Abstract
Chronic obstructive pulmonary disease (COPD) is a condition of progressive airflow obstruction occurring primarily as a result of tobacco use that accounts for substantial worldwide morbidity and mortality. Medical therapy, with the exception of oxygen and smoking cessation, does not appreciably alter the natural progression of the disease. In contrast, when performed in carefully selected candidates, lung transplantation can provide substantial benefits in physiology, function, quality of life, and survival. Strict selection criteria limit transplant to highly compliant candidates with advanced disease, but preserved functional status, who are capable of successfully undergoing the operation. Although either single or bilateral lung transplant may be offered in COPD, recent evidence suggests that bilateral transplant is the preferred operation due to superior long-term outcomes. Regardless of the type of transplant operation, however, all lung transplant recipients are susceptible to numerous complications including post-transplant infection and rejection. Despite these and other potential complications, advances in medical and surgical management now make lung transplantation a worthwhile therapeutic option in appropriately selected patients. In fact, lung transplant represents the only intervention that can substantially improve long-term outcomes in COPD patients with very advanced disease. Further work to refine recipient selection, improve lung allocation algorithms, and develop better treatments of chronic allograft dysfunction will lead to an even greater benefit to lung transplantation in this ill patient population.
Keywords: Chronic obstructive pulmonary disease, Lung transplant, Bilateral lung transplant, Single lung transplant
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive, debilitating lung disease estimated to cause in excess of 2.7 million deaths annually throughout the world. The burden of disease is predicted to increase over the coming decades translating into substantial loss of life and significant economic liability. In fact, COPD is the only major disease in which worldwide death rates are rising1. Effective treatments for this condition are lacking; aside from smoking cessation and oxygen therapy, medical management of COPD has not been shown to significantly alter the natural progression of the disease. Consequently, surgical options for the management of advanced COPD, including lung volume reduction surgery (LVRS) and particularly lung transplantation are increasingly utilized in carefully selected patients. This chapter aims to review the rationale and benefit of lung transplantation in COPD highlighting the need for meticulous candidate selection, and potential physiologic, quality of life, and survival benefits. In addition, we will underscore ongoing controversies such as the debate regarding single versus bilateral transplantation, consider common complications in the post-transplant period specific to COPD, and finally highlight future directions in the rapidly advancing discipline of lung transplantation for COPD.
Background
The first documented human lung transplant for COPD occurred in 1963 when Hardy and colleagues transplanted a single lung into a patient with advanced emphysema. The effort was unsuccessful, culminating in recipient death only 18 days after transplantation2. The following two decades would bring more failed attempts followed by successful transplantation first in idiopathic pulmonary fibrosis (IPF) during the mid 1980s, and ultimately by six successful bilateral lung transplant procedures in patients with end-stage emphysematous lung disease in 1986 3. Subsequent years realized the achievement of both single and bilateral lung transplant in patients with advanced COPD and this diagnosis group rapidly became the most common to undergo lung transplantation (Figure 1)4.
Figure 1. Indications for adult lung transplants by year.

(Reprinted with permission from Christie JD, Edwards LB, Aurora P, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult lung and heart/lung transplantation report – 2008. J Heart Lung Transplant 2008;27:957-969)
CF = Cystic fibrosis, IPF = Idiopathic pulmonary fibrosis, COPD = Chronic obstructive pulmonary disease, AT Def = alpha 1-antitrypsin deficiency, IPAH = Idiopathic pulmonary hypertension
In recent years, several interesting trends have been observed in the international experience with lung transplant for COPD. Registry data indicate that between January 1995 and June 2007, 36% of all lung transplants were performed for COPD accounting for over seven thousand transplants in total. Bilateral lung transplant has been used with increasing frequency in the last decade. Transplantation rates in patients aged 60 and older have also been noted to increase considerably, with this cohort now accounting for nearly 25% of all lung transplant recipients reflecting increasing numbers of lung transplants in both older COPD patients as well those with IPF 4. Finally, since the implementation of a new lung allocation system in May 2005 which favors transplant in the sickest patients, the percent of total transplants performed for COPD decreased for the first time in part due to the relatively lower wait list urgency applied to COPD as compared to other native lung diseases5. Despite the new allocation system, however, COPD remains a frequent indication for lung transplantation as shown in Figure 14.
Candidate Selection
Stringent candidate evaluation and selection is vital to support favorable post-transplant outcomes. Generally recommended absolute and relative contraindications to transplantation are outlined in Table 16. In COPD patients, specific attention should be given to sustained abstinence from tobacco and to comorbidities directly related to previous tobacco use, such as cardiovascular disease and malignancy. Although six months free of tobacco is required, our experience suggests patients who quit only when undergoing transplant evaluation are at a higher risk for relapse. Therefore, we strongly suggest all previous smokers undergo supervised smoking cessation therapy and remain free of any tobacco product for at least one year prior to transplantation. Medical therapy, including pulmonary rehabilitation, should be optimized in all patients prior to consideration for lung transplantation. Six-minute walk distance (6MWD) at transplant evaluation not only identifies patients with a high risk of death while waiting, but might also identify patients at higher risk for mortality post-transplant7. Our belief is that intensive rehabilitation prior to transplantation can translate into improved likelihood for a successful post-transplant outcome.
Table 1.
Recommended absolute and relative contraindications to lung transplantation
| Absolute Contraindications | Malignancy with the last 2 years* |
| Advanced dysfunction of another organ system | |
| Coronary artery disease not amenable to percutaneous intervention or bypass grafting | |
| Chronic, active hepatitis B | |
| Infection with HIV or hepatitis C | |
| Significant chest wall or spinal deformity | |
| Documented non-adherence or inability to maintain appropriate follow-up | |
| Untreated psychiatric comorbidity | |
| Absence of social support | |
| Substance addiction† | |
|
| |
| Relative Contraindications | Age > 65 years |
| Ongoing critical illness | |
| Severely limited functional status | |
| Colonization with highly resistant organisms | |
| BMI > 30kg/m2 | |
| Severe osteoporosis | |
Adapted from Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update – a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745–755
With the exception of squamous and basal cell skin cancers
Active or within the last 6 months
HIV = Human immunodeficiency virus, BMI = Body mass index
The International Society for Heart and Lung Transplantation (ISHLT) guidelines for lung transplantation in patients with COPD are listed in Table 26. Appropriate candidate selection is linked closely to predicted disease-related survival as preferably transplantation should be performed when post-transplant life expectancy is predicted to surpass survival without the procedure. Early lung transplant referral should be considered when there is intense symptom burden despite maximal medical therapy or when 2 to 3 year predicted survival is less than 50%6. While forecasting disease-related survival is imperfect, there are several indicators of poor short term outcomes in patients with COPD to be considered when selecting candidates for transplantation. As discussed in more detail below, factors such as hypercarbia, hypoxemia, pulmonary hypertension, and high BODE index (Body mass index, degree of airflow Obstruction, Dyspnea, and Exercise capacity measured by 6MWD) weigh heavily in the decision process 8–11.
Table 2.
ISHLT indications for lung transplantation in COPD
| ■ BODE index of 7 to 10 or at least one of the following: |
| ■ History of hospitalization for COPD exacerbation associated with acute hypercapnea (PaCO2 > 50mmHg) |
| ■ Pulmonary hypertension or cor pulmonale, or both, despite oxygen therapy |
| ■ FEV1 of less than 20% and either DLCO of less than 20% or homogenous distribution of emphysema |
Adapted from Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update – a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745–755
ISHLT = International Society for Heart and Lung Transplantation
BODE = Body mass index, degree of airflow Obstruction, Dyspnea, and Exercise capacity
FEV1 = Forced expiratory volume in one second
DLCO = Diffusing capacity of carbon monoxide
The BODE index is a multidimensional, validated index (with a scale from 0 to 10) used to predict death in patients with COPD. It has been demonstrated to be superior to using the forced expiratory volume in one second (FEV1) alone. In a prospective study by Celli and colleagues, a validation cohort consisting of 625 patients with COPD revealed those with a BODE index of 7 to 10 had documented mortality rates as high as 80% at 4 years with a median survival of only 3 years9. Additional data suggest that an increase in BODE index of greater than one point over a 6 to 24 month period is associated with increasing mortality10. Beyond the BODE index, evidence supports poor prognosis after hospitalization for COPD exacerbation complicated by acute hypercarbia (PaCO2 ≥ 50mmHg) with one study demonstrating a 2 year mortality of 49% among this population11. These findings, demonstrating poor short-term outcomes in specific patient subsets, provide rationale for the ISHLT guidelines for transplantation in COPD and emphasize that COPD is a complex disease in which objective measures of lung function (such as FEV1) incompletely reflect the true severity of disease. We fully advocate this multidisciplinary approach in which objective measures of lung function are used in conjunction with 6MWD, symptoms, and quality of life assessment to make informed decisions regarding optimal timing of transplantation.
In less severely ill COPD patients, LVRS may be considered as an alternative to lung transplantation, as discussed in a separate chapter. Importantly, in those who meet criteria for LVRS, this does not preclude future transplantation should it become necessary12. LVRS has been demonstrated to significantly improve FEV1 and total lung capacity, while reducing residual volume and re-optimizing diaphragmatic function in selected patients with predominately upper lobe disease13, 14. While there are no head-to-head trials evaluating superiority of either LVRS or lung transplantation in patients with COPD, the National Emphysema Treatment Trial identified a subset of high risk patients with considerable mortality increase after LVRS as compared to standard medical therapy15. This population includes those with homogenous emphysema, FEV1 of 20% of predicted or less, or diffusing capacity of carbon monoxide of 20% of predicted or less. Given these data, lung transplantation is the preferred surgical intervention for this particular population. Additionally, LVRS has not been demonstrated to reduce pulmonary artery pressures thus patients with significant secondary pulmonary hypertension should be considered preferentially for lung transplantation13.
COPD and Lung Allocation
The lung, perhaps more so than any other organ, is fragile making it easily susceptible to damage and poorly tolerant of prolonged ischemic times. Together these factors reduce the percentage of donor lungs suitable for transplantation thus severely limiting the donor organ pool and increasing time to transplantation for those on the waiting list16. Prior to 2005, donor lungs were allocated based exclusively on the recipient's accrued time on the transplant waiting list, without respect to severity of illness or medical urgency. The median waiting time to transplantation was in excess of 2 years with approximately 500 patients on the waiting list dying each year5. In order to address excess waiting list mortality, the Lung Allocation Score (LAS), a measure accounting for both medical urgency (predicted survival during the following year on the waiting list) and transplant benefit (predicted survival one year post-transplant), was developed to improve lung allocation in the United States and was subsequently implemented in May of 200517.
Since implementation of the LAS, median wait time to transplant has markedly decreased to less than 200 days and there has been a measurable reduction in waiting list mortality5. In part due to changed demographics of the referral population and in part due to the increased priority placed on risk of pre-transplant mortality in the LAS, since May 2005 there has been a preferential increase in transplants amongst older patients and those with IPF with a slight reduction in the number of transplants performed for COPD4, 5. As a result of the increased percentage of lung transplantation in older, sicker patients, a slight, non-significant, drop in overall post-transplant survival has been demonstrated18. Patients with COPD, however, maintained the greatest three and twelve month survival rates by diagnosis group (94% and 87% respectively)5. We believe the LAS does an effective job of ensuring that lung transplantation for COPD is reserved for those patients with the most advanced disease. Despite changes in allocation and decreased waiting times; early referral of potential candidates with COPD is still favored to allow appropriate time for thorough evaluation, rehabilitation, and education.
Post-transplant Outcomes in COPD
Favorable outcomes in the domains of physiology and function, health-related quality of life, and most importantly survival have been documented in patients who have undergone lung transplantation for COPD. Unlike cystic fibrosis or other septic lung diseases where bilateral transplant is mandatory, either single or bilateral transplant can be performed for COPD. In general, those with COPD sustain more marked improvements in many of the aforementioned domains with bilateral lung transplant (BLT) as opposed to single lung transplant (SLT). While BLT is increasingly performed for all native lung diseases4, debate continues as to the optimal transplant procedure of choice in the emphysema population as discussed in detail below.
Physiologic and Functional Benefit
Both SLT and BLT result in dramatic improvements in gas exchange, lung function, and exercise capacity in patients with COPD14, 16, 19–21. In correspondence with these improvements, registry data denote that more than 80% of survivors at 1, 3, and 5 years post-transplant report no activity limitation19. After an uncomplicated transplant procedure, hypoxemia and hypercarbia normalize readily. Typically, improvement in lung function peaks within six to nine months post-transplant16. In SLT, FEV1 increases to between 50 and 60% of predicted values while BLT results in normalization of FEV1 to between 80 and 90% of predicted values, although %predicted varies considerably depending on size matching, gender and ethnicity of the donor14, 19. Likewise, exercise capacity is estimated to double by 6 months post-transplant from 700 to 900 feet to between 1,700 and 1,900 feet14, 16, 19. Post-transplant exercise ability appears to be restricted primarily by aerobic capacity due to skeletal muscle dysfunction, not by ventilatory limitations. Although the specific cause of skeletal muscle dysfunction is unclear, deconditioning due to underlying chronic disease, post-transplant immunosuppressant use, and compromised nutrition could contribute20.
While short term physiologic benefit is undisputable and seems to favor BLT, Pochettino and colleagues have examined intermediate-term physiologic outcomes of SLT versus BLT21. Eighty-four SLTs and forty-six BLTs performed in patients with COPD were retrospectively reviewed with a mean follow-up time of 32.4 months. Study results indicate that BLT achieves a higher FEV1 and forced vital capacity than does SLT at all time points post-transplantation. This same study examined exercise capacity as assessed by 6MWD and similarly found that while post-transplant walk distance was enhanced in all patients; those who had undergone BLT sustained the greatest improvement at all post-transplantation time points21.
The onset of bronchiolitis obliterans syndrome (BOS), a condition of chronic allograft dysfunction, threatens the physiologic and functional improvements associated with lung transplantation for COPD. Although the precise etiology is uncertain, BOS is thought to reflect chronic immune mediated injury to the airways leading to progressive intraluminal fibrosis22. Consistent with this idea, the frequency and severity of prior acute rejection is the greatest risk factor for BOS23. Clinically, BOS is characterized by progressive airflow obstruction with decline in FEV1 to less than 80% of the post-transplant baseline in the absence of other possible explanatory factors22. Interestingly, it has been demonstrated that recipients of SLT develop BOS at earlier time points post-transplant than recipients of BLT24, 25. It remains uncertain if this difference reflects simply the higher starting FEV1 achieved with BLT or if there are in fact immunological variations in the host response to one versus two lungs. Regardless of the mechanism, it appears that BLT for COPD is protective against the erosion of lung function that occurs with BOS.
Quality of Life Benefit
Anticipation of improved health-related quality of life (HRQL) is another important consideration when making the decision to offer lung transplantation to patients with COPD. Indeed, improved HRQL after lung transplantation has been documented in many cross-sectional and longitudinal studies using a variety of validated instruments, and greater than 90% of lung transplant recipients report satisfaction with their decision to undergo transplantation14, 26–34. Although studies examining HRQL are generally limited by small sample sizes, results demonstrating sustained enhancement of this domain are relatively consistent. In one of the largest longitudinal prospective studies to assess HRQL in lung transplant patients, Rodrigue et al evaluated 66 patients who underwent SLT (41 patients) or BLT (25 patients)26. COPD was the indication for transplantation in 36%. Participants were assessed using the SF-36 Health Survey and the Transplant Symptom Frequency Questionnaire both pre and post-transplant.
As expected, pre-transplant HRQL was quite poor, and was significantly lower than a normative sample of adults with COPD. Post-transplant HRQL improved significantly in the areas of physical and role functioning, bodily pain, general health, mental health and vitality. Despite the marked incremental improvement, overall HRQL did not reach levels generally seen in healthy adults. In addition, while transplant improved reported affective distress, recipients reported increased neurocognitive and gastrointestinal symptoms and a change in physical appearance post-transplant. These symptoms, likely attributable to required immunosuppressive regimens, generally increase, as do other symptoms, with increased length of time since transplantation26. The onset of BOS was also noted to correlate with diminishing HRQL in transplant recipients and this finding is substantiated in several other studies26–28, 30, 35. As BOS develops at earlier time points post-transplant in recipients of SLT as compared to recipients of BLT24, 25, one might predict that HRQL would be worse among the SLT population. A small longitudinal trial demonstrated no significant difference in respiratory disease-specific HRQL between these two groups, however, did show a trend favoring BLT when comparing absolute mean differences in quality of life scores31. Notable limitations exist and the study did not consider quality-adjusted life years.
Survival Benefit
For many, survival benefit is the single most important outcome to weigh when considering lung transplantation in patients with advanced COPD and it is this expected benefit upon which the criteria for candidate selection are based. Registry data report a median survival of 5.2 years for all adult lung transplant recipients with one year, three year and five year survival for patients receiving BLT or SLT for COPD reported as 83.8% vs. 80.5%, 67.8% vs. 46.5%, and 30.1% vs. 17.7% respectively4. Improving survival has been noted in more recent time periods primarily due to decreased first-year mortality. This advance may correlate with refinement of surgical techniques and better peri-operative care. Long-term survival however, typically limited by infection and chronic rejection, has remained disappointingly unaffected. While patients with COPD, compared to other diagnosis groups, enjoy the best one year survival, lower ten year survival is noted, perhaps related to advanced age and the prevalence of comorbidities in this population4.
There is now increasing evidence that lung transplant confers measurable survival benefit in carefully selected patients with advanced emphysema36–39. Data on survival is primarily obtained from cohort studies; intrinsically flawed by the lack of control for confounding factors such as age, severity of illness at the time of transplantation, era during which transplant was performed, and transplant procedure. While early cohort data failed to demonstrate a survival advantage to lung transplant in patients with COPD 40, 41, more recent data consistently reveals quantifiable survival benefit within this population36–39. The reasons for this discrepancy are many. Early studies generally evaluated fewer patients, included a higher proportion of SLT, and were performed during an era in which overall post-transplant survival was inferior and recipient selection was less refined than in the present day. Additionally, more modern assessments have utilized robust, advanced statistical methods, such as propensity-based matching, to control for potential confounders and have included more BLT procedures.
Nevertheless, debate regarding the transplant procedure of choice in patients with COPD is ongoing. Survival after SLT versus BLT for advanced emphysema has never been compared in a randomized controlled trial and a trial of this nature is unlikely to be completed due to ethical considerations. Increased length of life after BLT is consistent with the observation that BOS, the leading cause of long-term death amongst transplant recipients, develops at later time points post-transplantation in BLT recipients when compared to recipients of SLT 24, 25. Additionally, SLT has been associated with other complications to include native lung hyperinflation, pneumothorax, and development of bronchogenic carcinoma within the native lung parenchyma42–45 which could contribute directly or indirectly to increased mortality. For those in whom other causes of functional decline have been excluded, LVRS of the native lung is a feasible option46. In addition to native lung hyperinflation, the development of bronchogenic lung cancer within the native lung is of particular concern in patients undergoing transplantation for COPD due to the nearly uniform exposure to tobacco in this population. Indeed SLT has been shown to confer a significant increase in the risk of developing primary post-transplant lung cancer as compared to BLT in patients with comparable native disease, age, and tobacco history45. Native lung cancers appear to be associated with a uniformly poor prognosis after transplant.
As shown in Figure 2, registry data suggest survival for SLT and BLT is similar within the first year post-transplant; however, over time BLT confers significant survival advantage in both those over and under age 504. Multiple recent studies have corroborated these results36–39. A major critique of older, conflicting studies has been that factors other than the type of transplant operation might have influenced the observed survival benefit among BLT recipients. Thabut et al used propensity based matching and simulation to address this point and demonstrate that bilateral transplant is associated with a significantly greater survival than single lung transplant in COPD (median 6·41 years (6·02–6·88) versus 4·59 years (4·41–4·76) (p<0·0001) after adjustment for baseline characteristics and disease severity. Based on these data it is estimated that 63.7% of BLT recipients would gain survival advantage compared to only 50.1% of SLT recipients39. An area of ongoing controversy remains if BLT offers this significant survival benefit in patients over the age of 60, as some studies suggest no additional benefit of BLT in these older patients; in part, however, this controversy is difficult to resolve because historically very few centers have performed BLT in older patients37, 38, 47.
Figure 2. Kaplan-Meier survival after lung transplantation for recipients with COPD for transplants performed between January 1990 and June 2006, stratified by procedure type and age.
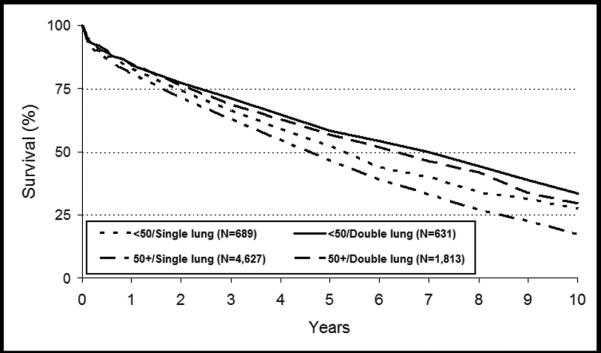
(Reprinted with permission from Christie JD, Edwards LB, Aurora P, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult lung and heart/lung transplantation report – 2008. J Heart Lung Transplant 2008;27:957-969)
In summary, BLT for COPD, especially in those under the age of 60, offers superior physiologic, functional, and survival outcomes and thus likely translates into increased quality-adjusted life years in this population. BLT is therefore the preferred transplant procedure in advanced COPD. Notwithstanding, the decision to offer BLT versus SLT must take into account the context of regional organ allocation policies, organ availability, and individual center experience performing the available transplant procedures.
Post-transplant Complications
Long-term outcomes after lung transplantation are inferior when compared to other solid organ transplants due to a number of lung-specific complications48. As a consequence of the transplant procedure itself, many innate protective responses are interrupted. The transplanted lungs are dennervated resulting in impaired cough reflex and abnormal mucocilliary clearance mechanisms These disruptions, in addition to the need for high levels of immunosuppression, culminate in increased infection risk predisposing to viral (most importantly cytomegalovirus), bacterial, and fungal infections19. Chronic exposure to immunosuppressive medications also leads to metabolic dysregulation resulting in increased risk of hypertension, hyperlipidemia, diabetes and cardiovascular disease. Chronic renal insufficiency also occurs in most patients and a subset go on to develop frank renal failure over time as a result of calcineurin inhibitor toxicity.
Despite the intense immunosuppression, acute rejection and chronic allograft dysfunction (BOS) are more frequent after lung transplant than with other solid organ transplants. While acute rejection is readily detected with transbronchial lung biopsy and responds reasonably to increased immunosuppression, BOS is less reliably distinguished but affects over 50% of recipients within 5 years post-transplant 4, 16. Indeed, BOS is generally progressive and directly or indirectly contributes to most late post-transplant deaths. Unfortunately, there are no effective, proven treatments for BOS and thus long-term transplant outcomes are likely to remain limited until a better understanding of the pathophysiology and treatment of BOS is achieved.
Conclusion and Future Directions
COPD remains an increasingly prevalent condition for which medical treatment is limited. Lung transplantation results in significant functional improvement, enhanced quality of life and survival advantage in rigorously selected patients with advanced emphysema. Both SLT and BLT are acceptable; however BLT offers patients greater potential for long-term survival and avoids possible native lung complications in COPD. Challenges to further growth of lung transplantation include the scarcity and fragility of suitable donor organs, algorithms for appropriate organ allocation, and the inadequacy of available treatments and preventive measures for BOS. Advances in donor lung preservation, enhanced understanding of the full impact of the LAS, and ongoing mechanistic studies of chronic allograft dysfunction should contribute to the continued growth and success of lung transplantation enabling even more patients with advanced lung disease due to COPD to enjoy the considerable benefits of lung transplantation.
Acknowledgements
No financial support was received for authorship of this manuscript. There are no relevant disclosures on behalf of the authors. Dr Palmer is supported by the NIH NHLBI 1P50-HL084917-01 and K24-091140-01.
Abbreviations
- COPD
Chronic obstructive pulmonary disease
- LVRS
Lung volume reduction surgery
- IPF
Idiopathic pulmonary fibrosis
- 6MWD
6-minute walk distance
- ISHLT
International Society for Heart and Lung Transplantation
- BODE
Body mass index, degree of airflow Obstruction, Dyspnea, and Exercise capacity
- FEV1
Forced expiratory volume in one second
- PaCO2
Partial pressure of arterial carbon dioxide
- mmHg
Millimeters of mercury
- LAS
Lung allocation score
- BLT
Bilateral lung transplant
- SLT
Single lung transplant
- BOS
Bronchiolitis obliterans syndrome
- HRQL
Health-related quality of life
References
- 1.Cruz AA, Mantzouranis E, Matricardi PM, et al. Global surveillance, prevention, and control of chronic respiratory diseases: a comprehensive approach. World Health Organization; 2007. [Accessed October, 2009]. Available at: http://www.who.int/gard/publications/GARD_Manual/en/index.html. [Google Scholar]
- 2.Hardy JD. Lung homotransplantation in man. JAMA. 1963;186:1065–1074. doi: 10.1001/jama.1963.63710120001010. [DOI] [PubMed] [Google Scholar]
- 3.Cooper JD, Patterson GA, Grossman R, Maurer J. Double-lung transplant for advanced chronic obstructive lung disease. Am Rev Respir Dis. 1989;139:303–307. doi: 10.1164/ajrccm/139.2.303. [DOI] [PubMed] [Google Scholar]
- 4.Christie JD, Edwards LB, Aurora P, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult lung and heart/lung transplantation report – 2008. J Heart Lung Transplant. 2008;27:957–969. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 5.McCurry KR, Shearon TH, Edwards LB, et al. Lung transplantation in the United States, 1998 – 2007. Am J Transplant. 2009;9:942–958. doi: 10.1111/j.1600-6143.2009.02569.x. [DOI] [PubMed] [Google Scholar]
- 6.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update – a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–755. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Martinu T, Babyak MA, O'Connell CF, et al. Baseline 6-min walk distance predicts survival in lung transplant candidates. Am J Transplant. 2008;8:1498–1505. doi: 10.1111/j.1600-6143.2008.02264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez FJ, Foster G, Curtis JL, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173:1326–1334. doi: 10.1164/rccm.200510-1677OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. NEJM. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 10.Martinez FJ, Han MK, Andrei AC, et al. Longitudinal change in the BODE index predicts mortality in severe emphysema. Am J Respir Crit Care Med. 2008;178:491–499. doi: 10.1164/rccm.200709-1383OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connors AF, Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) Am J Respir Crit Care Med. 1996;154:959–967. doi: 10.1164/ajrccm.154.4.8887592. [DOI] [PubMed] [Google Scholar]
- 12.Athan SD, Edwards LB, Barnett SD, Ahmad S, Burton NA. Outcomes of COPD lung transplant recipients after lung volume reduction surgery. Chest. 2004;126:1569–1574. doi: 10.1378/chest.126.5.1569. [DOI] [PubMed] [Google Scholar]
- 13.Mora JI, Hadjiliadis D. Lung volume reduction surgery and lung transplantation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3:629–635. doi: 10.2147/copd.s4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel N, DeCamp M, Criner GJ. Lung transplantation and lung volume reduction surgery versus transplantation in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:447–453. doi: 10.1513/pats.200707-107ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 16.Arcasoy SM, Kotloff RM. Lung transplantation. N Engl J Med. 1999;340:1081–1091. doi: 10.1056/NEJM199904083401406. [DOI] [PubMed] [Google Scholar]
- 17.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6:1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 18.Merlo CA, Weiss ES, Orens JB, et al. Impact of U.S. lung allocation score on survival after lung transplantation. Am J Heart Lung Transplant. 2009;28:769–775. doi: 10.1016/j.healun.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 19.Studer SM, Levy RD, McNeil K, Orens JB. Lung transplant outcomes: a review of survival, graft function, physiology, health-related quality of life and cost-effectiveness. Eur Respir J. 2004;24:674–685. doi: 10.1183/09031936.04.00065004. [DOI] [PubMed] [Google Scholar]
- 20.Chan KM, Martinez FJ, Chang AC. Nonmedical therapy for chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:137–145. doi: 10.1513/pats.200809-114GO. [DOI] [PubMed] [Google Scholar]
- 21.Pochettino A, Kotloff RM, Rosengard BR, et al. Bilateral versus single lung transplantation for chronic obstructive pulmonary disease: intermediate-term results. Ann Thorac Surg. 2000;70:1813–1819. doi: 10.1016/s0003-4975(00)01970-6. [DOI] [PubMed] [Google Scholar]
- 22.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 23.Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: a review of recent publications. J Heart Lung Transplant. 2002;21:271–281. doi: 10.1016/s1053-2498(01)00360-6. [DOI] [PubMed] [Google Scholar]
- 24.Hadjiliadis D, Davis RD, Palmer SM. Is transplant operation important in determining posttransplant risk of bronchiolitis obliterans syndrome in lung transplant recipients? Chest. 2002;122:1168–1175. doi: 10.1378/chest.122.4.1168. [DOI] [PubMed] [Google Scholar]
- 25.Hadjiliadis D, Chaparro C, Gutierrez C, et al. Impact of lung transplant operation on bronchiolitis obliterans syndrome in patients with chronic obstructive pulmonary disease. Am J Transplant. 2006;6:183–189. doi: 10.1111/j.1600-6143.2005.01159.x. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigue JR, Baz MA, Kanasky WF, MacNaughton KL. Does lung transplantation improve health-related quality of life? The University of Florida experience. J Heart Lung Transplant. 2005;24:755–63. doi: 10.1016/j.healun.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Gross CR, Savik K, Bolman M, III, Hertz MI. Long-term health status and quality of life outcomes of lung transplant recipients. Chest. 1995;108:1587–1593. doi: 10.1378/chest.108.6.1587. [DOI] [PubMed] [Google Scholar]
- 28.TenVergert EM, Essink-Bot ML, Geertsma A, van Enckevort PJ, de Boer WJ, van der Bij W. The effect of lung transplantation on health-related quality of life. Chest. 1998;113:358–364. doi: 10.1378/chest.113.2.358. [DOI] [PubMed] [Google Scholar]
- 29.Anyanwu AC, McGuire A, Rogers CA, Murday AJ. Assessment of quality of life in lung transplantation using a simple generic tool. Thorax. 2001;56:218–222. doi: 10.1136/thorax.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kugler C, Fischer S, Gottlieb J, et al. Health-related quality of life in two hundred-eighty lung transplant recipients. J Heart Lung Transplant. 2005;24:2262–2268. doi: 10.1016/j.healun.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Gerbase MW, Spilliopoulos A, Rochat T, Archinard M, Nicod L. Health-related quality of life following single or bilateral lung transplantation. Chest. 2005;128:1371–1378. doi: 10.1378/chest.128.3.1371. [DOI] [PubMed] [Google Scholar]
- 32.Snyder LD, Palmer SM. Quality, quantity, or both? Life after lung transplantation. Chest. 2005;128:1086–1087. doi: 10.1378/chest.128.3.1086. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigue JR, Baz MA. Are there sex differences in health-related quality of life after lung transplantation for chronic obstructive pulmonary disease? J Heart Lung Transplant. 2006;25:120–125. doi: 10.1016/j.healun.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Vasiliadis HM, Collet JP, Poirier C. Health-related quality-of-life determinants in lung transplantation. J Heart Lung Transplant. 2006;25:226–233. doi: 10.1016/j.healun.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Van den Berg JW, Geertsma A, van der Bij W, et al. Bronchiolitis obliterans syndrome after lung transplantation and health-related quality of life. Am J Respir Crit Care Med. 2000;161:1937–1941. doi: 10.1164/ajrccm.161.6.9909092. [DOI] [PubMed] [Google Scholar]
- 36.Titman A, Rogers CA, Bonser RS, Banner NR, Sharples LD. Disease-specific survival benefit of lung transplantation in adults: A national cohort study. Am J Transplant. 2009;9:1640–1649. doi: 10.1111/j.1600-6143.2009.02613.x. [DOI] [PubMed] [Google Scholar]
- 37.Meyer DM, Bennett LE, Novick RJ, Hosenpud JD. Single vs bilateral, sequential lung transplantation for end-stage emphysema: influence of recipient age on survival and secondary end-points. [DOI] [PubMed] [Google Scholar]
- 38.Thabut G, Christie JD, Ravaud P, et al. Survival after bilateral versus single lung transplantation for patients with chronic obstructive pulmonary disease: a retrospective analysis of registry data. Lancet. 2008;371:744–751. doi: 10.1016/S0140-6736(08)60344-X. [DOI] [PubMed] [Google Scholar]
- 39.Thabut G, Ravaud P, Christie JD, et al. Determinants of the survival benefit of lung transplantation in patients with chronic obstructive pulmonary disease. Am J Respir Care Med. 2008;177:1156–1163. doi: 10.1164/rccm.200708-1283OC. [DOI] [PubMed] [Google Scholar]
- 40.Hosenpud JD, Bennett LE, Keck BM, Edwards EB, Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet. 1998;351:24–27. doi: 10.1016/S0140-6736(97)06405-2. [DOI] [PubMed] [Google Scholar]
- 41.Stavem K, Bjortuft O, Borgan O, Geiran O, Boe J. Lung transplantation in patients with chronic obstructive pulmonary disease in a national cohort is without obvious survival benefit. J Heart Lung Transplant. 2006;25:75–84. doi: 10.1016/j.healun.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 42.Frost AE, Keller CA, Noon GP, Short D, Cagle PT. Outcome of the native lung after single lung transplant. Chest. 1995;107:981–984. doi: 10.1378/chest.107.4.981. [DOI] [PubMed] [Google Scholar]
- 43.Mal H, Brugiere O, Sleiman C, et al. Morbidity and mortality related to the native lung in single lung transplantation for emphysema. J Heart Lung Transplant. 2000;19:220–223. doi: 10.1016/s1053-2498(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 44.Weill D, Torres F, Hodges TN, Olmos JJ, Zamora MR. Acute native lung hyperinflation is not associated with poor outcomes after single lung transplant for emphysema. J Heart Lung Transplant. 1999;18:1080–1087. doi: 10.1016/s1053-2498(99)00079-0. [DOI] [PubMed] [Google Scholar]
- 45.Dickson RP, Davis RD, Rea JB, Palmer SM. High frequency of bronchogenic carcinoma after single-lung transplantation. J Heart Lung Transplant. 2006;25:1297–1301. doi: 10.1016/j.healun.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reece TB, Mitchell JD, Zamora MR, et al. Native lung volume reduction surgery relieve functional graft compression after single lung graft transplantation for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg. 2008;135:931–937. doi: 10.1016/j.jtcvs.2007.10.069. [DOI] [PubMed] [Google Scholar]
- 47.Nwakanma LU, Simpkins CE, Williams JA, et al. Impact of bilateral versus single lung transplantation on survival in recipients 60 years of age and older: analysis of United Network for Organ Sharing database. J Thorac Cardiovasc Surg. 2007;133:541–547. doi: 10.1016/j.jtcvs.2006.09.062. [DOI] [PubMed] [Google Scholar]
- 48.2008 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1998–2007. U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Rockville, MD: [Accessed October, 2009]. Available at: http://www.ustransplant.org/annual_reports/current/Chapter_I_AR_CD.htm?cp=2. [Google Scholar]


