Abstract
Objectives
The role of the norepinephrine transporter (NET) in cocaine dependence has never been demonstrated via in vivo imaging due to the lack of suitable NET radioligands. Here we report our preliminary studies evaluting the NET in individuals with cocaine dependence (COC) in comparison to healthy controls (HC) using (S,S)-[11C]methylreboxetine ([11C]MRB), the most promising C-11 labeled positron-emission tomography (PET) radioligand for NET developed to date.
Methods
Twenty two human volunteers (10 COC and 12 HC) underwent dynamic 11C-MRB-PET acquisition using a High Resolution Research Tomograph (HRRT). Binding potential (BPND) parametric images were computed using the simplified reference tissue model (SRTM2) with occipital cortex as reference region. BPND values were compared between the two groups.
Results
Locus coeruleus (LC), hypothalamus, and pulvinar showed a significant inverse correlation with age among HC (age range = 25–54 years; p = 0.04, 0.009, 0.03 respectively). The BPND was significantly increased in thalamus (27%; p < 0.02) and dorsomedial thalamic nuclei (30%; p < 0.03) in COC as compared to HC. Upon age normalization, the upregulation of NET in COC also reached significance in LC (63%, p < 0.01) and pulvinar (55%, p < 0.02) regions.
Conclusion
Our results suggest that (a) brain NET concentration declines with age in HC, and (b) there is a significant upregulation of NET in thalamus and dorsomedial thalamic nucleus in COC as compared to HC. Our results also suggest that the use of [11C]MRB and HRRT provides an effective strategy for studying alterations of the NET system in humans.
Keywords: Norepinephrine Transporter; Cocaine abuse; (S,S)-[11C]O-Methylreboxetine; MRB; PET
Introduction
Cocaine and other addictive drugs induce neuroadaptions in brain which may be mediated at the genetic level and may play a role in induction and persistence of drug dependence (Zhang et al., 2004; McClung and Nestler, 2003). The understanding of the nature of such molecular adaptations and their anatomical distribution can be crucial in understanding the neurobiology of cocaine addiction.
The human norepinephrine transporter (NET) is a 69kDa, transmembrane protein with 617 amino acids belonging to the Na+/Cl− dependent class of co-transporters (Mandela and Ordway, 2006; Pacholczyk et al., 1991). NET primarily clears norepinephrine from the synaptic cleft, but the NET protein has binding sites for both norepinephrine and dopamine. In fact, NET has a greater affinity for dopamine than norepinephrine (Pacholczyk et al., 1991), and whether dopamine transporter (DAT) or NET is the predominant protein clearing dopamine depends on the relative abundance of the two molecules in a given region (Moron et al., 2002).
Cocaine inhibits neurotransmitter uptake of human monoamine transporters, including the NET, DAT, and SERT (serotonin transporter), with comparable potency (with IC50 values of 910, 278, and 410 nM, respectively). Noradrenergic function has been implicated in the aversive effects of cocaine (McDougle et al., 1994; Rothman et al., 2001; Schank et al., 2006). Such aversive effects, in combination with its reinforcing/rewarding effects, may be important determinants of cocaine self-administration, (Freeman et al., 2008). Conversely, NET inhibition by cocaine has been shown to mediate the reinstatement of drug seeking independent of physiological or behavioral markers of stress (Platt et al., 2007), and mice lacking the norepinephrine transporter continue to self-administer cocaine and respond with heightened sensitivity to the locomotor effects of the drug (Xu et al., 2000). Given NE’s influence on cocaine aversion / reward, the modulation of brain noradrenergic systems offer one promising approach in the development of a cocaine pharmacotherapy [Weinshenker and Schroeder, 2007, review; Vocci & Elkashef, 2005, review).
Alterations in NET concentration in the central nervous system following cocaine exposure have been identified in rats, non-human primate models and post-mortem human studies in cocaine users (Beveridge et al., 2005; Macey et al., 2003; Mash et al., 2005). It has been shown that cocaine-induced reduction in glucose metabolism was attenuated in striatum but not in thalamus in DAT knock-out mice (Thanos et al., 2008), suggesting the involvement of NET in cocaine effects in thalamus. Although these observations point to potentially important dysregulations in NET and NET-mediated neuroenergetics in response to chronic cocaine, (Weinshenker and Schroeder, 2007), in vivo studies of NET regulation in clinical populations have yet to be explored. Furthermore, the NET has been a target of action of a number of drugs that are used long-term therapeutically. Investigations of how the NET and its function are regulated by long-term exposure to drugs are therefore crucial (Mandela and Ordway, 2006).
Cocaine-induced changes in dopamine transporter, dopamine receptors, glucose metabolism and distribution of radiolabeled cocaine itself have been extensively studied in vivo in cocaine abusers using positron emission tomography (PET) (Martinez et al., 2007; Volkow et al., 1995; Volkow et al., 1996a; Volkow et al., 1996b; Volkow et al., 1999; Volkow et al., 2000). While the cardiac effects of cocaine on NET have been studied in non-human primates (Fowler et al., 1994), as alluded to above, no brain studies of the NET system cocaine abusers have been conducted to date. The primary reason for this has been the lack of a suitable PET radioligand. However, the recent development of C-11 labeled reboxetine derivatives showing specific localization and highly favorable binding kinetics in rats, non-human primates and humans now make such studies feasible (Ding et al., 2003; Ding et al., 2006; Logan et al., 2007). The highest brain concentrations (S,S)-[11C]O-methylreboxetine ([11C]MRB) were observed in midbrain regions followed by the thalamus, while the lowest concentration was observed in basal ganglia and occipital cortex – observations consistent with the known distribution of NET in brain (Logan et al., 2007; Ghose et al., 2005).
High Resolution Research Tomograph (HRRT) is a dedicated brain and small animal scanner which has a resolution of up to 2.5–3.4 mm (de Jong et al., 2007). This resolution is better than the HR+, which has a full-width at half maximum (FWHM) resolution of 4 mm. Thus, the use of this scanner allows for the detailed delineation and quantification of radiotracer uptake in small nuclei and sub-nuclei of the brain (Heiss et al., 2004).
In this study, our aim was to determine the alterations in brain NET concentration due to aging and chronic cocaine use using [11C]MRB and HRRT.
Methods
Subjects
Twelve healthy control subjects (HC) (6 M, 6 F; mean ± SD age, 35 ± 10 years; range 25–54) and 10 cocaine dependent individuals (COC) (7M, 3F; 44 ± 3 years; range 39–49) were recruited for study. All subjects were physically healthy as determined by medical history, physical, neurological, ECG, and laboratory examinations. All COC subjects met DSM IV diagnostic criteria (6 ± 1 items; range 4–7) for cocaine dependence, and were chronic (18 ± 4 years), frequent (4 ± 2 times per week), and current smokers of the drug as confirmed by unstructured psychiatric interview and laboratory urine toxicology testing for cocaine metabolite (benzoylecgonine). Cocaine dependent subjects endorsed other drug use (N = 6 alcohol, 4 cannabis, 4 nicotine); however, dependence on drugs other than cocaine (excluding nicotine), cocaine use for less than one year, exclusive or predominant intranasal use, pregnancy or breast feeding, or present or past history of neurological or primary Axis I psychiatric disorder (e.g., schizophrenia, bipolar disorder, major depression, etc.) were exclusionary. Individuals were studied either as inpatients or outpatients, with cocaine abstinence at the time of PET scanning established by 24 hr inpatient supervision and/or negative urine toxicology testing, respectively. Since the urine toxicology test would detect trace amounts of the cocaine metabolite three to four days after cocaine use (despite the fact that there would be no cocaine existing in the body), it is reasonably assumed that the PET scan was performed at least three to four days after the last use of cocaine by abusers.
Following initial screening on the Clinical Neuroscience Research Unit, subjects participated in magnetic resonance imaging (MRI) and PET scanning on two different days.
MRI Scan
An MRI scan (3 T) was collected in each subject for the anatomical co-registration with functional PET images during image analysis. The acquisition sequence was a 3D fast spoiled grass (FSPGR) MR pulse sequence with an IR prep of 300 ms. (TE = 3.3 ms, flip angle = 17 degrees; slice thickness = 1.0 mm) optimized for delineating gray matter/white matter/CSF boundaries. The small voxel size (0.98 × 0.98 × 1.0 mm) provided high resolution volumetric images.
PET Imaging
[11C]MRB Synthesis
As described in previous reports (Ding et al, 2003), we developed the entire synthetic strategy for the nor precursor of the C-11-labeled tracer (S,S)-MRB, including the preparation and chiral separation of its enantiomers, enabling the preparation of (S,S)- and (R,R)-[11C]MRB and the conduct of initial comparative studies in baboons (Ding et al., 2003). Since (S,S)-[11C]MRB proved superior to any existing in vivo NET ligand (Ding et al., 2003; Ding et al., 2005; Logan et al., 2005), we designed an asymmetric synthesis resulting in a single enantiomerically pure precursor that can be used directly for the radiosynthesis of (S,S)-[11C]MRB, obviating the need of chiral separation by HPLC,. After recrystallization, the enantiomeric purity of the precursor was > 99 % as checked by chiral HPLC. This compound was then subjected to the same synthetic strategy described previously to obtain (S,S)-[11C]MRB. The detailed synthetic procedures for synthesizing the precursor, its chiral resolution, and radiosynthesis of individual enantiomers of [11C]MRB have been reported (Lin and Ding, 2004; Lin and Ding, 2005).
Subject preparation
Subject preparation consisted of intravenous and arterial catheterization, and immobilization of the head using a thermoplastic mask.
PET Acquisition
PET scans were acquired using an HRRT PET scanner (207 slices, resolution ~2.5 mm FWHM in 3D acquisition mode). A transmission scan using an orbiting Cs-137 point source was obtained before the emission scan. Motion correction was performed dynamically with measurements from the Vicra (NDI Systems, Waterloo, Ontario) used by a dedicated list-mode reconstruction algorithm (Carson et al., 2003). Dynamic PET scanning was performed using HRRT for 120 min following a bolus injection of 740 MBq (max. injection dose) of [11C]MRB. The extended scanning time in the current study (i.e., as compared to 90 min scanning time in our previous pilot studies in baboons and healthy humans (Ding et al., 2003, 2005 and 2006; Logan et al., 2007)) was used to improve the BP estimates; i.e., to reduce the noise. This is particularly important in quantitation of small regions with the HRRT. In the first phase of the study (5–10 min), the arterial input function was measured with an automated blood counting system (PBS-101, Veenstra Instruments, Joure, The Netherlands) using a continuous withdrawal system where the radioactivity in whole blood is measured with a calibrated radioactivity monitor. Subsequently, individual blood samples were taken at various time points. Samples were centrifuged to obtain plasma, which was counted. Selected samples were assayed by HPLC for the presence of unmetabolized, parent radiotracer. In addition, the fraction of plasma radioactivity unbound to protein was also determined. Subjects were asked to void immediately after the scan was completed to reduce radiation exposure to the bladder.
Kinetic Analysis
The values of binding potential (BPND), which is linearly proportional to the density of the available NET concentration, were computed using a simplified reference tissue model (2-parameter version: SRTM2) (Wu and Carson, 2002). The SRTM2 based values were validated against values obtained by multilinear method (MA1) (Gallezot et al., 2007). Occipital cortex was used as the reference region.
Image Analysis
An average image was generated for HC. New regions of interest (ROIs) for small regions were drawn on this averaged PET image for brainstem nuclei, including the locus coeruleus (LC), midbrain raphe, pontine raphe, red nucleus, thalamic (TH) subnuclei and hypothalamus (Hy). These new regions were incorporated in the Automated Anatomical Labeling (AAL) template (Tzourio-Mazoyer, et al., 2002) for the purpose of co-registration in Montreal Neurological Institute (MNI) space. The structure/volume and location of ROIs were determined based on the Talairach and Tournox atlas, the stereotactic atlas of the thalamus by Morel et al. (Morel et al., 1997) and an article on the cytoarchitecture of dorsal raphe by Baker et al. (Baker et al., 1990).
Statistical Analysis
The mean BPND values for a given ROI were compared on a between group basis using an unpaired Student’s t-test. Pearson’s correlation coefficient was used to describe the correlation between age and BPND. A two-tailed p-value < 0.05 was considered statistically significant.
Results
High resolution imaging of brain NET using [11C]MRB and HRRT
As seen in Figure 1, the HRRT is able to clearly delineate the fine-scale regional distribution of [11C]MRB in small brain regions known to have high NET concentrations, including the locus coeruleus (LC), midbrain raphe, pontine raphe, red nucleus, thalamic subnuclei and hypothalamus (Hy) (Figure 1). This allowed for quantitation of NET in these brain regions.
Figure 1.
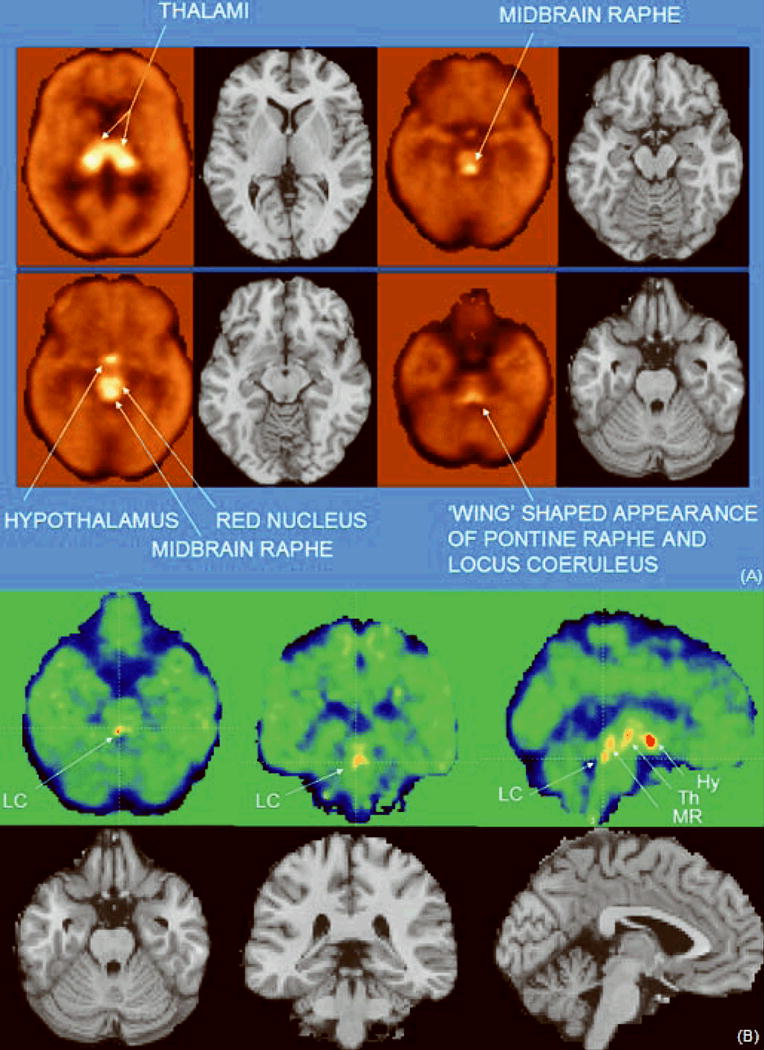
(A) Average [11C]MRB-PET images depicting the normal distribution of radiotracer in thalamus, midbrain raphe, hypothalamus, red nucleus, pontine raphe and locus coeruleus; B) Individual [11C]MRB-PET images showing high radiotracer uptake in locus coeruleus in a cocaine subject (LC = Locus Coeruleus, Hy = Hypothalamus, Th = Thalamus, MR = Midbrain Raphe)
Effect of age on [11C]MRB binding in healthy controls
Inverse correlations between age and [11C]MRB BPND were observed for all nine brain regions in healthy controls (r values −0.02 to −0.71), including statistically significant correlations for the LC, Hy, and pulvinar (N = 12; age range = 25–54 years; p-values 0.04, 0.009, 0.01 respectively; Figure 2). No significant correlation was observed between age and [11C]MRB BPND among cocaine subjects (N = 10, age range = 40–49 years).
Figure 2.
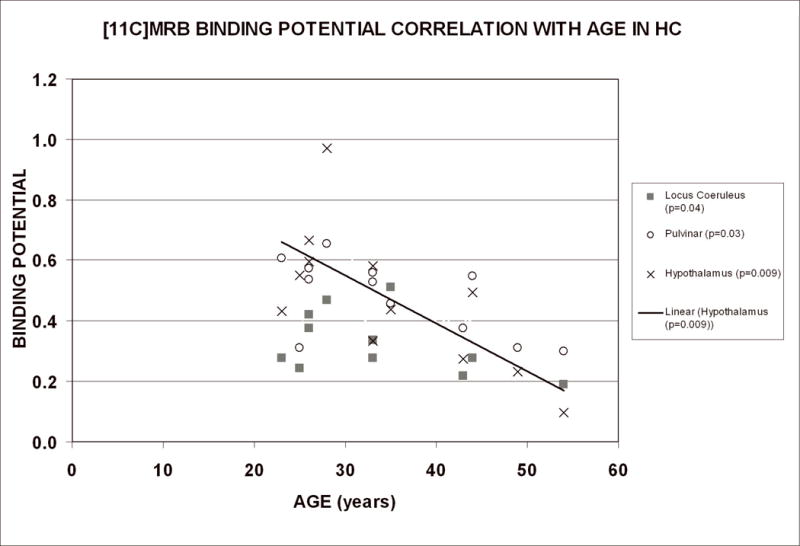
Effect of age on BPND values of [11C]MRB in healthy controls
Effect of cocaine dependence on [11C]MRB binding
A general trend of increases in BPND values for COC subjects as compared to HC was observed for most NET rich regions (BPND ≥ 0.4), including the TH (27%) and thalamic subnuclei [dorsomedial (30%), pulvinar (33%), and ventrolateral (22%)], Hy (8%), and LC (24%) (Table I and Figure 3). Among these regions, BPND increases were significance in TH and the dorsomedial thalamic nucleus (COC vs. HC = 0.59 ± 0.14 vs. 0.47 ± 0.09, p < 0.02; and 0.64 ± 0.19 vs. 0.49 ± 0.10, p < 0.03, respectively). Since the inverse correlations with age across regions (Figure 2), and given that COC subjects were, as a group, significantly older than HC in our sample (44 ± 3 years versus 35 ± 10 years, p < 0.02), we conducted an age-normalization analysis to rule-out potentially confounding effects of age on our observed group differences. Specifically, BPND in HC were age-adjusted using region-specific linear regression values and a mean HC age of 44 years (i.e., the same as for the COC group). Age-normalized results showed an increased and/or statistically strengthened effects of cocaine dependence on NET upregulation in 8 of 9 regions, including TH (37%; p = 0.003), LC (63%; p = 0.01), dorsomedial nucleus (41%; p = 0.007), pulvinar (55%; p = 0.02), and at trend levels, ventromedial nucleus (25%; p = 0.07). In contrast, a non-statistically significant trend of decrease BPND was noted in red nucleus in COC as compared to HC subjects (−25%; p = 0.12) (Table I).
Table I.
Comparison of [11C]MRB BPND in cocaine dependent subjects (COC) and healthy controls (HC)
| ROI | BPND in COC | BPND in HC1 | % Change in COC1 | p-value1 |
|---|---|---|---|---|
| Thalamus | 0.59 ± 0.14 | 0.47 ± 0.09 (0.43 ± 0.08) |
27% (37%) |
< 0.02** (0.003) |
| Dorsomedial Thalamic Nucleus |
0.64 ± 0.19 | 0.49 ± 0.10 (0.45 ± 0.09) |
30% (41%) |
< 0.03** (0.007) |
| Pulvinar | 0.64 ± 0.30 | 0.48 ± 0.13 (0.41 ± 0.10) |
33% (55%) |
0.11 (0.02*) |
| Ventrolateral Thalamic Nucleus |
0.55 ± 0.14 | 0.45 ± 0.13 (0.44 ± 0.13) |
22.00% (25%) |
0.10 (0.07) |
| Hypothalamus | 0.51 ± 0.35 | 0.47 ± 0.23 (0.33 ± 0.16) |
8% (56%) |
0.76 (0.12) |
| Locus Coeruleus | 0.37 ± 0.14 | 0.30 ± 0.14 (0.23 ± 0.11) |
24% (63%) |
0.24 (0.01*) |
| Pontine Raphe | 0.37 ± 0.22 | 0.40 ± 0.26 (0.36 ± 0.25) |
−7% (3%) |
0.79 (0.90) |
| Midbrain Raphe | 0.53 ± 0.26 | 0.61 ± 0.22 (0.51 ± 0.19) |
−12% (5%) |
0.49 (0.79) |
| Red Nucleus | 0.38 ± 0.12 | 0.50 ± 0.22 (0.50 ± 0.22) |
−25% (−25%) |
0.12 (0.12) |
Statistically significant p-value;
Statistically significant p-value after age-normalization;
values in parenthesis refer to results based on an age-normalization analysis
Figure 3.
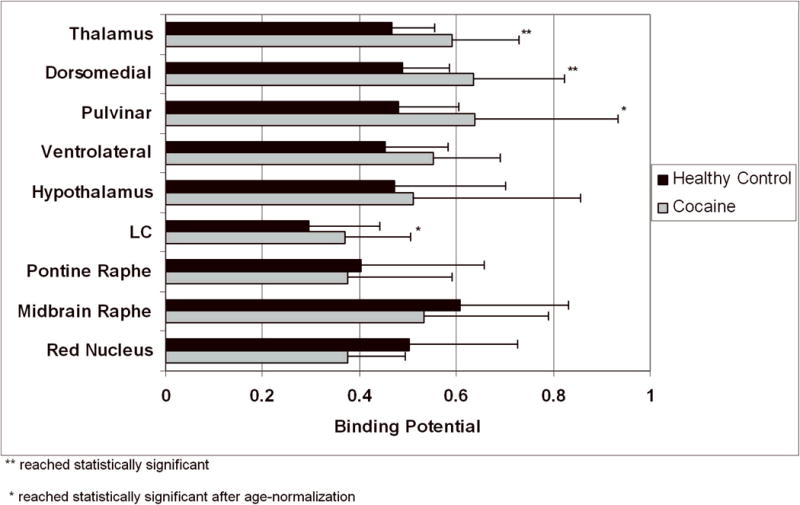
Effect of cocaine dependence on BPND values of [11C]MRB in humans
Discussion
Brain imaging studies of the NET in vivo have been hampered by the lack of suitable NET radioligands. After decades of search (see review article, Ding et al., 2006, and the references cited within), positron emitter labeled reboxetine analogues have been considered, to be, by far, the most promising PET tracers for studying brain NET system. We have shown that [11C]MRB possesses properties that are highly favorable for the imagine of brain NET as a PET tracer, including: (a) a regional distribution consistent with the known distribution of NET in the brain; (b) high test/retest reproducibility in non-human primates and humans; (c) excellent specificity and selectivity for NET over other monoamine transporters, namely DAT and SERT; and (d) the short half-life of its 11C label (t1/2 = 20 min), enabling multiple assessments (e.g., baseline and drug intervention) in the same subject on the same day. Its ability as a ligand to assess NET occupancy by pharmacologic drug doses was first evaluated in humans with atomoxetine (Logan et al., 2005). No obvious dose-dependent effect was observed, which was apparently due to the doses of atomoxetine used in this human study being too high. The occupancy of the three clinically relevant doses all reached saturation; i.e., the plateau portion of the corresponding dose-occupancy curve; as a result, there was no obvious dose-dependent effect. The occupancy of NET by atomoxetine was further evaluated by carrying out investigations in a non-human primate model using much lower atomoxetine doses. With refined kinetic modeling methods, an unequivocal dose-dependent occupancy of atomoxetine was obtained, definitively demonstrating the suitability of [11C]MRB for drug occupancy studies of the NET (Gallezot et al., 2008). Although the slow clearance of [11C]MRB in NET-rich regions such as thalamus posed a concern for kinetic modeling, the combination of a suitably designed infusion study paradigm, newly developed kinetic methodology, and high resolution HRRT afforded satisfactory quantitation of brain NET concentration. The detailed kinetic methodology and study results will be published elsewhere.
The current study investigated the role of NET in cocaine dependence using [11C]MRB and HRRT. An increased binding potential of [11C]MRB (uncorrected for potential age-effects) was observed in thalamus and dorsomedial thalamic nucleus in chronic cocaine users as compared to healthy controls. In addition, we noted an inverse relationship between age and NET concentration in healthy human subjects.
Observations of an increased binding of [11C]MRB in our chronic COC subjects is consistent with previously published data in rodents, rhesus monkeys and post-mortem human brains (Beveridge et al., 2005; Macey et al., 2003; Mash et al., 2005). Following chronic cocaine self-administration, [3H]nisoxetine binding was increased in rhesus monkeys in the basal nucleus of stria terminalis, basolateral amygdala, subnuclei of the hypothalamus, entorhinal cortex, hippocampal formation (parasubiculum), and brain stem nuclei (A1 nucleus and nucleus prepositus) (Beveridge et al., 2005; Macey et al., 2003). Increased NET protein expression, studied by immunoblotting, and increased [3H]nisoxetine binding were also noted in insular cortex in a human postmortem study of human cocaine abusers (Mash et al., 2005). Similarly, an upregulation of NET mRNA in the locus coeruleus was noted following chronic binge administration of cocaine in rats (Burchett and Bannon, 1997).
Thalamus is a NET-rich region of the brain (Biegon and Rainbow, 1983; Burchett and Bannon, 1997). [11C]Cocaine has been shown to bind in thalamus (Telang et al., 1999) (which is likely due to its binding to NET) and intermediate levels of radiolabeled cocaine have been noted in the dorsomedial and ventrolateral nuclei of thalamus. Intriguingly, both nuclei have also been implicated in the circuitry of cocaine addiction (Schmidt et al., 2005). Cocaine administration attenuated a decline in glucose metabolism in basal ganglia of DAT knock-out as compared to wild-type mice, whereas in thalamus, an attenuation of glucose metabolism persisted in both groups, suggesting its mediation by DAT-independent mechanisms) (Thanos et al., 2008). Abnormalities of thalamo-cortical pathways were recently reported in cocaine abusers using functional MRI (Tomasi et al., 2007). In our study, among the thalamic subnuclei, dorsomedial nucleus was most robustly dysregulated in our COC subjects. These results are intriguing given observations that the dorsomedial nucleus receives substantial afferent innervation from limbic-related brain areas and, in turn, provides major efferent projections to the pre-frontal cortex, including the medial and orbitofrontal cortex (Hurd and Fagergren, 2000). Excitotoxic lesions of the mediodorsal thalamic nucleus have also been shown to attenuate intravenous cocaine self-administration in rats (Weissenborn et al., 1998).
In addition, we noted a general trend of increased binding in COC as compared to HC for LC, Hy, pulvinar and ventrolateral thalamic nucleus. In fact, the effect in LC and pulvinar attained statistical significance following age-normalized comparisons. Moreover, the specificity of this effect in these regions, especially in LC, is highlighted by the absence of any increase (and, in fact, a decrease) in the binding potential in the adjacent regions such as midbrain raphe and red nucleus (Beveridge et al., 2005). This is quite intriguing as uniform increases observed in COC vs. HC across all regions might be interpreted as a potentially non-specific / global effect (i.e., not necessarily NET related); however, this is certainly not the case for the current study. The reasons for the downregulation in these specific regions are not known. A significant reduction NET protein and mRNA expression as well as NET function and [3H]-Norepinephrine binding in the spinal cords of rats after chronic cocaine exposure has been reported (Zhao et al., 2002). Rubro-spinal tract is a fiber bundle arising from the red nucleus and projecting to the spinal cord. Thus, whether the reduction of NET in midbrain raphe and red nucleus observed in our COC study is related to a reduction in spinal cord and the rubro-spinal tracts, needs further investigation.
The increased binding of [11C]MRB could be due to an increased synthesis of the NET protein, its increased surface expression, or both. Similarly, it is not clear whether [11C]MRB binds exclusively to the NET expressed on cell surfaces, or whether intracellular NET is a target as well. Increased NET expression observed in post-mortem human brains in cocaine abusers (Mash et al., 2005) points towards an increased synthesis of NET. On the other hand, cocaine-induced upregulation of phosphatases (Yuferov et al., 2003), which in turn maintain a check on NET internalization from the cell membrane (Jayanthi et al., 2004), points towards an increased expression of NET on the cell membrane. Indeed, cocaine is known to induce upregulation of transcription factors CREB and ΔFosB that, in turn, to upregulate a large number of proteins and receptors (McClung and Nestler, 2003). Furthermore, activation of Protein Kinase C-mediated phosphorylation leads to an internalization of NET mediated by lipid rafts (Jayanthi et al., 2004), and reduction in Vmax of [3H]norepinephrine uptake without affecting its Km (Mandela and Ordway, 2006) (Jayanthi et al., 2004). Thus, the absence of cocaine-induced upregulation of protein kinase C in thalamus (as demonstrated in rats) (Terwilliger et al., 1991) and the presence of upregulation of phosphatases by cocaine (Yuferov et al., 2003), can indirectly lead to an increased binding of [11C]MRB to NET on cell surface in thalamus. Many of the cocaine-induced modulations of the phosphorylation mechanism have been observed in regions other than thalamus, and the pathways involved are known to be differentially regulated in different brain regions (Terwilliger et al., 1991). Therefore, more studies are needed to investigate the specific effects of cocaine on NET in the thalamus. Similar to its role in regulating the NET, phosphorylation pathways are known to play an important role in regulating DAT, SERT and glutamate transporters as well.
Studies in animals have shown an increase of up to 52% in [3H]nisoxetine binding in bed nucleus of stria terminalis (Macey et al., 2003). Our results show an upregulation up to 33% in thalamus and its subnuclei; however, this may be an underestimation of the increase in binding of [11C]MRB in cocaine abusers. Indeed, age-normalized comparison revealed estimates, up to 54%, in locus coeruleus.
The reduction of [11C]MRB binding in healthy individuals with age is consistent with the reduction in [3H]nisoxetine binding in aging human brains as observed by Tejani-Butt and Ordway (Tejani-Butt and Ordway, 1992). The reduction in [11C]MRB binding in locus coeruleus may be secondary to a reduction in the absolute number of catecholaminergic neurons (Tejani-Butt and Ordway, 1992; Manaye et al., 1995; Vijayashankar and Brody, 1979). In these studies, up to a 50% reduction in catecholaminergic cell count in the locus coeruleus was observed with aging. Similarly, reductions in NET protein and messenger-RNA concentration in aging rat brains have been reported (Moll et al., 2000; Shores et al., 1999). A reduction in [3H]nisoxetine binding in locus coeruleus has been reported in age-related disorders like Alzheimer’s disease (Tejani-Butt et al., 1993). In fact, an age-related reduction in NET concentration has also been reported in sympathetic nerve terminals of the heart in humans and rodents (Leineweber et al., 2002; Snyder et al., 1998), perhaps a peripheral manifestation of the same age-related process that leads to a reduction in the NET concentration in the CNS, as demonstrated in our study.
Simplified reference tissue model-2 parameter version (SRTM2) was used as the method for BPND calculation in our study. Between the basal ganglia and occipital cortex, the latter was considered as a better reference region for this study because of the high degree of binding of cocaine to the basal ganglia and the high potential for cocaine induced alterations in the same region.
In our study, twelve-parameter linear method was used for co-registration of PET, MRI and the template ROIs. This could lead to a mis-registration, particularly, for small brain regions; however, a scatter-plot comparison of the degree of mis-registration did not reveal significant differences between the two groups. Future studies should focus on individualized ROI placement on regions such as locus coeruleus. Modified MR sequences, which potentially could better localize the position of locus coeruleus using the contrast generated by its neuromelanin content (Shibata et al., 2007), and the use of non-linear co-registration and partial volume correction methods are currently under investigation. A bigger sample size to confirm the results of the current study is needed. Additional studies to better understand the time course of these changes with respect to drug abstinence, potential relationships between degree of NET dysregulation and clinical outcome, as well as more sophisticated efforts to correlate regional changes in NET with symptoms/clinical features mediated by these same structures are also important.
Conclusion
Our results suggest that (a) NET concentration is reduced with age in healthy brain, particularly in locus coeruleus, hypothalamus, pulvinar and dorsomedial thalamic nucleus, and (b) there is a significant upregulation of NET in thalamus and dorsomedial thalamic nucleus in cocaine abusers as compared to healthy controls. Our results also suggest that the combination of [11C]MRB and HRRT is a valuable method of studying alterations of the NET system in humans.
Acknowledgments
This work was carried out at the Yale PET Center, Yale University School of Medicine and the Clinical Neuroscience Research Unit, Connecticut Mental Health Center. Support for this study was provided by National Institute on Drug Abuse (DA019062, R56DA19062, and K24DA017899), National Center of PTSD, The Patrick and Catherine Weldon Donaghue Medical Research Foundation (DF07-101), and the Connecticut Department of Mental Health and Addiction Services (DMHAS). This publication was made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. The authors thank the staff of the Yale University PET Center for their technical expertise and support, the staff of the Clinical Neuroscience Research Unit for their clinical services/support, Mindy McQueen and Andrea Rodrigues for subject recruitment, and Amy Turner for protocol coordination. We are also especially grateful to the individuals who volunteered for these studies.
References
- Baker KG, Halliday GM, Tork I. Cytoarchitecture of the human dorsal raphe nucleus. J Comp Neurol. 1990;301:147–61. doi: 10.1002/cne.903010202. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Effects of chronic cocaine self-administration on norepinephrine transporters in the nonhuman primate brain. Psychopharmacology (Berl) 2005;180:781–8. doi: 10.1007/s00213-005-2162-1. [DOI] [PubMed] [Google Scholar]
- Biegon A, Rainbow TC. Localization and characterization of [3H]desmethylimipramine binding sites in rat brain by quantitative autoradiography. J Neurosci. 1983;3:1069–76. doi: 10.1523/JNEUROSCI.03-05-01069.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchett SA, Bannon MJ. Serotonin, dopamine and norepinephrine transporter mRNAs: Heterogeneity of distribution and response to ‘binge’ cocaine administration. Brain Res Mol Brain Res. 1997;49:95–102. doi: 10.1016/s0169-328x(97)00131-9. [DOI] [PubMed] [Google Scholar]
- de Jong HW, van Velden FH, Kloet RW, Buijs FL, Boellaard R, Lammertsma AA. Performance evaluation of the ECAT HRRT: An LSO-LYSO double layer high resolution, high sensitivity scanner. Phys Med Biol. 2007;52:1505–26. doi: 10.1088/0031-9155/52/5/019. [DOI] [PubMed] [Google Scholar]
- Carson RE, Barker WC, Liow J-S, Adler S, Johnson CA. Design of a motion-compensation OSEM List-mode Algorithm for Resolution-Recovery Reconstruction of the HRRT. IEEE. 2003 Portland, OR, 2003, M16-6. [Google Scholar]
- Ding YS, Lin KS, Logan J. PET imaging of norepinephrine transporters. Curr Pharm Des. 2006;12:3831–45. doi: 10.2174/138161206778559687. [DOI] [PubMed] [Google Scholar]
- Ding YS, Lin KS, Logan J, Benveniste H, Carter P. Comparative evaluation of positron emission tomography radiotracers for imaging the norepinephrine transporter: (S,S) and (R,R) enantiomers of reboxetine analogs ([11C]methylreboxetine, 3-Cl-[11C]methylreboxetine and [18F]fluororeboxetine), (R)-[11C]nisoxetine, [11C]oxaprotiline and [11C]lortalamine. J Neurochem. 2005;94:337–51. doi: 10.1111/j.1471-4159.2005.03202.x. [DOI] [PubMed] [Google Scholar]
- Ding YS, Lin KS, Garza V, Carter P, Alexoff D, Logan J, Shea C, Xu Y, King P. Evaluation of a new norepinephrine transporter PET ligand in baboons, both in brain and peripheral organs. Synapse. 2003;50:345–52. doi: 10.1002/syn.10281. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Ding Y-S, Volkow ND, Martin T, MacGregor RR, Dewey S, King P, Pappas N, Alexoff D, Shea C, Gatley SJ, Schlyer DJ, Wolf AP. PET Studies of Cocaine Inhibition of the Myocardial Norepinephrine Uptake. Synapse. 1994;16:312–317. doi: 10.1002/syn.890160407. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Verendeev A, Riley AL. Noradrenergic antagonism enhances the conditioned aversive effects of cocaine. Pharmacol Biochem Behav. 2008;88:523–32. doi: 10.1016/j.pbb.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Gallezot JD, Planeta-Wilson B, Wang GK, Carson RE, Ding YS. Parametric imaging of the NET radioligand [C-11]MRB in humans: A test-retest study. J Nucl Med. 2007;48:159P. [Google Scholar]
- Gallezot JD, Weinzimmer D, Nabulsi N, Lin S-F, Fowles K, Maguire P, Carson RE, Ding Y-S. Evaluation of [C‐11]MRB for Receptor Occupancy Studies of Norepinephrine Transporters. Neuroimaging. 2008;41:T49. doi: 10.1016/j.neuroimage.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose S, Fujita M, Morrison P, Uhl G, Murphy DL, Mozley PD, Schou M, Halldin C, Innis R. Specific in vitro binding of (S,S)-[3H]MeNER to norepinephrine transporters. Synapse. 2005;56:100–4. doi: 10.1002/syn.20133. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Habedank B, Klein JC, Herholz K, Wienhard K, Lenox M, Nutt R. Metabolic rates in small brain nuclei determined by high-resolution PET. J Nucl Med. 2004;45:1811–5. [PubMed] [Google Scholar]
- Hurd YL, Fagergren P. Human cocaine- and amphetamine-regulated transcript (CART) mRNA is highly expressed in limbic- and sensory-related brain regions. J Comp Neurol. 2000;425:583–98. doi: 10.1002/1096-9861(20001002)425:4<583::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Samuvel DJ, Ramamoorthy S. Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters. evidence for localization in lipid rafts and lipid raft-mediated internalization. J Biol Chem. 2004;279:19315–26. doi: 10.1074/jbc.M311172200. [DOI] [PubMed] [Google Scholar]
- Leineweber K, Wangemann T, Giessler C, Bruck H, Dhein S, Kostelka M, Mohr FW, Silber RE, Brodde OE. Age-dependent changes of cardiac neuronal noradrenaline reuptake transporter (uptake1) in the human heart. J Am Coll Cardiol. 2002;40:1459. doi: 10.1016/s0735-1097(02)02168-x. [DOI] [PubMed] [Google Scholar]
- Lin KS, Ding YS. Synthesis and C-11 labeling of three potent norepinephrine transporter selective ligands ((R)-nisoxetine, lortalamine, and oxaprotiline) for comparative PET studies in baboons. Bioorg Med Chem. 2005;13:4658–66. doi: 10.1016/j.bmc.2005.04.062. [DOI] [PubMed] [Google Scholar]
- Lin KS, Ding YS. Synthesis, enantiomeric resolution, and selective C-11 methylation of a highly selective radioligand for imaging the norepinephrine transporter with positron emission tomography. Chirality. 2004;16:475–81. doi: 10.1002/chir.20055. [DOI] [PubMed] [Google Scholar]
- Logan J, Ding YS, Lin KS, Pareto D, Fowler J, Biegon A. Modeling and analysis of PET studies with norepinephrine transporter ligands: The search for a reference region. Nucl Med Biol. 2005;32:531–42. doi: 10.1016/j.nucmedbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Logan J, Wang GJ, Telang F, Fowler JS, Alexoff D, Zabroski J, Jayne M, Hubbard B, King P, Carter P, Shea C, Xu Y, Muench L, Schyler D, Learned-Coughlin S, Cosson V, Volkow ND, Ding YS. Imaging the norepinephrine transporter in humans with (S,S)-[11C]O-methyl reboxetine and PET: Problems and progress. Nucl Med Biol. 2007;34:667–79. doi: 10.1016/j.nucmedbio.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Smith HR, Nader MA, Porrino LJ. Chronic cocaine self-administration upregulates the norepinephrine transporter and alters functional activity in the bed nucleus of the stria terminalis of the rhesus monkey. J Neurosci. 2003;23:12–6. doi: 10.1523/JNEUROSCI.23-01-00012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaye KF, McIntire DD, Mann DM, German DC. Locus coeruleus cell loss in the aging human brain: A non-random process. J Comp Neurol. 1995;358:79–87. doi: 10.1002/cne.903580105. [DOI] [PubMed] [Google Scholar]
- Mandela P, Ordway GA. The norepinephrine transporter and its regulation. J Neurochem. 2006;97:310–33. doi: 10.1111/j.1471-4159.2006.03717.x. [DOI] [PubMed] [Google Scholar]
- Martinez D, Kim JH, Krystal J, Abi-Dargham A. Imaging the neurochemistry of alcohol and substance abuse. Neuroimaging Clin N Am. 2007;17:539–55. x. doi: 10.1016/j.nic.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Mash DC, Ouyang Q, Qin Y, Pablo J. Norepinephrine transporter immunoblotting and radioligand binding in cocaine abusers. J Neurosci Methods. 2005;143:79–85. doi: 10.1016/j.jneumeth.2004.09.013. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–15. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Black JE, Malison RT, Zimmermann RC, Kosten TR, Heninger GR, Price LH. Noradrenergic dysregulation during discontinuation of cocaine use in addicts. Archives of General Psychiatry. 1994;51:713–9. doi: 10.1001/archpsyc.1994.03950090045007. [DOI] [PubMed] [Google Scholar]
- Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Ruther E, Huether G. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Brain Res Dev Brain Res. 2000;119:251–7. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and stereotactic atlas of the human thalamus. J Comp Neurol. 1997;387:588–630. doi: 10.1002/(sici)1096-9861(19971103)387:4<588::aid-cne8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: Evidence from knock-out mouse lines. J Neurosci. 2002;22:389–95. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholczyk T, Blakely RD, Amara SG. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991;350:350–4. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Noradrenergic mechanisms in cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2007;322:894–902. doi: 10.1124/jpet.107.121806. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Schank J, Ventura R, Puglisi-Allegra S, Alcaro A, Cole C, Liles L, Seeman P, Weinshenker D. Dopamine beta-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. 2006. [DOI] [PubMed] [Google Scholar]
- Shibata E, Sasaki M, Tohyama K, Otsuka K, Sakai A. Reduced signal of locus ceruleus in depression in quantitative neuromelanin magnetic resonance imaging. Neuroreport. 2007;18:415–8. doi: 10.1097/WNR.0b013e328058674a. [DOI] [PubMed] [Google Scholar]
- Shores MM, White SS, Veith RC, Szot P. Tyrosine hydroxylase mRNA is increased in old age and norepinephrine uptake transporter mRNA is decreased in middle age in locus coeruleus of brown-norway rats. Brain Res. 1999;826:143–7. doi: 10.1016/s0006-8993(99)01200-7. [DOI] [PubMed] [Google Scholar]
- Snyder DL, Aloyo VJ, Wang W, Roberts J. Influence of age and dietary restriction on norepinephrine uptake into cardiac synaptosomes. J Cardiovasc Pharmacol. 1998;32:896–901. doi: 10.1097/00005344-199812000-00005. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt SM, Ordway GA. Effect of age on [3H]nisoxetine binding to uptake sites for norepinephrine in the locus coeruleus of humans. Brain Res. 1992;583:312–5. doi: 10.1016/s0006-8993(10)80041-1. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt SM, Yang J, Zaffar H. Norepinephrine transporter sites are decreased in the locus coeruleus in alzheimer’s disease. Brain Res. 1993;631:147–50. doi: 10.1016/0006-8993(93)91201-3. [DOI] [PubMed] [Google Scholar]
- Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–10. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. The effects of cocaine on regional brain glucose metabolism is attenuated in dopamine transporter knockout mice. Synapse. 2008;62:319–24. doi: 10.1002/syn.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: Implications in attention and perception. Psychiatry Res. 2007;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in spm using a macroscopic anatomical parcellation of the MNI MRI single subject brain. Neuroimage. 2002:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vijayashankar N, Brody H. A quantitative study of the pigmented neurons in the nuclei locus coeruleus and subcoeruleus in man as related to aging. J Neuropathol Exp Neurol. 1979;38:490–7. doi: 10.1097/00005072-197909000-00004. [DOI] [PubMed] [Google Scholar]
- Vocci F, Elkashef A. Pharmacotherapy and other treatments for cocaine abuse and dependence. Current Opinion in Psychiatry. 2005;18:265–270. doi: 10.1097/01.yco.0000165596.98552.02. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ. Cocaine addiction: Hypothesis derived from imaging studies with PET. J Addict Dis. 1996a;15:55–71. doi: 10.1300/J069v15n04_04. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Ding YS, Logan J, Dewey SL, Hitzemann R, Lieberman J. Relationship between psychostimulant-induced “high” and dopamine transporter occupancy. Proc Natl Acad Sci U S A. 1996b;93:10388–92. doi: 10.1073/pnas.93.19.10388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Dewey SL, Hitzemann R, Gifford AN, Pappas NR. Blockade of striatal dopamine transporters by intravenous methylphenidate is not sufficient to induce self-reports of “high”. J Pharmacol Exp Ther. 1999;288:14–20. [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, Dewey S, Ashby C, Liebermann J, Hitzemann R. Is methylphenidate like cocaine? studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry. 1995;52:456–63. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Franceschi D, Thanos PK, Wong C, Gatley SJ, Ding YS, Molina P, Schlyer D, Alexoff D, Hitzemann R, Pappas N. Cocaine abusers show a blunted response to alcohol intoxication in limbic brain regions. Life Sci. 2000;66:PL161–7. doi: 10.1016/s0024-3205(00)00421-5. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. There and back again: A tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–51. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Whitelaw RB, Robbins TW, Everitt BJ. Excitotoxic lesions of the mediodorsal thalamic nucleus attenuate intravenous cocaine self-administration. Psychopharmacology (Berl) 1998;140:225–32. doi: 10.1007/s002130050761. [DOI] [PubMed] [Google Scholar]
- Wu Y, Carson RE. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab. 2002;22:1440–52. doi: 10.1097/01.WCB.0000033967.83623.34. [DOI] [PubMed] [Google Scholar]
- Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, Wang YM, Caron MG. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci. 2000;3:465–71. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]
- Yuferov V, Kroslak T, Laforge KS, Zhou Y, Ho A, Kreek MJ. Differential gene expression in the rat caudate putamen after “binge” cocaine administration: Advantage of triplicate microarray analysis. Synapse. 2003;48:157–69. doi: 10.1002/syn.10198. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–54. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Flood P, Sun L. Chronic cocaine and amphetamine treatment is associated with changes in rat spinal norepinephrine transporters. Anesthesiology. 2002;96:A794. [Google Scholar]


