Abstract
Background
Soy and some of its constituents, such as isoflavones, have been shown to affect the inflammatory process in animal studies. The association between soy food intake and inflammatory markers has not been evaluated adequately in humans.
Objective
Our aim was to evaluate whether higher intake of soy foods was inversely associated with inflammatory markers in 1,005 middle-aged Chinese women.
Design
In this cross-sectional study, dietary intake of soy foods was assessed by a validated food frequency questionnaire and by a 24-hour recall when biospecimens were procured. A general linear model was used to estimate the geometric means of selected inflammatory markers, including interleukin-6 (IL-6), IL-1β, tumor necrosis factor-α (TNFα), soluble IL-6 receptor, soluble GP130, soluble TNF receptors 1 and 2, and C-reactive protein, across categories of soy food intake after adjusting for age, lifestyle and dietary factors, and history of infectious or inflammation-related diseases.
Results
We found that multivariable-adjusted geometric mean concentrations of IL-6 and TNFα were inversely associated with quintiles of soy food intake, with a difference between the highest and lowest quintiles of 25.5% for IL-6 (P for trend = 0.008) and 14% for TNFα (P for trend = 0.04). Similar inverse associations were found for TNFα (P for trend = 0.003), soluble TNF receptor 1 (P for trend=0.01), soluble TNF receptor 2 (P for trend=0.02), IL-1β (P for trend=0.05), and IL-6 (P for trend=0.04) when soy food consumption was assessed by the frequency of consumption in the preceding 24 hours. No significant associations were found for other markers studied.
Conclusions
This study suggests that soy food consumption is related to lower circulating levels of IL-6, TNFα, and soluble TNF receptors 1 and 2 in Chinese women.
Keywords: Soy, Inflammation, Interleukin-1β, Interleukin-6, Tumor necrosis factor-α
Low-grade chronic inflammation has been associated with increased risk of cardiovascular disease, type 2 diabetes mellitus, Alzheimer’s disease, and many types of cancer.1–8 Ahallmark of inflammation is increased production of proinflammatory cytokines, which induces a range of inflammatory enzymes.9 Clinical and epidemiologic studies suggest that dietary factors such as n-3 polyunsaturated fatty acids, antioxidant vitamins and minerals, and dietary fiber can exert health effects, in part, by modulating inflammation.1
Soy and its products are high in polyunsaturated fat, fiber, calcium, and vitamins, but low in saturated fat. In particular, soybeans and soy-based products are the richest dietary sources of isoflavones, the most common and extensively studied phytoestrogens in human diets.10 Consumption of soy foods has been shown to have beneficial effects on multiple aspects of human health, including reduced risk of inflammation-related diseases, such as cardiovascular disease, diabetes, and certain cancers.11–16 It has been hypothesized that the anti-inflammatory and antioxidative activity of soy constituents might explain some of its health benefits.16–19 Studies have shown that soy and its isoflavones can inhibit cell adhesion molecule expression in cultured endothelial cells,17,18 reduce production of proinflammatory cytokines, and decrease oxidative stress in animal models.20–23 Several recent clinical trials have found that a soy-rich diet substantially lowers levels of tumor necrosis factor-α (TNFα), interleukin (IL)-6, C-reactive protein (CRP), IL-18, and nitric oxide,15,24,25 although results are not entirely consistent.26,27 Most of these trials investigated the effects of specific supplemental soy components administered for a short period of time.
Inflammatory markers such as CRP, IL-6, TNFα, and soluble TNF receptor 2 (sTNF-R2) have been shown to be relatively stable within individuals over time.6,28–32 Intraclass correlation coefficients for these markers ranged from 0.48 to 0.77 in our study population.28 This range is similar to intraclass correlation coefficients for serum cholesterol level (intraclass correlation coefficient = 0.60),6 which is generally accepted as being measured reasonably well by a single blood sample. In this study, we evaluated the association between concentrations of selected inflammatory markers and dietary intake of soy foods measured by a validated food frequency question-naire (FFQ) and by a 24-hour recall of soy food consumption among 1,005 middle-aged Chinese women, hypothesizing that intake of soy foods would be inversely associated with circulating levels of inflammatory markers.
METHODS
Study Participants
This cross-sectional analysis was conducted among 1,005 healthy participants of the Shanghai Women’s Health Study, a population-based cohort study. The design and methods of the Shanghai Women’s Health Study have been described in depth elsewhere.33,34 Briefly, at the baseline survey conducted between 1997 and 2000, 74,941 women aged 40 to 70 years were recruited from seven urban communities of Shanghai (participation rate: 92.7%). All women completed a detailed baseline survey that collected information on demographic characteristics, lifestyle and dietary habits, medical history, and other exposures. Anthropometric measurements, including current weight, height, and circumferences of the waist and hip, were also taken.35 The study was approved by the relevant Institutional Review Boards for human research in both China and the United States. Written informed consent was obtained from all study participants.
Seventy-six percent of cohort members (n = 56,942) donated a 10-mL blood sample at baseline. After collection, samples were kept at 0 to 4°C and processed within 6 hours. Immediately after processing, all samples were stored at −70°C until laboratory analyses were conducted.
Measurement of Inflammatory Markers and Their Receptors
The sample preparation was performed at Vanderbilt Survey and Biospecimen Shared Resource. Cytokines and their receptors were assayed by using the Millipore’s MILLIPLEX MAP High Sensitivity Human Cytokine multiplex kit (Millipore Corporation) for IL-1β, IL-6, and TNFα28 and the MILLIPLEX MAP Human Soluble Cytokine Receptor Panel multiplex kits (Millipore Corporation) for soluble IL-6 receptor (sIL-6R), soluble GP130 (sGP130, a regulator of IL-6/sIL-6R complex signaling), soluble TNF-R1, and sTNF-R236 at the Hormone Assay & Analytical Services Core, Vanderbilt University, following manufacturer’s instructions. Plasma samples and standards were assayed in duplicate. All laboratory assays were performed in 2009 to 2010. High-sensitivity CRP measurements were performed using ACE High Sensitivity C-Reactive Protein Reagent (ACI-22) on an ACE Clinical Chemistry System (Alfa Wassermann, Inc).28 A second batch of samples was analyzed using the CRP (HS) Wide Range kit (Pointe Scientific) on ACE Clinical Chemistry System following the manufacturer’s protocol. We adjusted for batch in all analyses. Limits of detection were as follows: TNFα, 0.05 pg/mL; sTNF-R1, 9.6 pg/mL; sTNF-R2,16.9 pg/mL; IL-1β,0.06 pg/mL; IL-6,0.10 pg/ mL; sGP130,7.2 pg/mL; sIL-6R, 3.6 pg/mL; and CRP, 0.1 mg/L Intra-assay coefficients of variation were 11.8% for TNFα, 7.5% for sTNF-R1, 5.5% for sTNF-R2,17.4% for IL-1 β,15.5% for IL-6, 3.6% for sGP130, and 3.8% for sIL-6R in this study; inter-assay coefficients of variation were <21% in our validation study conducted in the same core laboratory.28
Assessment of Diet
Habitual dietary intakes during the preceding 12 months were collected during in-person interviews using a validated FFQ.33 For each food item or food group, participants were asked how frequently (daily, weekly, monthly, yearly, or never) they consumed the food or food groups, followed by a question on the amount consumed in liang (1 liang = 50 g) per unit of time. For seasonal foods (mainly vegetables and fruits), an in-season consumption pattern was elicited. The FFQ included a comprehensive list of soy foods (11 soy food items) commonly consumed in Shanghai, including soy milk, tofu, fried tofu, dried or pressed tofu, fresh green soy beans, dry soy beans, soy sprouts, and other soy products. Nutrient intakes, including soy protein and isoflavones, were calculated by summing nutrient intake amounts for each food item or food group. This was calculated by multiplying the reported amount of food intake by the nutrient content of the food item reported in the Chinese Food Composition Tables (2002).37 Because the water content of soy foods varies widely (96.4% for soy milk, 82.8% for tofu, 65.2% for dried/pressed tofu, 10.2% for dry soybeans), we also calculated total intake of the dry weight of soy foods.37 Neither soy protein isolate nor isoflavone supplements are commonly consumed in our study population13 and they were not included in the study. In addition to the FFQ survey, information on the frequency of soy food consumption in the preceding 24 hours was collected in person by trained interviewers when biospecimens were procured.
Statistical Analysis
Participants with less than the detectable limits of inflammatory markers were excluded from the analysis (TNFα, n = 4; sTNF-R1, n = 5; sTNF-R2, n = 1; IL-1β, n=102; IL-6, n=71; sGP130, n = 1; sIL-6R, n = 3; and CRP, n = 90), as were participants with outliers according to a box plot (sTNF-R1, n = 1; sTNF-R2, n = 3; sGP130, n = 1; and sIL-6R, n = 6). Log-transformation was conducted to normalize the distribution of inflammatory markers studied. Geometric means of these markers were obtained based on the least square means estimated using a general linear model according to quintiles of soy food, soy protein, and isoflavone intake assessed by the FFQ or frequency (0,1, or ≥2 times) of soy food consumption in the 24 hours before the blood sample collection. Tests for trend were performed by entering the categorical variables as continuous variables in the linear regression model. We also used a restricted cubic spline linear regression analysis38 to evaluate the association between selected biomarkers and soy food intake on a continuous basis. Knots were placed at the 5th, 50th, and 95th percentiles of the distribution of soy food intake. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for a cross-sectional analysis were followed (http://www.strobe-statement.org/).
Categorical covariates adjusted for in multivariable models included education (college and beyond vs less than college), occupation (professional and clerical vs manual laborer), history of infectious or inflammation-related diseases (including coronary heart diseases, hypertension, diabetes mellitus, tuberculosis, bronchitis, asthma, gastritis, ulcerative colitis, and hepatitis; yes or no), menopausal status (pre- or postmenopausal), cigarette smoking (yes or no), alcohol consumption (yes or no), aspirin and other nonsteroid anti-inflammatory drug use (yes or no), vitamin supplement use (yes or no), season of interview (Spring vs other seasons), and assay batch. Continuous covariates adjusted for included age, body mass index (BMI; calculated as kg/m2), physical activity (measured as metabolic equivalent hours per week per year),39 total calories, and total fruit and vegetable intakes. Overall metabolic equivalents from physical activity were calculated as the sum of metabolic equivalents from recreational activity and nonrecreational activity. Nonrecreational activity included walking, cycling, stair climbing, and household activities.
In sensitivity analyses, participants with CRP >10 mg/L were excluded to reduce the influence of potential acute infectious conditions on our results,40 and participants with less than the detectable limits of markers were included to check whether nondetection differed by soy food intake. We also evaluated potential effect modification by age, BMI, menopausal status, and self-reported history of infectious or inflammation-related diseases. Tests for interaction were performed by including cross-product terms of covariates and soy food intake in the main effect model. Multicollinearity was not a concern because the variance inflation factors for all variables were <3 (the variance inflation factors were about 1 for most covariates).41 All statistical tests were two-sided and performed using SAS statistical software (version 9.2, 2010, SAS Institute).
RESULTS
Characteristics of study participants by quintiles of total soy food intake are presented in Table 1. Women with high intake of soy foods were likely to be older and consume more vegetables. There were no significant differences in other variables across categories of soy food intake, including BMI, waist-to-hip ratio, physical activity, cigarette smoking, or history of infectious or inflammation-related diseases. In addition, there were no significant differences in season of interview across categories of soy food intake. Apart from age, the characteristics of this selected subset of the cohort were not significantly different from the rest of the cohort (data not shown).
Table 1.
Characteristics of study participants according to total soy food intake: Shanghai Women’s Health Studya
| Quintiles of Soy Food Intake (g/day)b |
||||||
|---|---|---|---|---|---|---|
| 0.01–8.6 (n=201 |
8.7–14.0 (n=201 |
14.1–19.2 (n=201 |
19.3–27.4 (n=201 |
27.5–95.0 (n=201 |
P for trendc |
|
| mean±standard deviation | ||||||
| Age (y) | 57.5±9.2 | 56.7 ±9.2 | 58.0±8.8 | 59.0±8.1 | 59.2±8.4 | 0.03 |
| % | ||||||
| Education, college, and beyond | 9.9 | 10.9 | 11.1 | 12.7 | 9.3 | 0.79 |
| Household income >30,000 yuan/yd | 13.2 | 15.8 | 12.6 | 11.4 | 16.8 | 0.58 |
| Professional occupation | 22.9 | 25.2 | 26.6 | 25.5 | 23.9 | 0.91 |
| Cigarette smoking | 3.6 | 2.8 | 3.9 | 2.8 | 5.1 | 0.71 |
| Alcohol consumption | 2.1 | 3.2 | 2.4 | 1.9 | 4.7 | 0.40 |
| Post-menopause | 76.2 | 73.4 | 75.7 | 71.7 | 76.7 | 0.14 |
| Postmenopausal hormone use | 1.5 | 2.1 | 2.6 | 1.9 | 4.4 | 0.45 |
| Aspirin and other nonsteroidal anti-inflammatory drug use | 4.6 | 1.2 | 2.9 | 4.2 | 4.1 | 0.32 |
| History of infectious and/or inflammation-related diseasese | 56.6 | 60.5 | 55.9 | 57.3 | 67.5 | 0.11 |
| Season of interview | ||||||
| Spring | 45.07 | 36.39 | 37.35 | 37.56 | 36.93 | 0.80 |
| Summer | 24.35 | 28.95 | 30.91 | 27.54 | 34.75 | |
| Fall | 20.72 | 23.72 | 20.62 | 22.22 | 19.07 | |
| Winter | 9.86 | 10.94 | 11.12 | 12.68 | 9.25 | |
| mean±standard error of estimate | ||||||
| Body mass indexf | 24.7 ±0.2 | 24.6 ±0.2 | 24.7±0.2 | 24.7±0.2 | 24.5 ±0.2 | 0.66 |
| Waist-to-hip ratio | 0.825 ±0.004 | 0.823 ±0.004 | 0.816±0.004 | 0.824±0.004 | 0.823 ±0.004 | 0.83 |
| Physical activity (METg h/wk/y) | 106.1 ±3.0 | 102.6±3.0 | 111.5±3.0 | 107.0±3.0 | 110.1 ±3.0 | 0.20 |
| Dietary intake | ||||||
| Fruit (g/day) | 239.3±11.3 | 225.411.4 | 234.0±11.3 | 252.6±11.3 | 237.0±11.4 | 0.54 |
| Vegetables (g/day) | 238.3 ±10.8 | 267.3 ±10.8 | 272.0±10.8 | 299.5 ±10.8 | 344.7 ±10.8 | <0.0001 |
| Red meat (g/day) | 43.6±2.0 | 45.4±2.0 | 46.0±2.0 | 46.7±2.0 | 47.4±2.0 | 0.17 |
| Total calories (kcal/day) | 1,665.3 ±28.6 | 1,683.2 ±28.7 | 1,678.7 ±28.6 | 1,697.5±28.6 | 1,592.1 ±28.7 | 0.15 |
| Soy protein (g/day) | 3.1 ±0.2 | 5.8±0.2 | 7.8±0.2 | 10.6±0.2 | 17.0±0.2 | <0.0001 |
| Isoflavones (mg/day) | 9.9±0.7 | 19.4±0.7 | 26.8 ±0.7 | 37.0±0.7 | 59.6±0.7 | <0.0001 |
Except for mean age, data were standardized to age distribution; data on dietary variables, except for total calories, were further standardized to total calorie intake
The amount of soy food intake was assessed on a dry-weight basis.
Linear regression models were used for continuous variables and the Cochran-Mantel-Haenszel χ2 test for categorical variables.
US$1=8 yuan, at the time of recruitment.
History of infectious and/or inflammation-related diseases included coronary heartdiseases, hypertension, diabetes mellitus, tuberculosis, bronchitis, asthma, gastritis, ulcerative colitis, and hepatitis
Calculated as kg/m2
MET=metabolic equivalent.
After adjustment for age, batch of biomarker measurement, and total energy intake, we found that women in higher quintiles compared with the lowest quintile of soy food intake had lower concentrations of IL-6, TNFα, and IL-1 β (P for trend <0.05 for IL-6 and TNFα; P for trend=0.05 for IL-1 β) (data not shown). Further adjustment for lifestyle and other dietary factors did not appreciably alter the results for IL-6 and TNFα, but attenuated the association with IL-1 β (Table 2). The fully adjusted geometric mean of IL-6 was inversely associated with quintiles of soy food intake (4.39, 4.00, 3.77, 3.39, and 3.27 pg/mL for the lowest to highest quintiles, respectively; P for trend = 0.008), with a difference between the highest and lowest quintiles of 25.5%. Similar inverse associations were observed for TNFα across quintiles of soy food intake (6.37, 6.13, 5.82, 5.48, and 5.46 pg/mL for the lowest to highest quintiles, respectively; P for trend=0.04). Women in the highest quintile of soy food intake had 14% lower TNFα levels compared with women in the lowest quintile. Inverse associations with concentrations of IL-6 and TNFα were also found for dietary intake of soy protein and isoflavones (Table 2).
Table 2.
Multivariable-adjusted concentrations of inflammatory markers in women according to quintiles of daily intake of soy foods, soy protein, and isoflavones, the Shanghai Women’s Health Studya
| By Quintiles of Soy Food Intakeb |
By Quintiles of Soy Protein Intakeb |
By Quintiles of Isoflavone Intakeb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker | n | Geometric mean±SEE |
Differenced (%) |
P for trend |
n | Geometric mean±SEE |
Differenced (%) |
P for trend |
n | Geometric mean±SEE |
Differenced (%) |
P for trend |
| Interleukin-6 (IL-6) (pg/mL) | 183 | 4.39±1.09 | Reference | 0.008 | 183 | 4.30±1.09 | Reference | 0.007 | 182 | 4.35±1.09 | Reference | 0.006 |
| 188 | 4.00±1.09 | −8.88 | 187 | 4.09±1.09 | −4.88 | 190 | 4.17± 1.09 | −4.14 | ||||
| 186 | 3.77±1.09 | −14.12 | 184 | 3.66±1.09 | −14.88 | 183 | 3.69±1.09 | −15.17 | ||||
| 185 | 3.39±1.09 | −22.78 | 189 | 3.74±1.09 | −13.02 | 187 | 3.34±1.09 | −23.22 | ||||
| 188 | 3.27±1.09 | −25.51 | 187 | 3.05±1.09 | −29.07 | 188 | 3.29±1.09 | −24.37 | ||||
| Soluble GP130 (pg/mL) | 193 | 177,089±1.02 | Reference | 0.69 | 193 | 176,510± 1.02 | Reference | 0.64 | 191 | 177,825 ±1.02 | Reference | 0.69 |
| 185 | 183,599±1.02 | 3.68 | 185 | 185,664 ±1.02 | 5.19 | 186 | 181,821 ±1.02 | 2.25 | ||||
| 188 | 179,428 ±1.02 | 1.32 | 187 | 177,329±1.02 | 0.46 | 187 | 179,603 ±1.02 | 1.00 | ||||
| 186 | 178,571 ±1.02 | 0.84 | 190 | 180,048 ±1.02 | 2.00 | 187 | 180,083 ±1.02 | 1.27 | ||||
| 192 | 176,811 ±1.02 | −0.16 | 189 | 176,039±1.02 | −0.27 | 193 | 176,105 ±1.02 | −0.97 | ||||
| Soluble IL-6 receptor (pg/mL) | 193 | 21,574±1.04 | Reference | 0.99 | 193 | 210,159± 1.04 | Reference | 0.96 | 191 | 21,867 ±1.04 | Reference | 0.96 |
| 187 | 21,664±1.04 | 0.41 | 187 | 22,635 ±1.04 | 7.70 | 188 | 20,813 ±1.04 | −4.82 | ||||
| 188 | 21,684±1.04 | 0.51 | 187 | 21,560±1.04 | 2.59 | 187 | 22,546 ±1.04 | 3.11 | ||||
| 187 | 21,553±1.04 | −0.10 | 191 | 21,483 ±1.04 | 2.22 | 188 | 21,105± 1.04 | −3.48 | ||||
| 192 | 21,644±1.04 | 0.32 | 189 | 21,480±1.04 | 2.21 | 193 | 21,824 ±1.04 | −0.20 | ||||
| Tumor necrosis factor-α (TNFα) (pg/mL) | 201 | 6.37±1.05 | Reference | 0.04 | 201 | 6.31 ±1.05 | Reference | 0.03 | 201 | 6.26±1.05 | Reference | 0.04 |
| 200 | 6.13± 1.05 | −3.77 | 201 | 6.28±1.05 | −0.48 | 200 | 6.37±1.05 | 1.76 | ||||
| 200 | 5.82±1.05 | −8.63 | 198 | 5.72±1.05 | −9.35 | 200 | 5.85 ±1.05 | −6.55 | ||||
| 199 | 5.48±1.05 | −8.79 | 201 | 5.91 ±1.05 | −6.34 | 198 | 5.54±1.05 | −11.50 | ||||
| 197 | 5.46±1.06 | −13.97 | 196 | 5.39±1.05 | −14.58 | 198 | 5.60±1.06 | −10.54 | ||||
| Soluble TNF receptor 1 (pg/mL) | 193 | 1,076.68±1.03 | Reference | 0.87 | 193 | 1,076.26±1.03 | Reference | 0.94 | 191 | 1,091.90± 1.03 | Reference | 0.79 |
| 187 | 1,193.36±1.03 | 10.84 | 187 | 1,210.03±1.03 | 12.43 | 188 | 1,172.05±1.03 | 7.34 | ||||
| 188 | 1,101.54±1.03 | 2.31 | 188 | 1,083.47 ±1.03 | 0.67 | 188 | 1,088.54 ±1.03 | −0.31 | ||||
| 189 | 1,140.33±1.03 | 5.91 | 190 | 1,154.32±1.03 | 7.25 | 188 | 1,148.99± 1.03 | 5.23 | ||||
| 193 | 1,108.40±1.03 | 2.95 | 192 | 1,098.32±1.03 | 2.05 | 195 | 1,116.48±1.03 | 2.25 | ||||
| Soluble TNF receptor 2 (pg/mL) | 193 | 4,350.67 ±1.02 | Reference | 0.16 | 193 | 4,344.28 ±1.02 | Reference | 0.048 | 191 | 4,345.17±1.02 | Reference | 0.15 |
| 188 | 4,391.28± 1.02 | 0.93 | 188 | 4,471.24 ±1.02 | 2.92 | 189 | 4,363.22± 1.02 | 0.42 | ||||
| 189 | 4,307.66±1.02 | −0.99 | 188 | 4,284.54 ±1.02 | −1.38 | 188 | 4,353.63 ±1.02 | 0.19 | ||||
| 188 | 4,287.66±1.02 | −1.45 | 191 | 4,321.26± 1.02 | −0.53 | 189 | 4,305.27 ±1.02 | −0.92 | ||||
| 194 | 4,161.54± 1.02 | −4.35 | 192 | 4,083.33 ±1.02 | −6.01 | 195 | 4,134.38± 1.02 | −4.85 | ||||
| IL-1β (pg/mL) | 183 | 1.28±1.09 | Reference | 0.11 | 182 | 1.26±1.09 | Reference | 0.12 | 183 | 1.27 ±1.09 | Reference | 0.17 |
| 182 | 1.46 ±1.09 | 14.06 | 182 | 1.47 ±1.09 | 16.67 | 184 | 1.55±1.09 | 22.05 | ||||
| 183 | 1.24±1.09 | −3.13 | 180 | 1.25 ±1.09 | −0.79 | 182 | 1.12±1.09 | −11.81 | ||||
| 181 | 1.16±1.09 | −9.38 | 185 | 1.17±1.09 | −7.14 | 179 | 1.14±1.09 | −10.24 | ||||
| 170 | 1.13±1.10 | −11.72 | 170 | 1.12±1.09 | −11.11 | 171 | 1.21 ±1.09 | −4.72 | ||||
| C-reactive protein (CRP) (mg/L) | 151 | 1.20±1.10 | Reference | 0.51 | 150 | 1.13±1.10 | Reference | 0.34 | 151 | 1.08±1.09 | Reference | 0.49 |
| 171 | 1.08±1.09 | −10.00 | 171 | 1.17±1.09 | 3.54 | 169 | 1.30±1.09 | 20.37 | ||||
| 151 | 1.25 ±1.09 | 4.17 | 155 | 1.20±1.09 | 6.19 | 152 | 1.15±1.09 | 6.48 | ||||
| 155 | 1.20±1.09 | 0.00 | 154 | 1.14±1.09 | 0.88 | 156 | 1.18±1.09 | 9.26 | ||||
| 162 | 1.25 ±1.09 | 4.17 | 160 | 1.33±1.09 | 17.70 | 162 | 1.25 ±1.09 | 15.74 | ||||
Adjusted for age, education, occupation, cigarette smoking, alcohol consumption, body mass index, vitamin supplement use, menopausal status, aspirin and other nonsteroidal anti-inflammatory drug use, season of interview, total intake of fruits and vegetables, total energy intake, physical activity, history of infectious and/or inflammation-related diseases, and assay batch in general linear regression models.
Quintile cutoffs for soy food intake assessed on a dry-weight basis: 0.01— 8.6,14.0,19.2, and 27.4— 95.0 g/day; quintile cutoffs for soy protein intake: 0.01 — 4.3,6.8,9.5, and 13.2— 47.5 g/day; quintile cutoffs for isoflavone intake: 0.02—14.0, 23.0,33.1, and 46.4— 147.2 mg/day.
SEE=standard error of estimate.
Difference (%)=(geometric mean of inflammatory marker in each quintile— geometric mean in the lowest quintile)/geometric mean in the lowest quintile of soy foods, soy protein, or isoflavone intake.
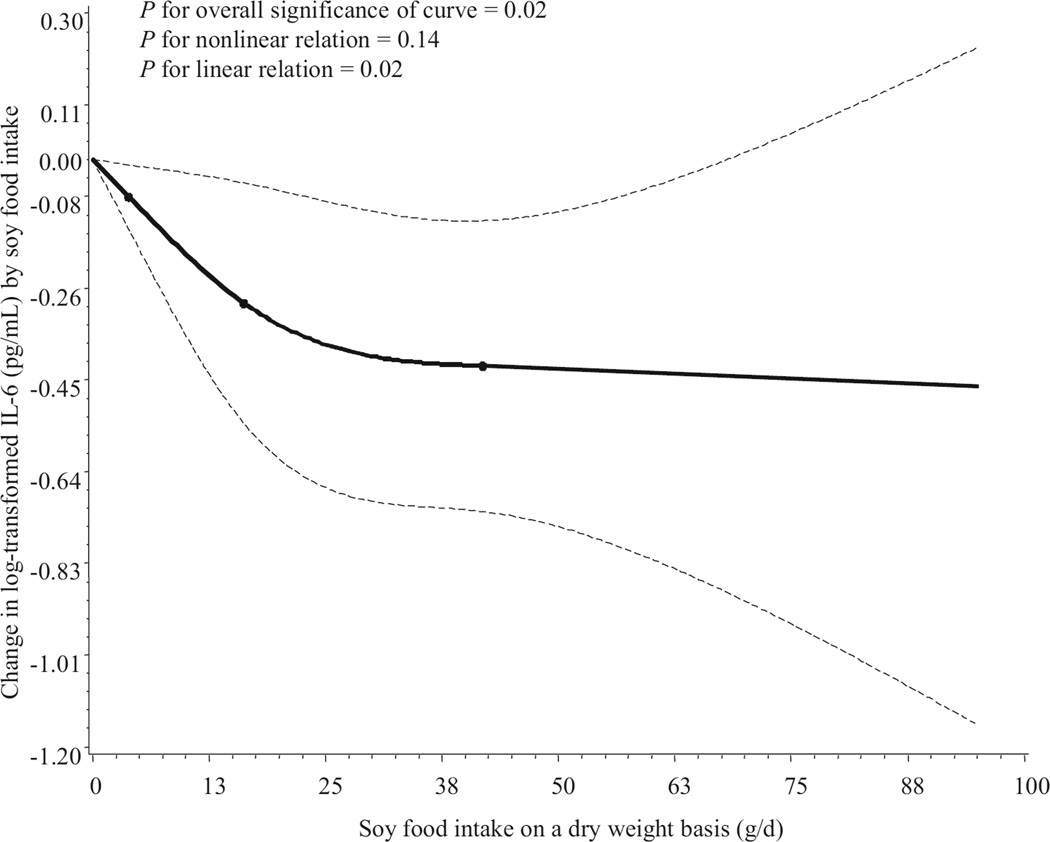
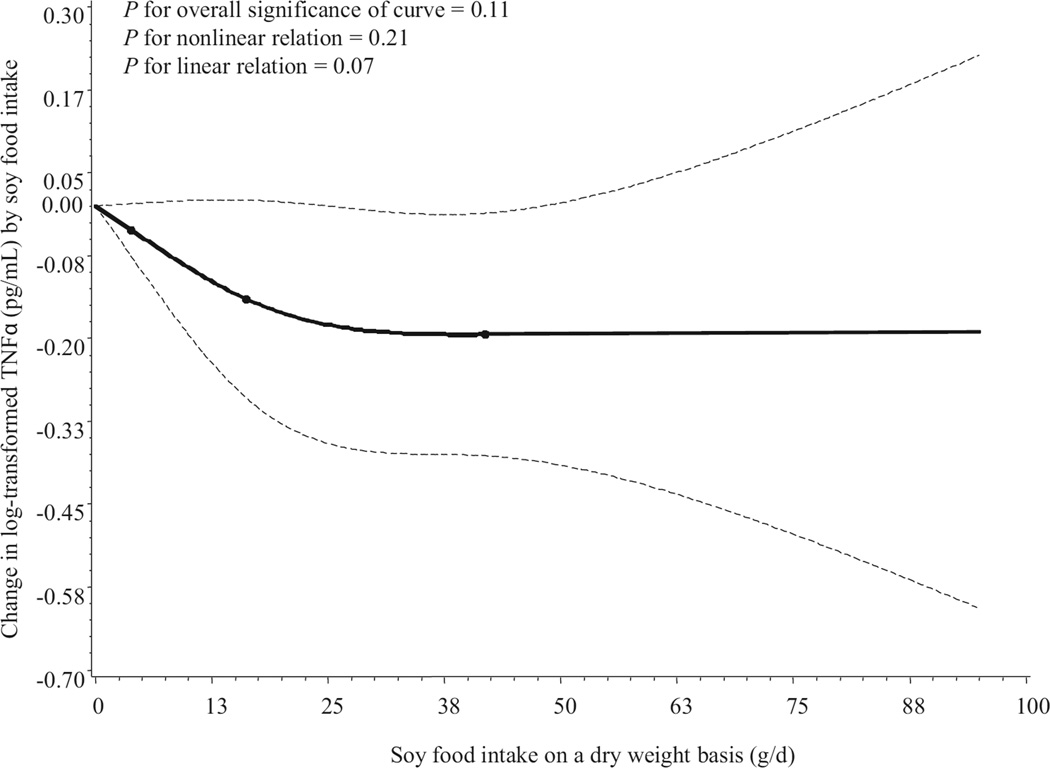
When intake of soy foods was analyzed on a continuous basis in a restricted cubic spline linear regression model, the concentrations of IL-6 were inversely associated with soy food intake (Figure 1, available online at www.andjrnl.org), with an apparent monotonic trend (P for overall significance = 0.02 and P for linear relation = 0.02). A similar inverse association between TNFα level and soy food intake was found, but TNFα levels remained unchanged beyond 28 g/day dry weight of soy food intake (at the 80th percentile of the distribution of intake). Tests for overall significance and linear trend were not statistically significant (Figure 2, available online at www.andjrnl.org).
Figure 1.
Smoothed plot of changes in logarithmically transformed interleukin-6 (IL-6) levels (pg/mL) according to intake of soy foods. The amount of soy food intake was assessed on a dry-weight basis (g/day). Changes in IL-6 concentration relative to that in nonconsumers of soy foods were estimated by restricted cubic-spline linear regression analysis with knots placed at the 5th, 50th, and 95th percentiles of intake, after adjustment for age, education, occupation, cigarette smoking, alcohol consumption, body mass index, vitamin supplement use, menopausal status, aspirin and other nonsteroidal anti-inflammatory drug use, seasons of interview, total intake of fruits and vegetables, total energy intake, physical activity, history of infectious and/or inflammation-related diseases, and assay batch. Point estimates are indicated by a solid line and 95% confidence intervals by dashed lines.
Figure 2.
Smoothed plot of changes in logarithmically transformed tumor necrosis factor-α (TNFα) concentration (pg/mL) according to intake of soy foods. The amount of soy food intake was assessed on a dry-weight basis (g/day). Changes in TNFα concentration relative to that in nonconsumers of soy foods were estimated by restricted cubic-spline linear regression analysis with knots placed at the 5th, 50th, and 95th percentiles of intake, after adjustment for age, education, occupation, cigarette smoking, alcohol consumption, body mass index, vitamin supplement use, menopausal status, aspirin, and other nonsteroidal anti-inflammatory drug use, seasons of interview, total intake of fruits and vegetables, total energy intake, physical activity, history of infectious and/or inflammation-related diseases, and assay batch. Point estimates are indicated by a solid line and 95% confidence intervals by dashed lines.
Similar inverse associations were found for TNFα (P for trend = 0.003), sTNF-R1 (P for trend=0.01), sTNF-R2 (P for trend=0.02), IL-1 β (P for trend=0.05), and IL-6 (P for trend=0.04) when soy food consumption was assessed by the frequency of consumption in the preceding 24 hours (Table 3). Geometric mean concentrations of inflammatory markers in women consuming soy foods two or more times vs none in the preceding 24 hours were 18.7% lower forTNFα, 16.3% lower for sTNF-R1, 9.4% lower for sTNF-R2,11.9% lower for IL-1β, and 29.1% lower for IL-6, after adjusting for socioeconomic and lifestyle factors, health conditions, and other factors collected for the preceding 24 hours, including cigarette smoking and use of antibiotics, vitamin supplements, and nonsteroidal anti-inflammatory drugs. The association with soy food consumption in the preceding 24 hours was attenuated, but persisted significantly after further adjustment for usual intake of soy foods assessed by the FFQ (data not shown). No significant associations were found between soy food consumption and levels of sIL-6R, sGP130, or CRP (Table 2 and Table 3).
Table 3.
Concentrations of inflammatory markers by the frequency of soy consumption in the 24 hours before sample collection, the Shanghai Women’s Health Studya
| Age-, Batch-, and Energy-Adjusted Model |
Multivariable-Adjusted Model |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Marker | n | Soy intake frequency | Geometric mean | SEEb | Differencec (%) | P for trend | Geometric mean | SEE | Differencec(%) | P for trend |
| Interleukin-6 (IL-6) (pg/mL) | 547 | 0 | 3.94 | 1.05 | Reference | 0.03 | 3.92 | 1.05 | Reference | 0.04 |
| 327 | 1 | 3.65 | 1.06 | −7.36 | 3.64 | 1.06 | −7.14 | |||
| 57 | ≥ 2 | 2.69 | 1.16 | −31.73 | 2.78 | 1.16 | −29.08 | |||
| Soluble GP130 (pg/mL) | 551 | 0 | 179,425 | 1.01 | Reference | 0.57 | 179,476 | 1.01 | Reference | 0.55 |
| 334 | 1 | 179,344 | 1.02 | −0.05 | 179,413 | 1.02 | −0.04 | |||
| 60 | ≥ 2 | 173,666 | 1.04 | −3.21 | 173,216 | 1.04 | −3.49 | |||
| Soluble IL-6 receptor (pg/mL) | 553 | 0 | 21,977 | 1.02 | Reference | 0.14 | 21,923 | 1.02 | Reference | 0.23 |
| 335 | 1 | 21,281 | 1.03 | −3.16 | 21,365 | 1.03 | −2.55 | |||
| 60 | ≥ 2 | 20,140 | 1.06 | −8.36 | 20,366 | 1.07 | −7.10 | |||
| Tumor necrosis factor-α (TNFα) (pg/mL) | 581 | 0 | 6.24 | 1.03 | Reference | 0.003 | 6.25 | 1.03 | Reference | 0.003 |
| 354 | 1 | 5.59 | 1.04 | −10.42 | 5.55 | 1.04 | −11.20 | |||
| 63 | ≥ 2 | 4.98 | 1.09 | −20.19 | 5.08 | 1.09 | −18.72 | |||
| Soluble TNF receptor 1 (pg/mL) | 552 | 0 | 1,144.28 | 1.02 | Reference | 0.01 | 1,145.26 | 1.02 | Reference | 0.01 |
| 337 | 1,121.25 | 1.02 | −2.01 | 1,119.43 | 1.02 | −2.26 | ||||
| 62 | ≥ 2 | 961.22 | 1.05 | −16.00 | 958.98 | 1.05 | −16.27 | |||
| Soluble TNF receptor 2 (pg/mL) | 555 | 0 | 4,369.81 | 1.01 | Reference | 0.01 | 4,367.76 | 1.01 | Reference | 0.02 |
| 337 | 1 | 4,239.39 | 1.02 | −2.98 | 4,249.58 | 1.02 | −2.71 | |||
| 61 | ≥ 2 | 3,954.80 | 1.04 | −9.50 | 3,957.87 | 1.04 | −9.38 | |||
| IL-1β (pg/mL) | 529 | 0 | 1.34 | 1.05 | Reference | 0.06 | 1.34 | 1.05 | Reference | 0.05 |
| 319 | 1 | 1.14 | 1.07 | −14.93 | 1.12 | 1.07 | −16.42 | |||
| 52 | ≥ 2 | 1.16 | 1.17 | −13.43 | 1.18 | 1.17 | −11.94 | |||
| C-reactive protein (CRP) (mg/L) | 463 | 0 | 1.25 | 1.05 | Reference | 0.20 | 1.24 | 1.05 | Reference | 0.25 |
| 281 | 1 | 1.12 | 1.07 | −10.40 | 1.13 | 1.07 | −8.87 | |||
| 47 | 2 | 1.10 | 1.18 | −12.00 | 1.13 | 1.17 | −8.87 | |||
Adjusted for age, education, occupation, alcohol consumption, body mass index, menopausal status, season of interview, total intake of fruits and vegetables, total energy intake, physical activity, history of infectious and/or inflammation-related diseases and assay batch in general linear regression models, as well as factors collected for the preceding 24 hours, including cigarette smoking and use of antibiotics, vitamin supplements, and nonsteroidal anti-inflammatory drugs.
SEE=standard error of estimate
Difference (%)=(geometric mean of inflammatory marker in each group—geometric mean in the group with no consumption of soyfood)/geometric mean in the group with no consumption of soy food
In stratified analyses, the observed inverse associations between soy food intake and levels of selected inflammatory biomarkers were not modified by age, BMI, menopausal status, postmenopausal hormone use, or history of infectious or inflammation-related diseases (data not shown).
To reduce the influence of potential acute infection on measures of these biomarkers, we conducted a sensitivity analysis by excluding participants with CRP >10 mg/L (n = 29, 2.89%). The exclusion did not markedly change the results for IL-6 (P for trend=0.02), but the inverse association for TNFα became weaker (P for trend=0.06). With this exclusion, the inverse association of IL-1 β level with soy food intake became statistically significant; adjusted geometric means were inversely associated with quintiles of soy food intake (1.35, 1.46, 1.23, 1.11, and 1.09 pg/mL for the lowest to highest quintiles, respectively; P for trend=0.02). In another sensitivity analysis, inclusion of participants with less than the detectable limits of markers (assigning one half the detectable limits to the missing data) did not markedly change the results for TNFα (P for trend=0.04) or IL-1β (P for trend = 0.02). However, the inverse association for IL-6 became weaker (P for trend=0.10) (Table 4, available online at www.andjrnl.org.andjrnl.org).
Table 4.
Multivariable-adjusted concentrations of inflammatory markers in women according to quintiles of daily intake of soy foods, including both detectable and undetectable measurements of the biomarkers studied, the Shanghai Women′s Health Studyab
| By Quintiles of Soy Food Intakec |
By Quintiles of Soy Protein Intakec |
By Quintiles of Isoflavone Intakec |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker | n | Geometric mean±SEEd |
Differencee (%) |
P for trend |
n | Geometric mean± SEE |
Differencee (%) |
P for trend |
n | Geometric mean± SEE |
Differencee (%) |
P for trend |
| Interleukin-6 (IL-6) (pg/mL) | 201 | 3.06±1.11 | Reference | 0.10 | 201 | 2.97±1.11 | Reference | 0.14 | 201 | 2.94±1.11 | Reference | 0.11 |
| 201 | 3.07±1.11 | 0.33 | 201 | 3.09±1.11 | 4.04 | 201 | 3.34±1.11 | 13.61 | ||||
| 201 | 2.73±1.11 | −10.78 | 201 | 2.58±1.11 | −13.13 | 201 | 2.53±1.11 | −13.95 | ||||
| 201 | 2.37±1.11 | −22.55 | 201 | 2.79±1.11 | −6.06 | 200 | 2.49±1.11 | −15.31 | ||||
| 197 | 2.60±1.11 | −15.03 | 197 | 2.39±1.11 | −19.53 | 198 | 2.55±1.11 | −13.27 | ||||
| Soluble GP130 (pg/ mL) | 193 | 178,614±1.05 | Reference | 0.10 | 193 | 177,455 ±1.05 | Reference | 0.08 | 191 | 178,289 ±1.05 | Reference | 0.35 |
| 189 | 175,758±1.05 | −1.60 | 189 | 178,552±1.05 | 0.62 | 190 | 174,372±1.05 | −2.20 | ||||
| 189 | 178,277 ±1.05 | −0.19 | 189 | 168,497 ±1.05 | −5.05 | 189 | 169,803 ±1.05 | −4.76 | ||||
| 190 | 158,609 ±1.05 | −11.20 | 191 | 176,964 ±1.05 | −0.28 | 191 | 159,876±1.05 | −10.33 | ||||
| 195 | 164,563 ±1.05 | −7.87 | 194 | 154,741 ±1.05 | −12.80 | 195 | 172,932±1.05 | −3.00 | ||||
| Soluble IL-6 receptor (pg/mL) | 193 | 21,711 ±1.06 | Reference | 0.20 | 193 | 21,090 ±1.06 | Reference | 0.16 | 191 | 21,895 ±1.06 | Reference | 0.50 |
| 189 | 21,112±1.06 | −2.76 | 189 | 22,142± 1.06 | 4.99 | 190 | 20,298 ±1.06 | −7.30 | ||||
| 189 | 21,386±1.06 | −1.50 | 189 | 20,258 ±1.06 | −3.94 | 189 | 21,110±1.06 | −3.59 | ||||
| 190 | 19,113±1.06 | −11.97 | 191 | 21,324 ±1.06 | 1.11 | 191 | 18,875 ±1.06 | −13.79 | ||||
| 195 | 20,209 ±1.06 | −6.92 | 194 | 18,801 ±1.06 | −10.85 | 195 | 21,367 ±1.06 | −2.41 | ||||
| Tumor necrosis factor-α (TNFα) (pg/mL) | 201 | 6.40±1.06 | Reference | 0.04 | 201 | 6.33 ±1.06 | Reference | 0.02 | 201 | 6.27 ±1.06 | Reference | 0.03 |
| 201 | 5.98±1.06 | −6.56 | 201 | 6.29±1.06 | −0.63 | 201 | 6.22±1.06 | −0.80 | ||||
| 201 | 5.67 ±1.06 | −11.41 | 201 | 5.30±1.06 | −16.27 | 201 | 5.69±1.06 | −9.25 | ||||
| 201 | 5.49±1.06 | −14.22 | 201 | 5.89±1.06 | −6.95 | 200 | 5.25 ±1.06 | −16.27 | ||||
| 197 | 5.45 ±1.06 | −14.84 | 197 | 5.22±1.06 | −17.54 | 198 | 5.56±1.06 | −11.32 | ||||
| Soluble TNF receptor 1 (pg/mL) | 193 | 1,073.42±1.05 | Reference | 0.70 | 193 | 1,072.69±1.05 | Reference | 0.56 | 191 | 1,085.90± 1.05 | Reference | 0.95 |
| 189 | 1,130.10±1.04 | 5.28 | 189 | 1,146.93 ±1.04 | 6.92 | 190 | 1,110.69±1.04 | 2.28 | ||||
| 189 | 1,080.03 ±1.04 | 0.62 | 189 | 1,063.62 ±1.04 | −0.85 | 189 | 1,069.93 ±1.04 | −1.47 | ||||
| 190 | 1,100.16±1.04 | 2.49 | 191 | 1,117.95 ±1.04 | 4.22 | 191 | 1,048.08 ±1.04 | −3.48 | ||||
| 195 | 1,055.66±1.05 | −1.65 | 194 | 1,040.14±1.05 | −3.03 | 195 | 1,122.93 ±1.04 | 3.41 | ||||
| Soluble TNF receptor 2 (pg/mL) | 193 | 4,361.23 ±1.03 | Reference | 0.11 | 193 | 4,353.58± 1.03 | Reference | 0.04 | 191 | 4,349.28±1.03 | Reference | 0.21 |
| 189 | 4,269.46 ±1.03 | −2.10 | 189 | 4,347.65 ±1.03 | −0.14 | 190 | 4,242.99±1.03 | −2.44 | ||||
| 189 | 4,307.56±1.03 | −1.23 | 189 | 4,217.71 ±1.03 | −3.12 | 189 | 4,273.97 ±1.03 | −1.73 | ||||
| 190 | 4,112.79± 1.03 | −5.70 | 191 | 4,298.21 ±1.03 | −1.27 | 191 | 4,150.09± 1.03 | −4.58 | ||||
| 195 | 4,093.58±1.03 | −6.14 | 194 | 3,937.65 ±1.03 | −9.55 | 195 | 4,126.07 ±1.03 | −5.13 | ||||
| IL-1β (pg/mL) | 201 | 0.93±1.11 | Reference | 0.02 | 201 | 0.90±1.11 | Reference | 0.04 | 201 | 0.91 ±1.11 | Reference | 0.02 |
| 201 | 1.02±1.11 | 9.68 | 201 | 1.03 ±1.11 | 14.44 | 201 | 1.12±1.11 | 23.08 | ||||
| 201 | 0.89±1.11 | −4.30 | 201 | 0.85 ±1.11 | −5.56 | 201 | 0.80±1.11 | −12.09 | ||||
| 201 | 0.79±1.11 | −15.05 | 201 | 0.85 ±1.11 | −5.56 | 200 | 0.77±1.11 | −15.38 | ||||
| 197 | 0.68±1.12 | −26.88 | 197 | 0.67 ±1.12 | −25.56 | 198 | 0.72±1.11 | −20.88 | ||||
| C-reactive protein (CRP) (mg/L) | 169 | 0.89±1.11 | Reference | 0.44 | 169 | 0.85 ±1.11 | Reference | 0.30 | 170 | 0.80±1.11 | Reference | 0.39 |
| 179 | 0.96±1.11 | 7.87 | 179 | 1.02±1.10 | 20.00 | 177 | 1.13±1.10 | 41.25 | ||||
| 163 | 0.99±1.10 | 11.24 | 167 | 0.96±1.10 | 12.94 | 164 | 0.92± 1.10 | 15.00 | ||||
| 167 | 0.95±1.10 | 6.74 | 164 | 0.94±1.11 | 10.59 | 167 | 0.97± 1.10 | 21.25 | ||||
| 170 | 1.02±1.11 | 14.61 | 169 | 1.05 ±1.11 | 23.53 | 170 | 1.01 ±1.11 | 26.25 | ||||
Adjusted for age, education, occupation, cigarette smoking, alcohol consumption, body mass index, vitamin supplement use, menopausal status, aspirin and other nonsteroidal anti-inflammatory drug use, season of interview, total intake of fruits and vegetables, total energy intake, physical activity, history of infectious and/or inflammation-related diseases, and assay batch in general linear regression models.
Assigned one half the detectable limits to undetectable measures.
Quintile cutoffs for soy food intake assessed on a dry-weight basis: 0.01 —8.6,14.0,19.2, and 27.4—95.0 g/day; quintile cutoffs for soy protein intake: 0.01 —4.3,6.8,9.5, and 13.2—47.5 g/day; quintile cutoffs for isoflavone intake: 0.02—14.0, 23.0,33.1, and 46.4—147.2 mg/day.
SEE=standard error of estimate.
Difference (%)=(geometric mean of inflammatory marker in each quintile—geometric mean in the lowest quintile)/geometric mean in the lowest quintile of soy food, soy protein, or isoflavone intake.
DISCUSSION
In this large, population-based, cross-sectional study of Chinese women, we found that concentrations of IL-6, TNFα, and sTNF-R1 and 2 were inversely associated with intake of soy foods. These associations were independent of age, lifestyle factors, and history of infectious or inflammation-related diseases and were not explained by differences in other dietary factors, such as intakes of fruits, non–soy vegetables, or total calories.
To our knowledge, this is the first population-based study of soy food intake and inflammatory markers. Clinical trials of dietary supplementation with various soy foods or specific constituents of soy have been conducted and reported mixed results.15,16,24–26 Consumption of soy milk or soy nuts by postmenopausal women was associated with decreases in circulating levels of TNFα and CRP.24,25 Pasta enriched with isoflavone aglycons from soy germ reduced levels of CRP in middle-aged adults with hypercholesterolemia15 and 8-iso-PGF2α in patients with type 2 diabetes.16 However, taking purified phytoestrogens in the form of tablets or isolated soy protein did not substantially affect levels of inflammatory markers.26,42 This inconsistency might be explained, in part, by variations in the types, doses, or periods of intervention in these studies.
The potential mechanisms through which soy and its constituents affect inflammatory biomarkers remain to be clarified. In vitro data suggest that the effects of soy on modulating inflammatory markers such as TNFα are likely attributable to two phytoestrogens in soy, daidzein and genistein.24 Soy phytoestrogens, which are structurally similar to 17β-estradiol,43 may resemble hormone-replacement therapy regimens, reducing cell adhesion molecules and inflammatory markers.43 Soy daidzein and genistein have been found to inhibit prostaglandin E2 expression in a dose-dependent manner20,21 and improve oxidative stress.16,21 In addition, soy foods contribute 37% of total intake of polyunsaturated fat in our study population (data not shown). Diets rich in certain polyunsaturated fats, such as linolenic acid, have been shown to lower levels of inflammatory markers.44,45 For example, supplements with linolenic acid appear to reduce IL-6 levels in patients with dyslipidemia.45
The study population is well suited to the investigation of the soy and inflammation association because of its high, yet diverse, levels of soy food intake. As with any nutritional epidemiology study, measurement error in assessing soy food intake is a possible concern. However, the FFQ used in the study was found to have reasonably good validity for the measurement of usual dietary intake of soy foods.33 Soy food intake assessed by the FFQ and multiple 24-hour dietary recalls was moderately correlated (r=0.49). In addition, the inverse associations between inflammatory markers and usual intake of soy foods assessed by the FFQ were consistently observed in another analysis of the frequency of soy food consumption in the preceding 24 hours. Another concern is the exclusion of participants with less than detectable limits of markers from our primary analyses. The purpose of the exclusion was to avoid the influence of imprecise measurements of markers on the results. When including participants with below detectable limits of markers in a sensitivity analysis, the results for most markers studied were not markedly changed, although the association with IL-6 became slightly weaker for the highest quintile of intake. In addition, we could not completely rule out the possibility of residual confounding due to unmeasured or inaccurately measured covariates, although careful adjustment for a wide range of potential confounding factors did not appreciably change the results.
CONCLUSIONS
We found that soy food consumption was related to lower circulating levels of IL-6, TNFα, and sTNF-R1 and 2, pivotal cytokines in the inflammatory cascade, which have been associated with many chronic diseases.1–8,46,47 Women in the highest quintile of soy food intake had 26% lower levels of IL-6 and 14% lower levels of TNFa compared with women in the lowest quintile, which can have major implications for public health. For example, a 27% reduction in circulating levels of IL-6 between two extreme quartiles of the marker was associated with a 37% lower risk of lung cancer,48 a 32% reduction in IL-6 level was related to a 25% reduction in risk of type 2 diabetes,49 and a 15% reduction in TNFα level was associated with an 18% decreased risk of acute myocardial infarction.50 Further investigation of the soy-inflammation association and related potential health benefits are warranted.
ACKNOWLEDGEMENTS
We are grateful to the participants and research staff of the Shanghai Women’s Health Study for their contributions to the study. We thank Bethanie Rammer and Jacqueline Stern for their assistance in preparing the manuscript. We also thank Regina Courtney and Rodica Gal-Chris for sample preparations.
FUNDING/SUPPORT
This study was supported by US Public Health Service grants R01CA122364 and R37CA070867 and, in part, by R01HL095931, the National Institutes of Health intramural program (N02 CP1101066), and the Vanderbilt-Ingram Cancer Center (P30 CA68485).
Footnotes
STATEMENT OF POTENTIAL CONFLICT OF INTEREST
No potential conflict of interest was reported by the authors.
References
- 1.Galland L. Diet and inflammation. Nutr Clin Pract. 2010;25(6):634–640. doi: 10.1177/0884533610385703. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aleksandrova K, Jenab M, Boeing H, et al. Circulating C-reactive protein concentrations and risks of colon and rectal cancer: A nested case-control study within the European Prospective Investigation into Cancer and Nutrition. Am J Epidemiol. 2010;172(4):407–418. doi: 10.1093/aje/kwq135. [DOI] [PubMed] [Google Scholar]
- 4.Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004;291(5):585–590. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- 5.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 6.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14):1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 7.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8(suppl 2):S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridker PM, Silvertown JD. Inflammation, C-reactive protein, and atherothrombosis. J Periodontol. 2008;79(8 suppl):1544–1551. doi: 10.1902/jop.2008.080249. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236(1):13–23. doi: 10.1016/j.canlet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Messina MJ. Legumes and soybeans: Overview of their nutritional profiles and health effects. Am J Clin Nutr. 1999;70(3 suppl):439S–450S. doi: 10.1093/ajcn/70.3.439s. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Shu XO, Gao YT, et al. Soy food consumption is associated with lower risk of coronary heart disease in Chinese women. J Nutr. 2003;133(9):2874–2878. doi: 10.1093/jn/133.9.2874. [DOI] [PubMed] [Google Scholar]
- 12.Yang G, Shu XO, Jin F, et al. Longitudinal study of soy food intake and blood pressure among middle-aged and elderly Chinese women. Am J Clin Nutr. 2005;81(5):1012–1017. doi: 10.1093/ajcn/81.5.1012. [DOI] [PubMed] [Google Scholar]
- 13.Yang G, Shu XO, Li H, et al. Prospective cohort study of soy food intake and colorectal cancer risk in women. Am J Clin Nutr. 2009;89(2):577–583. doi: 10.3945/ajcn.2008.26742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SA, Shu XO, Li H, et al. Adolescent and adult soy food intake and breast cancer risk: Results from the Shanghai Women’s Health Study. Am J Clin Nutr. 2009;89(6):1920–1926. doi: 10.3945/ajcn.2008.27361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clerici C, Setchell KD, Battezzati PM, et al. Pasta naturally enriched with isoflavone aglycons from soy germ reduces serum lipids and improves markers of cardiovascular risk. J Nutr. 2007;137(10):2270–2278. doi: 10.1093/jn/137.10.2270. [DOI] [PubMed] [Google Scholar]
- 16.Clerici C, Nardi E, Battezzati PM, et al. Novel soy germ pasta improves endothelial function, blood pressure, and oxidative stress in patients with type 2 diabetes. Diabetes Care. 2011;34(9):1946–1948. doi: 10.2337/dc11-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottstein N, Ewins BA, Eccleston C, et al. Effect of genistein and daidzein on platelet aggregation and monocyte and endothelial function. Br J Nutr. 2003;89(5):607–616. doi: 10.1079/BJN2003820. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee TK, Nathan L, Dinh H, Reddy ST, Chaudhuri G. 17-epi-estriol, an estrogen metabolite, is more potent than estradiol in inhibiting vascular cell adhesion molecule 1 (VCAM-1) mRNA expression. J Biol Chem. 2003;278(14):11746–11752. doi: 10.1074/jbc.M207800200. [DOI] [PubMed] [Google Scholar]
- 19.Raschke M, Rowland IR, Magee PJ, Pool-Zobel BL. Genistein protects prostate cells against hydrogen peroxide-induced DNA damage and induces expression of genes involved in the defence against oxidative stress. Carcinogenesis. 2006;27(11):2322–2330. doi: 10.1093/carcin/bgl082. [DOI] [PubMed] [Google Scholar]
- 20.Dia VP, Berhow MA, Gonzalez De ME. Bowman-Birk inhibitor and genistein among soy compounds that synergistically inhibit nitric oxide and prostaglandin E2 pathways in lipopolysaccharide-induced macrophages. J Agric Food Chem. 2008;56(24):11707–11717. doi: 10.1021/jf802475z. [DOI] [PubMed] [Google Scholar]
- 21.Hamalainen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isor-hamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007;2007:45673. doi: 10.1155/2007/45673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rimbach G, Weinberg PD, de Pascual-Teresa S, et al. Sulfation of genistein alters its antioxidant properties and its effect on platelet aggregation and monocyte and endothelial function. Biochim Biophys Acta. 2004;1670(3):229–237. doi: 10.1016/j.bbagen.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Curran EM, Judy BM, Newton LG, et al. Dietary soy phytoestrogens and ER-alpha signalling modulate interferon gamma production in response to bacterial infection. Clin Exp Immunol. 2004;135(2):219–225. doi: 10.1111/j.1365-2249.2003.02368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y, Cao S, Nagamani M, Anderson KE, Grady JJ, Lu LJ. Decreased circulating levels of tumor necrosis factor-alpha in postmenopausal women during consumption of soy-containing isoflavones. J Clin En-docrinol Metab. 2005;90(7):3956–3962. doi: 10.1210/jc.2005-0161. [DOI] [PubMed] [Google Scholar]
- 25.Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Hu FB, Willett WC. Soy consumption, markers of inflammation, and endothelial function: A cross-over study in postmenopausal women with the metabolic syndrome. Diabetes care. 2007;30(4):967–973. doi: 10.2337/dc06-2126. [DOI] [PubMed] [Google Scholar]
- 26.Maskarinec G, Steude JS, Franke AA, Cooney RV. Inflammatory markers in a 2-year soy intervention among premenopausal women. J Inflamm Lond. 2009;6:9. doi: 10.1186/1476-9255-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beavers KM, Jonnalagadda SS, Messina MJ. Soy consumption, adhesion molecules, and pro-inflammatory cytokines: A brief review of the literature. Nutr Rev. 2009;67(4):213–221. doi: 10.1111/j.1753-4887.2009.00191.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee SA, Kallianpur A, Xiang YB, et al. Intra-individual variation of plasma adipokine levels and utility of single measurement of these biomarkers in population-based studies. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2464–2470. doi: 10.1158/1055-9965.EPI-07-0374. [DOI] [PubMed] [Google Scholar]
- 29.Picotte M, Campbell CG, Thorland WG. Day-to-day variation in plasma interleukin-6 concentrations in older adults. Cytokine. 2009;47(3):162–165. doi: 10.1016/j.cyto.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Karakas M, Baumert J, Greven S, Ruckerl R, Peters A, Koenig W. Reproducibility in serial C-reactive protein and interleukin-6 measurements in post-myocardial infarction patients: Results from the AIRGENE study. Clin Chem. 2010;56(5):861–864. doi: 10.1373/clinchem.2010.143719. [DOI] [PubMed] [Google Scholar]
- 31.Linkov F, Gu Y, Arslan AA, et al. Reliability of tumor markers, chemo-kines, and metastasis-related molecules in serum. Eur Cytokine Netw. 2009;20(1):21–26. doi: 10.1684/ecn.2009.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho GY, Xue XN, Burk RD, Kaplan RC, Cornell E, Cushman M. Variability of serum levels of tumor necrosis factor-alpha, interleukin 6, and soluble in-terleukin 6 receptor over 2 years in young women. Cytokine. 2005;30(1):1–6. doi: 10.1016/j.cyto.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Shu XO, Yang G, Jin F, et al. Validity and reproducibility of the food frequency questionnaire used in the Shanghai Women’s Health Study. Eur J Clin Nutr. 2004;58(1):17–23. doi: 10.1038/sj.ejcn.1601738. [DOI] [PubMed] [Google Scholar]
- 34.Zheng W, Chow WH, Yang G, et al. The Shanghai Women’s Health Study: Rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162(11):1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Shu XO, Yang G, et al. Abdominal adiposity and mortality in Chinese women. Arch Intern Med. 2007;167(9):886–892. doi: 10.1001/archinte.167.9.886. [DOI] [PubMed] [Google Scholar]
- 36.Chaturvedi AK, Kemp TJ, Pfeiffer RM, et al. Evaluation of multiplexed cytokine and inflammation marker measurements: A methodologic study. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1902–1911. doi: 10.1158/1055-9965.EPI-11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang YX, Wang GY, Pan XC. China Food Composition Tables 2002. Beijing, China: Beijing University Medical Press; 2002. [Google Scholar]
- 38.Harrell FJ., Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, Survival Analysis. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- 39.Matthews CE, Shu XO, Yang G, et al. Reproducibility and validity of the Shanghai Women’s Health Study physical activity questionnaire. Am J Epidemiol. 2003;158(11):1114–1122. doi: 10.1093/aje/kwg255. [DOI] [PubMed] [Google Scholar]
- 40.Dhingra R, Gona P, Nam BH, et al. C-reactive protein, inflammatory conditions, and cardiovascular disease risk. Am J Med. 2007;120(12):1054–1062. doi: 10.1016/j.amjmed.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox J. Regression Diagnostics: An Introduction (Quantitative Applications in the Social Sciences) Newbury Park, CA: Sage Publications; 1991. [Google Scholar]
- 42.Blum A, Lang N, Peleg A, et al. Effects of oral soy protein on markers of inflammation in postmenopausal women with mild hypercholesterolemia. Am Heart J. 2003;145(2):e7. doi: 10.1067/mhj.2003.115. [DOI] [PubMed] [Google Scholar]
- 43.Steinberg FM, Guthrie NL, Villablanca AC, Kumar K, Murray MJ. Soy protein with isoflavones has favorable effects on endothelial function that are independent of lipid and antioxidant effects in healthy postmenopausal women. Am J Clin Nutr. 2003;78(1):123–130. doi: 10.1093/ajcn/78.1.123. [DOI] [PubMed] [Google Scholar]
- 44.Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA. 2004;292(12):1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 45.Rallidis LS, Paschos G, Liakos GK, Velissaridou AH, Anastasiadis G, Zampelas A. Dietary alpha-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. Atherosclerosis. 2003;167(2):237–242. doi: 10.1016/s0021-9150(02)00427-6. [DOI] [PubMed] [Google Scholar]
- 46.Wong HL, Rabkin CS, Shu XO, et al. Systemic cytokine levels and subsequent risk of gastric cancer in Chinese Women. Cancer Sci. 2011;102(10):1911–1915. doi: 10.1111/j.1349-7006.2011.02033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giovannini S, Onder G, Liperoti R, et al. Interleukin-6, C-reactive protein, and tumor necrosis factor-alpha as predictors of mortality in frail, community-living elderly individuals. J Am Geriatr Soc. 2011;59(9):1679–1685. doi: 10.1111/j.1532-5415.2011.03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pine SR, Mechanic LE, Enewold L, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst. 2011;103(14):1112–1122. doi: 10.1093/jnci/djr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu S, Tinker L, Song Y, et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med. 2007;167(15):1676–1685. doi: 10.1001/archinte.167.15.1676. [DOI] [PubMed] [Google Scholar]
- 50.Biswas S, Ghoshal PK, Mandal SC, Mandal N. Relation of anti- to pro-inflammatory cytokine ratios with acute myocardial infarction. Korean J Intern Med. 2010;25(1):44–50. doi: 10.3904/kjim.2010.25.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]