Abstract
The mitochondrial oxidative phosphorylation (OxPhos) system generates the vast majority of cellular energy, but is also involved in the generation of reactive oxygen species (ROS), and apoptosis. Cytochrome c (Cytc) and cytochrome c oxidase (COX) represent the terminal step of the electron transport chain (ETC), the proposed rate-limiting reaction in mammals. Cytc and COX show unique regulatory features including allosteric regulation, isoform expression, and regulation through cell signaling pathways. This chapter focuses on the latter and discusses all mapped phosphorylation sites based on the crystal structures of COX and Cytc. Several signaling pathways have been identified that target COX including protein kinase A and C, receptor tyrosine kinase, and inflammatory signaling. In addition, four phosphorylation sites have been mapped on Cytc with potentially large implications due to its multiple functions including apoptosis, a pathway that is overactive in stressed cells but inactive in cancer. The role of COX and Cytc phosphorylation is reviewed in a human disease context, including cancer, inflammation, sepsis, asthma, and ischemia/reperfusion injury as seen in myocardial infarction and ischemic stroke.
Keywords: Apoptosis, Asthma, Cancer, Inflammation, Ischemia-reperfusion, Noonan syndrome, Oxidative phosphorylation, Sepsis, Stroke
X.1 Introduction
The mitochondrial oxidative phosphorylation machinery (OxPhos) is essential for cell function, maintenance, and survival. OxPhos in mammals provides more than 90% of cellular energy. OxPhos consists of the electron transport chain (ETC), which generates the mitochondrial proton motive force by pumping protons across the inner mitochondrial membrane, and ATP synthase (complex V), which couples the backflow of protons into the matrix with the synthesis of ATP from ADP and phosphate. The ETC consists of three proton-pumping complexes, NADH dehydrogenase (complex I), bc1-complex (complex III), and cytochrome c oxidase (COX; complex IV), and non-proton pumping succinate dehydrogenase (complex II), as well as the non-protein two-electron carrier ubiquinone and the small one-electron carrier cytochrome c (Cytc). Electrons enter the ETC mainly through complex I from NADH. In addition, complex II feeds electrons derived from succinate directly into the ubiquinone/ubiquinol pool, linking the Krebs cycle with OxPhos.
OxPhos dysfunction is devastating as can be seen in patients with “traditional” mitochondrial diseases, caused, for example, by mutations in the mitochondrial DNA or nuclear encoded assembly factors of OxPhos complexes. The tissues that are most prominently affected are those that rely most heavily on aerobic energy production including skeletal muscle, brain, and the visual system. More recently, mitochondrial dysfunction has been implicated in an increasing number of human diseases, including the most common pathologies such as cardiovascular disease, diabetes, cancer, and ischemia/reperfusion injury as seen in myocardial infarction and stroke. These “nontraditional” mitochondrial diseases can be better understood when viewed in light of cellular signaling pathways and regulatory control mechanisms, which are often dysregulated in those pathologies. Recent studies have begun to establish a connection between cell signaling and OxPhos, and more than 20 phosphorylation sites have been mapped on the OxPhos complexes (Hüttemann et al. 2007). There is strong evidence that additional sites are phosphorylated, and given the hydrophobic nature of the many membrane-spanning subunits of the OxPhos complexes, several additional phosphorylation sites will likely be revealed in the future due to technological improvements, specifically in mass spectrometry (MS) and related methodologies such as enrichment of phosphopeptides (Thingholm et al. 2006; Walther et al. 2010).
X.2 Composition and function of cytochrome c oxidase and cytochrome c
This chapter focuses on the regulation of cytochrome c oxidase (COX) and cytochrome c (Cytc) by cell signaling pathways. To gain a better insight into this topic we will first briefly discuss the basic properties of both enzymes.
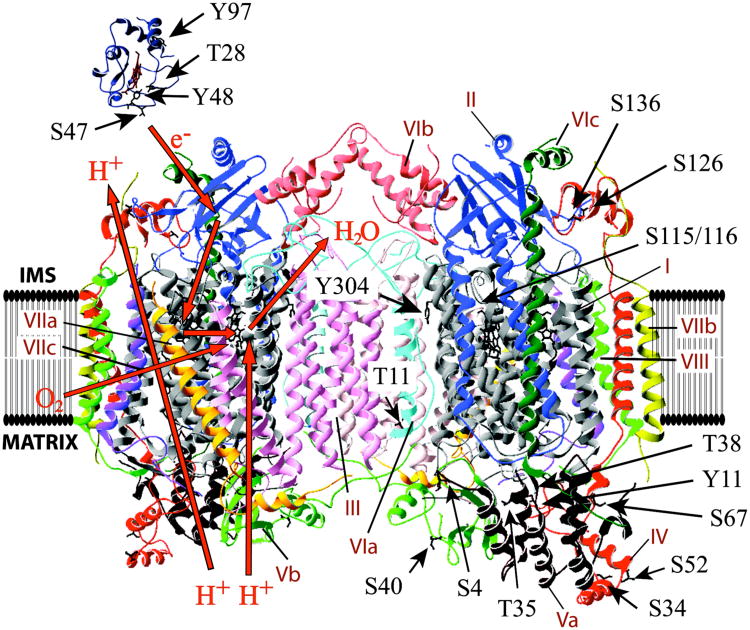
Mammalian COX contains 13 subunits per monomer, and cow heart COX has been crystallized as a dimer suggesting that this is the functional form in mammals (Tsukihara et al. 1996). Each COX monomer contains two heme and two copper redox centers, which are located in catalytic subunits I and II. Of the 13 subunits, three are encoded by mitochondrial DNA and the remaining ten by nuclear DNA (Fig. 1).
Figure 1.
Identified phosphorylation sites on cytochrome c and cytochrome c oxidase. Crystal structure data of horse heart cytochrome c (Sanishvili et al. 1995) and cow heart COX (Tsukihara et al. 1996) were processed with the program Swiss-PDBViewer 3.7. Identified phosphorylated amino acids in mammals are shown in sticks. See Table 1 for a detailed description of the sites including phospho-epitopes and references. Note that subunit IV residue Thr52 in rabbit corresponds to Ser52 in cow.
Mammalian Cytc is a one-electron carrier that shuttles electrons from bc1-complex to COX. It contains 104 amino acids and a heme group, which is covalently attached to cysteines 14 and 17 (Fig. 1, top left). Cytc is highly positively charged with a pI of 9.6 and is located in the mitochondrial intermembrane space, where it is associated with negatively charged phospholipids of the inner membrane, in particular cardiolipin.
Cytc and COX represent the last step of the ETC, and catalyze the successive transfer of four electrons to molecular oxygen, which is reduced to water. At the same time, COX pumps protons across the inner mitochondrial membrane, with a stoichiometry of 1H+/1e− under normal conditions. The precise mechanism of how electron transfer is coupled to proton pumping and the location of the proton exit pathways remain unclear and are a matter of heated debate (von Ballmoos et al. 2011; von der Hocht et al. 2011; Yoshikawa et al. 2011). In addition to the pumped protons, the consumption of “chemical” protons from the matrix side for the water formation reaction contributes to the generation of the proton motive force.
The reaction catalyzed by COX with a free energy of ΔGº′= −100 kJ/mol (Hinkle et al. 1991) is essentially irreversible. In mammals, it is the proposed rate-limiting step of the ETC in intact cells (Villani et al. 1997; Villani et al. 1998; Acin-Perez et al. 2003; Piccoli et al. 2006; Dalmonte et al. 2009; Pacelli et al. 2011), but not in isolated mitochondria. The latter phenomenon may be explained by unsuitable mitochondria isolation methods, which disrupt cellular structures and signaling networks, leading to a loss of regulatory properties of COX and Cytc, likely via dephosphorylation reactions. It has been shown that not only mitochondrial morphology changes dramatically after isolation but also that, in contrast to the other ETC complexes, specifically COX activity is significantly increased (Picard et al. 2011), which may account for a loss of ETC flux control by COX in isolated mitochondria.
Based on the central role of the terminal step of the ETC it is not surprising that a multitude of regulatory mechanisms are in place in addition to reversible phosphorylation. One other mechanism is the expression of tissue-specific and developmentally-regulated isoforms. Cytc occurs as somatic and testis-specific isoforms in rodents (Goldberg et al. 1977), but not in humans, where the genomic region syntenic to the testes-isoform in rodents now contains a non-transcribed pseudogene (Hüttemann et al. 2003; Zhang et al. 2003). The slight differences in amino acid composition between the isoforms affect its function. In comparison with the somatic isoform, testes Cytc shows a threefold increased activity to reduce hydrogen peroxide; however, it also shows a fourfold increased ability to trigger apoptosis (Liu et al. 2006).
For COX, six subunit isoforms have been identified in mammals to date. Those are heart/skeletal-muscle specific isoforms of subunit VIa, VIIa, and VIII, a lung-specific isoform of subunit IV, a testes-specific isoform of subunit VIb and a third isoform of subunit VIII (see Hüttemann et al. (2011b) for a current review). All isoforms are encoded by separate genes. Expression of tissue-specific isoforms may not only directly affect the activities of COX and Cytc but it can also provide a platform for tissue-specific cell signaling. For example, a phosphorylation site was mapped in the heart/skeletal muscle-specific subunit VIa of COX (Tsukihara et al. 2003) (Table 1). The corresponding epitope in the liver-type isoform is distinct suggesting that cell signaling is adapted to tissue-specific signals and needs.
Table 1. Identified phospho-epitopes in mammalian cytochrome c and cytochrome c oxidase1.
| Enzyme | Species & tissue | Phosphorylated amino acid2 | Phospho-epitope3 | Reference | Method |
|---|---|---|---|---|---|
| Cytc | Human skeletal muscle | Thr28 | EKGGKHKTGPNLHGL | (Zhao et al. 2010) | HT-MS4 |
| Cytc | Human skeletal muscle | Ser47 | TGQAPGYSYTAANKN | (Zhao et al. 2010) | HT-MS |
| Cytc | Cow liver | Tyr48 | GQAPGFSYTDANKKN | (Yu et al. 2008a) | MS |
| Cytc | Cow heart | Tyr97 | EREDLIAYLKKATNE | (Lee et al. 2006) | MS |
|
| |||||
| COX | Cow liver | Tyr304, SU I | MDVDTRAYFTSATMI | (Lee et al. 2005) | MS, cAMP-dependent |
| COX | Cow liver | Tyr304, SU I | MDVDTRAYFTSATMI | (Samavati et al. 2008) | Phospho-epitope-specific antibody; TNFα-dependent |
| COX | Rabbit heart | Ser115 and Ser116, SU I | SLHLAGVSSILGAINF | (Fang et al. 2007) | MS; after I/R5 |
| COX | Cow heart | Ser126, SU II | DSYMIPTSELKPGEL | (Hüttemann et al. 2011a) | MS |
| COX | Cow liver | Tyr11, SU IV-1 | SVVKSEDYALPSYVD | (Lee et al. 2006) | MS |
| COX | Cow heart | Ser34, SU IV-1 | VAHVKNLSASQKALK | (Helling et al. 2008) | MS |
| COX | HeLa cells | Ser67, SU IV-1 | YRIKFKESFAEMNRG | (Olsen et al. 2010) | HT-MS |
| COX | Rabbit heart | Thr52, SU IV-1 | KAPWGSLTRDEKVEL | (Fang et al. 2007) | MS; after I/R HT-MS |
| COX | HeLa cells | Ser136, SU IV-1 | NPIQGLASKWDYEKN | (Olsen et al. 2010) | MS |
| COX | Cow heart | Ser4, SU Va | SHGSHETDEEF | (Helling et al. 2008) | MS |
| COX | Cow heart | Thr35, SU Va | ELRKGMNTLVGYDLV | (Helling et al. 2008) | HT-MS |
| COX | HeLa cells | Thr35 and Thr38, SU Va | LRKGINTLVTYDMVPE | (Olsen et al. 2010) | |
| COX | Rabbit heart | Ser40, SU Vb | MLPPKAASGTKEDPN | (Fang et al. 2007) | MS; after I/R |
| COX | Cow heart | Thr11, SU VIa | AKGDHGGTGARTWRF | (Tsukihara et al. 2003) | Crystal structure |
Identified phosphorylation sites are presented in an ascending amino acid and subunit (SU) order.
Numbering according to mature peptide based on cow.
Sequence is based on the species where the phosphorylation site was identified.
High throughput mass spectrometry sequencing.
Ischemia/reperfusion.
Another mechanism is allosteric regulation via binding of ATP and ADP, a built-in energy sensor in COX that adapts COX activity to energy demand (see Chap. 11). Interestingly, ATP also binds to Cytc, contributing to the inhibition of the reaction between Cytc and COX (Ferguson-Miller et al. 1976) but no such regulation has yet been reported for the other OxPhos complexes. Additional regulatory mechanisms acting on COX include 1) competitive binding of nitric oxide, making COX one of the most important targets for NO signaling within the cell (Martinez-Ruiz et al. 2011); 2) binding of small molecules such as thyroid hormone T2, leading to an activation of COX even in the presence of allosteric inhibitor ATP (Arnold et al. 1998) and the fatty acid palmitate, which reduces the H+/e− stoichiometry in liver COX, making the COX reaction less efficient (Lee et al. 2001); and 3) direct protein-protein interactions with nitric oxide synthase (Persichini et al. 2005), the androgen receptor (Beauchemin et al. 2001), the epidermal growth factor receptor (EGFR) (Boerner et al. 2004), and Smad4, a downstream executer of TGF-β signaling, which binds to COX during apoptosis (Pang et al. 2011).
X.3 Regulation of mitochondrial OxPhos via phosphorylation of cytochrome c oxidase and cytochrome c
Single-celled and particularly multicellular organisms employ mechanisms that allow the exchange of information within and between cells and with the cellular environment. Post-translational modifications such as phosphorylations allow rapid modification of protein function, adapting it to cellular changes. Since the mitochondrial OxPhos system is a central functional unit primarily linked to energy production it is a logical target of cell signaling. Other functions such as ROS production and the participation of Cytc in apoptosis also require tight regulation.
Phosphorylation sites have been identified on all mammalian OxPhos complexes. For the vast majority, signaling pathways, kinases and phosphatases mediating these phosphorylations remain unknown or uncertain (Hüttemann et al. 2007). Future work should focus on the functional consequences of individual phosphorylations, their dynamics, i.e., regarding both extent and change over time, and the identification of kinases and phosphatases acting on OxPhos. Among OxPhos complexes, phosphorylation of COX and Cytc have been studied more deeply and will be discussed here, including their potential role in pathological conditions.
X.3.1 Phosphorylation of cytochrome c
Cytc is a pivotal component of both apoptosis and electron transfer. There are good reasons to believe that such an important molecule would be the target of cell signaling pathways, but such pathways had never been discovered, even though Cytc had been studied for more than a century. To discover phosphorylations of Cytc, if they existed, it would be necessary to isolate Cytc from animal tissue under stringent conditions that would preserve the physiological phosphorylation state. Carrying out isolation of Cytc from cow heart tissue using such stringent conditions, we found by mass spectrometry (MS) that it was phosphorylated on Tyr97 (Fig. 1) (Lee et al. 2006). We then investigated the effects of that phosphorylation. Previous investigators had found that the heme iron-Met80 absorption band (normally at 695 nm) is an important indicator of functional intactness of Cytc. We found that this band was shifted to 687 nm upon phosphorylation of Tyr97. Phosphorylated Cytc showed functional differences: a shifted Km of COX for Cytc from 2.5 μM compared to 5.5 μM and enhanced sigmoidal kinetics indicating an inhibition in the reaction with COX.
Cytc isolated from cow liver tissue, we later found, is phosphorylated too, but on a different residue, Tyr48 (Yu et al. 2008a) (Fig. 1). This finding surprised us at first, but is consistent with what is known about differential regulation of COX from tissue type to tissue type. The effects of this phosphorylation were distinct from those found in Tyr97 phosphorylation in cow heart in that in liver Cytc there were no spectral changes, and Tyr48 phosphorylation produced a hyperbolic response, similar to the unphosphorylated Cytc. However, at maximal turnover COX activity was more than 50% reduced with Tyr48-phosphorylated Cytc. Thus, phosphorylation in both cases (Tyr97 in heart, Tyr48 in liver) causes partial, but not full, inhibition of the reaction between Cytc and COX.
The functional explanation for inhibition of mitochondrial respiration by Cytc phosphorylation may be that it provides ample mitochondrial membrane potential Δψm for the production of ATP without the production of excessive free radicals that are concomitant with high Δψm levels. However, excess capacity is needed in certain conditions as a trigger of type II apoptosis, which is initiated by a transient hyperpolarization of Δψm, followed by a burst of ROS as a signal to commit to cell death, a model that we will revisit below.
Experiments with phosphomimetic mutant Cytc indicates that Cytc phosphorylation at Tyr48 may have important functional effects for apoptosis, the second key role of Cytc. We replaced Tyr48 with Glu, which mimics the negative charge of the phosphate group (Pecina et al. 2010). The mutant Cytc showed a 45 mV reduction of its midpoint redox potential compared to wild-type unphosphorylated Cytc. The reaction kinetics of phosphomimetic Cytc with COX were similar to that of the Tyr48 Cytc with COX, suggesting that the phosphomimetic form is a good model for Tyr48-phosphorylated Cytc. Strikingly, the ability of Cytc to trigger downstream caspase activation, a requirement of apoptosis, was completely lost in the phosphomimetic mutant. The possibility that phosphorylation of Cytc regulates programmed cell death has potentially important therapeutic implications for diseases like cancer, in which apoptosis is inhibited. Once the kinases and phosphatases are identified that act on Cytc, these enzymes could be specifically targeted to promote apoptosis by dephosphorylation of Cytc in conditions such as cancer. In addition, phosphorylation of Cytc could be induced in conditions of stress such as ischemia/reperfusion injury where cell survival strategies would be beneficial.
A fraction of Cytc is normally bound to the mitochondria-specific lipid cardiolipin, tethering Cytc to the inner mitochondrial membrane. It has been suggested that the release of Cytc from cardiolipin is one of the first steps of the participation of Cytc in apoptosis, and that this is mediated via cardiolipin peroxidase activity of Cytc (Kagan et al. 2009). Upon oxidation of cardiolipin by Cytc in the presence of ROS or lipid peroxides that serve as substrates, Cytc binding affinity is reduced, leading to a dissociation of Cytc from cardiolipin. Interestingly, peroxidase activity of Tyr48Glu phosphomimetic Cytc was inducible only at high cardiolipin concentrations, unlike controls, suggesting that Cytc phosphorylation may suppress the cardiolipin oxidation reaction. (Pecina et al. 2010). This could be a second safeguard mechanism to ensure that apoptosis is well regulated, including through modulation of Cytc attachment to the inner mitochondrial membrane and thus its release.
Two more phosphorylation sites (Thr28 and Ser47; Fig. 1,Table 1) have recently been mapped on Cytc in skeletal muscle tissue by high throughput phosphoproteomic MS analysis (Zhao et al. 2010). Although their function is unknown, these phosphorylations suggest regulation by a different pathway than those operating in heart and liver.
It should be kept in mind that there are other functions of Cytc than the well-known roles of Cytc in electron transport and apoptosis. Cytc also scavenges ROS under healthy conditions (Korshunov et al. 1999; Wang et al. 2003), and generates ROS through the p66shc pathway (Giorgio et al. 2005).
X.3.2 Phosphorylation of cytochrome c oxidase
The first indication of phosphorylation of COX was presented by Steenaart and Shore (1997), who found that COX was phosphorylated on subunit IV-1 by labeling mitochondrial proteins with radioactive ATP. Since then 14 phosphorylation sites have been mapped on COX (Table 1; Fig. 1) and several signaling pathways have been studied in some depth. Although many pieces of the puzzle are still missing it is clear that phosphorylation of COX can decisively regulate its activity.
X.3.2.1 The cAMP-dependent pathway
In comparison to other signaling pathways PKA signaling has received the most attention. In vitro work first suggested that in cow heart COX incubated with PKA, cAMP, and γ-32ATP, subunit Vb, perhaps subunit II or III, and subunit I are all phosphorylated (Bender et al. 2000; Lee et al. 2002). However, in vitro work does not usually include various auxiliary components such as scaffolding proteins (e.g., A-kinase anchoring proteins for PKA), and therefore phosphorylation may lack specificity (Welch et al. 2010). Based on our experience, from observation of phosphorylation patterns of COX after in vitro incubation with several kinases, which did not match results obtained in vivo after stimulation or inhibition of the corresponding signaling pathway, we caution the reader against pursuing an in vitro approach.
Our laboratory pursued an in vivo approach to elucidating the issue of cAMP-dependent phosphorylation of COX in liver tissue, where cAMP is a starvation signal triggered by the hormone glucagon. High cAMP levels were generated by treatment with phosphodiesterase inhibitor theophylline. Vanadate and fluoride, two unspecific tyrosine and serine/threonine phosphatase inhibitors, respectively, were included during the purification of mitochondria and subsequently of COX. The treated enzyme, but not the untreated control, showed phosphorylation on Tyr304 of subunit I (Lee et al. 2005). Tyr304 is located adjacent to the oxygen-binding center on COX, and structural modifications near that site can be expected to exhibit functional consequences. Experiments revealed that Tyr304 phosphorylated COX was inhibited strongly to completely at up to 10 μM Cytc substrate concentrations, even in the presence of allosteric activator ADP (Lee et al. 2005). A similar inhibitory effect was seen with other agents that increase cAMP levels, i.e., the adenylyl cyclase activator forskolin and the physiological starvation hormone glucagon. Functionally, this finding makes sense because in starved conditions it would be adaptive for energy production via the electron transport chain to be decreased. Mechanistically, since PKA does not phosphorylate tyrosine residues it appears that a tyrosine kinase downstream of PKA phosphorylates COX.
A similar inhibitory effect along with lowered ATP levels resulted when cow lung tissue was treated with theophylline (Lee et al. 2005). The concentrations of theophylline were those used in asthma therapy, for which theophylline has long been used effectively. The efficacy of theophylline, then, may be in part related to its effect on cAMP, which would then lead to COX phosphorylation. The general mechanism of action would be the following. Theophylline treatment increases cAMP levels, which causes COX phosphorylation, thus reducing COX activity and decreasing ATP production. Decreased ATP production would lower the ability of the airway to constrict, which is an energy-intensive process, and thus would counter the airway constriction that is a hallmark of asthma. If the energy hypothesis is correct, mitochondrial OxPhos could be specifically targeted for asthma treatment, preferably by agents that act only on lung.
It is known that the response of COX to cAMP signaling is tissue-specific. In neuronal tissue, theophylline treatment leads to COX activation (Hüttemann et al. 2010), whereas in heart theophylline and also 3-isobutyl-1-methylxanthine (IBMX, a heart specific cAMP inducer) had no significant effects on COX activity in our hands. Other phosphorylations have been found in ischemic rabbit heart, and the possibility was raised that these could be a result of cAMP/PKA signaling (Prabu et al. 2006) because the phosphorylations were not found in the presence of kinase inhibitor H89. However, H89 does not affect only PKA and instead inhibits multiple kinases (Bain et al. 2007). The sites were later identified by MS and are Ser115 and Ser116 of subunit I, Thr52 of subunit IV, and Ser40 of subunit Vb (Fang et al. 2007). Since the above sites are not PKA consensus sequences PKA is probably not directly involved in these phosphorylations.
Although COX subunit I Tyr304 phosphorylation, located towards the intermembrane side, inhibits the enzyme in mammalian liver, a distinct effect was found by the Manfredi group in their investigation of a carbon dioxide/bicarbonate regulated adenylyl cyclase, which localizes to the mitochondrial matrix (Acin-Perez et al. 2009). This enzyme links nutrient availability to OxPhos activity through sensing of CO2 generated by the citric acid cycle. The study was performed in HeLa cells, and COX subunits I and IV were phosphorylated. The authors proposed that this phosphorylation is mediated by a matrix-localized PKA, although the phosphorylation sites need to be mapped to further confirm PKA involvement. The effect of such signaling is activation of COX (Acin-Perez et al. 2009). Another example of matrix-localized PKA signaling to COX had been reported earlier: the Riα regulatory subunit of PKA can bind to matrix-localized subunit Vb of COX (Yang et al. 1998).
X.3.2.2 Other signaling pathways
Various other studies suggest that COX is a target of signaling pathways without identification of the phosphorylation sites. For example, in addition to its primary cytosolic localization, non-receptor tyrosine kinase Src has been shown to localize to the mitochondrial intermembrane space (Salvi et al. 2002). In osteoblasts Src targets COX subunit II for phosphorylation, leading to increased COX activity (Miyazaki et al. 2003).
X.3.2.3 Signaling pathways unknown
Additional phosphorylation sites have been mapped on COX for which the signaling pathways involved and the functional consequences are unknown. Those sites are Tyr11 of subunit IV-1 in isolated cow liver (Lee et al. 2006), Ser67 and Ser136 of subunit IV-1, Thr35 and Thr38 of subunit Va in human HeLa cells by high-throughput MS (Olsen et al. 2010), Ser34 of subunit IV-1 and Ser4 and Thr35 of subunit Va in cow heart (Helling et al. 2008), Thr11 of subunit VIa heart isoform, which was identified in the cow heart crystal structure (Tsukihara et al. 2003), and Ser126 on catalytic subunit II, which was identified in three independent COX isolations (Hüttemann et al. 2011a) (Table 1; Fig. 1).
X.3.3 Calcium signaling
Finally, a promising avenue of research would be to investigate the direct or indirect action of calcium on COX and Cytc phosphorylation. Calcium has been proposed to be the strongest signal for mitochondrial activation (Robb-Gaspers et al. 1998). Calcium also plays a key role during conditions of cellular stress, where it leads to hyperactive ETC complexes and increased Δψm levels, leading to excessive ROS production as discussed in section X.4.1.1. Mammalian COX contains a calcium-sodium exchange site in subunit I (Kirichenko et al. 1998; Kirichenko et al. 2005). In addition to a possible direct functional effect on COX, which remains to be studied, calcium may indirectly affect COX and Cytc through changes in the phosphorylation state. It was shown in pig heart mitochondria that calcium leads to dephosphorylation of most mitochondrial proteins (Hopper et al. 2006). Since dephosphorylation of COX at subunit I Tyr304, and of Cytc at Tyr48 and Tyr97 results in increased ETC activity, this may explain some of the activating effects of calcium signaling.
X.3.4 Mitochondrial tyrosine phosphatase Shp-2 and Noonan syndrome
Protein tyrosine phosphatase Shp-2 was the first identified tyrosine phosphatase with mitochondrial co-localization. It was identified in the intermembrane space and the outer mitochondrial membrane in addition to the cytoplasm in rat brain (Salvi et al. 2004). Shp-2 is mostly associated with Ras/mitogen-activated signaling, where it acts as a positive modulator. Mutations in the gene encoding Shp-2 (PTPN11) account for about half of the cases of Noonan syndrome (Tartaglia et al. 2001), a relatively common autosomal dominant disorder characterized by congenital heart defects, dysmorphic facial features, webbed neck, short stature, chest deformity, and variable cognitive deficits (Tartaglia et al. 2011). In these Noonan patients with PTPN11 mutations Shp-2 shows increased basal phosphatase activity (Neel et al. 2003). Because COX and Cytc are currently the only OxPhos components with mapped tyrosine phosphorylation sites, three of which localize to the IMS (Fig. 1) as does Shp-2, they might be targets of Shp-2. We thus analyzed patient and mouse cell lines with Noonan syndrome mutations and observed significantly increased COX activity (Lee et al. 2010). COX and Cytc protein levels were downregulated in the mutant cells, suggesting a compensatory mechanism to counterbalance increased COX activity. In addition, mutant cells showed 30% decreased ATP and increased ROS levels, both of which may interfere with organ development. Currently it is unknown if any of the identified tyrosine phosphorylation sites on COX or Cytc are targets of Shp-2, but the observation above, i.e., the combination of significantly increased COX activity with reduced COX and Cytc protein levels, suggests changes in protein phosphorylation as a mechanistic explanation.
X.4 Role of cytochrome c oxidase and cytochrome c in human disease
COX and Cytc have been implicated in numerous diseases, some of which we briefly discussed above. This final section focuses on three distinct pathological conditions, ischemia/reperfusion injury, cancer, and inflammation, all of which involve COX and/or Cytc in a highly specific and distinct manner.
X.4.1 Cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and the role of protein kinase C signaling
Loss of blood flow and thus delivery of oxygen and nutrients to tissues causes extensive damage. Different cell types have varying sensitivities or resistance to ischemic damage, depending on the extent to which the tissue relies on OxPhos for ATP production. While tissue damage caused by ischemia can be extensive, of greater clinical interest is the additional damage induced by restoration of blood flow, or reperfusion injury (Oliver et al. 1990). Currently, the only treatment for ischemia is prompt restoration of blood flow. However, paradoxically, the act of restoring oxygen supply to tissue that has undergone ischemic stress causes profound damage (Campbell et al. 1986; Zweier et al. 1988; Aronowski et al. 1997; Fellman et al. 1997; Tilney et al. 1997). This, in turn, results in worsened morbidity and mortality in a host of clinical disorders, including stroke, cardiac arrest/resuscitation, myocardial infarction, acute tubular necrosis, and neonatal hypoxic/ischemic encephalopathy (Zweier et al. 1988; Schumer et al. 1992; Aronowski et al. 1997; Fellman et al. 1997; Roger et al. 2011). Importantly, reperfusion injury can, in theory, be treated therapeutically.
X.4.1.1 The connection between respiratory activity, the mitochondrial membrane potential, and the production of reactive oxygen species
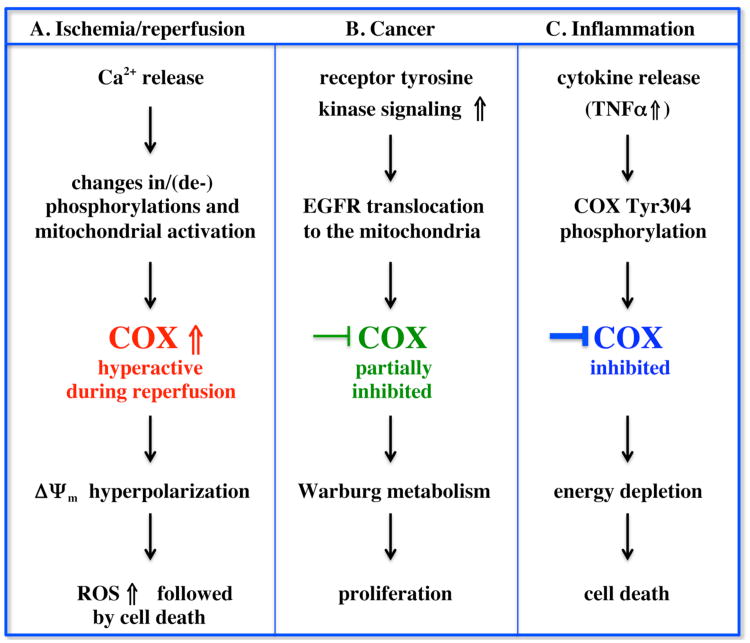
Mitochondria play a central role in reperfusion injury, primarily as a major source of ROS. As we discussed in detail previously (Hüttemann et al. 2008; Hüttemann et al. 2011b), mitochondrial ROS are generated by OxPhos, specifically at high Δψm. One of the proposed mechanisms of increased ROS at high Δψm is an overall reduction of electron flux in the ETC because high Δψm levels inhibit the proton pumping activity of complexes I, III, and COX from further proton pumping. This causes an increased half-life of the ubisemiquinone radical intermediates, which now have more time to transfer their unpaired electron to oxygen (Liu et al. 1999). As the proposed rate-limiting step of the ETC in intact cells (see Introduction), Cytc and COX can be considered primary regulators of mitochondrial ROS generation (Piantadosi et al. 1996). Here we put forth a model of ROS generation during reperfusion in multiple tissues, and present studies suggesting the validity of this model. Specifically, we propose that altered phosphorylation of COX and Cytc, caused by ischemia, results in high ‘uncontrolled’ respiratory rates, high Δψm levels, and thus a reperfusion-induced ROS burst upon restoration of blood flow (Fig. 2A).
Figure 2.
Role of COX in human disease. In our proposed model the activities of COX and Cytc are regulated by cell signaling pathways under healthy conditions. Both proteins are phosphorylated in vivo leading to controlled respiration. This generates healthy mitochondrial membrane potentials (Δψm), allowing efficient energy production but preventing the production of ROS, which are only generated at pathologically high Δψm levels. During pathological conditions the phosphorylation patterns change, leading to an imbalance of ROS and ATP production. A) Ischemic stroke and myocardial infarction are common human pathologies and the only current treatment is rapid resumption of blood flow, i.e., reperfusion. Reperfusion causes the major part of ischemia/reperfusion-related injury through the following sequence of events. During episodes of ischemia nutrients and oxygen become depleted causing excessive calcium release and alterations of PKC signaling. This results in changes of phosphorylation and/or dephosphorylation of COX and/or Cytc (Cytc is not shown). In the ischemic phase COX does not turn over due to lack of substrates. In the reperfusion phase, oxygen and nutrients are reintroduced leading to a rapid reestablishment of Δψm. Because COX is still in a hyperactive state, Δψm increases further, leading to a Δψm hyperpolarization and the production of excessive ROS. ROS in turn serve as a signal for triggering apoptosis leading to cell death. B) Many cancers are characterized by hyperactive receptor tyrosine kinase signaling including EGFR signaling. Here, activation of the pathway leads to an internalization of the receptor and translocation to the mitochondria where EGFR directly interacts with and phosphorylates COX subunit II, leading to a partial inhibition of COX activity. This step allows shifting of aerobic energy metabolism to Warburg metabolism: increased glycolytic and pentose phosphate pathway activity now provide essential building blocks for the cell, enabling rapid proliferation. C) Acute inflammation as in sepsis leads to the release of cytokines including TNFα resulting in phosphorylation of Tyr304 on catalytic subunit I. COX is strongly inhibited leading to decreased Δψm levels, and eventually depletion of ATP. In septic patients this model would explain organ failure and death through energetic failure.
Our model proposes that cells modify the activity of OxPhos by posttranslational modifications on COX and/or Cytc under normal physiologic conditions. Under conditions of energy depletion, cells attempt to augment their energy production by the OxPhos system by altering the phosphorylation status of these complexes. However, under conditions of pathologic energy depletion, such as the ischemic state, energy exhaustion is coupled to oxygen deprivation. In this state, the terminal substrate for OxPhos is absent, and no aerobic ATP production is possible. We propose that the apparent dysfunction of this normal response to energy depletion is the proximal cause of ROS generation upon reperfusion.
The specific role of COX and Cytc phosphorylation in the setting of ischemia/reperfusion remains largely unknown at present. However, many studies have identified alterations in COX activity during the progression of reperfusion injury suggesting a role for cell signaling that alters OxPhos activity. During ischemia, intracellular calcium concentrations increase when ATP-dependent pumps fail (Rosenthal et al. 1987), and mitochondria actively sequester calcium during early restoration of blood flow (Zaidan et al. 1994). Increased mitochondrial calcium is a potent signal for phosphatase activation, and calcium induces dephosphorylation of most mitochondrial proteins (Robb-Gaspers et al. 1998; Hopper et al. 2006). Interestingly, multiple studies have observed increased mitochondrial respiration, and COX activity, upon calcium sequestration by mitochondria (Rosenthal et al. 1987; Fiskum et al. 2004). In vitro, calcium causes COX hyperactivation and loss of allosteric inhibition by ATP, and the Kadenbach group showed that calcium did not act directly on COX (Bender et al. 2000). In contrast, they observed an indirect effect, most likely through changes in post-translational modifications of COX induced by calcium. Additionally, mitochondria treated with calcium had increased state 4 respiration (Vlessis et al. 1990), which may be explained by COX dephosphorylation. Dephosphorylation of COX in vitro results in loss of the ability of ATP to allosterically inhibit COX (Hüttemann et al. 2008). This effect, when occurring in the setting of ischemia/reperfusion injury would result in a condition where COX could generate high Δψm and subsequently generate ROS, triggering death processes (Fig. 2A).
As discussed above all functionally studied phosphorylations of Cytc inhibit respiration. Therefore, if calcium-induced dephosphorylation of Cytc were to occur, this would contribute to increased OxPhos flux and thus the hyperpolarization of Δψm. Recent studies from our group found that Cytc phosphorylation is lost when brain is rendered ischemic (unpublished). This dephosphoryated Cytc would also have the full capability to induce apoptosis (Pecina et al. 2010).
X.4.1.2 Protein kinase C
In the context of ischemia/reperfusion injury, PKC signaling has been studied in some detail. PKC is a stress-activated kinase involved in modulating calcium uptake by mitochondria, production of free radicals, and the induction of apoptosis. Importantly, several PKC subtypes have been implicated in regulating these deleterious events following ischemia/reperfusion injury in multiple tissues. It is generally believed that PKC signaling serves to protect the cells during episodes of ischemia/reperfusion but the intricate connection between calcium and PKC signaling may complicate the picture.
There is clear evidence of a direct action of PKC on mitochondria. For example, following ischemia/reperfusion in the heart, PKCε translocates to the mitochondrial inner membrane (Budas et al. 2010). In a model of global brain ischemia, PKCβ translocates to the mitochondria in neurons resistant to cell death, while in brain regions that go on to die (the CA1 hippocampus), no translocation of PKCβ was seen (Kowalczyk et al. 2011). Interestingly, PKCβ was found in the mitochondria, associated with components of the electron transport chain. Indeed, inhibition of PKC family kinases leads to increased Δψm and ROS production (Lu et al. 2011). An important protective effect of PKCε in ischemia/reperfusion injury may be due to its interaction with the calcium-sensing receptor, leading to a reduction of calcium release (Dong et al. 2010). This would prevent calcium-activated dephosphorylation and hyperactivation of mitochondrial proteins. Interestingly, administration of compounds that induce PKC activation provides protection from ischemia/reperfusion injury of the heart (Sivaraman et al. 2009) and brain (Della-Morte et al. 2011). The compound tribulosin protects the heart from ischemia/reperfusion injury through activation of PKCε and parallel activation of superoxide dismutase (Zhang et al. 2010). Another selective PKCε activator, psivarepsilon-RACK, demonstrated significant neuroprotection from reperfusion-induced neuronal cell death (Della-Morte et al. 2011). Translocation of PKCδ to the mitochondria does not occur during ischemia, but was observed within 5 min of reperfusion after 30 min ischemia in rat hearts, followed by a decrease in mitochondrial respiration and an increase in superoxide radical production (Churchill et al. 2005).
The above studies underscore the potential contribution of PKC signaling in reversible phosphorylation of OxPhos complexes, and the central role PKC may play in the pathophysiology of ischemia/reperfusion injury. As discussed in section X.3.2.1, ischemia directly affects COX and leads to phosphorylation of several subunits (Prabu et al. 2006), and it is possible that this phosphorylation is mediated by a yet-to-be-identified PKC isozyme. In rat heart, ischemia/reperfusion resulted in changes of the immunoreactivity of several COX subunits, especially subunit I, and the authors proposed that those subunits are lost from the holoenzyme (Yu et al. 2008b). An alternative and perhaps more likely explanation might be masking of the epitope recognized by the antibody by phosphorylation.
The ischemia-triggered phosphorylations lead to a partial inhibition of COX activity (Prabu et al. 2006), which would be protective during reperfusion. However, other studies have shown that the opposite effect, i.e., activation of COX, is possible. In rat neonatal cardiac myocytes activation of PKC with diacylglycerol or 4β-PMA caused phosphorylation of COX subunit IV in vitro and resulted in about two- to fourfold increased COX activity (Ogbi et al. 2004; Ogbi et al. 2006). PKCε is a possible candidate for this phosphorylation because the authors showed that it co-immunoprecipitated with COX (Guo et al. 2007).
Based on the extent of the stress impact, calcium signaling may prevail leading to an overall activation of the ETC proton pumps. Based on our model (Fig. 2A), upon restoration of blood flow, if oxygen reaches a COX enzyme that has been post-translationally modified to increase its activity, these alterations would initially aid in the restoration of Δψm, and the reestablishment of cellular energy levels (Ekholm et al. 1993). However, in this hyperactive state, the proton pumps would not be inhibited by physiological Δψm levels <140 mV and would continue to generate pathologically high Δψm levels >140 mV, which would lead to ROS generation (Liu et al. 1999). The majority of mitochondrial ROS are created during this early reperfusion interval (Fabian et al. 1995), and the ETC is a primary source of ROS during reperfusion (Piantadosi et al. 1996). Reperfusion of ischemic brain results in a rapid restoration of Δψm, followed by a transient hyperpolarization of Δψm and substantial ROS generation (Liu et al. 2009). These findings position COX and Cytc as potential regulatory sites that can indirectly control ROS generation by regulating overall ETC flux, thereby controlling Δψm and ROS. It is thus possible that therapeutic interventions targeting COX and/or Cytc phosphorylations may be neuroprotective in the context of ischemia/reperfusion injury.
In summary, we propose a model of reperfusion-induced ROS generation and cell death where post-translational modifications of Cytc and/or COX play a critical role in controlling the eventual fate of the cell. Specifically, we propose that cell signaling systems, most notably PKC signaling, retain Cytc and/or COX in a phosphorylated ‘controlled’ state. However, ischemic stress can result in excessive calcium release and subsequent dephosphorylation of OxPhos complexes, thus tipping the balance from controlled respiration to Δψm hyperpolarization, subsequent ROS generation, and cell death (Fig. 2A). Therefore, regulation of OxPhos phosphorylation may represent a novel method to minimize reperfusion injury following ischemic events in multiple tissues.
X.4.2 Cancer and inflammation: Cytochrome c oxidase and cytochrome c as functional targets
In this section we will discuss mechanisms underlying cancer and inflammation and discuss the emerging link between the two (Fig. 2B and C). We will start at the macroscopic level by identifying similarities between cancer and inflammation and eventually focus on COX and Cytc at the molecular level.
X.4.2.1 Inflammation as a promoter of cancer
Metabolism changes during carcinogenesis, and most solid tumors show a 25-60% reduction of mitochondrial mass compared to healthy differentiated tissue (Pedersen et al. 1978). During carcinogenesis cells shift their metabolism from aerobic energy production to glycolysis. The shift takes place even in the presence of oxygen and is therefore referred to as aerobic glycolysis, and it is known as the Warburg effect (Warburg et al. 1924; Warburg et al. 1956). Since Warburg's discovery, considerable work has been done on the role of mitochondria in cancer. Generally, two primary mitochondrial cancer promoting factors have been tied to cancer, via metabolic switching to provide building blocks for the growing cells (Weinberg et al. 2009), and/or via increased mitochondrial ROS production resulting in the emergence of some cells with oncogenic mutations (Ralph et al. 2010).
Inflammation is an immune response initiated by the vascular system to fight various compounds including pathogens, irritants, and even cells of the organism itself. It is involved acutely and chronically in numerous pathological conditions, such as sepsis, asthma, and rheumatoid arthritis. More recently, inflammation has also become a widely accepted component in different stages of tumor development (Rakoff-Nahoum et al. 2006; Mantovani et al. 2008). Grivennikov and colleagues (2010b) proposed a two-step model in which reactive oxygen and nitrogen species (RONS) produced by inflammatory cells first cause mutations in neighboring cells. Tumor initiation is further amplified by cytokine-mediated increased RONS production in pre-malignant cells. The second step, tumor promotion, is accompanied by immune cell-mediated cytokine production, which activates key transcription factors in pre-malignant cells, including NF-κB and STAT3. This induces pro-tumorigenic processes, including survival, proliferation, growth, angiogenesis, and invasion. Since it has turned out that key factors such as ROS are found in both inflammation and cancer and are connected to mitochondria, this has stimulated examination of the links that may tie them together (Kamp et al. 2011).
The observations that tumors often arise at a site of chronic inflammation, and that they contain inflammatory cells, are more than 100 years old. Recent interest in this topic was promoted by several types of observations, including substantial epidemiological evidence, such as the beneficial effect on cancer prevalence of chronic use of non-steroidal anti-inflammatory drugs, and the unraveling and manipulating in animal models of the molecular pathways. The considerable epidemiological evidence includes a wide array of chronic infections, such as by Helicobacter pylori, exposure to a wide range of irritants that trigger inflammation, such as tobacco smoke, and autoimmune conditions. Strong evidence also comes from studies of inflammatory bowel disease. Patients with ulcerative colitis, an inflammatory bowel disease, have a 5-7–fold increased risk of colorectal cancer; this risk is reduced by 80% by administration of cyclooxygenase-2 inhibitors (Kamp et al. 2011).
Germ-line mutations are rare causes of cancer; about 90% of cancers results from a combination of environmental factors and somatic mutations. Detailed studies have uncovered two pathways of inflammatory connection to cancer. An extrinsic pathway, which raises cancer risk, is provided by a chronic site-specific inflammatory condition such as pancreatitis or inflammatory bowel disease (Mantovani et al. 2008). In the intrinsic pathway oncogenes are activated, which is the driving force in an environment provided by the extrinsic pathway. Studies of RAS family mutations and MYC show the early induction of chemokines and inflammatory cytokines as part of the remodeling of the tissue microenvironment.
Inflammation has been shown to participate in all of the recognized stages in tumorigenesis – initiation, promotion, and metastasis. Initiation usually consists of accumulation of multiple mutations in the same cell. This is more likely in an inflammatory microenvironment, either via RONS produced by activated inflammatory cells or via cytokines produced by inflammatory cells that stimulate RONS production in neighboring epithelial cells. In tumor promotion, an initiated cell transforms into a tumor. Initiated cells are aided by inflammation both to proliferate and to survive, such as via the ROS-stimulated production of HIF-1α (Hamanaka et al. 2010). In addition, inflammatory mediators such as STAT3 and NF-κB promote the HIF-1α stimulation of angiogenesis that is needed by growing tumors to provide adequate blood supply (Grivennikov et al. 2010b). Lastly, since more than 90% of cancer mortality results from metastasis, this component is of the greatest clinical importance. Metastasis is clearly linked to inflammation. Many initiated cells express chemokine receptors on their surface (Balkwill et al. 2004). During metastasis, such cells utilize chemokines to aid their migration to distant sites, and to aid their survival upon arriving (Kim et al. 2005; Kim et al. 2009). Furthermore, both autocrine and paracrine signaling by cytokines like TNFα upregulate receptor expression and thereby increase invasive capacity and facilitate metastasis (Kulbe et al. 2005).
The epidemiological connection between inflammation and cancer stimulated numerous studies designed to elicit the mechanistic basis. As signaling pathways were unearthed, it became clear that central mediators of inflammation-associated cancer are RONS. Among those, ROS are the most studied reactive species and arise primarily from NADPH oxidase in phagocytes but elsewhere largely (>90%) from metabolism via the mitochondrial electron transport chain.
Work being pursued from another direction has turned out to be germane. The Warburg hypothesis connected metabolism and cancer in the original version by noticing that tumors are more glycolytic than normal tissues and that, therefore, metabolism was a component of cancer. This observation has also stimulated numerous studies, which have led to a more nuanced picture than the original hypothesis that takes into account a number of metabolic adaptations made by tumors to promote their growth. Except for the situation where hypoxia is found centrally in solid tumors, glycolysis appears not to be utilized for ATP production but for producing intermediates through the pentose phosphate pathway for nucleotide and phospholipid synthesis (Hamanaka et al. 2010; Weinberg et al. 2010). Mitochondrial metabolism per se in normoxic conditions may be dispensable, as suggested by a study that utilized a mutation in a complex III gene to block electron transport. Cells containing this mutation reduced but did not abolish a proxy for tumor growth, whereas ρ° cells did not show anchorage independent growth in a Kras tumor model (Weinberg et al. 2010). An important function of mitochondria appears to be ROS production as a signal for cell proliferation.
A provisional consolidated picture that emerges from considering these dual connections is that inflammation-stimulated ROS, produced in mitochondria under stimulation by cytokines and hypoxia, can act as a tumor promoter. Furthermore, such ROS would be amplified by ROS already produced as growth stimulators by emerging tumors. The increased ROS level could therefore act also as a mutagen to initiated feedforward cycles of cellular decline, thereby playing a role in both the extrinsic and the intrinsic pathways (Mantovani et al. 2008).
X.4.2.2 Cancer signaling targets cytochrome c oxidase
In addition to metabolic changes, cancers manage to evade apoptosis, and both changes are likely caused, at least in part, by receptor tyrosine kinase signaling that is upregulated in many cancers. Increased epidermal growth factor receptor (EGFR) signaling is implicated in numerous cancers including breast, colon, and lung cancers, and it is the first example of a tyrosine kinase receptor with a direct effect on COX. After stimulation with EGF it was shown in breast cancer cell lines that EGFR translocates to the mitochondria where it physically interacts with COX subunit II (Boerner et al. 2004). Only the activated, Tyr845-phosphorylated EGFR receptor binds to COX together with Src kinase leading to an increase in COX subunit II phosphorylation in vitro as was shown after incubation with [γ-32P]ATP (Demory et al. 2009). Although the phosphorylation site remains to be identified, those findings are in line with the Warburg effect because COX activity was decreased by 60% after cells were treated with EGF (Fig. 2B). It is possible that other receptor tyrosine kinases may follow a similar mechanism and that they target other OxPhos components. The only other OxPhos complex where tyrosine phosphorylation has been shown to date is ATP synthase (Ko et al. 2002): NIH3T3 and kidney cells treated with platelet-derived growth factor (PDGF) displayed tyrosine phosphorylation of the δ-subunit of ATP synthase.
Future studies of receptor tyrosine kinase signaling on multiple OxPhos components might reveal a concerted mode of action, i.e., the parallel targeting of several enzymes for phosphorylation to adapt OxPhos activity to cancer-specific energy metabolism. Specifically, Cytc might be targeted for phosphorylation for two reasons: since the functionally studied Cytc phosphorylations on tyrosines 48 and 97 both lead to an inhibition of respiration as discussed above, increased phosphorylation would contribute to the Warburg effect. In addition, Cytc phosphorylation may interfere with apoptosis as suggested by studies with phosphomimetic Cytc, which was not able to trigger any measureable caspase activation (Pecina et al. 2010). Since cancers manage to evade apoptosis, increased phosphorylation of Cytc via cancer signaling might provide a mechanism for the suppression of apoptosis.
X.4.2.3 Inflammatory signaling targets cytochrome c oxidase
The effect of acute inflammation on mitochondrial function has been studied in some detail. Acute inflammation as seen in sepsis is a major medical problem and leading cause of mortality in intensive care units with 210,000 deaths annually in the US alone (Hotchkiss et al. 2003). It can be caused by pathogenic infections of the blood and is therefore often referred to as blood poisoning. Sepsis can affect various organs such as the brain, heart, and liver, and it can result multiple organ dysfunction syndrome (MODS) (Ruggieri et al. 2010).
Genetic evidence strongly suggests that mitochondria play a key role in sepsis. The mitochondrial DNA composition is a genetic predictor for survival after sepsis. Mitochondrial DNA can be grouped into evolutionarily related DNA families, i.e., mitochondrial DNA haplogroups. Interestingly, septic patients belonging to haplogroup H, which is common in Europeans, have a more than twofold higher chance of survival compared to patients with other haplogroups (Baudouin et al. 2005).
Somewhat similarly to cancer, acute inflammation is accompanied by metabolic changes and a suppression of mitochondrial respiration. Thus septic patients show increased rates of lactate production and blood lactate levels (Revelly et al. 2005). Increased systemic delivery of oxygen during the course of sepsis does not improve outcome (Hayes et al. 1994), suggesting that oxygen consumption rather than uptake and delivery is impaired, a condition referred to as cytopathic hypoxia (Fink et al. 2002). Therefore, the ETC and specifically COX seem to be a logical target of inflammatory signaling. Indeed, in endotoxin-treated rats, a commonly used animal model for sepsis, ETC complexes I, II, and COX were downregulated both at the transcript and protein levels within 24 h after treatment (Callahan et al. 2005). A recent study with 96 septic patients analyzed COX in platelets. The authors demonstrated a highly significant positive correlation between survival and COX activity and amount (Lorente et al. 2011). The Levy group demonstrated in a cecal ligation sepsis animal model that oxidation of Cytc by COX was competitively and reversibly inhibited, whereas in later stages it became irreversible and noncompetitive (Levy et al. 2004). Others have further shown that cellular energy levels are significantly reduced in septic animals (Astiz et al. 1988), which can be explained with the suppression of mitochondrial respiration. In septic rats, a 70% reduction of tissue ATP appears to be a critical threshold for cellular survival, since a further reduction appears to be incompatible with sustaining cellular functions, resulting in death (Duvigneau et al. 2008).
The above reports point to alterations in COX function during the course of sepsis. To gain a better molecular understanding we tested the effect of tumor necrosis factor α (TNFα) on COX. TNFα is a pro-inflammatory cytokine that is strongly induced during sepsis and is a promoter of the septic state (Duvigneau et al. 2008). TNFα alters cellular metabolism by inducing lactate production in vitro and in vivo (Lee et al. 1987; Tracey et al. 1987), indicating a metabolic switch from respiration to glycolysis, as seen in septic patients.
We therefore analyzed the effect of TNFα on cow and mouse liver tissue as well as mouse hepatocytes in culture. TNFα treatment of liver homogenates caused a 60% reduction of COX activity within 5 min after treatment (Samavati et al. 2008). To identify the molecular mechanism explaining this effect we isolated COX from cow liver with and without TNFα treatment. Further analysis revealed phosphorylation of subunit I tyrosine 304 after TNFα treatment, the same site that was targeted for phosphorylation by the cAMP-dependent pathway in liver as discussed above (Fig. 1). TNFα treatment resulted in a reduction of Δψm and a 35% and 64% decrease of cellular ATP levels in mouse liver tissue and H2.35 cells, respectively (Samavati et al. 2008).
The reader may ask, why does such an inflammatory mechanism with potentially disastrous consequences exist in humans? We recently proposed the following scenario (Hüttemann et al. 2011a): The septic state is an extreme inflammatory condition that affects major parts of or an entire organism, and thus is not localized to a restricted small area, a much more common inflammatory situation. Sepsis is very rare compared to conditions of localized inflammation, such as small wounds that may occur on a daily basis. Here shutdown of cellular processes and specifically OxPhos is understandable because several pathogens take over the host infrastructure and energy production system. For example, Chlamydiae bacteria express several nucleotide transporters that facilitate the uptake of molecules such as ATP (Knab et al. 2011). As a result cutting off essential metabolites locally at the infected area will help the organism fight the pathogen. If inflammation gets out of control and becomes a systemic reaction MODS and death can occur due to energy failure of entire organs (Fig. 2C).
X.5 Conclusion
In contrast to the other ETC complexes, COX and Cytc show all three main regulatory mechanisms found in key metabolic enzymes: isoform expression, allosteric control, and phosphorylation. This supports the suggested rate-limiting role of this step in the ETC and thus makes COX and Cytc prime target candidates for therapeutic interventions in the future in the numerous pathological conditions where mitochondrial energy and ROS production are dysregulated. Identification of kinases and phosphatases that act on COX and Cytc will be a central step in this endeavor and allow specific manipulation of signaling pathways.
Functional consequences of cell signaling also have to be carefully analyzed in order to gain a better understanding of the structure-function relationships and the effect at the physiological or organismal level. For example, from what is known about inflammatory and cancer signaling to date, both pathways affect COX at the molecular level and both pathways lead to an inhibition of COX activity (Fig. 2B and C). COX is a target via phosphorylation on subunits I or II triggered by TNFα and EGFR, respectively. The resultant changes in COX kinetics are different, however, since TNFα causes a shift from hyperbolic to sigmoidal kinetics with very low COX activities at low Cytc substrate concentrations (Samavati et al. 2008), whereas EGFR signaling does not change the hyperbolic kinetics but decreases maximal turnover by 60% (Demory et al. 2009). Thus, inflammatory signaling can function as an off-switch whereas growth factor signaling leads to a general partial inhibition of COX, in support of the Warburg hypothesis.
Acknowledgments
This work was supported by grant GM089900 from the National Institutes of Health, a Department of Defence USAMRAA National Oncogenomic and Molecular Imaging Center contract through the Karmanos Cancer Institute, Detroit, the Center for Molecular Medicine and Genetics, and the Cardiovascular Research Institute, Wayne State University School of Medicine, Detroit.
References
- Acin-Perez R, Bayona-Bafaluy MP, Bueno M, Machicado C, Fernandez-Silva P, Perez-Martos A, Montoya J, Lopez-Perez MJ, Sancho J, Enriquez JA. An intragenic suppressor in the cytochrome c oxidase I gene of mouse mitochondrial DNA. Hum Mol Genet. 2003;12:329–339. doi: 10.1093/hmg/ddg021. [DOI] [PubMed] [Google Scholar]
- Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metabolism. 2009;9:265–276. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S, Goglia F, Kadenbach B. 3,5-Diiodothyronine binds to subunit Va of cytochrome-c oxidase and abolishes the allosteric inhibition of respiration by ATP. Eur J Biochem. 1998;252:325–330. doi: 10.1046/j.1432-1327.1998.2520325.x. [DOI] [PubMed] [Google Scholar]
- Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cerebr Blood F Met. 1997;17:1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Astiz M, Rackow EC, Weil MH, Schumer W. Early impairment of oxidative metabolism and energy production in severe sepsis. Circ Shock. 1988;26:311–320. [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F. Cancer and the chemokine network. Nature reviews. Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- Baudouin SV, Saunders D, Tiangyou W, Elson JL, Poynter J, Pyle A, Keers S, Turnbull DM, Howell N, Chinnery PF. Mitochondrial DNA and survival after sepsis: a prospective study. Lancet. 2005;366:2118–2121. doi: 10.1016/S0140-6736(05)67890-7. [DOI] [PubMed] [Google Scholar]
- Beauchemin AM, Gottlieb B, Beitel LK, Elhaji YA, Pinsky L, Trifiro MA. Cytochrome c oxidase subunit Vb interacts with human androgen receptor: a potential mechanism for neurotoxicity in spinobulbar muscular atrophy. Brain Res Bull. 2001;56:285–297. doi: 10.1016/s0361-9230(01)00583-4. [DOI] [PubMed] [Google Scholar]
- Bender E, Kadenbach B. The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation. FEBS Lett. 2000;466:130–134. doi: 10.1016/s0014-5793(99)01773-1. [DOI] [PubMed] [Google Scholar]
- Boerner JL, Demory ML, Silva C, Parsons SJ. Phosphorylation of Y845 on the epidermal growth factor receptor mediates binding to the mitochondrial protein cytochrome c oxidase subunit II. Mol Cell Biol. 2004;24:7059–7071. doi: 10.1128/MCB.24.16.7059-7071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budas GR, Churchill EN, Disatnik MH, Sun L, Mochly-Rosen D. Mitochondrial import of PKCepsilon is mediated by HSP90: a role in cardioprotection from ischaemia and reperfusion injury. Cardiovasc Res. 2010;88:83–92. doi: 10.1093/cvr/cvq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan LA, Supinski GS. Downregulation of diaphragm electron transport chain and glycolytic enzyme gene expression in sepsis. J Appl Physiol. 2005;99:1120–1126. doi: 10.1152/japplphysiol.01157.2004. [DOI] [PubMed] [Google Scholar]
- Campbell CA, Przyklenk K, Kloner RA. Infarct size reduction: a review of the clinical trials. J Clin Pharmacol. 1986;26:317–329. doi: 10.1002/j.1552-4604.1986.tb03532.x. [DOI] [PubMed] [Google Scholar]
- Churchill EN, Szweda LI. Translocation of deltaPKC to mitochondria during cardiac reperfusion enhances superoxide anion production and induces loss in mitochondrial function. Arch Biochem Biophys. 2005;439:194–199. doi: 10.1016/j.abb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Dalmonte ME, Forte E, Genova ML, Giuffre A, Sarti P, Lenaz G. Control of respiration by cytochrome c oxidase in intact cells: role of the membrane potential. J Biol Chem. 2009;284:32331–32335. doi: 10.1074/jbc.M109.050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Morte D, Raval AP, Dave KR, Lin HW, Perez-Pinzon MA. Post-ischemic activation of protein kinase C epsilon protects the hippocampus from cerebral ischemic injury via alterations in cerebral blood flow. Neurosci Lett. 2011;487:158–162. doi: 10.1016/j.neulet.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demory ML, Boerner JL, Davidson R, Faust W, Miyake T, Lee I, Hüttemann M, Douglas R, Haddad G, Parsons SJ. Epidermal growth factor receptor translocation to the mitochondria: Regulation and effect. J Biol Chem. 2009;284:36592–36604. doi: 10.1074/jbc.M109.000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Teng Z, Lu FH, Zhao YJ, Li H, Ren H, Chen H, Pan ZW, Lv YJ, Yang BF, Tian Y, Xu CQ, Zhang WH. Post-conditioning protects cardiomyocytes from apoptosis via PKC(epsilon)-interacting with calcium-sensing receptors to inhibit endo(sarco)plasmic reticulum-mitochondria crosstalk. Mol Cell Biochem. 2010;341:195–206. doi: 10.1007/s11010-010-0450-5. [DOI] [PubMed] [Google Scholar]
- Duvigneau JC, Piskernik C, Haindl S, Kloesch B, Hartl RT, Hüttemann M, Lee I, Ebel T, Moldzio R, Gemeiner M, Redl H, Kozlov AV. A novel endotoxin-induced pathway: upregulation of heme oxygenase 1, accumulation of free iron, and free iron-mediated mitochondrial dysfunction. Lab Invest. 2008;88:70–77. doi: 10.1038/labinvest.3700691. [DOI] [PubMed] [Google Scholar]
- Ekholm A, Katsura K, Kristian T, Liu M, Folbergrova J, Siesjo BK. Coupling of cellular energy state and ion homeostasis during recovery following brain ischemia. Brain Res. 1993;604:185–191. doi: 10.1016/0006-8993(93)90367-v. [DOI] [PubMed] [Google Scholar]
- Fabian RH, DeWitt DS, Kent TA. In vivo detection of superoxide anion production by the brain using a cytochrome c electrode. J Cerebr Blood F Met. 1995;15:242–247. doi: 10.1038/jcbfm.1995.30. [DOI] [PubMed] [Google Scholar]
- Fang JK, Prabu SK, Sepuri NB, Raza H, Anandatheerthavarada HK, Galati D, Spear J, Avadhani NG. Site specific phosphorylation of cytochrome c oxidase subunits I, IVi1 and Vb in rabbit hearts subjected to ischemia/reperfusion. FEBS Lett. 2007;581:1302–1310. doi: 10.1016/j.febslet.2007.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellman V, Raivio KO. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr Res. 1997;41:599–606. doi: 10.1203/00006450-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Ferguson-Miller S, Brautigan DL, Margoliash E. Correlation of the kinetics of electron transfer activity of various eukaryotic cytochromes c with binding to mitochondrial cytochrome c oxidase. J Biol Chem. 1976;251:1104–1115. [PubMed] [Google Scholar]
- Fink MP. Bench-to-bedside review: Cytopathic hypoxia. Crit Care. 2002;6:491–499. doi: 10.1186/cc1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskum G, Rosenthal RE, Vereczki V, Martin E, Hoffman GE, Chinopoulos C, Kowaltowski A. Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J Bioenerg Biomembr. 2004;36:347–352. doi: 10.1023/B:JOBB.0000041766.71376.81. [DOI] [PubMed] [Google Scholar]
- Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Goldberg E, Sberna D, Wheat TE, Urbanski GJ, Margoliash E. Cytochrome c: immunofluorescent localization of the testis-specific form. Science. 1977;196:1010–1012. doi: 10.1126/science.193188. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010a;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010b;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Nguyen T, Ogbi M, Tawfik H, Ma G, Yu Q, Caldwell RW, Johnson JA. Protein kinase C-epsilon coimmunoprecipitates with cytochrome oxidase subunit IV and is associated with improved cytochrome-c oxidase activity and cardioprotection. Am J Physiol Heart Circ Physiol. 2007;293:H2219–H2230. doi: 10.1152/ajpheart.01306.2006. [DOI] [PubMed] [Google Scholar]
- Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trend Biochem Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. New Engl J Med. 1994;330:1717–1722. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]
- Helling S, Vogt S, Rhiel A, Ramzan R, Wen L, Marcus K, Kadenbach B. Phosphorylation and kinetics of mammalian cytochrome c oxidase. Mol Cell Proteomics. 2008;7:1714–1724. doi: 10.1074/mcp.M800137-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle PC, Kumar MA, Resetar A, Harris DL. Mechanistic stoichiometry of mitochondrial oxidative phosphorylation. Biochemistry. 1991;30:3576–3582. doi: 10.1021/bi00228a031. [DOI] [PubMed] [Google Scholar]
- Hopper RK, Carroll S, Aponte AM, Johnson DT, French S, Shen RF, Witzmann FA, Harris RA, Balaban RS. Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry. 2006;45:2524–2536. doi: 10.1021/bi052475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. The New England journal of medicine. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- Hüttemann M, Helling S, Sanderson TH, Sinkler C, Samavati L, Mahapatra G, Varughese A, Lu G, Liu J, Ramzan R, Vogt S, Grossman LI, Doan JW, Marcus K, Lee I. Regulation of mitochondrial respiration and apoptosis through cell signaling: Cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim Biophys Acta. 2011a doi: 10.1016/j.bbabio.2011.07.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttemann M, Jaradat S, Grossman LI. Cytochrome c oxidase of mammals contains a testes-specific isoform of subunit VIb – the counterpart to testes-specific cytochrome c? Mol Reprod Dev. 2003;66:8–16. doi: 10.1002/mrd.10327. [DOI] [PubMed] [Google Scholar]
- Hüttemann M, Lee I, Pecinova A, Pecina P, Przyklenk K, Doan JW. Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J Bioenerg Biomembr. 2008;40:445–456. doi: 10.1007/s10863-008-9169-3. [DOI] [PubMed] [Google Scholar]
- Hüttemann M, Lee I, Samavati L, Yu H, Doan JW. Regulation of mitochondrial oxidative phosphorylation through cell signaling. Biochim Biophys Acta. 2007;1773:1701–1720. doi: 10.1016/j.bbamcr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Hüttemann M, Nantwi KD, Lee I, Liu J, Mohiuddin S, Petrov T. Theophylline treatment improves mitochondrial function after upper cervical spinal cord hemisection. Exp Neurol. 2010;223:523–528. doi: 10.1016/j.expneurol.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttemann M, Pecina P, Rainbolt M, Sanderson TH, Kagan VE, Samavati L, Doan JW, Lee I. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis. Mitochondrion. 2011b;11:369–381. doi: 10.1016/j.mito.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Bayir HA, Belikova NA, Kapralov O, Tyurina YY, Tyurin VA, Jiang J, Stoyanovsky DA, Wipf P, Kochanek PM, Greenberger JS, Pitt B, Shvedova AA, Borisenko G. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic Biol Med. 2009;46:1439–1453. doi: 10.1016/j.freeradbiomed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp DW, Shacter E, Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology. 2011;25:400–410. 413. [PubMed] [Google Scholar]
- Kim J, Takeuchi H, Lam ST, Turner RR, Wang HJ, Kuo C, Foshag L, Bilchik AJ, Hoon DS. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichenko A, Vygodina T, Mkrtchyan HM, Konstantinov A. Specific cation binding site in mammalian cytochrome oxidase. FEBS Lett. 1998;423:329–333. doi: 10.1016/s0014-5793(98)00117-3. [DOI] [PubMed] [Google Scholar]
- Kirichenko AV, Pfitzner U, Ludwig B, Soares CM, Vygodina TV, Konstantinov AA. Cytochrome c oxidase as a calcium binding protein. Studies on the role of a conserved aspartate in helices XI-XII cytoplasmic loop in cation binding. Biochemistry. 2005;44:12391–12401. doi: 10.1021/bi050376v. [DOI] [PubMed] [Google Scholar]
- Knab S, Mushak TM, Schmitz-Esser S, Horn M, Haferkamp I. Nucleotide parasitism by Simkania negevensis (Chlamydiae) J Bacteriol. 2011;193:225–235. doi: 10.1128/JB.00919-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko YH, Pan W, Inoue C, Pedersen PL. Signal transduction to mitochondrial ATP synthase: evidence that PDGF-dependent phosphorylation of the delta-subunit occurs in several cell lines, involves tyrosine, and is modulated by lysophosphatidic acid. Mitochondrion. 2002;1:339–348. doi: 10.1016/s1567-7249(01)00036-8. [DOI] [PubMed] [Google Scholar]
- Korshunov SS, Krasnikov BF, Pereverzev MO, Skulachev VP. The antioxidant functions of cytochrome c. FEBS Lett. 1999;462:192–198. doi: 10.1016/s0014-5793(99)01525-2. [DOI] [PubMed] [Google Scholar]
- Kowalczyk JE, Kawalec M, Beresewicz M, Debski J, Dadlez M, Zablocka B. Protein kinase C beta in postischemic brain mitochondria. Mitochondrion. 2011 doi: 10.1016/j.mito.2011.06.002. in press. [DOI] [PubMed] [Google Scholar]
- Kulbe H, Hagemann T, Szlosarek PW, Balkwill FR, Wilson JL. The inflammatory cytokine tumor necrosis factor-alpha regulates chemokine receptor expression on ovarian cancer cells. Cancer Res. 2005;65:10355–10362. doi: 10.1158/0008-5472.CAN-05-0957. [DOI] [PubMed] [Google Scholar]
- Lee I, Bender E, Kadenbach B. Control of mitochondrial membrane potential and ROS formation by reversible phosphorylation of cytochrome c oxidase. Mol Cell Biochem. 2002;234-235:63–70. [PubMed] [Google Scholar]
- Lee I, Kadenbach B. Palmitate decreases proton pumping of liver-type cytochrome c oxidase. Eur J Biochem. 2001;268:6329–6334. doi: 10.1046/j.0014-2956.2001.02602.x. [DOI] [PubMed] [Google Scholar]
- Lee I, Pecinova A, Pecina P, Neel BG, Araki T, Kucherlapati R, Roberts AE, Hüttemann M. A suggested role for mitochondria in Noonan syndrome. Biochim Biophys Acta. 2010;1802:275–283. doi: 10.1016/j.bbadis.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Salomon AR, Ficarro S, Mathes I, Lottspeich F, Grossman LI, Hüttemann M. cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. J Biol Chem. 2005;280:6094–6100. doi: 10.1074/jbc.M411335200. [DOI] [PubMed] [Google Scholar]
- Lee I, Salomon AR, Yu K, Doan JW, Grossman LI, Hüttemann M. New prospects for an old enzyme: mammalian cytochrome c is tyrosine-phosphorylated in vivo. Biochemistry. 2006;45:9121–9128. doi: 10.1021/bi060585v. [DOI] [PubMed] [Google Scholar]
- Lee MD, Zentella A, Vine W, Pekala PH, Cerami A. Effect of endotoxin-induced monokines on glucose metabolism in the muscle cell line L6. Proc Natl Acad Sci U S A. 1987;84:2590–2594. doi: 10.1073/pnas.84.9.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RJ, Vijayasarathy C, Raj NR, Avadhani NG, Deutschman CS. Competitive and noncompetitive inhibition of myocardial cytochrome c oxidase in sepsis. Shock. 2004;21:110–114. doi: 10.1097/01.shk.0000108400.56565.ab. [DOI] [PubMed] [Google Scholar]
- Liu RR, Murphy TH. Reversible cyclosporin A-sensitive mitochondrial depolarization occurs within minutes of stroke onset in mouse somatosensory cortex in vivo: a two-photon imaging study. J Biol Chem. 2009;284:36109–36117. doi: 10.1074/jbc.M109.055301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SS. Cooperation of a “reactive oxygen cycle” with the Q cycle and the proton cycle in the respiratory chain--superoxide generating and cycling mechanisms in mitochondria. J Bioenerg Biomembr. 1999;31:367–376. doi: 10.1023/a:1018650103259. [DOI] [PubMed] [Google Scholar]
- Liu Z, Lin H, Ye S, Liu QY, Meng Z, Zhang CM, Xia Y, Margoliash E, Rao Z, Liu XJ. Remarkably high activities of testicular cytochrome c in destroying reactive oxygen species and in triggering apoptosis. Proc Natl Acad Sci U S A. 2006;103:8965–8970. doi: 10.1073/pnas.0603327103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente L, Martin MM, Lopez-Gallardo E, Iceta R, Sole-Violan J, Blanquer J, Labarta L, Diaz C, Jimenez A, Lafuente N, Hernandez M, Mendez F, Medina N, Ferrer-Aguero JM, Ferreres J, MC LL, Mora ML, Lubillo S, Sanchez-Palacios M, Montoya J, Ruiz-Pesini E. Platelet cytochrome c oxidase activity and quantity in septic patients. Crit Care Med. 2011;39:1289–1294. doi: 10.1097/CCM.0b013e31820ee20c. [DOI] [PubMed] [Google Scholar]
- Lu N, Wang W, Liu J, Wong CW. Protein kinase C epsilon affects mitochondrial function through estrogen-related receptor alpha. Cell Signal. 2011 doi: 10.1016/j.cellsig.2011.04.010. in press. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Martinez-Ruiz A, Cadenas S, Lamas S. Nitric oxide signaling: classical, less classical, and nonclassical mechanisms. Free Radic Biol Med. 2011;51:17–29. doi: 10.1016/j.freeradbiomed.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Neff L, Tanaka S, Horne WC, Baron R. Regulation of cytochrome c oxidase activity by c-Src in osteoclasts. J Cell Biol. 2003;160:709–718. doi: 10.1083/jcb.200209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- Ogbi M, Chew CS, Pohl J, Stuchlik O, Ogbi S, Johnson JA. Cytochrome c oxidase subunit IV as a marker of protein kinase Cε function in neonatal cardiac myocytes: implications for cytochrome c oxidase activity. Biochem J. 2004;382:923–932. doi: 10.1042/BJ20040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbi M, Johnson JA. Protein kinase Cε interacts with cytochrome c oxidase subunit IV and enhances cytochrome c oxidase activity in neonatal cardiac myocyte preconditioning. Biochem J. 2006;393:191–199. doi: 10.1042/BJ20050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver CN, Starke-Reed PE, Stadtman ER, Liu GJ, Carney JM, Floyd RA. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci U S A. 1990;87:5144–5147. doi: 10.1073/pnas.87.13.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, Gnad F, Cox J, Jensen TS, Nigg EA, Brunak S, Mann M. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal. 2010;3:ra3. doi: 10.1126/scisignal.2000475. [DOI] [PubMed] [Google Scholar]
- Pacelli C, Latorre D, Cocco T, Capuano F, Kukat C, Seibel P, Villani G. Tight control of mitochondrial membrane potential by cytochrome c oxidase. Mitochondrion. 2011;11:334–341. doi: 10.1016/j.mito.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Pang L, Qiu T, Cao X, Wan M. Apoptotic role of TGF-beta mediated by Smad4 mitochondria translocation and cytochrome c oxidase subunit II interaction. Exp Cell Res. 2011;317:1608–1620. doi: 10.1016/j.yexcr.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Pecina P, Borisenko GG, Belikova NA, Tyurina YY, Pecinova A, Lee I, Samhan-Arias AK, Przyklenk K, Kagan VE, Hüttemann M. Phosphomimetic substitution of cytochrome c tyrosine 48 decreases respiration and binding to cardiolipin and abolishes ability to trigger downstream caspase activation. Biochemistry. 2010;49:6705–6714. doi: 10.1021/bi100486s. [DOI] [PubMed] [Google Scholar]
- Pedersen PL. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- Persichini T, Mazzone V, Polticelli F, Moreno S, Venturini G, Clementi E, Colasanti M. Mitochondrial type I nitric oxide synthase physically interacts with cytochrome c oxidase. Neurosci Lett. 2005;384:254–259. doi: 10.1016/j.neulet.2005.04.085. [DOI] [PubMed] [Google Scholar]
- Piantadosi CA, Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke. 1996;27:327–332. doi: 10.1161/01.str.27.2.327. [DOI] [PubMed] [Google Scholar]
- Picard M, Taivassalo T, Ritchie D, Wright KJ, Thomas MM, Romestaing C, Hepple RT. Mitochondrial structure and function are disrupted by standard isolation methods. PloS one. 2011;6:e18317. doi: 10.1371/journal.pone.0018317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli C, Scrima R, Boffoli D, Capitanio N. Control by cytochrome c oxidase of the cellular oxidative phosphorylation system depends on the mitochondrial energy state. Biochem J. 2006;396:573–583. doi: 10.1042/BJ20060077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabu SK, Anandatheerthavarada HK, Raza H, Srinivasan S, Spear JF, Avadhani NG. Protein kinase A-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury. J Biol Chem. 2006;281:2061–2070. doi: 10.1074/jbc.M507741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S. Why cancer and inflammation? Yale J Biol Med. 2006;79:123–130. [PMC free article] [PubMed] [Google Scholar]
- Ralph SJ, Rodriguez-Enriquez S, Neuzil J, Saavedra E, Moreno-Sanchez R. The causes of cancer revisited: “mitochondrial malignancy” and ROS-induced oncogenic transformation - why mitochondria are targets for cancer therapy. Mol Aspects Med. 2010;31:145–170. doi: 10.1016/j.mam.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Revelly JP, Tappy L, Martinez A, Bollmann M, Cayeux MC, Berger MM, Chiolero RL. Lactate and glucose metabolism in severe sepsis and cardiogenic shock. Crit Care Med. 2005;33:2235–2240. doi: 10.1097/01.ccm.0000181525.99295.8f. [DOI] [PubMed] [Google Scholar]
- Robb-Gaspers LD, Burnett P, Rutter GA, Denton RM, Rizzuto R, Thomas AP. Integrating cytosolic calcium signals into mitochondrial metabolic responses. Embo J. 1998;17:4987–5000. doi: 10.1093/emboj/17.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal RE, Hamud F, Fiskum G, Varghese PJ, Sharpe S. Cerebral ischemia and reperfusion: prevention of brain mitochondrial injury by lidoflazine. J Cereb Blood Flow Metab. 1987;7:752–758. doi: 10.1038/jcbfm.1987.130. [DOI] [PubMed] [Google Scholar]
- Ruggieri AJ, Levy RJ, Deutschman CS. Mitochondrial dysfunction and resuscitation in sepsis. Critical Care Clinics. 2010;26:567–575. x–xi. doi: 10.1016/j.ccc.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi M, Brunati AM, Bordin L, La Rocca N, Clari G, Toninello A. Characterization and location of Src-dependent tyrosine phosphorylation in rat brain mitochondria. Biochim Biophys Acta. 2002;1589:181–195. doi: 10.1016/s0167-4889(02)00174-x. [DOI] [PubMed] [Google Scholar]
- Salvi M, Stringaro A, Brunati AM, Agostinelli E, Arancia G, Clari G, Toninello A. Tyrosine phosphatase activity in mitochondria: presence of Shp-2 phosphatase in mitochondria. Cell Mol Life Sci. 2004;61:2393–2404. doi: 10.1007/s00018-004-4211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavati L, Lee I, Mathes I, Lottspeich F, Hüttemann M. Tumor necrosis factor α inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J Biol Chem. 2008;283:21134–21144. doi: 10.1074/jbc.M801954200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanishvili R, Volz KW, Westbrook EM, Margoliash E. The low ionic strength crystal structure of horse cytochrome c at 2.1 Å resolution and comparison with its high ionic strength counterpart. Structure. 1995;3:707–716. doi: 10.1016/s0969-2126(01)00205-2. [DOI] [PubMed] [Google Scholar]
- Sivaraman V, Hausenloy DJ, Kolvekar S, Hayward M, Yap J, Lawrence D, Di Salvo C, Yellon DM. The divergent roles of protein kinase C epsilon and delta in simulated ischaemia-reperfusion injury in human myocardium. J Mol Cell Cardiol. 2009;46:758–764. doi: 10.1016/j.yjmcc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Steenaart NA, Shore GC. Mitochondrial cytochrome c oxidase subunit IV is phosphorylated by an endogenous kinase. FEBS Lett. 1997;415:294–298. doi: 10.1016/s0014-5793(97)01145-9. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Gelb BD, Zenker M. Noonan syndrome and clinically related disorders. Best practice & research. J Clin Endocrinol Metab. 2011;25:161–179. doi: 10.1016/j.beem.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M, Mehler EL, Goldberg R, Zampino G, Brunner HG, Kremer H, van der Burgt I, Crosby AH, Ion A, Jeffery S, Kalidas K, Patton MA, Kucherlapati RS, Gelb BD. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- Thingholm TE, Jorgensen TJ, Jensen ON, Larsen MR. Highly selective enrichment of phosphorylated peptides using titanium dioxide. Nature Protocols. 2006;1:1929–1935. doi: 10.1038/nprot.2006.185. [DOI] [PubMed] [Google Scholar]
- Tilney NL, Guttmann RD. Effects of initial ischemia/reperfusion injury on the transplanted kidney. Transplantation. 1997;64:945–947. doi: 10.1097/00007890-199710150-00001. [DOI] [PubMed] [Google Scholar]
- Tracey KJ, Lowry SF, Fahey TJ, Albert JD, Fong Y, Hesse D, Beutler B, Manogue KR, Calvano S, Wei H, Cerami A, Shires GT. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987;164:415–422. [PubMed] [Google Scholar]
- Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 Å. Science. 1996;272:1136–1144. doi: 10.1126/science.272.5265.1136. [DOI] [PubMed] [Google Scholar]
- Tsukihara T, Shimokata K, Katayama Y, Shimada H, Muramoto K, Aoyama H, Mochizuki M, Shinzawa-Itoh K, Yamashita E, Yao M, Ishimura Y, Yoshikawa S. The low-spin heme of cytochrome c oxidase as the driving element of the proton-pumping process. Proc Natl Acad Sci U S A. 2003;100:15304–15309. doi: 10.1073/pnas.2635097100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani G, Attardi G. In vivo control of respiration by cytochrome c oxidase in wild-type and mitochondrial DNA mutation-carrying human cells. Proc Natl Acad Sci U S A. 1997;94:1166–1171. doi: 10.1073/pnas.94.4.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani G, Greco M, Papa S, Attardi G. Low reserve of cytochrome c oxidase capacity in vivo in the respiratory chain of a variety of human cell types. J Biol Chem. 1998;273:31829–31836. doi: 10.1074/jbc.273.48.31829. [DOI] [PubMed] [Google Scholar]
- Vlessis AA, Widener LL, Bartos D. Effect of peroxide, sodium, and calcium on brain mitochondrial respiration in vitro: potential role in cerebral ischemia and reperfusion. J Neurochem. 1990;54:1412–1418. doi: 10.1111/j.1471-4159.1990.tb01977.x. [DOI] [PubMed] [Google Scholar]
- von Ballmoos C, Gennis RB, Adelroth P, Brzezinski P. Kinetic design of the respiratory oxidases. Proc Natl Acad Sci U S A. 2011;108:11057–11062. doi: 10.1073/pnas.1104103108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Hocht I, van Wonderen JH, Hilbers F, Angerer H, MacMillan F, Michel H. Interconversions of P and F intermediates of cytochrome c oxidase from Paracoccus denitrificans. Proc Natl Acad Sci U S A. 2011;108:3964–3969. doi: 10.1073/pnas.1100950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther TC, Mann M. Mass spectrometry-based proteomics in cell biology. The J Cell Biol. 2010;190:491–500. doi: 10.1083/jcb.201004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZB, Li M, Zhao Y, Xu JX. Cytochrome c is a hydrogen peroxide scavenger in mitochondria. Protein Pept Lett. 2003;10:247–253. doi: 10.2174/0929866033479013. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Warburg O, Posener K, Negelein E. Über den Stoffwechsel der Carcinomzelle. Biochem Z. 1924;152:309–344. [Google Scholar]
- Weinberg F, Chandel NS. Mitochondrial metabolism and cancer. Ann N Y Acad Sci. 2009;1177:66–73. doi: 10.1111/j.1749-6632.2009.05039.x. [DOI] [PubMed] [Google Scholar]
- Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch EJ, Jones BW, Scott JD. Networking with AKAPs: context-dependent regulation of anchored enzymes. Molecular Interventions. 2010;10:86–97. doi: 10.1124/mi.10.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WL, Iacono L, Tang WM, Chin KV. Novel function of the regulatory subunit of protein kinase A: regulation of cytochrome c oxidase activity and cytochrome c release. Biochemistry. 1998;37:14175–14180. doi: 10.1021/bi981402a. [DOI] [PubMed] [Google Scholar]
- Yoshikawa S, Muramoto K, Shinzawa-Itoh K. The O2 reduction and proton pumping gate mechanism of bovine heart cytochrome c oxidase. Biochim Biophys Acta. 2011;1807:1279–1286. doi: 10.1016/j.bbabio.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Yu H, Lee I, Salomon AR, Yu K, Hüttemann M. Mammalian liver cytochrome c is tyrosine-48 phosphorylated in vivo, inhibiting mitochondrial respiration. Biochim Biophys Acta. 2008a;1777:1066–1071. doi: 10.1016/j.bbabio.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Nguyen T, Ogbi M, Caldwell RW, Johnson JA. Differential loss of cytochrome-c oxidase subunits in ischemia-reperfusion injury: exacerbation of COI subunit loss by PKC-epsilon inhibition. Am J Physiol. 2008b;294:H2637–2645. doi: 10.1152/ajpheart.91476.2007. [DOI] [PubMed] [Google Scholar]
- Zaidan E, Sims NR. The calcium content of mitochondria from brain subregions following short-term forebrain ischemia and recirculation in the rat. J Neurochem. 1994;63:1812–1819. doi: 10.1046/j.1471-4159.1994.63051812.x. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li H, Yang SJ. Tribulosin protects rat hearts from ischemia/reperfusion injury. Acta Pharmacologica Sinica. 2010;31:671–678. doi: 10.1038/aps.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Gerstein M. The human genome has 49 cytochrome c pseudogenes, including a relic of a primordial gene that still functions in mouse. Gene. 2003;312:61–72. doi: 10.1016/s0378-1119(03)00579-1. [DOI] [PubMed] [Google Scholar]
- Zhao X, Leon IR, Bak S, Mogensen M, Wrzesinski K, Hojlund K, Jensen ON. Phosphoproteome analysis of functional mitochondria isolated from resting human muscle reveals extensive phosphorylation of inner membrane protein complexes and enzymes. Mol Cell Proteomics. 2010:M110.000299. doi: 10.1074/mcp.M110.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988;263:1353–1357. [PubMed] [Google Scholar]