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


