Abstract
Protein degradation is a key variable in controlling protein abundance in cells. Here, we compare classical methods to measure protein degradation rates with a novel GFP reporter library based method that characterizes degradation of thousands of individual proteins by flow cytometry. While no method is perfect, we conclude that chimeric gene reporter approaches should be applied cautiously due principally to GFP (or other tag) interference with organelle targeting or incorporation of chimeric proteins into macromolecular assemblies that results in spuriously high degradation rates.
Metabolic stability greatly influences the abundance of proteins in cells. Historically, protein degradation has been studied using methods that measure overall protein degradation or focus on a few individual proteins. With recent technical advances, it is possible to measure degradation rates of large numbers of defined proteins, with the ultimate goal of proteome-wide determination of protein stabilities under various conditions.
In reporter-dependent methods, open reading frames (ORFs) are expressed individually as fusion proteins with fluorescent protein or epitope tags, and their stabilities assayed based on tag detection. In reporter-independent methods, the fate of nascent proteins is followed by tagging them with isotopically labeled amino acids, chasing with unlabelled amino acids, and measuring the loss of the labeled cohort.
Yen et al. 1 describe a novel version of the reporter-dependent approach which they term global protein stability profiling (GPSP). After transducing cells with a biscistronic retroviral vector encoding red fluorescent protein (RFP) and a cDNA of the gene of interest fused to GFP, they determine the RFP/GFP ratio for each library member by flow cytometry, and convert values to a half-life using a panel of GFP variants with biochemically defined half-lives.
Doherty et al. 2 update the classical method of radioisotope amino acid pulse-cold amino acid chase studies by employing dynamic SILAC (stable isotope labeling with amino acids in cell culture), using [13C6] Arg labeling to measure the degradation of 576 proteins by quantitative mass spectrometry on proteins identified in 1D-SDS-PAGE gel slices..
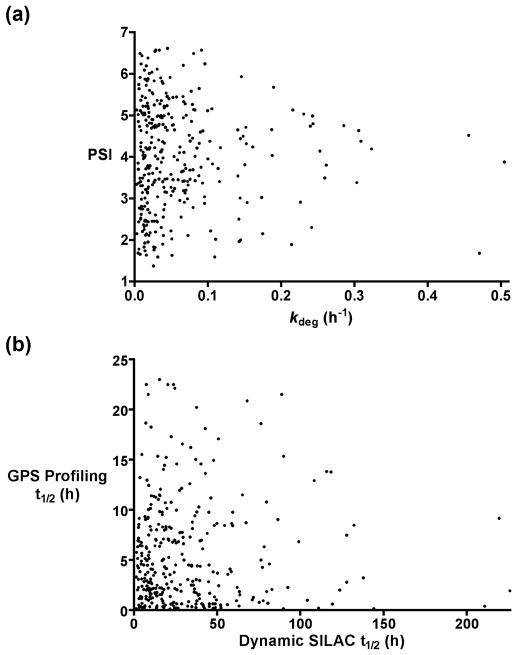
To evaluate the validity of these methods, we examined the extent to which their findings correlate. We identified the 339 gene products shared between SILAC and GPSP data sets. The output of the SILAC study kdeg, fraction of degradation min−1, whereas GPSP provides a protein stability index (PSI) from 1–7 (less stable to more stable). If both approaches accurately measure protein stability, there should be a clear negative correlation between kdeg and PSI.
Surprisingly, a scatter plot comparison of these two parameters demonstrates no significant correlation (Figure 1a, in Figure 1b convert the kdeg and PSI values to half-lives). What could account for such a dramatic difference?
Figure 1.
Comparisons of protein stability constants determined by dynamic SILAC versus global protein stability (GPSP) profiling. Correlation analysis of protein stability values determined by SILAC and global protein stability (GPSP) profiling (a) Proteins common to the dynamic SILAC 2 and GPSP 1 data sets were identified (339 in total) along with their associated degradation rate (kdeg) and protein stability index (PSI) value, respectively. The kdeg and corresponding PSI value for each protein is shown on a scatter plot. In this plot, data set correlation would be indicated by a scatter plot with negative slope. As is apparent, no significant correlation is seen (Spearman’s rank correlation coefficient, ρ = 0.028, p = 0.61). To clarify the graphical presentation, 14 outlier values (kdeg < 0.00307 and kdeg > 0.5042) that were included in the statistical analysis are not shown on the scatter plot. (b) Comparison of the half-life values calculated from kdeg based on 2 and from PSI using a modification of the regression analysis in 1. While one would predict a positive correlation between the two half-life measurements, again, no significant correlation is seen (Spearman’s rank correlation coefficient, ρ = −0.28, p = 0.61). The same outlier values from (a) are not shown in the scatter plot, but were included in the statistical analysis. All statistical analyses were performed with GraphPad Prism 4. It should be noted that this analysis required recalculation of the protein half-lives initially reported by Yen et al. Following their method yields a linear regression where PSI values of less than 2.96 yield negative half-life values, a biological impossibility. We instead fit the published PSI data to the half-life values of the various eGFP-ornithine decarboxylase degron fusions used as standards by these authors. The best fit is described by the equation t1/2 = 0.0243 x (PSI)3.6281, which gives an R2 of 0.9974 for the provided data.
One possibility is that the SILAC and GPSP studies were conducted using different human cell lines, A549 lung adenocarcinoma cells and 293T embryonic kidney cells, respectively. While some proteins, no doubt, are degraded in a cell type specific manner, this is unlikely to apply to most proteins, particularly, essential housekeeping proteins. Rechsteiner et al. 3 measured metabolic stabilities of six proteins injected into five different cell lines and found less than 4-fold difference in half-lives for each of the proteins. Qian et al. 4 observed similar overall protein degradation rates in HeLa, 293, and hamster E36 cells by standard [35S]-Met pulse chase radiolabeling. Furthermore, cell type-specific differences would not be expected to yield a statistically random scatter of degradation profiles.
Notably, the SILAC data agree with the many reported pulse-chase studies performed in cultured cells with radiolabeled amino acids 5, 6. By contrast, GPSP half-lives are typically ~10-fold shorter than determined by radiolabeled pulse chase studies. This suggests that one of the approaches exhibits intrinsic bias, and calls for consideration of the strengths and weaknesses associated with each method.
Amino acid isotope pulse chase experiments
The most direct approach to study protein degradation is to label nascent proteins and follow their fate using either amino acid analogs that can be identified by their chemical properties, or isotopically labeled forms of the natural amino acids that can be identified by their mass or radioactivity. Since the precise chemical properties of amino acid side chains tremendously influence protein folding, amino acid analogs will induce misfolding to some extent, limiting this approach to isotopic labeling.
Isotopic amino acid tagging makes a number of assumptions, however, that if unwarranted undermine its accuracy
The isotopic label is exclusively present in the intended amino acid and not chemical analogs that are incorporated into proteins and induce misfolding This is not a problem for SILAC, since the read out is limited to the predicted mass of the labeled amino acid. While this is a real concern for radiolabeled preparations, incorporation of a radiolabeled analog would diminish half-lives and not increase them, as would be required to explain the difference with GPSP.
Other contaminants present in the isotope label do not alter metabolic stability
For example, commercially available radiolabeled amino acids prepared from bacteria contain bacterial components that trigger cellular innate immune receptors with unpredictable effects on protein stabilities 7.
Reutilization of labeled amino acids from protein turnover increases the apparent half- lives of proteins
This is rarely a concern using cultured cells where a saturating level of unlabelled amino acid is easy to achieve during the chase when rapidly incorporated labels, commonly [35S]-Met, [35S]-Cys, [3H]-Leu, or [3H]-Tyr are employed.
The method used to identify individual protein species (typically antibody based recovery from detergent lysates for radiolabeling, mass spectrometry for SILAC) accurately quantitates all forms of the labeled protein
This is a significant problem for radiolabeling, since misfolded proteins are typically poorly solubilized with the mild cell lysis conditions needed to maintain antigenicity. Further, misfolding may prevent antibody binding, resulting either in failure to detect nascent protein (and an overestimation of protein stability) or in the mistaken conclusion that the protein has been degraded (and an underestimation of stability). This is less of a problem for SILAC, but still an issue, since misfolded/aggregated proteins may not be solubilized or may migrate aberrantly in SDS-PAGE.
Given the uncertainties associated with isotope pulse-labeling (indeed with any single technique), it is essential to corroborate its findings with other techniques used to study protein degradation.
Post-synthetic Radiolabeling
Proteins can also be labeled for by post-translational modification of side chains with radioisotopes (e.g. Tyr/Lys radioiodination) or other tags (e.g. Lys-biotinylation). Modified amino acids are typically not recognized by tRNA aminoacyl synthetases and are therefore not reincorporated into proteins, eliminating the confounding effects of reutilization. An important limitation is that labeling can damage the protein, leading to underestimation of protein stability.
Plasma membrane proteins
Plasma membrane proteins offer unique post-synthetic labeling targets since they can be selectively labeled extracellularly. Chu and Doyle 8 radioiodinated rat hepatoma plasma membrane proteins and found that eleven prominently labeled proteins exhibited half-lives ranging from 16 to 100 h, highly similar to values obtained in the same study by traditional 35S-Met pulse-chase labeling. Similarly, Hare and Taylor 9 used biotinylation to selectively label cell surface proteins of 3T3 fibroblasts and rat hepatoma cells, reporting that the vast majority of proteins exhibited half-lives greater than 75 hours.
Cytosolically delivered proteins
Rechsteiner and colleagues developed a method for cytosolically introducing radioiodinated proteins based on target cells fusion with protein-loaded erythrocyte ghosts 10–12 and found good agreement with pulse chase labeling methods for dozens of proteins measured 13, 14. Although the injection approach provides useful information, it is best suited for abundant proteins with half lives of at least hours..
The reasonable agreement between metabolic stabilities measured by isotopic pulse labeling vs. post-synthetic labeling or microinjection cross validates these methods for measuring protein half-lives..
“Cycloheximide-Chase” Immunoblotting
Given an immunoblotting mAb or antiserum, it is relatively easy to accurately quantitate antigen decay following addition and continued incubation (“chase”) with cycloheximide, a rapidly acting eukaryotic protein synthesis inhibitor (with the caveat that the data should be related to a standard curve to account for non-linearity in the immunoblot signal). A significant advantage of this method is its insensitivity to highly denaturing extraction buffers that allow for maximal recovery of proteins from cells, often including misfolded, and even aggregated forms.
The method is ill suited, however, for long-lived proteins due to the effects of prolonged protein synthesis inhibition on overall cell function. There are also possible rapid effects on degradation pathways themselves. For example, increased levels of free amino acids, aminoacyl-tRNAs or nucleotides could affect proteolytic systems or the conformations of specific substrates. If key components of the degradation pathway are themselves short-lived, their substrates may actually be stabilized by cycloheximide 14.
Nearly all of the myriad cycloheximide-chase studies focus on one or a few chosen proteins. Belle et al. 15, however, combined this approach with a reporter-dependent methodology to measure the half-lives of more than 3700 yeast gene ORFs encoding a tandem affinity purification (TAP) tag, a protein consisting of 184 amino acids. The mean and median half-lives of 3,751 yeast proteins were 43 min with a range from less than 4 min to greater than 400 min. Significantly, a comparison of the stabilities of tagged and untagged versions for 24 proteins revealed that two-thirds of the tagged proteins were degraded more rapidly than their wild-type forms, demonstrating that TAP-tagging, like GPSP, displays an intrinsic bias to decreasing protein stability.
GFP interferes with Protein Function
Based on their broad agreement with other approaches, it is clear that isotope based amino acid pulse chase studies, while imperfect, provide a reasonably accurate measure of protein turnover, and if anything, generally underestimate protein stability. How then, to explain the consistent overestimation of protein turnover that appears characteristic of reporter-dependent methods, such as GPSP?
Examining the most divergent proteins between SILAC and GPSP studies (Table 1) provides insight into this question. Ribosomal proteins, which represent 10% of the proteins shared between the studies, account for 35% of the top decile of most divergent proteins, with GPSP reporting much shorter half lives than dynamic SILAC and other methods 16, 17., The stability of ribosomal subunits is dependent on assembly into functional ribosomes 16,18. The likely explanation, then, is that GPSP underestimates protein stability by interfering with proteins assembly into macromolecular structures either structurally or stoichiometrically (i.e. by over expression, which is well known to result in the degradation of free subunits, whose stability depends on their integration). Consistent with this interpretation, many proteins in the subset of most divergent proteins between the data sets are components of larger macromolecular assemblies. Many of the other proteins in the top decile exhibit organelle-specific targeting for proper localization and function (into mitochondria, the endoplasmic reticulum, and the nucleus), consistent with mistargeting of the GFP-fusion proteins.
Table 1.
Examples from the top 10% of “worst offenders” showing divergent stability between the dynamic SILAC and GPS profiling data sets.
| Proteins | SILAC stability percentilea | GPS stability percentilea | Notes | Entrez Gene IDs |
|---|---|---|---|---|
| L14, L15, L23, L23A, L27, L28, L35, S10, S13, S14, S15A, S16 | 88th (75th-99th) | 11th (2nd-21st) | Ribosomal proteins | 9045, 6138, 9349, 6147, 6155, 6158, 11224, 6204, 6207, 6208, 6210, 6217 |
| F0 complex subunit G and F1 complex subunit O | 98th and 99th | 7th and 18th | Components of ATP synthase | 10632, 539 |
| Cytochrome c oxidase subunit Va | 88th | 17th | Mitochondrial inner membrane protein | 9377 |
| GRP94 | 97th | 9th | ER chaperone | 7184 |
| RRC1 | 7th | 91st | Ran Guanine exchange factor | 1104 |
Stability is expressed as a percentile of the rank ordered stability of the 339 proteins shared between the SILAC and GPS data sets. High percentiles correspond to stable proteins, while low percentiles correspond to unstable proteins. For the ribosomal proteins, the mean percentile of the group is shown with the percentile range in parentheses.
GFP-fusion, while a simple and powerful technique is fraught with artifacts as expounded by Snapp 19. To wit:
GFP tagging impairs protein biogenesis
Table 1 gives reason to suspect that GFP reporter fusion sterically blocks incorporation of ribosomal, ATP synthase, and cytochrome oxidase subunits into their respective multimeric complexes. The eGFP tag could also prevent chaperones from binding and promoting proper folding the ORF-encoded protein, leading to artifactual destabilization. Conversely, high local concentrations of eGFP, such as in oligomers, can cause the eGFP tag to dimerize and potentially stabilize otherwise labile complexes 19.
GFP tagging blocks subcellular targeting signals
The discrepancies highlighted in Table 1 strongly suggest GFP-interference with intracellular trafficking. The N- and C-termini of proteins contain the majority of sub-cellular localization signals. Methods based on cassette-termini tagging will likely alter the localization and thereby stability of the chimeric product. Snapp 19 highlights the magnitude of this problem: 7500 of 30,000 annotated proteins are targeted to the ER or mitochondria, and would be expected to misfold if not properly exported from the cytosol. Yen et al. noted that membrane proteins were notably less stable in their GPSP data set. They also reported that C-terminally tagged versions of the proteins were equally unstable, and use randomly selected ORFs mAb epitope tagged at the C-terminus to validate their GPSP data. The concern remains that tagging either terminus similarly increases degradation rates.
GFP expression intrinsically interferes with polyubiquitylation and polyubiquitin-dependent signals
Baens et al. 20 reported that eGFP expressed either alone or as fusion proteins inhibits polyubiquitylation and modifies multiple cell signaling pathways. The authors cite other examples where eGFP disrupts cellular physiology by unknown mechanisms. Obviously, this will exert unpredictable effects on the stability of individual gene products.
A significant fraction of GFP is rapidly cleaved
In extensively using eGFP either alone or in multiple gene fusion contexts and expression scenarios, JWY’s laboratory has found that that approximately 25% of GFP is rapidly cleaved (probably autocatalytically 21) resulting in degradation of the fusion protein (e.g. Qian et al. 4). If this process is affected by the nature of the fusion partner, it will variably affect the RFP/GFP ratio, and result in spurious degradation rates. Moreover, the cleaved fusion protein has a high chance of misfolding and acting in a dominant negative manner, interfering with cell function and accelerating degradation of folded versions of the fusion protein.
Differential DRiP Fraction Lead to Spurious Values
Numerous studies point to the conclusion that a significant fraction of nascent proteins do not achieve their stable functional conformation, and are rapidly degraded 4, 22–26. Such defective ribosomal products (DRiPs) provide the majority of antigenic peptides for the MHC class I immunosurveillance system 27, 28. While little is known about differences in the DRiP fraction among gene products, this could vary significantly. GPSP substrates with a high intrinsic DRiP fraction, or a DRiP fraction increased by GFP fusion will score with aberrantly rapid degradation rates since the reference protein is only affected by its own intrinsic DRiP fraction, which should be constant. (It should be possible to study this effect by measuring the decay in the GFP signal via flow cytometry following addition of cycloheximide to cells. Gene products with a higher DRiP fraction will demonstrate less GFP decay than predicted by their steady state levels.)
Based on these considerations, we suggest that reporter-dependent methods for assessing intrinsic protein stability, such as GPSP, are ill suited for proteome-wide assessments of protein stability. At the same time, this approach is clearly applicable for exploring cell type differences in protein stability and for absolute measurements of proteins whose stabilities match those measured by alternative techniques. Further, Yen & Elledge 29 demonstrate the value of GPSP for high throughput screening to identify ubiquitin ligase substrates, pointing the way to applications in which relative stabilities yield important insights and information.
In comparison, SILAC is more laborious and requires expertise in a highly demanding technology based on expensive instrumentation. It does, however, provide an accurate measure of stability of hundreds to low thousands of proteins and is the method of choice for measuring absolute protein degradation rates in different cells and under different conditions.
Pressing Questions in Proteolysis
Renewed appreciation for the importance of proteolysis has accompanied the explosion in research on the ubiquitin-proteasome pathway in the past decade. In addition to gene product specific issues, a number of basic questions remain to be addressed.
What is the basis for the typical first order degradation kinetics of proteins?
Classic studies in the 60’s correlated protein metabolic stability in cells with their in vitro resistance to endoprotease digestion. This is consistent with the idea that stability is inversely proportional to protein dynamics. This correlation was observed using a limited set of proteins chosen for ease of purification, and it is important to update and extend these findings to more proteins and additional parameters of protein stability. Which of the myriad ubiquitin ligases are involved in normal protein turnover and how they select their substrates? The contribution of protein “aging”, i.e. post-translational damage (e.g. oxidative damage) to stochastic recognition based on spontaneous unfolding remains a central question.
Why are 20% or more of translation products degraded so rapidly 4, 22–25
Is this due to translation of many defective polypeptides?. What are the contributions of pioneer translation in nonsense mediated decay and translation of short mRNAs generated transcriptionally 30 or via microRNA mediated cleavage 31? What exactly are cells translating (i.e. defining the translatome)? What are the inefficiencies in folding, assembling, or modifying otherwise normal proteins? Why are nascent proteins destined to become native proteins more sensitive to chemical or physical denaturation for the first hour post-synthesis 32? Do proteins need to pass a final quality control step before being integrated into the cell, as originally suggested by Wheatley 33?
To what extent is translation specialized based on the requirements of individual gene products?
Ribosomes are known to be highly heterogeneous 34. Mauro and Edelman’s ribosome filter hypothesis, posits that sequence specific targeting of mRNAs to ribosome subsets contributes to controlling gene expression 35. This is supported by the report of Komili et al.36, that duplicated ribosomal genes function to specialize ribosomes for translation of specific mRNAs. Could ribosome specialization also entail recruitment of chaperones and protein modification machinery tailored for classes of gene products? Does this contribute to the failure of genome wide gene fusion methods to recapitulate physiological biogenesis of the gene products of interest?
How do findings with cultured cells relate to cells in their natural state in organisms?
The original pulse chase radiolabeling studies of protein stability were largely performed in living animals 13, 14, 37. Use of cultured cells offered obvious advantages in labor, expense, and experimental manipulation. Mammalian cells, however, did not evolve to grow in culture, and it is essential in future studies to develop methods for quantitatively studying protein degradation (and biogenesis) in vivo.
References
- 1.Yen HC, et al. Global protein stability profiling in mammalian cells. Science (New York, N Y. 2008;322:918–923. doi: 10.1126/science.1160489. [DOI] [PubMed] [Google Scholar]
- 2.Doherty MK, et al. Turnover of the human proteome: determination of protein intracellular stability by dynamic SILAC. J Proteome Res. 2009;8:104–112. doi: 10.1021/pr800641v. [DOI] [PubMed] [Google Scholar]
- 3.Rechsteiner M, et al. Use of microinjection techniques to study intracellular proteolysis. In: Glaumann H, Ballard FJ, editors. Lysosomes. Academic Press; 1987. pp. 487–515. [Google Scholar]
- 4.Qian SB, et al. Characterization of rapidly degraded polypeptides in mammalian cells reveals a novel layer of nascent protein quality control. J Biol Chem. 2006;281:392–400. doi: 10.1074/jbc.M509126200. [DOI] [PubMed] [Google Scholar]
- 5.Poole B, Wibo M. Protein degradation in cultured cells. The effect of fresh medium, fluoride, and iodoacetate on the digestion of cellular protein of rat fibroblasts 40. Journal of Biological Chemistry. 1973;248:6221–6226. [PubMed] [Google Scholar]
- 6.Rock KL, et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 7.Lelouard H, et al. Transient aggregation of ubiquitinated proteins during dendritic cell maturation 3. Nature. 2002;417:177–182. doi: 10.1038/417177a. [DOI] [PubMed] [Google Scholar]
- 8.Chu FF, Doyle D. Turnover of plasma membrane proteins in rat hepatoma cells and primary cultures of rat hepatocytes. J Biol Chem. 1985;260:3097–3107. [PubMed] [Google Scholar]
- 9.Hare JF, Taylor K. Mechanisms of plasma membrane protein degradation: recycling proteins are degraded more rapidly than those confined to the cell surface. Proc Natl Acad Sci U S A. 1991;88:5902–5906. doi: 10.1073/pnas.88.13.5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlegel RA, Rechsteiner MC. Red cell-mediated microinjection of macromolecules into mammalian cells. Methods Cell Biol. 1978;20:341–354. doi: 10.1016/s0091-679x(08)62026-9. [DOI] [PubMed] [Google Scholar]
- 11.Rogers SW, Rechsteiner M. Degradation of structurally characterized proteins injected into HeLa cells. Tests of hypotheses. J Biol Chem. 1988;263:19850–19862. [PubMed] [Google Scholar]
- 12.Neff NT, et al. Degradation of proteins microinjected into IMR-90 human diploid fibroblasts. J Cell Biol. 1981;91:184–194. doi: 10.1083/jcb.91.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg AL, Dice JF. Intracellular protein degradation in mammalian and bacterial cells 4. Annu Rev Biochem. 1974;43:835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg AL, St John AC. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- 15.Belle A, et al. Quantification of protein half-lives in the budding yeast proteome. Proc Natl Acad Sci U S A. 2006;103:13004–13009. doi: 10.1073/pnas.0605420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warner JR. In the absence of ribosomal RNA synthesis, the ribosomal proteins of HeLa Cells are synthesized normally and degraded rapidly. Journal of Molecular Biology. 1977;115:315–333. doi: 10.1016/0022-2836(77)90157-7. [DOI] [PubMed] [Google Scholar]
- 17.Lastick SM, McConkey EH. Exchange and stability of HeLa ribosomal proteins in vivo. J Biol Chem. 1976;251:2867–2875. [PubMed] [Google Scholar]
- 18.Granneman S, Tollervey D. Building ribosomes: even more expensive than expected? Curr Biol. 2007;17:R415–417. doi: 10.1016/j.cub.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Snapp EL. Fluorescent proteins: a cell biologist’s user guide. Trends in Cell Biology. 2009;19:649–655. doi: 10.1016/j.tcb.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baens M, et al. The dark side of EGFP: defective polyubiquitination. PLoS One. 2006;1:e54. doi: 10.1371/journal.pone.0000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barondeau DP, et al. Understanding GFP posttranslational chemistry: structures of designed variants that achieve backbone fragmentation, hydrolysis, and decarboxylation. J Am Chem Soc. 2006;128:4685–4693. doi: 10.1021/ja056635l. [DOI] [PubMed] [Google Scholar]
- 22.Wheatley DN, et al. Kinetics of degradation of “short-” and “long-lived” proteins in cultured mammalian cells. Cell Biology International Reports. 1980;4:1081–1090. doi: 10.1016/0309-1651(80)90045-4. [DOI] [PubMed] [Google Scholar]
- 23.Schubert U, et al. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 24.Vabulas RM, Hartl FU. Protein synthesis upon acute nutrient restriction relies on proteasome function. Science (New York, N Y. 2005;310:1960–1963. doi: 10.1126/science.1121925. [DOI] [PubMed] [Google Scholar]
- 25.Turner GC, Varshavsky A. Detecting and measuring cotranslational protein degradation in vivo. Science (New York, N Y. 2000;289:2117–2120. doi: 10.1126/science.289.5487.2117. [DOI] [PubMed] [Google Scholar]
- 26.Fuertes G, et al. Role of proteasomes in the degradation of short-lived proteins in human fibroblasts under various growth conditions. The International Journal of Biochemistry & Cell Biology. 2003;35:651–664. doi: 10.1016/s1357-2725(02)00382-5. [DOI] [PubMed] [Google Scholar]
- 27.Yewdell JW, et al. Making sense of mass destruction: quantitating MHC class I antigen presentation. Nat Rev Immunol. 2003;3:952–961. doi: 10.1038/nri1250. [DOI] [PubMed] [Google Scholar]
- 28.Yewdell JW, Nicchitta CV. The DRiP hypothesis decennial: support, controversy, refinement and extension. Trends in Immunology. 2006;27:368–373. doi: 10.1016/j.it.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Yen HC, Elledge SJ. Identification of SCF ubiquitin ligase substrates by global protein stability profiling. Science (New York, N Y. 2008;322:923–929. doi: 10.1126/science.1160462. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y, Ganem D. Making sense of antisense: seemingly noncoding RNAs antisense to the master regulator of KSHV lytic replication do not regulate that transcript but serve as mRNAs encoding small peptides. J Virol. doi: 10.1128/JVI.02705-09. JVI.02705–02709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu W, et al. Both treated and untreated tumors are eliminated by short hairpin RNA-based induction of target-specific immune responses. Proceedings of the National Academy of Sciences. 2009;106:8314–8319. doi: 10.1073/pnas.0812085106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medicherla B, Goldberg AL. Heat shock and oxygen radicals stimulate ubiquitin-dependent degradation mainly of newly synthesized proteins. J Cell Biol. 2008;182:663–673. doi: 10.1083/jcb.200803022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheatley DN, et al. Significance of the rapid degradation of newly synthesized proteins in mammalian cells: a working hypothesis. J Theor Biol. 1982;98:283–300. doi: 10.1016/0022-5193(82)90265-x. [DOI] [PubMed] [Google Scholar]
- 34.Dinman JD. The eukaryotic ribosome: current status and challenges. J Biol Chem. 2009;284:11761–11765. doi: 10.1074/jbc.R800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauro VP, Edelman GM. The ribosome filter hypothesis. Proc Natl Acad Sci U S A. 2002;99:12031–12036. doi: 10.1073/pnas.192442499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komili S, et al. Functional specificity among ribosomal proteins regulates gene expression. Cell. 2007;131:557–571. doi: 10.1016/j.cell.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schimke RT, Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]