Abstract
Purpose
The management of patients with International Neuroblastoma Staging System (INSS) stage 3 neuroblastoma (NB) is not consistent worldwide. We describe a single centre approach at Memorial Sloan-Kettering Cancer Centre (MSKCC) from 1991 to 2007 that minimizes therapy except for those patients with MYCN-amplified NB.
Methods
In this retrospective analysis of 69 patients, tumour MYCN was not amplified in 53 and amplified in 16. Event-free survival (EFS) and overall survival (OS) were determined by Kaplan–Meier analysis.
Results
Fourteen patients with non-MYCN-amplified tumours were treated with surgery alone (group A) and the remaining 39 (group B) with surgery following chemotherapy that was initiated and administered at non-MSKCC institutions. Chemotherapy was discontinued after surgery in 38/39 of the latter. The 10-year EFS and OS for all patients with MYCN-non-amplified NB were 74.9 ± 16.9% and 92.6 ± 5.5%, respectively. There was no difference in OS between groups A and B (p = 0.2; 10-year OS for groups A and B was 84.6 ± 14% and 97.1 ± 2.9%, respectively). Patients with MYCN-amplified disease (group C) underwent dose-intensive induction, tumour resection and local radiotherapy: 13 achieved complete or very good partial remission, and 10 received myeloablative chemotherapy. 11/16 patients also received 3F8-based immunotherapy: 10 remain free of disease. The 10-year EFS and OS for patients with MYCN-amplified neuroblastoma treated with immunotherapy were both 90.9 ± 8.7%.
Conclusion
Patients with MYCN-non-amplified stage 3 NB can be successfully treated with surgery without the need for radiotherapy or continuation of chemotherapy. Combination of dose-intensive chemotherapy, surgery, radiotherapy and immunotherapy was associated with a favourable outcome for most patients with MYCN-amplified stage 3 NB.
Keywords: Stage 3 neuroblastoma, Immunotherapy, Prognosis
1. Introduction
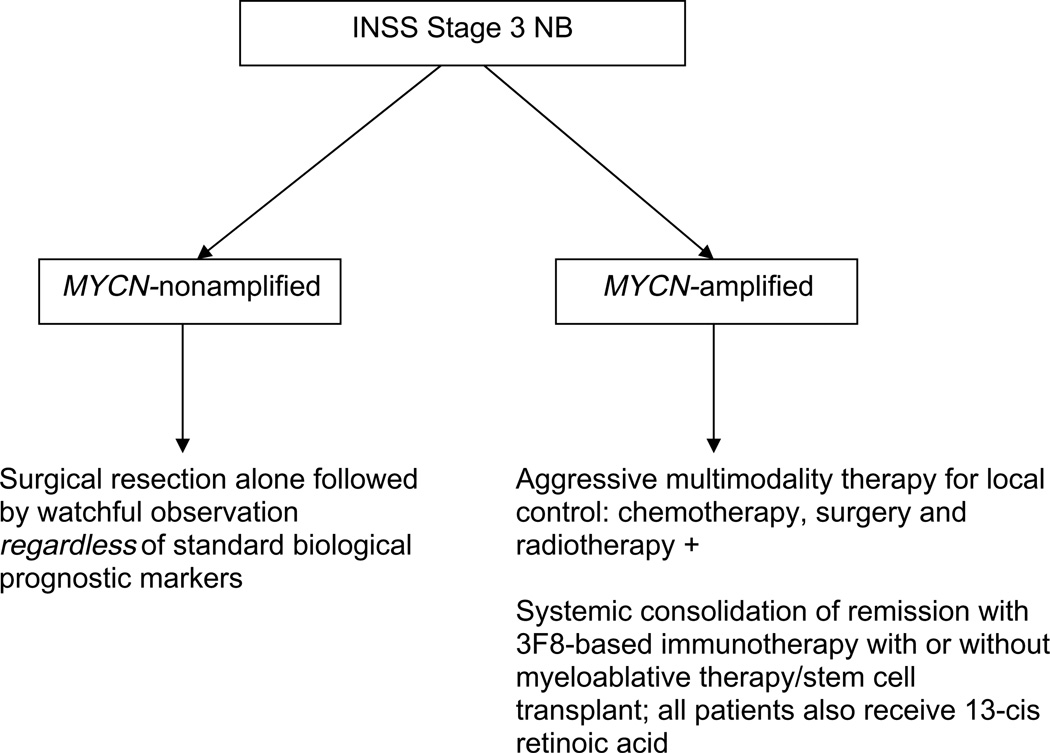
The International Neuroblastoma Staging System (INSS),1 established in 1993 replacing the Evans and Paediatric Oncology Group (POG) systems, defined stage 3 neuroblastoma (NB) as an unresectable tumour that extends across the midline either itself, or with associated involved lymph nodes. Approximately 15% of all NB patients have stage 3 disease at diagnosis.2 Clinical investigators include stage 3 patients in reports on locoregional NB and on high-risk, predominantly metastatic disease, and outcomes related specifically to patients with stage 3 disease are not always specifically presented.3–7 Biological and clinical prognostic markers help stratify risk and guide therapy.3,8 MYCN-amplified stage 3 NB, which accounts for ~25% of cases, is considered high-risk and is treated with aggressive multimodality programmes which use dose-intensive or dose-dense chemotherapy, surgery, radiotherapy (RT), myeloablative chemotherapy with autologous stem cell transplant (SCT) and 13-cis-retinoic acid. That aggressive strategy is also widely used, including by the Children’s Oncology Group (COG), for MYCN-non-amplified stage 3 NB with the adverse prognostic markers of unfavourable histology and age >18 months. In contrast, moderatedose chemotherapy is standard of care for stage 3 NB with favourable biology. At Memorial Sloan-Kettering Cancer Centre (MSKCC), 3F8-based immunotherapy (ClinicalTrials.gov NCT00002560 or NCT00072358) is routinely used in addition to aggressive multimodality therapy for MYCN-amplified stage 3 NB. However, for MYCN-non-amplified stage 3 NB, regardless of other standard prognostic factors, our strategy is surgical resection followed by observation without exposing patients to any cytotoxic therapy (Fig. 1).9,10 We now report an analysis of the long-term outcome of these approaches for all INSS stage 3 NB patients treated at MSKCC.
Fig. 1.
Memorial Sloan-Kettering Cancer Centre (MSKCC) algorithm for management of patients with International Neuroblastoma (NB) Staging System (INSS) stage 3 NB.
2. Patients and methods
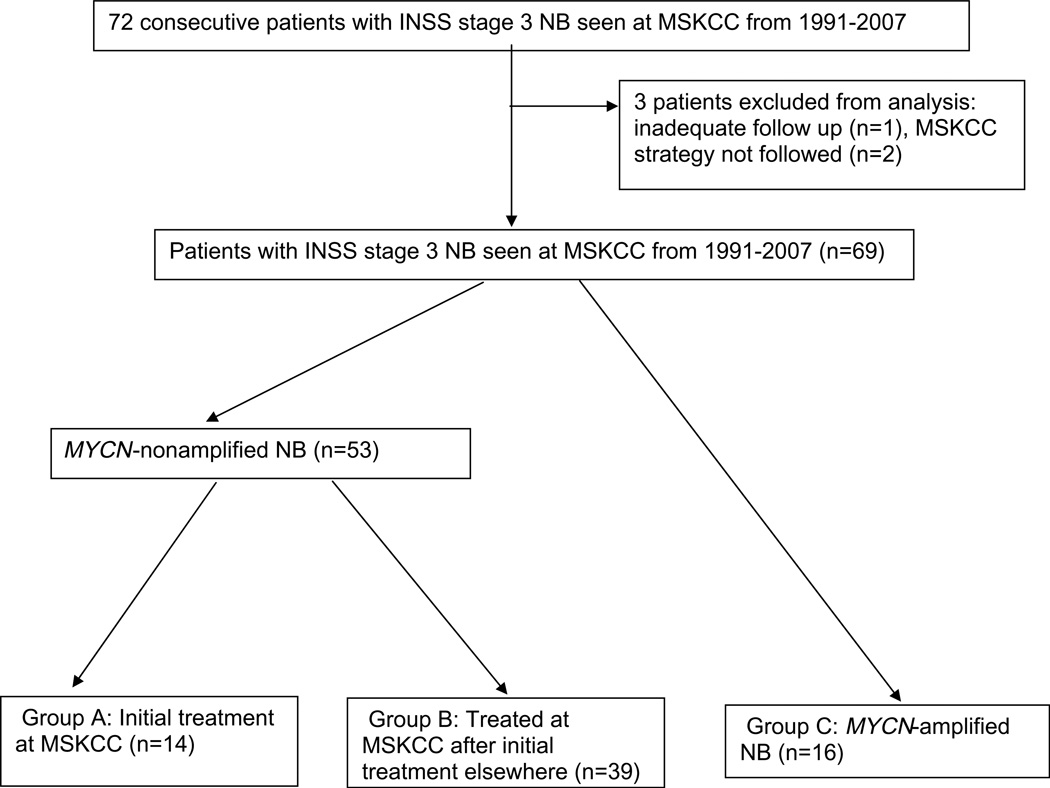
The subjects of this retrospective report are 69 consecutive patients with INSS stage 3 NB treated at MSKCC between 1991 and 2007; patients who came to MSKCC after relapse were not included in this analysis. Although 72 patients with stage 3 NB were seen at MSKCC from 1991 to 2007, three were excluded from the analysis: one because of lack of follow-up information and two because MSKCC strategy was not followed after tumour resection (Fig. 2). Institutional Review Board approval was obtained for review of patient records. Disease status was assessed by computed tomography or magnetic resonance imaging, meta-iodobenzylguanidine (MIBG) scan, urine catecholamines and bilateral bone marrow (BM) biopsies and aspirates. In accordance with hospital rules, informed written consents for treatments were obtained from guardians after they understood the side-effects of each agent and the possibility of unforeseen toxicities. The International NB Response Criteria1 were used: complete response (CR), no evidence of NB; very good partial response (VGPR), volume of primary mass reduced by >90%, no evidence of distant NB (including normal MIBG) except for skeletal residua, catecholamines normal; partial response (PR), >50% decrease in measurable disease and 61 positive BM site; mixed response, >50% decrease of any lesion with <50% decrease in any other; no response, <50% decrease but <25% increase in any lesion and progressive disease (PD), new lesion or >25% increase in an existing lesion. Biological parameters evaluated included serum ferritin, serum lactate dehydrogenase, histology assessed by Shimada classification, DNA index and MYCN gene amplification. The latter was measured by fluorescent in situ hybridization and/or Southern blot analysis. Tumours were considered to be MYCN-amplified if they had ≥ 5 times the copy number (i.e. ≥ 10 copies) of the MYCN gene per cell. Radiology reports were reviewed for image-defined surgical risk factors currently used by the International NB Research Group (INRG) to classify localised NB.11,12 Tumours were accordingly classified as L1 or L2.
Fig. 2.
Distribution of patients with stage 3 NB.
2.1. Treatment groups
Regardless of other standard biological markers, intended treatment for the 53 patients with MYCN-non-amplified NB consisted of surgery to resect the tumour and involved regional lymph nodes followed by close observation without any further therapy (Table 1). Gross total resection was attempted, though not at the expense of mutilation or sacrifice of vital organs. We adopted this approach regardless of prior chemotherapy or RT. For the purposes of analysis, the 53 patients with MYCN-non-amplified NB were divided into two groups (Table 1): 14 treated with surgery alone without prior chemotherapy or RT (group A); and 39 who had received chemotherapy ± RT elsewhere before referral to MSKCC (group B). The 16 patients with MYCN-amplified tumours (group C; Table 2) were considered to have high-risk NB. Treatment consisted of dose-intensive chemotherapy and surgery followed by local RT and consolidation of CR/VGPR with myeloablative chemotherapy + SCT, 3F8-based anti-GD2 immunotherapy (ClinicalTrials.gov NCT00002560 or NCT00072358) or both.13,14 All CR/VGPR patients in group C also received 13-cis-retinoic acid.
Table 1.
Characterisitcs and outcome of patients with MUYCN-non-amplified NB
| Group A: initial treatment at MSKCC (n = 14) |
Group B: treated at MSKCC after initial treatment elsewhere (n = 39) |
|
|---|---|---|
| M:F ratio | 9:5 | 17:22 |
| Age:median (range) in years | 1.3 (0.1–5.1) | 1.6 (0.1–8.8) |
| Number of patients >24 months | 5 | 16 |
| Number of patients >12 months | 10 | 29 |
| Number of patients <6 months | 1 | 3 |
| Primary site | ||
| Cervical | 1 | 0 |
| Mediastinal | 3 | 4 |
| Retroperitoneal | 5 | 21 |
| Adrenal | 3 | 10 |
| Pelvic | 2 | 4 |
| Epidural disease | 7 | 14 |
| L2 classification by INRG | 13 | 35 |
| Serum LDH (mean ± SD) U/L | 354 ± 154 | 814 ± 687 (n = 20) |
| Serum ferritin (mean ± SD) ng/mL | 64 ± 78 | 64 ± 52 (n = 17) |
| Hyperdiploidy | 11/12 | 24/31 |
| Favourable histology | 12/13 | 28/34 |
| Patients with diploid tumours AND unfavourable histology | 0/12 | 0/29 |
| Pre-surgery chemotherapy | 0 | 39 |
| Intermediate-dose | 34 | |
| COG protocol 3961 | 26 | |
| Protocol 3961 – 1 cycle | 1 | |
| Protocol 3961 – 2 cycles | 4 | |
| Protocol 3961 – 4 cycles | 11 | |
| Protocol 3961 – 8 cycles | 10 | |
| Other intermediate-dose | 8 | |
| High-dose | 0 | 4 |
| High-dose + SCT | 0 | 1 |
| Pre-surgery radiotherapy | 0 | 3 |
| Gross total resection | 5 | 25 |
| Relapses | 3 | 0 |
| Deaths | 1 | 1 |
| Median follow-up (months) | 58.8 | 46.5 |
| 5 year EFS | 84.6 ± 10% | 97.1 ± 3% |
| 10-year OS | 84.6 ± 14% | 97.1 ± 3% |
Abbreviations: COG: Children’s Oncology Group; EFS: event-free survival; INRG: International Neuroblastoma Research Group; MSKCC: Memorial Sloan-Kettering Cancer Centre; OS: overall survival; SCT: stem cell transplant; SD: standard deviation.
Table 2.
Characterisitcs and outcome of patients with MUYCN-non-amplified NB(Group C)
| No. | Age at diagnosis (years)/sex |
Primary site | Post-induction status |
Myeloablative chemotherapy |
Current clinical status |
Time since diagnosis (months) |
|---|---|---|---|---|---|---|
| Patients treated with 3F8 | ||||||
| 1 | 0.8/F | Abdomen | CR | None | NED | 57 |
| 2 | 1.2/M | Abdomen | PD | None | DOD; local + liver relapse |
11 |
| 3 | 1.5/F | Abdomen | CR | Thiotepa, carboplatin, topotecan33 | NED | 92 |
| 4 | 1.6/F | Abdomen | CR | Carboplatin, etoposide, melphalan8 | NED | 39 |
| 5 | 1.6/F | Mediastinum | CR | Carboplatin, etoposide, melphalan8 | NED | 95 |
| 6 | 1.9/F | Abdomen | CR | Thiotepa, carboplatin, topotecan33 | NED | 97 |
| 7 | 1.9/M | Abdomen | CR | Carboplatin, etoposide, melphalan8 | NED | 58 |
| 8 | 2.6/M | Abdomen | CR | None | NED | 48 |
| 9 | 2.9/M | Abdomen | CR | None (received 131I-3F813) | NED | 126 |
| 10 | 4.1/F | Abdomen | CR | Tandem regimen5 | NED | 45 |
| 11 | 4.3/F | Abdomen | CR | Thiotepa, carboplatin, topotecan33 | NED | 100 |
| Patients not receiving 3F8 | ||||||
| 12 | 0.7/M | Abdomen | CR | None | DOD; local + skeletal relapse |
19 |
| 13 | 1.5/F | Abdomen | PR | None | DOD: local + skeletal relapse |
12 |
| 14 | 2.0/M | Abdomen | CR | Carboplatin, etoposide, melphalan8 | NED | 63 |
| 15 | 2.5/M | Abdomen | PR | Carboplatin, etoposide, melphalan8 | NED | 89 |
| 16 | 4.3/M | Abdomen | CR | Carboplatin, etoposide, melphalan8 | DOD; local + liver relapse |
20 |
Abbreviations: CR: complete remission; DOD: died of disease; NED: no evaluable disease; PD: progressive disease; PR: partial remission.
2.2. Statistical analysis
Event-free survival (EFS) and overall survival (OS) were estimated using Kaplan–Meier analyses. The log-rank test was used to determine the prognostic significance of selected variables. Events were defined as relapse, PD, secondary malignancy or death. Cut off date for follow-up was 1st March 2008 or time to last contact with patient.
3. Results
3.1. Outcome of patients with MYCN-non-amplified NB
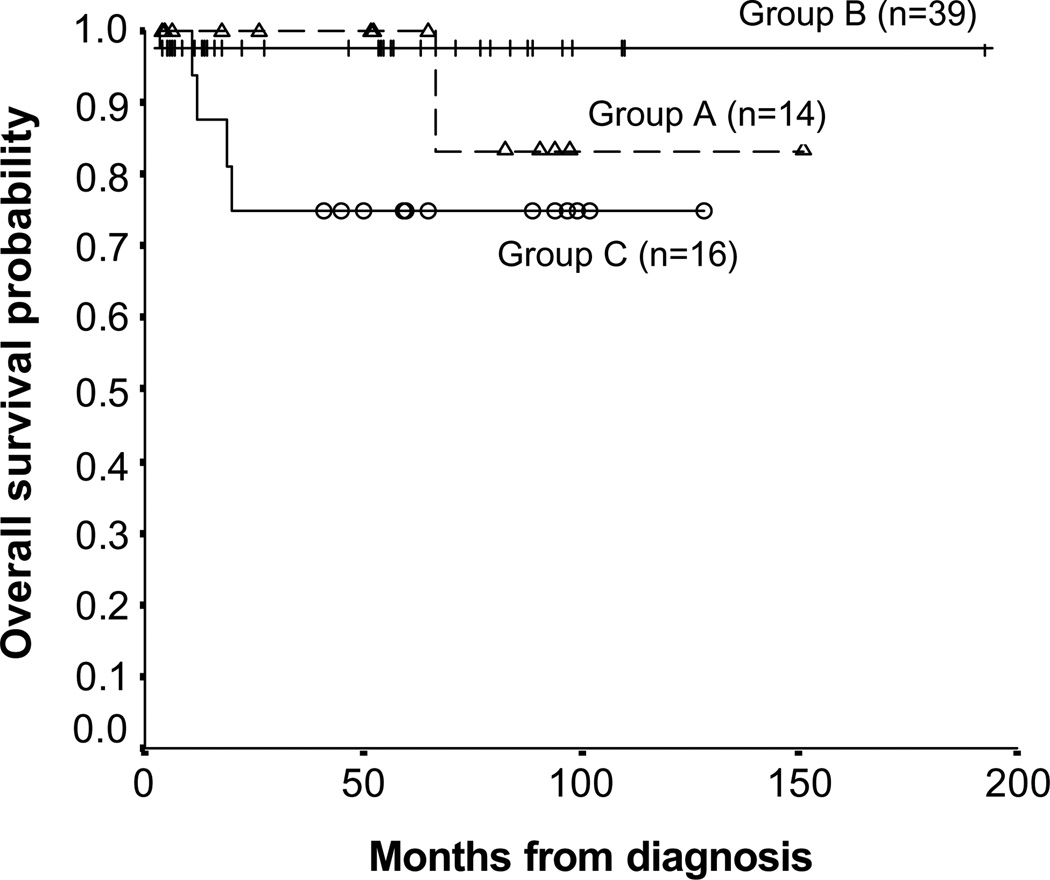
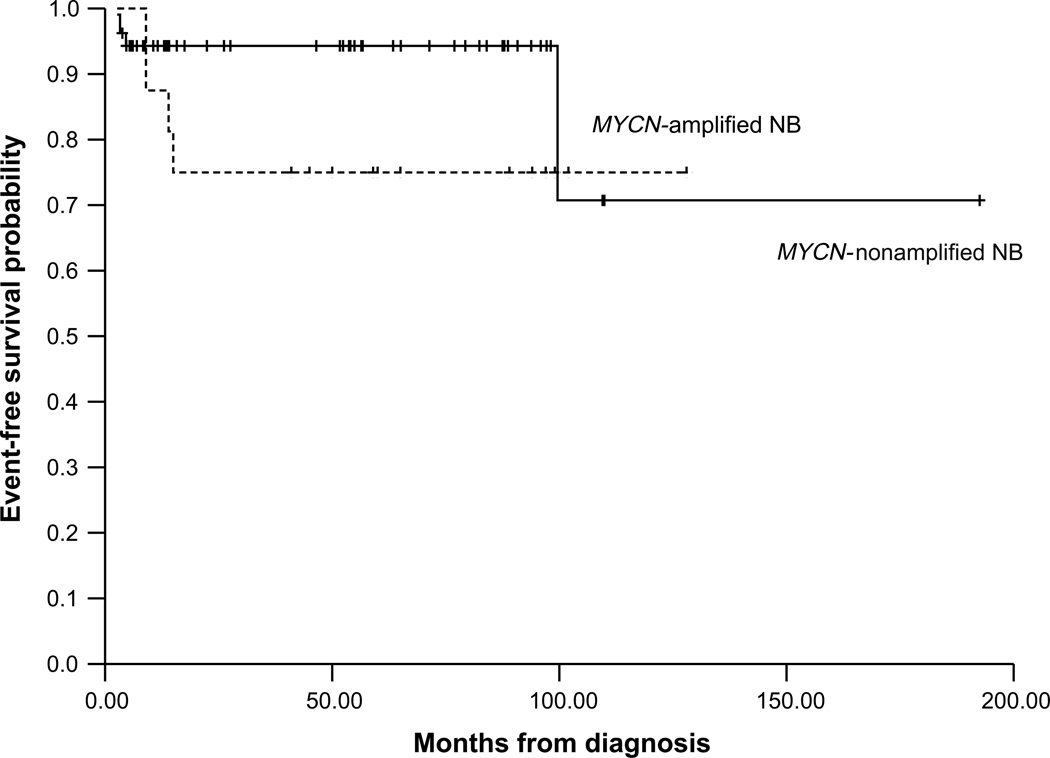
OS and EFS for all patients with MYCN-non-amplified NB were 92.6 ± 5.5% and 74.9 ± 16.9%, respectively, 10 years post-diagnosis (Figs. 3 and 4).
Fig. 3.
Kaplan–Meier analysis for overall survival in patients with stage 3 NB: group A: patients with MYCN-non-amplified NB treated with surgical resection without chemotherapy; group B: MYCN-non-amplified NB patients treated with chemotherapy at non-MSKCC institutions prior to surgical resection and group C: patients with MYCN-amplified NB.
Fig. 4.
Kaplan–Meier analysis for event-free survival in patients with stage 3 NB.
In group A (Table 1), 13/14 patients had L2 NB. Gross total tumour resection with the preservation of vital organs was performed in five (36%) patients: all remain event-free survivors at 52–97 (median, 92) months from surgery. The other nine group A patients had minimal (n = 7) or extensive (n = 2) visible residual post-operative disease (one had residual cervical disease involving the brachial plexus, and one had NB closely approximated to the superior mesenteric artery). No patient underwent nephrectomy; three underwent laminectomy. Six of the nine remain event-free survivors at 5–82 (median, 26) months from surgery, but three had PD. A patient with pelvic and epidural disease had local PD 2 months after initial surgery and then again 2 months after debulking of the recurrence; he is currently receiving dose-intensive chemotherapy. In the above patient with residual cervical disease, progression was detected and resected 96 months from diagnosis; she received no cytotoxic therapy and remains an event-free survivor 48+ months later. The third patient had local PD at 5, 50 and 56 months from diagnosis; despite dose-intensive chemotherapy and RT after the third local recurrence, metastatic BM disease emerged and he died of NB 90 months postdiagnosis. The 10-year OS for patients treated with surgery alone was 84.6 ± 14% (Fig. 3) at a median follow-up of 59 (range 3.8–151) months.
Among the 39 patients in group B (Table 1), 35 had L2 tumours based on pre-surgical risk criteria. Before referral to MSKCC, five received dose-intensive chemotherapy and 34 (85%) modest doses of chemotherapy, including 26 patients who were treated with 1–8 (median 4) cycles of the POG/COG intermediate-risk protocol which uses modest doses of carboplatin, etoposide, cyclophosphamide and doxorubicin. Gross total resection was achieved in 25/39 (64%); laminectomy was performed in 4 patients. Fourteen patients were left with minimal (n = 7) or extensive (n = 7) visible residual NB. All patients with incomplete resection had residual disease restricted to the epidural area. Surgical complications included two nephrectomies and one intraoperative death. After surgery, all patients were observed without cytotoxic therapy except for one patient who, at diagnosis, had massive abdominal disease, including peritoneal implants, and post-operatively received whole abdominal RT and a cycle of low-dose chemotherapy. With a follow-up of 6–145 (median, 47) months from diagnosis, there have been no further adverse events. The 10-year OS and EFS were 97.1 ± 2.9% (Fig. 3).
Although chemotherapy prior to surgery was associated with superior EFS (p = 0.02 for group B versus group A) it did not confer OS advantage (p = 0.5). Biological parameters did not predict EFS in either group and the same was true for all patients with MYCN-non-amplified tumours: p = 0.59 for favourable histology versus unfavourable histology; p = 0.63 for diploid tumours versus hyperdiploid tumours; p = 0.47 for age >18 months versus age <18 months; p = 0.67 for age >24 months versus age <24 months. Image-defined risk factors also did not influence OS (p = 0.65 for L1 disease versus L2 disease). Of note two of three patients with PD had hyperdiploid tumours with favourable histology. EFS was significantly better for patients undergoing gross total resection compared with incomplete resection (p = 0.02), but there was no difference in OS (p = 0.06).
3.2. Outcome of patients with MYCN-amplified NB
In group C (Table 2), all 16 patients (M:F = 8:8; median age = 1.9 years; range 0.8–4.3 years) received dose-intensive induction chemotherapy, 11 with either MSKCC N713 chemotherapy or a similar COG A3973 protocol.15 Thirteen achieved CR with chemotherapy and surgery, two patients had PD (one local despite prior surgery, one local and distant) during induction, and one patient was in PR with residual retroperitoneal tumour post-SCT. Patients in CR were consolidated systemically with myeloablative chemotherapy/SCT+3F8-based immunotherapy (n = 7), 131I-3F8+3F8 (n = 1), 3F8 alone (n = 2), or myeloablative chemotherapy/SCT alone (n = 2). All CR/VGPR patients were also treated with 13-cis-retinoic acid. RT was used for local control in all patients except in one, whose family refused any consolidative therapy (he developed osteomedullary relapse 14 months from diagnosis). The patient with PR subsequently had residual tumour resected at MSKCC, with no post-operative cytotoxic therapy, and remains an event-free survivor 89+ months from diagnosis. Both the 10-year EFS and OS were 75 ± 10.8% for all 16 group C patients at a median follow-up of 60 (range 12–128) months (Fig. 3), and 90.9 ± 8.7% for the 11 treated with 3F8. Eleven of 13 patients who achieved CR survive without the evidence of disease including 7/7 treated with myeloablative chemotherapy/SCT+3F8, 1/1 treated with 131I-3F8+3F8, 2/2 treated with 3F8 alone and 1/2 treated with myeloablative chemotherapy/SCT alone. The four patients who relapsed did so at primary (4/4) as well as in distant sites (2 in bones, 2 in liver). They died shortly after progression, with a median time from relapse to death of 3.8 months (range 2.5–5.5 months) despite salvage chemotherapy.
The 10-year OS for all patients with MYCN-non-amplified NB was significantly better than that for MYCN-amplified NB (p = 0.03).
4. Discussion
To date few published reports16 have focused exclusively on the outcome of INSS stage 3 NB. In this retrospective study, we report favourable prognoses for patients with both MYCN-non-amplified and MYCN-amplified stage 3 NB, adopting two divergent therapeutic strategies: a surgical emphasis while reducing exposure to cytotoxic therapy for the former group, and multimodality therapy consisting of dose-intensive induction chemotherapy, surgery, 13-cis-retinoic acid, myeloablative chemotherapy and/or 3F8-based anti-GD2 immunotherapy for the latter. We have previously elaborated on our rationale to avoid chemotherapy or RT in patients with MYCN-non-amplified locoregional disease.9,10 Briefly, our considerations included the limited proliferative and metastatic potential of residual post-operative disease, the severity of the long-term side-effects of cytotoxic therapy,17 the relative lack of chemoresponsiveness of some localised NB, and the curability of recurrent low-risk localised NB.18 In the past reports we included only 4 patients with INSS stage 3 disease19; the current report extends the series to 14 patients, all treated with surgery alone. The intent of surgery was to achieve a gross total resection of the tumour and associated lymph nodes, but not at the expense of vital organs: nephrectomy was not required in any of the 14 patients in group A. In fact major surgical complications: nephrectomies in two patients and one perioperative death, were encountered only in group B. Although 3/14 group A patients experienced PD, only one died of NB: chemotherapy could be avoided in 12. Relapse in one patient was an unusually late event (90 months post-diagnosis), highlighting the need for continued follow-up. OS and EFS were >80%, similar to the EFS reported in the largest group of Evans stage III patients, all of whom received cytotoxic therapy in addition to attempted surgical resection.8 Survival probabilities for our patients were comparable to those reported in two other preliminary reports on stage 3 NB: INRG analysis of MYCN-non-amplified patients treated on diverse protocols worldwide,20 and COG results of protocol A3961.21 The number of patients undergoing surgery and chemotherapy was not defined in these reports. However, given the present management approaches for stage 3 NB at non-MSKCC institutions, it is likely that a large majority of patients were treated with both surgery and chemotherapy.
Among the 39 group B patients with MYCN-non-amplified tumours first seen at MSKCC for surgical resection after chemotherapy elsewhere, further cytotoxic therapy was withheld in 97% (38/39), in many cases despite initial recommendations for chemotherapy post-surgery. Most (87%) had received intermediate-dose chemotherapy, although eight had been treated with dose-intensive chemotherapy ± RT to residual disease before surgery. Variations in pre-surgical treatments prevent definitive conclusions being drawn, but the excellent outcome (10-year EFS > 97%) suggests that the intensity and duration of prior chemotherapy were not prognostic. In addition, post-surgery chemotherapy and RT were not necessary for MYCN-non-amplified stage 3 patients, even when resection was incomplete as in 43%.
In keeping with referral patterns to our tertiary centre, 91% of patients with MYCN-non-amplified NB had image-defined pre-surgical risk factors (INRG L2 disease): gross total tumour resection was feasible in 57% (30 of 53) patients. Not surprisingly, as has been reported in a prior study on localized NB,11,12 the presence of L2 disease correlated with completeness of resection (data not shown); however, it was not prognostic for OS. The main reason for failure to achieve complete resection was the presence of residual epidural NB (92% of patients with incomplete resection had only residual epidural tumour). The OS of >90% reflects the low malignant potential of these tumours previously described in reports detailing the good prognosis for patients with incompletely resected loco-regional NB.22 Nevertheless, it should be emphasised that the bulk of tumours were removed, and residual disease was, in almost all cases, restricted to small amounts in the epidural space. Neither histological classification nor ploidy status was prognostic in this series, partly because of the relatively few events (2 deaths and 4 relapses in 53 patients with MYCN-non-amplified NB) encountered. Other prognostic markers including chromosome 1p and 11q deletions23,24 are currently being studied in a national COG study. Although our data are encouraging, the strategy of withholding cytotoxic therapy for all newly diagnosed non-MYCN-amplified stage 3 patients needs to be validated by a prospective multi-institutional study.
In contrast to our approach for patients with non-MYCN-amplified NB, patients with MYCN-amplified disease were treated aggressively with dose-intensive chemotherapy13,15 in addition to surgery and RT. CR/VGPR was achieved in 81% similar to our previously reported results in stage 4 patients.25 Patients who achieved CR received RT to the primary site.26 Most patients received systemic consolidation of CR/VGPR with myeloablative chemotherapy+3F8. The ability of 3F8 to eliminate microscope BM disease was previously demonstrated among patients with stage 4 NB.27 In this study, the relative roles of myeloablative chemotherapy and 3F8 in maintaining remission in patients with MYCN-amplified stage 3 NB cannot be adequately assessed because the majority of patients also underwent myeloablative chemotherapy for the remission of consolidation. However, two patients who received 3F8 alone remain alive and disease-free 57+ and 48+ months from diagnosis; and group C patients treated with 3F8 appeared to have a better prognosis than those not receiving 3F8 (p = 0.02 by Fisher’s exact test) suggesting that myeloablative chemotherapy may not be necessary for a favourable outcome in all patients with stage 3 MYCN-amplified NB.
Few prior studies have specifically described outcome for MYCN-amplified stage 3 disease. The 4-year EFS was 32% for 29 patients with MYCN-amplified Evans stage III disease, most of whom were also classified as INSS stage 3, treated on the Children Cancer Group Protocol CCG-3891.8 In contrast, the 6-year OS for 10 stage 3 MYCN-amplified patients on the French NBL 94 study was 80%.28 These differences may be explained by small sample sizes and possible under-staging of patients on the earlier CCG-3891 on which most patients were enrolled prior to 1990 when 123I-MIBG scans were not uniformly used for staging. For our 16 patients, both the 10-year EFS and OS were 75 ± 10.8%. Unfavourable outcomes were related to failure to achieve CR/VGPR (2/3 patients not achieving CR/VGPR had PD and died of disease) and less commonly to failure to maintain remission (2/13 patients who entered CR/VGPR subsequently relapsed and died of disease). These data indicate that aggressive chemotherapeutic and surgical approaches to achieve remission followed by RT to primary site are critical for cure from MYCN-amplified NB. Furthermore, systemic consolidation of remission appears important given the development of distant metastases in the majority of patients who relapsed. Although not included in this analysis, 12 patients with MYCN-amplified stage 3 NB were treated at MSKCC after relapse; median time to relapse was 5.8 months (range 0–20.4 months) from end of therapy. Sites of relapse included primary site (10/12), distant skeletal sites (3), liver (1), central nervous system (1) and distant soft tissues (1). Six patients received 3F8-based immunotherapy: 4 developed further progression and died. Two patients remain free of disease 36+ and 84+ months after relapse. Six patients did not receive immunotherapy and all died. Median OS with 3F8 was 19 ± 2.9 months compared to 8.5 ± 0.2 months for those not receiving antibody (p = 0.007). This experience in relapsed MYCN-amplified stage 3 patients also supports the hypothesis that stage 3 patients have the propensity to develop distant metastases and that 3F8 immunotherapy may be helpful in this regard.
In summary, we conclude that stage 3 NB, irrespective of risk groups, can achieve OS >80% at long follow-up. Patients with MYCN-non-amplified NB appeared to have a good prognosis when treated surgically without any cytotoxic therapy. In contrast to a prior report on 227 patients treated on three German cooperative trials,29 older age did not appear to impart a poorer prognosis for our patients. The relatively small numbers of patients >2 years at diagnosis (66 on the German studies and 21 in this report) and varying treatment approaches preclude direct comparison between the two reports. Other biological markers such as ploidy and histology also did not add to prognostication. Furthermore, this excellent prognosis makes it unlikely that other markers currently being considered for patient stratification will significantly impact prognosis for the majority of patients with INSS stage 3 NB. Indeed it can be hypothesised that young infants with MYCN-non-amplified locoregional NB may have tumours with ‘stage 4S’ biology with the potential for spontaneous involution. A recent study described an observation alone approach for such patients,30 however, most of the small number of patients with stage 3 NB appeared to have been eventually treated with surgery and/or chemotherapy. Of note, only 4/53 patients with MYCN-non-amplified NB in our study were <6 months at diagnosis. Conversely, patients with MYCN-amplified tumours can be successfully treated with a regimen of dose-intensive chemotherapy, aggressive surgery, RT and systemic consolidation of remission. The individual roles of myeloablative chemotherapy and 3F8 immunotherapy to maintain long-term remission in patients with MYCN-amplified NB need to be further investigated. The use of 3F8 antibody instead of myeloablative chemotherapy offers the potential advantage of fewer treatment-related toxicities.31,32
Acknowledgements
This work was supported in part by grants from the National Cancer Institute (CA61017, CA72868, CA134274, CA106450), Bethesda, MD; Hope Street Kids, Alexandria, VA; the Katie’s Find A Cure Fund, New York, NYand the Robert Steel Foundation, New York, NY.
Footnotes
Conflicts of interest statement
None declared.
Contributor Information
Shakeel Modak, Email: modaks@mskcc.org.
Brian H. Kushner, Email: kushnerb@mskcc.org.
Michael P. LaQuaglia, Email: laquaglim@mskcc.org.
Kim Kramer, Email: kramerk@mskcc.org.
Nai-Kong V. Cheung, Email: cheungn@mskcc.org.
REFERENCES
- 1.Brodeur G, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 2.Brodeur GM, Maris JM. Neuroblastoma. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2006. pp. 933–970. [Google Scholar]
- 3.Bagatell R, Rumcheva P, London WB, et al. Outcomes of children with intermediate-risk neuroblastoma after treatment stratified by MYCN status and tumour cell ploidy. J Clin Oncol. 2005;23:8819–8827. doi: 10.1200/JCO.2004.00.2931. [DOI] [PubMed] [Google Scholar]
- 4.Rubie H, Hartmann O, Michon J, et al. N-myc gene amplification is a major prognostic factor in localized neuroblastoma: results of a french NBL study. J Clin Oncol. 1997;15:1171–1182. doi: 10.1200/JCO.1997.15.3.1171. [DOI] [PubMed] [Google Scholar]
- 5.George RE, Li S, Medeiros-Nancarrow C, et al. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J Clin Oncol. 2006;24:2891–2896. doi: 10.1200/JCO.2006.05.6986. [DOI] [PubMed] [Google Scholar]
- 6.Berthold F, Boos J, Burdach S, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005;6:649–658. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 7.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. New Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 8.Matthay KK, Perez C, Seeger RC, et al. Successful treatment of stage III neuroblastoma based on prospective biologic staging: a children’s cancer group study. J Clin Oncol. 1998;16:1256–1264. doi: 10.1200/JCO.1998.16.4.1256. [DOI] [PubMed] [Google Scholar]
- 9.Cheung NKV, Kushner BH, LaQuaglia MP, et al. Survival from non-stage 4 neuroblastoma without cytotoxic therapy: an analysis of clinical and biologic markers. Eur J Cancer. 1997;33:2117–2121. doi: 10.1016/s0959-8049(97)00281-5. [DOI] [PubMed] [Google Scholar]
- 10.Kushner BH, Cheung NK, LaQuaglia MP, et al. Survival from locally invasive or widespread neuroblastoma without cytotoxic therapy. J Clin Oncol. 1996;14:373–381. doi: 10.1200/JCO.1996.14.2.373. [DOI] [PubMed] [Google Scholar]
- 11.Cecchetto G, Mosseri V, De Bernardi B, et al. Surgical risk factors in primary surgery for localized neuroblastoma: the LNESG1 study of the European International Society of Paediatric Oncology Neuroblastoma Group. J Clin Oncol. 2005;23:8483–8489. doi: 10.1200/JCO.2005.02.4661. [DOI] [PubMed] [Google Scholar]
- 12.Simon T, Hero B, Benz-Bohm G, von Schweinitz D, Berthold F. Review of image defined risk factors in localized neuroblastoma patients: results of the GPOH NB97 trial. Pediatr Blood Cancer. 2008;50:965–969. doi: 10.1002/pbc.21343. [DOI] [PubMed] [Google Scholar]
- 13.Cheung NK, Kushner BH, LaQuaglia M, et al. N7: a novel multi-modality therapy of high risk neuroblastoma (NB) in children diagnosed over 1 year of age. Med Pediatr Oncol. 2001;36:227–230. doi: 10.1002/1096-911X(20010101)36:1<227::AID-MPO1055>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 14.Kushner BH, Kramer K, Cheung NKV. Phase II trial of the anti-G(D2) monoclonal antibody 3F8 and granulocyte-macrophage colony-stimulating factor for neuroblastoma. J Clin Oncol. 2001;19:4189–4194. doi: 10.1200/JCO.2001.19.22.4189. [DOI] [PubMed] [Google Scholar]
- 15.Kreissman SG, Villablanca JG, Diller L, et al. Response and toxicity to a dose-intensive multi-agent chemotherapy induction for high-risk neuroblastoma: a Children’s Oncology Group (COG A3973) study. J Clin Oncol. 2007;25:9505. [Google Scholar]
- 16.Garaventa A, Boni L, Lo Piccolo MS, et al. Localized unresectable neuroblastoma: results of treatment based on clinical prognostic factors. Ann Oncol. 2002;13:956–964. doi: 10.1093/annonc/mdf165. [DOI] [PubMed] [Google Scholar]
- 17.Laverdiere C, Cheung NK, Kushner BH, et al. Long-term complications in survivors of advanced stage neuroblastoma. Pediatr Blood Cancer. 2005;45:324–332. doi: 10.1002/pbc.20331. [DOI] [PubMed] [Google Scholar]
- 18.De Bernardi B, Conte M, Mancini A, et al. Localized resectable neuroblastoma: results of the second study of the Italian Cooperative Group for Neuroblastoma. J Clin Oncol. 1995;13:884–893. doi: 10.1200/JCO.1995.13.4.884. [DOI] [PubMed] [Google Scholar]
- 19.Kushner BH, LaQuaglia MP, Ambros PF, et al. Survival from locally invasive or metastatic neuroblastoma (NB) without cytotoxic therapy (Rx) Proc Am Soc Clin Oncol. 1993;12:413. doi: 10.1200/JCO.1996.14.2.373. [DOI] [PubMed] [Google Scholar]
- 20.Park JE, London W, Maris JM, et al. Prognostic markers for stage 3 neuroblastoma (NB): a report from the International Neuroblastoma Risk Group (INRG) project. Proc ASCO. 2008 [Google Scholar]
- 21.Baker DL, Schmidt M, Cohn SL, et al. A phase III trial of biologically-based therapy reduction for intermediate risk neuroblastoma. Proc ASCO. 2007;25(S):9504. [Google Scholar]
- 22.Strother D, van Hoff J, Rao PV, et al. Event-free survival of children with biologically favourable neuroblastoma based on the degree of initial tumour resection: results from the Paediatric Oncology Group. Eur J Cancer. 1997;33:2121–2125. doi: 10.1016/s0959-8049(97)00293-1. [DOI] [PubMed] [Google Scholar]
- 23.Attiyeh EF, London WB, Mosse YP, et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma. New Engl J Med. 2005;353:2243–2253. doi: 10.1056/NEJMoa052399. [DOI] [PubMed] [Google Scholar]
- 24.Maris JM, Weiss MJ, Guo C, et al. Loss of heterozygosity at chromosome 1p36 independently predicts for disease progression but not decreased overall survival probability in neuroblastoma patients: a children’s cancer study group study. J Clin Oncol. 2000;18:1888–1899. doi: 10.1200/JCO.2000.18.9.1888. [DOI] [PubMed] [Google Scholar]
- 25.Cheung NK, Kushner BH, LaQuaglia M, et al. N7: a novel multi-modality therapy of high risk neuroblastoma (NB) in children diagnosed over 1 year of age. Med Pediatr Oncol. 2001;36:227–230. doi: 10.1002/1096-911X(20010101)36:1<227::AID-MPO1055>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 26.Wolden SL, Gollamudi SV, Kushner BH, et al. Local control with multimodality therapy for stage 4 neuroblastoma. Int J Radiat Oncol Biol Phys. 2000;46:969–974. doi: 10.1016/s0360-3016(99)00399-5. [DOI] [PubMed] [Google Scholar]
- 27.Cheung NK, Sowers R, Vickers AJ, Cheung IY, Kushner BH, Gorlick R. FCGR2A polymorphism is correlated with clinical outcome after immunotherapy of neuroblastoma with anti-GD2 antibody and granulocyte macrophage colonystimulating factor. J Clin Oncol. 2006;24:2885–2890. doi: 10.1200/JCO.2005.04.6011. [DOI] [PubMed] [Google Scholar]
- 28.Laprie A, Michon J, Hartmann O, et al. High-dose chemotherapy followed by locoregional irradiation improves the outcome of patients with international neuroblastoma staging system stage II and III neuroblastoma with MYCN amplification. Cancer. 2004;101:1081–1089. doi: 10.1002/cncr.20453. [DOI] [PubMed] [Google Scholar]
- 29.Simon T, Spitz R, Faldum A, Hero B, Berthold F. New definition of low-risk neuroblastoma using stage, age, and 1p and MYCN status. J Pediatr Hematol Oncol. 2004;26:791–796. [PubMed] [Google Scholar]
- 30.Hero B, Simon T, Spitz R, et al. Localized infant neuroblastomas often show spontaneous regression: results of the prospective trials NB95-S and NB97. J Clin Oncol. 2008;26:1504–1510. doi: 10.1200/JCO.2007.12.3349. [DOI] [PubMed] [Google Scholar]
- 31.Cheung NK, Lazarus H, Miraldi FD, et al. Ganglioside GD2 specific monoclonal antibody 3F8: a phase I study in patients with neuroblastoma and malignant melanoma. J Clin Oncol. 1987;5:1430–1440. doi: 10.1200/JCO.1987.5.9.1430. [DOI] [PubMed] [Google Scholar]
- 32.Matthay KK, Seeger RC, Reynolds CP, et al. Allogeneic versus autologous purged bone marrow transplantation for neuroblastoma: a report from the Children’s Cancer Group. J Clin Oncol. 1994;12:2382–2389. doi: 10.1200/JCO.1994.12.11.2382. [DOI] [PubMed] [Google Scholar]
- 33.Kushner BH, Cheung NK, Kramer K, Dunkel IJ, Calleja E, Boulad F. Topotecan combined with myeloablative doses of thiotepa and carboplatin for neuroblastoma, brain tumours, and other poor-risk solid tumours in children and young adults. Bone Marrow Transplant. 2001;28:551–556. doi: 10.1038/sj.bmt.1703213. [DOI] [PubMed] [Google Scholar]