Abstract
In this paper, the Escherichia coli cell is considered as a system designed for rapid growth, but limited by the medium. We propose that this very design causes the cell to become subsaturated with precursors and catalytic components at all levels of macromolecular biosynthesis and leads to a molecular sharing economy at a high level of competition inside the cell. Thus, the promoters compete with each other in the binding of a limited amount of free RNA polymerase and the ribosome binding sites on the mRNA chains compete with each other for the free ribosomes. The macromolecular chain elongation reactions sequester a considerable proportion of the total amount of RNA polymerase and ribosomes in the cells. We propose that the degree of subsaturation of the macromolecular biosynthetic apparatus renders a variable fraction of RNA polymerase and ribosomes unavailable for the initiation of new chain synthesis and that this, at least in part, determines the composition of the cell as a function of the growth rate. Thus, at rapid growth, the high speed of the elongation reactions enables the cell to increase the concentrations of free RNA polymerase and ribosomes for initiation purposes. Furthermore, it is proposed that the speed of RNA polymerase movement is adjusted to the performance speed of the ribosomes. Mechanistically, this adjustment of the coupling between transcription and translation involves transcriptional pause sites along the RNA chains, the adjustment of the saturation level of RNA polymerase with the nucleoside triphosphate substrates, and the concentration of ppGpp, which is known to inhibit RNA chain elongation. This model is able to explain the stringent response and the control of stable RNA and of ribosome synthesis in steady states and in shifts, as well as the rate of overall protein synthesis as a function of the growth rate.
Full text
PDF

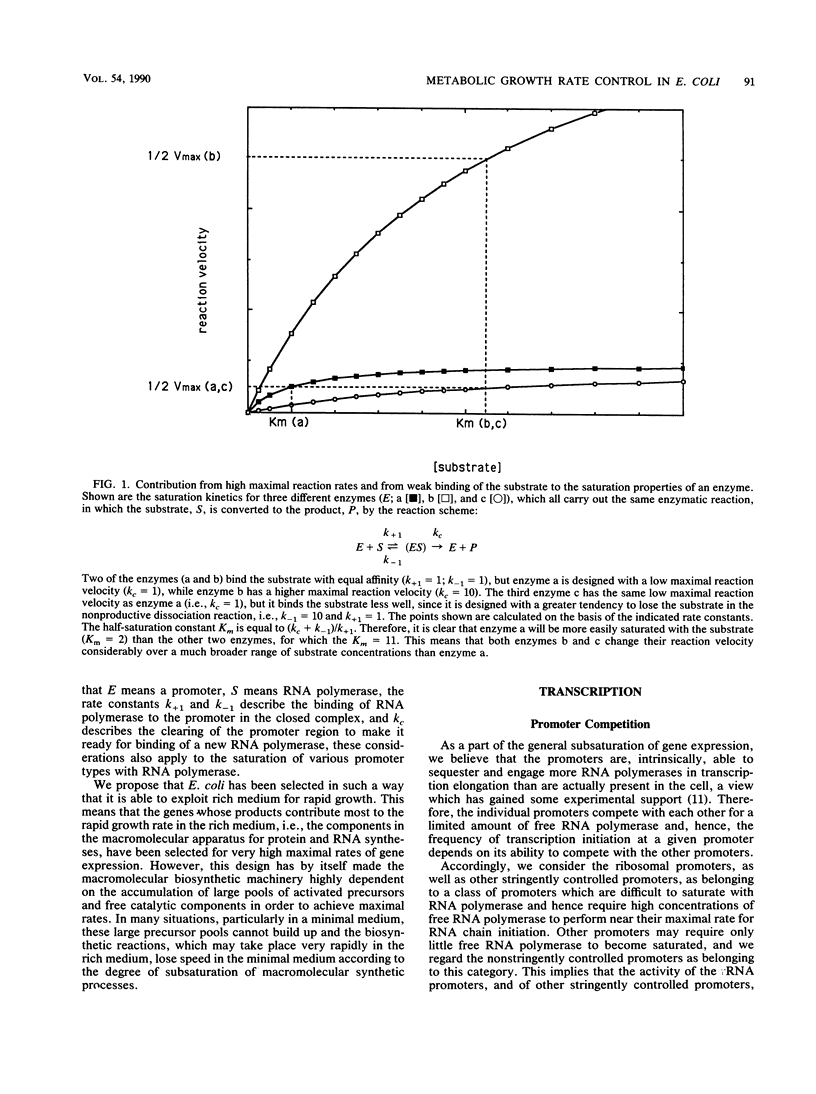

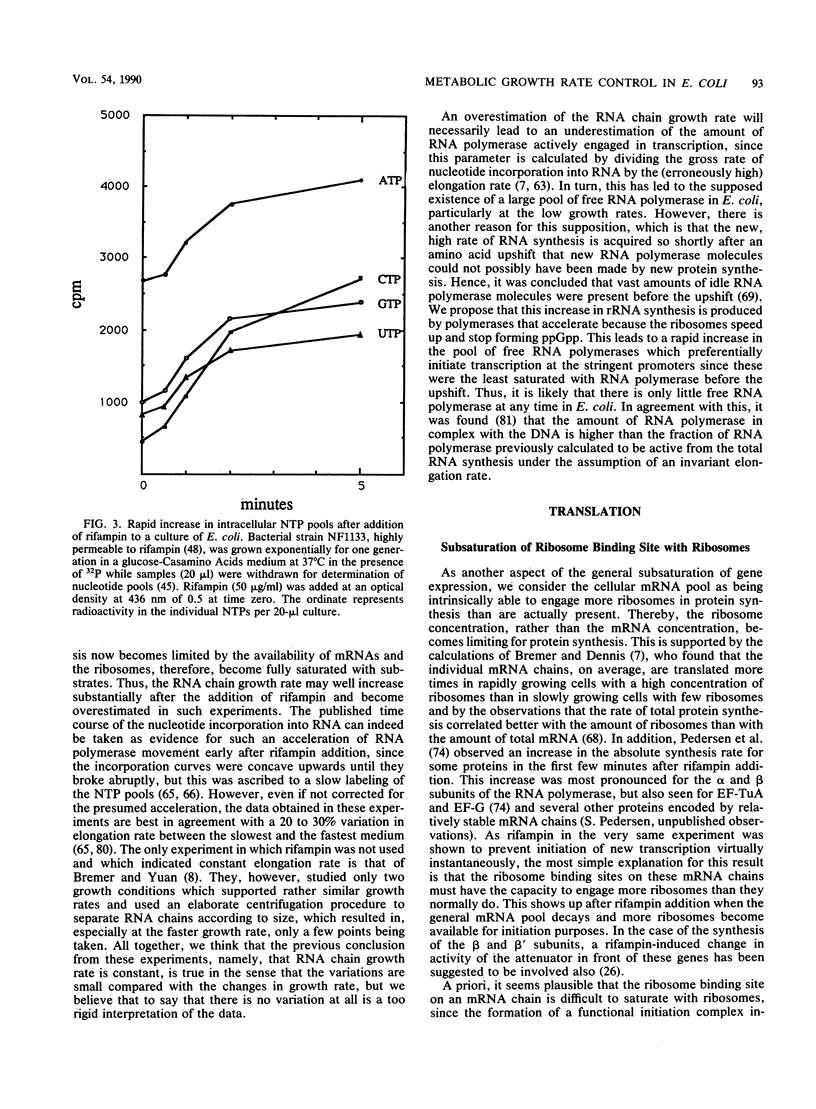







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Andersen K. B., von Meyenburg K. Are growth rates of Escherichia coli in batch cultures limited by respiration? J Bacteriol. 1980 Oct;144(1):114–123. doi: 10.1128/jb.144.1.114-123.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracchini E., Bremer H. Stringent and growth control of rRNA synthesis in Escherichia coli are both mediated by ppGpp. J Biol Chem. 1988 Feb 25;263(6):2597–2602. [PubMed] [Google Scholar]
- Baracchini E., Glass R., Bremer H. Studies in vivo on Escherichia coli RNA polymerase mutants altered in the stringent response. Mol Gen Genet. 1988 Aug;213(2-3):379–387. doi: 10.1007/BF00339606. [DOI] [PubMed] [Google Scholar]
- Bedwell D. M., Nomura M. Feedback regulation of RNA polymerase subunit synthesis after the conditional overproduction of RNA polymerase in Escherichia coli. Mol Gen Genet. 1986 Jul;204(1):17–23. doi: 10.1007/BF00330181. [DOI] [PubMed] [Google Scholar]
- Blumenthal R. M., Lemaux P. G., Neidhardt F. C., Dennis P. P. The effects of the relA gene on the synthesis of aminoacyl-tRNA synthetases and other transcription and translation proteins in Escherichia coli A. Mol Gen Genet. 1976 Dec 22;149(3):291–296. doi: 10.1007/BF00268530. [DOI] [PubMed] [Google Scholar]
- Bremer H., Yuan D. RNA chain growth-rate in Escherichia coli. J Mol Biol. 1968 Dec 14;38(2):163–180. doi: 10.1016/0022-2836(68)90404-x. [DOI] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Churchward G., Bremer H., Young R. Transcription in bacteria at different DNA concentrations. J Bacteriol. 1982 May;150(2):572–581. doi: 10.1128/jb.150.2.572-581.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Norris T. E., Koch A. L. Chain elongation rate of messenger and polypeptides in slowly growing Escherichia coli. J Mol Biol. 1971 Aug 28;60(1):1–19. doi: 10.1016/0022-2836(71)90442-6. [DOI] [PubMed] [Google Scholar]
- Cole J. R., Nomura M. Changes in the half-life of ribosomal protein messenger RNA caused by translational repression. J Mol Biol. 1986 Apr 5;188(3):383–392. doi: 10.1016/0022-2836(86)90162-2. [DOI] [PubMed] [Google Scholar]
- Cole J. R., Nomura M. Translational regulation is responsible for growth-rate-dependent and stringent control of the synthesis of ribosomal proteins L11 and L1 in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4129–4133. doi: 10.1073/pnas.83.12.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. R., Olsson C. L., Hershey J. W., Grunberg-Manago M., Nomura M. Feedback regulation of rRNA synthesis in Escherichia coli. Requirement for initiation factor IF2. J Mol Biol. 1987 Dec 5;198(3):383–392. doi: 10.1016/0022-2836(87)90288-9. [DOI] [PubMed] [Google Scholar]
- Dalbow D. G., Young R. Synthesis time of beta-galactosidase in Escherichia coli B/r as a function of growth rate. Biochem J. 1975 Jul;150(1):13–20. doi: 10.1042/bj1500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Fill N. P. Transcriptional and post-transcriptional control of RNA polymerase and ribosomal protein genes cloned on composite ColE1 plasmids in the bacterium Escherichia coli. J Biol Chem. 1979 Aug 25;254(16):7540–7547. [PubMed] [Google Scholar]
- Dennis P. P., Nomura M. Stringent control of ribosomal protein gene expression in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3819–3823. doi: 10.1073/pnas.71.10.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Nomura M. Stringent control of the transcriptional activities of ribosomal protein genes in E. coli. Nature. 1975 Jun 5;255(5508):460–465. doi: 10.1038/255460a0. [DOI] [PubMed] [Google Scholar]
- Dickson R. R., Gaal T., deBoer H. A., deHaseth P. L., Gourse R. L. Identification of promoter mutants defective in growth-rate-dependent regulation of rRNA transcription in Escherichia coli. J Bacteriol. 1989 Sep;171(9):4862–4870. doi: 10.1128/jb.171.9.4862-4870.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G., Elford R. M., Holmes W. M. Fusion of the Escherichia coli tRNALeu1 promoter to the galK gene: analysis of sequences necessary for growth-rate-dependent regulation. Cell. 1982 Oct;30(3):855–864. doi: 10.1016/0092-8674(82)90290-2. [DOI] [PubMed] [Google Scholar]
- Engbaek F., Kjeldgaard N. O., Maaloe O. Chain growth rate of -galactosidase during exponential growth and amino acid starvation. J Mol Biol. 1973 Mar 25;75(1):109–118. doi: 10.1016/0022-2836(73)90532-9. [DOI] [PubMed] [Google Scholar]
- Forchhammer J., Lindahl L. Growth rate of polypeptide chains as a function of the cell growth rate in a mutant of Escherichia coli 15. J Mol Biol. 1971 Feb 14;55(3):563–568. doi: 10.1016/0022-2836(71)90337-8. [DOI] [PubMed] [Google Scholar]
- Fournier M. J., Peterkofsky A. Formation of chromatographically unique species of transfer ribonucleic acid during amino acid starvation of relaxed-control Escherichia coli. J Bacteriol. 1975 May;122(2):538–548. doi: 10.1128/jb.122.2.538-548.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L. P., Zengel J. M., Lindahl L. Genetic dissection of stringent control and nutritional shift-up response of the Escherichia coli S10 ribosomal protein operon. J Mol Biol. 1985 Oct 20;185(4):701–712. doi: 10.1016/0022-2836(85)90055-5. [DOI] [PubMed] [Google Scholar]
- Fukuda R., Nagasawa-Fujimori H. Mechanism of the rifampicin induction of RNA polymerase beta and beta' subunit synthesis in Escherichia coli. J Biol Chem. 1983 Feb 25;258(4):2720–2728. [PubMed] [Google Scholar]
- Gaal T., Barkei J., Dickson R. R., deBoer H. A., deHaseth P. L., Alavi H., Gourse R. L. Saturation mutagenesis of an Escherichia coli rRNA promoter and initial characterization of promoter variants. J Bacteriol. 1989 Sep;171(9):4852–4861. doi: 10.1128/jb.171.9.4852-4861.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gausing K. Regulation of ribosome production in Escherichia coli: synthesis and stability of ribosomal RNA and of ribosomal protein messenger RNA at different growth rates. J Mol Biol. 1977 Sep 25;115(3):335–354. doi: 10.1016/0022-2836(77)90158-9. [DOI] [PubMed] [Google Scholar]
- Glaser G., Sarmientos P., Cashel M. Functional interrelationship between two tandem E. coli ribosomal RNA promoters. Nature. 1983 Mar 3;302(5903):74–76. doi: 10.1038/302074a0. [DOI] [PubMed] [Google Scholar]
- Glass R. E., Jones S. T., Ishihama A. Genetic studies on the beta subunit of Escherichia coli RNA polymerase. VII. RNA polymerase is a target for ppGpp. Mol Gen Genet. 1986 May;203(2):265–268. doi: 10.1007/BF00333964. [DOI] [PubMed] [Google Scholar]
- Gourse R. L., Nomura M. Level of rRNA, not tRNA, synthesis controls transcription of rRNA and tRNA operons in Escherichia coli. J Bacteriol. 1984 Dec;160(3):1022–1026. doi: 10.1128/jb.160.3.1022-1026.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourse R. L., Takebe Y., Sharrock R. A., Nomura M. Feedback regulation of rRNA and tRNA synthesis and accumulation of free ribosomes after conditional expression of rRNA genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1069–1073. doi: 10.1073/pnas.82.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourse R. L., de Boer H. A., Nomura M. DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, antitermination. Cell. 1986 Jan 17;44(1):197–205. doi: 10.1016/0092-8674(86)90498-8. [DOI] [PubMed] [Google Scholar]
- Greenblatt J., Li J. Interaction of the sigma factor and the nusA gene protein of E. coli with RNA polymerase in the initiation-termination cycle of transcription. Cell. 1981 May;24(2):421–428. doi: 10.1016/0092-8674(81)90332-9. [DOI] [PubMed] [Google Scholar]
- Hall B., Gallant J. Defective translation in RC - cells. Nat New Biol. 1972 May 31;237(74):131–135. doi: 10.1038/newbio237131a0. [DOI] [PubMed] [Google Scholar]
- Hansen M. T., Bennett P. M., von Meyenburg K. Intracistronic polarity during dissociation of translation from transcription in Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):589–604. doi: 10.1016/0022-2836(73)90225-8. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz R. J., Li J., Greenblatt J. An elongation control particle containing the N gene transcriptional antitermination protein of bacteriophage lambda. Cell. 1987 Nov 20;51(4):631–641. doi: 10.1016/0092-8674(87)90132-2. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Kepes A. Initiation, elongation and inactivation of lac messenger RNA in Escherichia coli studied studied by measurement of its beta-galactosidase synthesizing capacity in vivo. J Mol Biol. 1971 Sep 28;60(3):453–472. doi: 10.1016/0022-2836(71)90181-1. [DOI] [PubMed] [Google Scholar]
- Jensen K. F., Fast R., Karlström O., Larsen J. N. Association of RNA polymerase having increased Km for ATP and UTP with hyperexpression of the pyrB and pyrE genes of Salmonella typhimurium. J Bacteriol. 1986 Jun;166(3):857–865. doi: 10.1128/jb.166.3.857-865.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. F., Houlberg U., Nygaard P. Thin-layer chromatographic methods to isolate 32P-labeled 5-phosphoribosyl-alpha-1-pyrophosphate (PRPP): determination of cellular PRPP pools and assay of PRPP synthetase activity. Anal Biochem. 1979 Oct 1;98(2):254–263. doi: 10.1016/0003-2697(79)90138-6. [DOI] [PubMed] [Google Scholar]
- Jensen K. F. Hyper-regulation of pyr gene expression in Escherichia coli cells with slow ribosomes. Evidence for RNA polymerase pausing in vivo? Eur J Biochem. 1988 Aug 15;175(3):587–593. doi: 10.1111/j.1432-1033.1988.tb14232.x. [DOI] [PubMed] [Google Scholar]
- Jensen K. F., Neuhard J., Schack L. RNA polymerase involvement in the regulation of expression of Salmonella typhimurium pyr genes. Isolation and characterization of a fluorouracil-resistant mutant with high, constitutive expression of the pyrB and pyrE genes due to a mutation in rpoBC. EMBO J. 1982;1(1):69–74. doi: 10.1002/j.1460-2075.1982.tb01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks-Robertson S., Gourse R. L., Nomura M. Expression of rRNA and tRNA genes in Escherichia coli: evidence for feedback regulation by products of rRNA operons. Cell. 1983 Jul;33(3):865–876. doi: 10.1016/0092-8674(83)90029-6. [DOI] [PubMed] [Google Scholar]
- Johnsen K., Molin S., Karlström O., Maaloe O. Control of protein synthesis in Escherichia coli: analysis of an energy source shift-down. J Bacteriol. 1977 Jul;131(1):18–29. doi: 10.1128/jb.131.1.18-29.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justesen J., Lund T., Skou Pedersen F., Kjeldgaard N. O. The physiology of stringent factor (ATP:GTP 3'-diphosphotransferase) in Escherichia coli. Biochimie. 1986 May;68(5):715–722. doi: 10.1016/s0300-9084(86)80165-1. [DOI] [PubMed] [Google Scholar]
- Kajitani M., Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase. Differential stringent control of the multiple promoters from ribosomal RNA and protein operons. J Biol Chem. 1984 Feb 10;259(3):1951–1957. [PubMed] [Google Scholar]
- Kassavetis G. A., Chamberlin M. J. Pausing and termination of transcription within the early region of bacteriophage T7 DNA in vitro. J Biol Chem. 1981 Mar 25;256(6):2777–2786. [PubMed] [Google Scholar]
- Kingston R. E., Chamberlin M. J. Pausing and attenuation of in vitro transcription in the rrnB operon of E. coli. Cell. 1981 Dec;27(3 Pt 2):523–531. doi: 10.1016/0092-8674(81)90394-9. [DOI] [PubMed] [Google Scholar]
- Kingston R. E., Nierman W. C., Chamberlin M. J. A direct effect of guanosine tetraphosphate on pausing of Escherichia coli RNA polymerase during RNA chain elongation. J Biol Chem. 1981 Mar 25;256(6):2787–2797. [PubMed] [Google Scholar]
- Landick R., Carey J., Yanofsky C. Translation activates the paused transcription complex and restores transcription of the trp operon leader region. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4663–4667. doi: 10.1073/pnas.82.14.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. C., Squires C. L., Squires C. Antitermination of E. coli rRNA transcription is caused by a control region segment containing lambda nut-like sequences. Cell. 1984 Oct;38(3):851–860. doi: 10.1016/0092-8674(84)90280-0. [DOI] [PubMed] [Google Scholar]
- Liljenström H., von Heijne G. Translation rate modification by preferential codon usage: intragenic position effects. J Theor Biol. 1987 Jan 7;124(1):43–55. doi: 10.1016/s0022-5193(87)80251-5. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Zengel J. M. Ribosomal genes in Escherichia coli. Annu Rev Genet. 1986;20:297–326. doi: 10.1146/annurev.ge.20.120186.001501. [DOI] [PubMed] [Google Scholar]
- Little R., Ryals J., Bremer H. rpoB mutation in Escherichia coli alters control of ribosome synthesis by guanosine tetraphosphate. J Bacteriol. 1983 May;154(2):787–792. doi: 10.1128/jb.154.2.787-792.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher D. L., Dennis P. P. In vivo transcription of E. coli genes coding for rRNA, ribosomal proteins and subunits of RNA polymerase: influence of the stringent control system. Mol Gen Genet. 1977 Oct 20;155(2):203–211. doi: 10.1007/BF00393161. [DOI] [PubMed] [Google Scholar]
- Matzura H., Hansen B. S., Zeuthen J. Biosynthesis of the beta and beta' subunits of RNA polymerase in Escherichia coli. J Mol Biol. 1973 Feb 15;74(1):9–20. doi: 10.1016/0022-2836(73)90350-1. [DOI] [PubMed] [Google Scholar]
- Miura A., Krueger J. H., Itoh S., de Boer H. A., Nomura M. Growth-rate-dependent regulation of ribosome synthesis in E. coli: expression of the lacZ and galK genes fused to ribosomal promoters. Cell. 1981 Sep;25(3):773–782. doi: 10.1016/0092-8674(81)90185-9. [DOI] [PubMed] [Google Scholar]
- Molin S., Von Meyenburg K., Maaloe O., Hansen M. T., Pato M. L. Control of ribosome synthesis in Escherichia coli: analysis of an energy source shift-down. J Bacteriol. 1977 Jul;131(1):7–17. doi: 10.1128/jb.131.1.7-17.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nene V., Glass R. E. Relaxed mutants of Escherichia coli RNA polymerase. FEBS Lett. 1983 Mar 21;153(2):307–310. doi: 10.1016/0014-5793(83)80630-9. [DOI] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Oostra B. A., Kok K., Van Vliet A. J., Ab G., Gruber M. A mutation in the RNA polymerase beta' subunit causing depressed ribosomal RNA synthesis in Escherichia coli. Mol Gen Genet. 1981;183(1):54–58. doi: 10.1007/BF00270138. [DOI] [PubMed] [Google Scholar]
- Pedersen F. S., Lund E., Kjeldgaard N. O. Codon specific, tRNA dependent in vitro synthesis of ppGpp and pppGpp. Nat New Biol. 1973 May 2;243(122):13–15. [PubMed] [Google Scholar]
- Pedersen S. Escherichia coli ribosomes translate in vivo with variable rate. EMBO J. 1984 Dec 1;3(12):2895–2898. doi: 10.1002/j.1460-2075.1984.tb02227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S., Reeh S. Functional mRNA half lives in E. coli. Mol Gen Genet. 1978 Nov 9;166(3):329–336. doi: 10.1007/BF00267626. [DOI] [PubMed] [Google Scholar]
- Poulsen P., Jensen K. F. Effect of UTP and GTP pools on attenuation at the pyrE gene of Escherichia coli. Mol Gen Genet. 1987 Jun;208(1-2):152–158. doi: 10.1007/BF00330436. [DOI] [PubMed] [Google Scholar]
- Reeh S., Pedersen S., Friesen J. D. Biosynthetic regulation of individual proteins in relA+ and relA strains of Escherichia coli during amino acid starvation. Mol Gen Genet. 1976 Dec 22;149(3):279–289. doi: 10.1007/BF00268529. [DOI] [PubMed] [Google Scholar]
- Roland K. L., Liu C. G., Turnbough C. L., Jr Role of the ribosome in suppressing transcriptional termination at the pyrBI attenuator of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7149–7153. doi: 10.1073/pnas.85.19.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland K. L., Powell F. E., Turnbough C. L., Jr Role of translation and attenuation in the control of pyrBI operon expression in Escherichia coli K-12. J Bacteriol. 1985 Sep;163(3):991–999. doi: 10.1128/jb.163.3.991-999.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Mosteller R. D., Yanofsky C. Tryptophan messenger ribonucleic acid elongation rates and steady-state levels of tryptophan operon enzymes under various growth conditions. J Mol Biol. 1970 Aug;51(3):541–550. doi: 10.1016/0022-2836(70)90007-0. [DOI] [PubMed] [Google Scholar]
- Ryals J., Little R., Bremer H. Temperature dependence of RNA synthesis parameters in Escherichia coli. J Bacteriol. 1982 Aug;151(2):879–887. doi: 10.1128/jb.151.2.879-887.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmientos P., Cashel M. Carbon starvation and growth rate-dependent regulation of the Escherichia coli ribosomal RNA promoters: differential control of dual promoters. Proc Natl Acad Sci U S A. 1983 Nov;80(22):7010–7013. doi: 10.1073/pnas.80.22.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbi E., Rudd K. E., Cashel M. Basal ppGpp level adjustment shown by new spoT mutants affect steady state growth rates and rrnA ribosomal promoter regulation in Escherichia coli. Mol Gen Genet. 1988 Aug;213(2-3):214–222. doi: 10.1007/BF00339584. [DOI] [PubMed] [Google Scholar]
- Schleif R. Control of production of ribosomal protein. J Mol Biol. 1967 Jul 14;27(1):41–55. doi: 10.1016/0022-2836(67)90350-6. [DOI] [PubMed] [Google Scholar]
- Schmidt M. C., Chamberlin M. J. Amplification and isolation of Escherichia coli nusA protein and studies of its effects on in vitro RNA chain elongation. Biochemistry. 1984 Jan 17;23(2):197–203. doi: 10.1021/bi00297a004. [DOI] [PubMed] [Google Scholar]
- Shand R. F., Blum P. H., Mueller R. D., Riggs D. L., Artz S. W. Correlation between histidine operon expression and guanosine 5'-diphosphate-3'-diphosphate levels during amino acid downshift in stringent and relaxed strains of Salmonella typhimurium. J Bacteriol. 1989 Feb;171(2):737–743. doi: 10.1128/jb.171.2.737-743.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmund C. D., Morgan E. A. Nus A protein affects transcriptional pausing and termination in vitro by binding to different sites on the transcription complex. Biochemistry. 1988 Jul 26;27(15):5622–5627. doi: 10.1021/bi00415a034. [DOI] [PubMed] [Google Scholar]
- Stephens J. C., Artz S. W., Ames B. N. Guanosine 5'-diphosphate 3'-diphosphate (ppGpp): positive effector for histidine operon transcription and general signal for amino-acid deficiency. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4389–4393. doi: 10.1073/pnas.72.11.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen M. A., Kurland C. G., Pedersen S. Codon usage determines translation rate in Escherichia coli. J Mol Biol. 1989 May 20;207(2):365–377. doi: 10.1016/0022-2836(89)90260-x. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Conserved features of coordinately regulated E. coli promoters. Nucleic Acids Res. 1984 Mar 26;12(6):2605–2618. doi: 10.1093/nar/12.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Hicks K. L., Donahue J. P. Attenuation control of pyrBI operon expression in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1983 Jan;80(2):368–372. doi: 10.1073/pnas.80.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi M., Nomura M. Effects of induction of rRNA overproduction on ribosomal protein synthesis and ribosome subunit assembly in Escherichia coli. J Bacteriol. 1988 Nov;170(11):5042–5050. doi: 10.1128/jb.170.11.5042-5050.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi M., de Boer H. A., Nomura M. Feedback regulation of rRNA synthesis. A mutational alteration in the anti-Shine-Dalgarno region of the 16 S rRNA gene abolishes regulation. J Mol Biol. 1987 Dec 5;198(3):547–550. doi: 10.1016/0022-2836(87)90299-3. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Kelley R. L., Horn V. Repression is relieved before attenuation in the trp operon of Escherichia coli as tryptophan starvation becomes increasingly severe. J Bacteriol. 1984 Jun;158(3):1018–1024. doi: 10.1128/jb.158.3.1018-1024.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



