Abstract
Established cell lines are utilized extensively to study tumor biology and preclinical therapeutic development; however, they may not accurately recapitulate the heterogeneity of their corresponding primary disease. B-cell tumor cells are especially difficult to maintain under conventional culture conditions, limiting access to samples that faithfully represent this disease for preclinical studies. Here, we used primary canine diffuse large B-cell lymphoma to establish a culture system that reliably supports the growth of these cells. CD40 ligand, either expressed by feeder cells or provided as a soluble two-trimeric form, was sufficient to support primary lymphoma cells in vitro. The tumor cells retained their original phenotype, clonality and known karyotypic abnormalities after extended expansion in culture. Finally, we illustrate the utility of the feeder cell-free culture system for comparable assessment of cytotoxicity using dog and human B-cell malignancies. We conclude this system has broad applications for in vitro preclinical development for B-cell malignancies.
Keywords: Diffuse large B-cell lymphoma, CD40L, Primary cell culture, Canine model, Cytotoxicity assay
INTRODUCTION
Established tumor cell lines are useful for evaluation of therapeutics in many types of cancer, but one drawback is that they may not accurately recapitulate the heterogeneity of their corresponding primary disease. For example, available diffuse large B-cell lymphoma (DLBCL) cell lines have been established from cells that survived conventional culture conditions without Epstein–Barr virus infection, a process that is inefficient (low probability of success) and likely stochastic (high selective pressure for single clones that adapt to growth in vitro). Thus, it has been challenging to establish a diverse complement of cell lines that are truly representative of DLBCL, and we surmised that developing a culture method to support primary DLBCL cells would be valuable to improve translation of pre-clinical data.
CD40 ligand (CD40L, also known as CD154) is a member of the tumor necrosis factor family and is preferentially expressed on activated CD4+ T cells, basophils and mast cells. CD40, the receptor for CD40L, is expressed in various types of cells, including dendritic cells, macrophages, B-cells, epithelial cells, endothelial cells and some malignant cells. In B cells, cross-linking of CD40 by CD40L drives differentiation, proliferation, and immunoglobulin class switching, while it also prevents apoptosis [1,2]. Aberrant CD40 signaling is suspected to play an important role in the oncogenic processes of various types of B-cell malignancies, for example the activation of NF-κB transcription factors is one of the best-studied CD40 signaling pathways that promotes survival of DLBCL (and other B-lymphoma) cells [3–5]. Furthermore, B-cell specific expression of constitutively active CD40 was shown to promote B-cell lymphomagenesis in transgenic mice via JNK, Erk, and the non-canonical NF-κB pathway [6].
B-cell culture using CD40 stimulation, so-called the “CD40 system”, has been described as an effective method to grow malignant B-cells in vitro[7–9]; however, the precise role of CD40 activation, and particularly its effects on growth and apoptosis of malignant B-cells, remains unresolved [7–12]. One of the factors that may influence the effects of CD40 in this system is the type of ligand used (i.e., recombinant CD40L, agonistic antibodies, or CD40L-expressing feeder cells). Specifically, conformation of CD40L is important to induce robust stimulation [9,13,14], and feeder cells may alter or modulate CD40 signaling by engaging other stimulatory or inhibitory molecules on the B cell surface. The functional consequences of CD40 signaling also appear to be highly dependent on the type of B-cell malignancies, and in this regard, the utility of the “CD40 system” to maintain primary DLBCL cells has not been fully studied.
The high-quality dog genome sequence has demonstrated the close phylogenetic relationship between dogs and humans [15], emphasizing the potential benefit of canine models in identifying disease genes and evaluating response to novel therapies. Dogs spontaneously develop tumors, including B-cell lymphomas that share many biological, behavioral, genetic and cytogenetic features with their human counterparts, making the dog a good model for studies in tumor biology [16,17]. Furthermore, as these tumors develop in outbred dogs that share the same environmental risk factors with humans, and as the disease course is generally accelerated in dogs due to their shorter lifespan, pet dogs with cancer can be used as clinically relevant models for pre-clinical drug development that will inform subsequent human studies [18–20]. We hypothesized that development of a robust system to grow and maintain primary malignant canine B-cells in culture would provide a valuable tool for pre-clinical development and further enable the spontaneous canine B-cell lymphoma model.
For this study, we utilized human K562 cells transduced with human CD40L (KtCD40L) [21] as feeder cells to maintain primary dog DLBCL cells in vitro. Furthermore, we adapted the “CD40 system” using recombinant soluble human (shu)CD40L to demonstrate the application of this system to assess in vitro cytotoxicity of a targeted therapeutic.
MATERIALS AND METHODS
Primary tumor samples and cell culture
Sterile lymph node biopsy samples were collected from dogs with lymphoma and classified histologically as described [22]. All procedures were done with approval from institutional animal care and use committees at participating veterinary hospitals. Tumor cells classified as DLBCL (n = 9) were cryopreserved in liquid nitrogen until use. Bone marrow samples of human primary B-cell acute lymphoblastic leukemia (B-ALL) samples (n = 4) were obtained from the Leukemia and Myelodysplastic Syndrome Tissue Bank of the Masonic Cancer Center, University of Minnesota with Institutional Review Board approval.
KtCD40L cells were obtained from Dr. Robert Vonderheide (University of Pennsylvania). These cells were maintained under selection with hygromycin B (Invivogen, San Diego, CA) and irradiated before use as described [21]. B-lymphoma cells were plated at 1 × 106 cells/mL with 2 × 105 cells/mL of irradiated KtCD40L (5:1 ratio), and KtCD40L-B-lymphoma cell colonies were dispersed and restimulated with freshly irradiated KtCD40L every 5–7 days. As a substitute for KtCD40L, a shuCD40L (megaCD40L; Enzo Life Science, Plymouth Meeting, PA), which forms a hexamer and effectively stimulates B-cells in vitro[13], was used to maintain DLBCL cells in the feeder cell-free culture. For CD40 blocking experiments, shuCD40L was incubated with antihuman CD40L-IgA (Invivogen) for 30 minutes before use. The human DLBCL cell line SU-DHL4 was obtained from Dr. Lee Honigberg (Pharmacyclics Inc., Sunnyvale, CA). The human T-cell leukemia cell line, Jurkat, was from the ATCC (Manassas, VA). All cells were maintained in RPMI1640 (Gibco/BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (Atlas Biologicals, Fort Collins, CO), 2-mercaptoethanol (Gibco/BRL), HEPES, L-glutamine, sodium pyruvate (Mediatech Inc., Manassas, VA), non-essential amino acid (Sigma Aldrich, St. Louis, MO), and Primocin (Invivogen, San Diego, CA) at 37°C in a humidified 5% CO2 atmosphere.
Flow cytometry
Flow cytometry was performed as described [22]. Briefly, primary DLBCL cells were incubated with dog immunoglobulin G (IgG; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) to prevent non-specific binding of antibodies to Fc receptors. Cells were stained using phycoerythrin (PE) or fluorescein isothiocyanate (FITC) conjugated antibodies against dog CD5, human CD14, dog CD21, dog CD45 (Serotec, Raleigh, NC), human CD22, human CD40 (Abcam, Cambridge, MA), and mouse IgG1 as a negative control (eBioscience, San Diego, CA). For analysis of CD40 expression in dog tumors, cells were incubated with anti-human CD22 labeled using Zenon Alexa-Fluor 647 probes (Invitrogen-Molecular Probes, Carlsbad, CA) and human CD154 (CD40L)-muCD8/Biotin (Ancell, Bayport, MN) with streptavidin-FITC (eBioscience). Tumor cells were gated based on their light scatter properties and dead cells were excluded using 7-amino-actinomycin D (7-AAD; eBioscience) staining. Flow cytometry was performed using a LSRII cytometer (BD Immunocytometry Systems, San Jose, CA) and results were analyzed using FlowJo software (Tree Star, Ashland, OR).
Polymerase chain reaction (PCR) for clonal antigen receptor rearrangement (PARR)
The PARR analysis was performed as described [22]. Briefly, DNA samples were prepared from tumor cells and used for PCR to detect clonal immunoreceptor gene rearrangements using dye-labeled primers for Ig heavy chain (IgH), T-cell receptor gamma chain (TCRγ), and the constant region of IgM (Cµ; positive control). The size of PCR products was analyzed using a Genetic Analyzer 3130xl with the GeneScan 600 LIZ size standard (Applied Biosystems, Foster City, CA).
Evaluation of DNA copy number aberrations in primary and cultured tumor cells
Array comparative genomic hybridization (aCGH) analysis was used to define regions of recurrent DNA copy number imbalance in primary tumor cells, as described previously [23]. For each tumor, a canine bacterial artificial chromosome (BAC) clone was selected that was located within a region of recurrent genomic gain defined by aCGH analysis. Each clone has been shown previously to map to a unique chromosomal location consistent with its position in the dog genome sequence assembly [24], and was obtained from the CHORI-82 dog BAC library (www.chori.org). These clones were used as probes in fluorescence in situ hybridization (FISH) analysis of DLBCL cells as described previously [25]. The copy number status of the dog BAC probe was determined in each of 30 cells from the pre- and post-culture cell populations. KtCD40L cells within mixed-dog-human cell populations were identified using a BAC clone from the RP11 human BAC library in the same FISH assay, and human cells were excluded from probe enumeration analysis. All dog and human BAC probes were also hybridized to clinically healthy donors to demonstrate the expected copy number (n = 2) in normal cells, and to confirm absence of probe hybridization signals across species. Probe signals were scored by two independent investigators and these data were then compared between pre- and post-culture cell populations from the same DLBCL case.
Cytotoxicity/proliferation assay
Cell proliferation and viability were determined by the MTS assay using CellTiter 96® AQueous One Solution Cell Proliferation Assay Kit (Promega, Madison, WI). Briefly, 5 × 104 cells were resuspended in 100 μL of medium containing 100 ng/mL shuCD40L (for primary tumor cells) in 96-well plates. Two immunotoxins, CD22KDEL (anti-CD22 scFv fused to truncated Pseudomonas exotoxin) and Bic3 (anti-CD3ε scFv fused to DT390) [26,27], kindly provided by Dr. Daniel Vallera (University of Minnesota), were added to the cultures. After 72 hours, 20 μL of MTS solution was added to each well and cells were incubated for another 4 hours before measuring absorbance at 490 nm using a Wallac Victor2 1420 Multilabel Counter (Perkin Elmer, Waltham, MA). To determine IC50, the cytotoxicity assay was performed in log serial dilutions (from 0.01 to 100 nM) and IC50 was calculated using Prism 4 software (GraphPad Software, Inc., La Jolla, CA).
Transcript Profiling
Primary dog B-cell and T-cell lymphoma samples (n = 29) were profiled using Affymetrix Canine 2.0 cDNA microarrays as previously described [28]. GC-RMA normalization was carried out using Genedata Refiner software (Genedata, Lexington, MA), and the data were annotated based on pathological classification as B-cell or T-cell lymphoma. The levels of transcripts of interest were then compared between B-cell and T-cell lymphomas using the Genedata Analyst software package.
Statistical considerations
Statistical significance between more than 2 experimental groups was evaluated with one-way ANOVA with Bonferroni multicomparison posttest correction using Prism 4 software.
RESULTS
Expression of CD40 in DLBCL cells
We first retrospectively analyzed CD40, the receptor for CD40L, gene expression in 29 canine primary lymphoma samples, including 11 DLBCLs, using our gene expression profile data sets of canine lymphomas. Figure 1A shows expression of prototypical B-cell and T-cell differentiation genes (CD20, CD21, CD79a, and CD22 vs, CD3, CD4, and CD8, respectively) correlated with the pathological lymphoma phenotypes. Among these samples, B-cell lymphomas consistently expressed higher levels of CD40 relative to T-cell lymphomas. We also analyzed the expression of CD40 in primary dog DLBCL (n = 3) using flow cytometry with a recombinant human CD40L chimeric protein. Figure 1B shows one representative result that demonstrated virtually all the CD22+ tumor cells expressed CD40 (for example, compare the mean fluorescence intensity of the 2-dimensional dot plots on the right vs. the left in Figure 1B). Together, these data demonstrate that canine B-cell lymphomas, including DLBCL, routinely express the receptor for CD40L (CD40).
Figure 1.
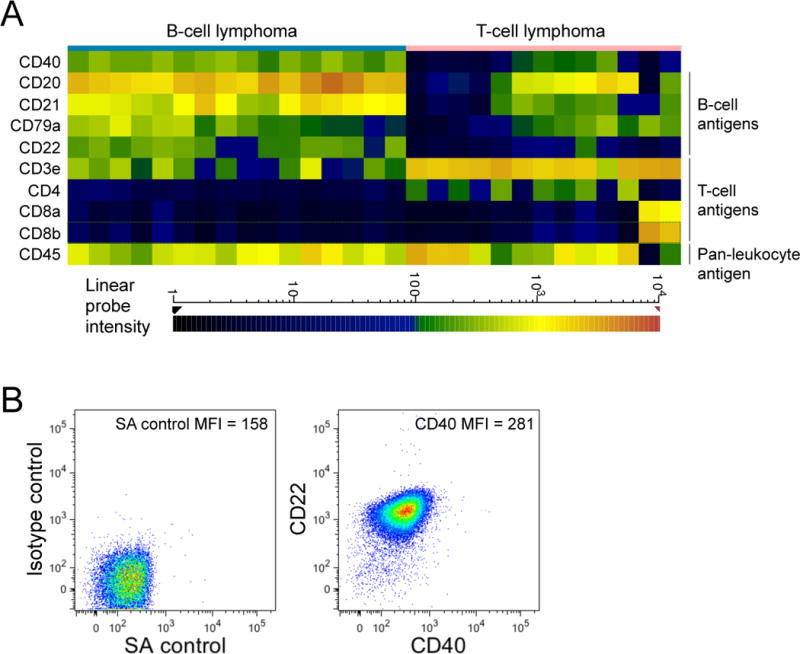
Expression of CD40 in primary dog B cell lymphomas. (A) A heat map of selected gene expression in dog B-cell and T-cell lymphomas. Expressions of genes, chosen for prototypical expression in B-cells and T-cells, were shown as a heat map where color represents fluorescence intensity of hybridized probes as indicated at the bottom and therefore the amount of gene transcript in any given sample. Generally, Red/Orange represents high expression, Yellow/Green represents moderate expression and Blue/Black represents little or no expression. (B) DLBCL samples were stained for CD22 and CD40 (using CD40L-CD8 fusion protein). A representative result using an isotype control antibody and streptavidin (SA control) is shown as a negative control (left panel). One representative sample of 3 different primary dog B-cell lymphomas is shown, including the mean fluorescence intensity (MFI) of the FITC channel.
KtCD40L supports primary B-cell lymphoma cells in culture
We used irradiated KtCD40L feeder cells to examine the potential for membrane-bound CD40L to support DLBCL cells in culture. Canine primary DLBCL cells and irradiated KtCD40L cells were cultured alone or in combination. DLBCL cells cultured without feeder cells, and irradiated KtCD40L cultured alone, died within 24–72 hr (Figure 2A and B). DLBCL cells also did not survive when they were cultured with the parental K562 cells (data not shown). In contrast, DLBCL cells co-cultured with KtCD40L formed large clusters (Figure 2A, middle panel), composed of large and more complex cells that had greater viability than DLBCL cells cultured alone (Figure 2B). Compared to primary tumor cells, KtCD40L cells were larger and more complex, making them easily distinguishable from tumor cells using light scatter parameters (Figure 2B). Under these conditions, growth of the primary DLBCL cells was not further enhanced by addition of canine recombinant IL-4 or human recombinant B-cell activating factor (BAFF, data not shown). The growth kinetics and the duration of cell survival varied among tumors (Figure 2C), reflecting the heterogeneous nature of the primary B-lymphoma cells.
Figure 2.
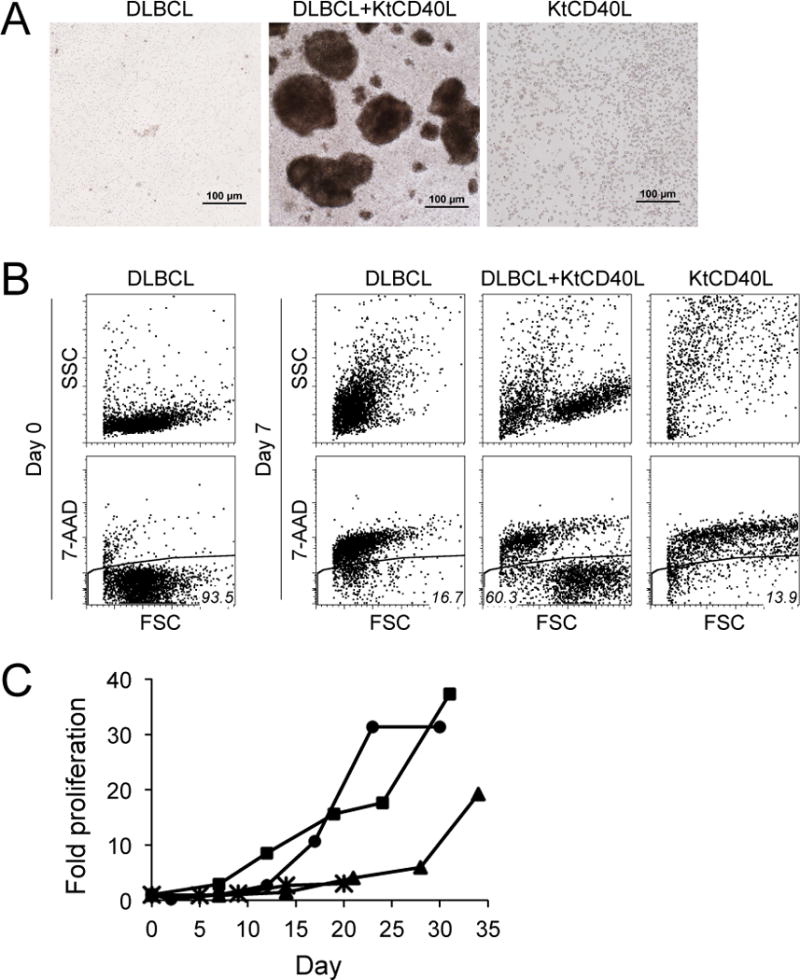
In vitro culture of primary dog DLBCL cells with KtCD40L. (A) Photomicrographs of DLBCL cells alone (left), DLBCL cells cultured with KtCD40L (middle), and KtCD40L alone (right), each shown after seven days in culture. Bars = 100 μm (B) Flow analysis of DLBCL cells before and after culture with KtCD40L for 7 days. Estimates of cell size and complexity were obtained from flow cytometric light scatter properties (upper panels). Viability was determined by 7-AAD exclusion (lower panels). FSC; forward scatter, SSC; side scatter (C) Growth curves of primary dog DLBCL cells (n = 4) cultured with KtCD40L. DLBCL cell numbers were determined at each time point using hemacytometer with dead cell exclusion by trypan blue staining.
Phenotypic characteristics of DLBCL cells cultured with KtCD40L
The phenotype of DLBCL cells cultured with KtCD40L feeder cells was analyzed using flow cytometry. Cultured DLBCL cells maintained expression of prototypical B-cell markers (CD21 and CD22) as well as CD45 for > 1 month in culture, and they did not express myeloid (CD14) or T-cell (CD5) markers, suggesting these cells were indeed expanded from the B-cell lymphoma population (Figure 3A). PARR analysis was performed using two DLBCL samples and showed that cultured tumor cells had a clonal IgH rearrangement, which was identical to that observed in the original tumor (Figure 3B). Concurrently, Cμ amplification product (a positive control) was observed, but TCRγ product was not observed from the same sample (data not shown). The canine-specific IgH, Cµ, and TCRγ primer pairs did not produce relevant PCR products when DNA extracted from KtCD40L was tested in the assay (data not shown). In addition, FISH analysis of one DLBCL using a dog-specific probe demonstrated CFA31 trisomy in 51% of tumor cells at the time culture was initiated (mean copy number = 2.46) and in 48% of cells after 40 days in culture with KtCD40L (mean copy number = 2.47, Figure 3C). Similar evidence for tumor persistence in KtCD40L co-culture was obtained from a second DLBCL sample where CFA9 trisomy was observed in 38% of the original tumor cells (mean copy number = 2.41) and in 40% of the cultured tumor cells (mean copy number = 2.33). Together, these data suggest stimulation using CD40L in a membrane-bound context can maintain primary dog DLBCL cells in vitro.
Figure 3.
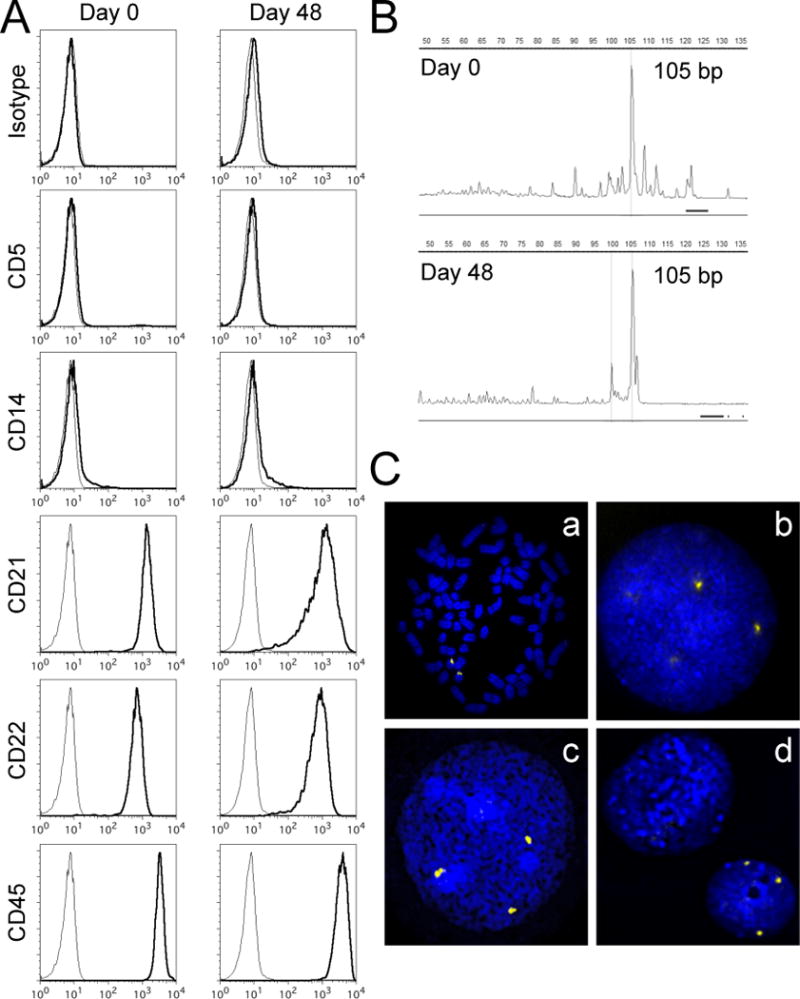
Phenotypic characteristics of DLBCL cells cultured with KtCD40L. (A) DLBCL cells cultured with KtCD40L for 48 days were phenotyped using flow cytometry. Gray lines represent unstained samples. One representative sample of three DLBCL cases analyzed is shown. (B) Clonal IgH gene rearrangements of the same size (105 bp) were observed in tumor cells before (Day 0) and after (Day 48) the culture. One representative sample of two DLBCL cases analyzed is shown. (C) Examples of FISH images obtained from one DLBCL case with trisomy of CFA 31. Control dog metaphase chromosomes (a) and an interphase nucleus (b) probed with a marker for CFA 31 (yellow signal), exhibiting the expected chromosomal location and normal copy number status (n = 2) of this region. Three copies of this locus were evident in 51% of DLBCL cells prior to culture (c), and in 48% of DLBCL cells after culture with KtCD40L for 40 days (d). A KtCD40L cell (upper left without a probe signal) and a DLBCL cell (lower right with probe signals) are shown together in (d).
Recombinant soluble CD40L supports dog DLBCL cells in culture
Next, we examined the capacity of shuCD40L multimeric complexes (megaCD40L) to support primary dog DLBCL cells in culture. DLBCL cells (n = 4) were cultured with increasing concentrations of shuCD40L and viable cells quantified at 24 hours intervals using the MTS assay. Primary dog DLBCL cells proliferated in the presence of CD40L in a dose dependent manner and continued to expand for 72 hours in culture (Figure 4A). As was true with cells grown with KTCD40L feeder cells, the growth kinetics varied among tumor samples, reflecting the heterogeneity of primary tumors. Anti-CD40L neutralizing antibody inhibited DLBCL cell proliferation and survival in a dose dependent manner, with 1 μg/mL (7 μM) of anti-CD40L-IgA being sufficient to achieve complete inhibition in cells stimulated using 100 ng/mL (2.5 μM) CD40L (Figure 4B), indicating that DLBCL cell proliferation in culture was specifically mediated by shuCD40L. Furthermore, we demonstrated the shuCD40L culture system could effectively support growth of human primary B-ALL cells (n = 2) that expressed CD40 (Figure 4C and 4D). Together, these results show that shuCD40L supports growth and survival of primary B-cell malignancies from dogs and humans in culture.
Figure 4.
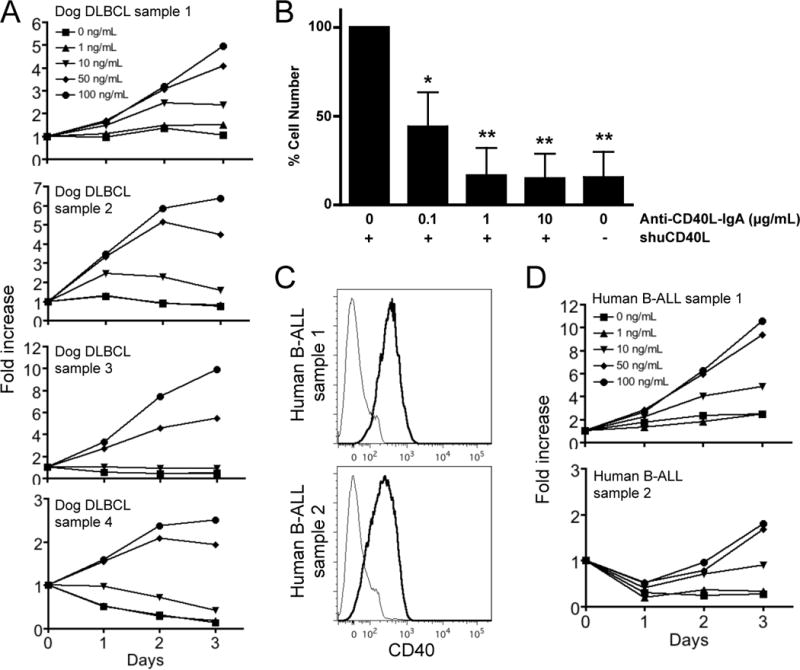
Feeder cell-free culture of primary DLBCL cells with recombinant shuCD40L. (A) The MTS cell proliferation assay was performed at 24-hour intervals on primary dog DLBCL cells stimulated with increasing amounts of shuCD40L. Data from 4 independent primary dog DLBCL samples are shown. Data were normalized to results from 5 × 104 cells (0 hour) to show fold increase of cell numbers during the culture period. (B) MTS cell proliferation assay was performed on primary DLBCL cells stimulated with 100 ng/mL shuCD40L in the presence of increasing amounts of anti-human CD40L-IgA. Three different primary DLBCL samples were tested for 72 hours in duplicate conditions and data were normalized to results of cells cultured with 100 ng/mL shuCD40L without neutralizing antibodies. Data shown represent the mean ± SD (n = 3) and statistical significance was tested against the condition of 100 ng/mL shuCD40L without neutralizing antibodies using ANOVA (*P < 0.01, **P < 0.001). (C) Human B-ALL samples were stained for CD22 and CD40. CD22+ tumor cells (> 90% CD22+ cells in both samples) were gated and CD40 expression was analyzed. Gray lines represent staining with an isotype control antibody. (D) The MTS cell proliferation assay using primary human B-ALL cells was performed as described above. Data from 2 independent samples are shown.
CD40L culture system demonstrates efficacy of targeted therapeutics
One major advantage for the primary culture system is the capability to test efficacy of targeted compounds in vitro. We validated the system using the anti-CD22 and anti-CD3 immunotoxins, 22KDEL and Bic3. We initially confirmed the specificity of these toxins in the human B-cell and T-cell tumor cell lines DHL-4 and Jurkat (Table I). Next, we evaluated the effect of these toxins on four distinct primary canine DLBCL samples grown in the presence of shuCD40L (Figure 5A). 22KDEL effectively killed each culture of primary dog DLBCL cells, while Bic3 showed minimal cytotoxicity. The IC50 of 22KDEL against the primary dog DLBCL was 1.95, 3.83, 10.9, and 39.2 (Table I). To extend these findings to samples of human origin, we also examined two human primary B-ALL samples using the shuCD40L culture system. As a result, shuCD40L supported B-ALL cells in culture and provided a reliable platform for cytotoxicity testing: Figure 5B and Table I show that B-ALL cells were efficiently killed by 22KDEL (IC50 of 0.11 and 0.002 nM), and they were largely resistant to Bic3.
Table I.
IC50 of 22KDEL and Bic3 for tumor cells
| 22KDEL IC50 (nM) | Bic3 IC50 (nM) | |
|---|---|---|
| DHL-4 | 0.02 | > 100 |
| Jurkat | > 50 | 0.06 |
| Dog DLBCL | ||
| Sample 1 | 1.95 | > 100 |
| Sample 2 | 3.83 | > 100 |
| Sample 3 | 39.22 | > 100 |
| Sample 4 | 10.95 | > 100 |
| Human B-ALL | ||
| Sample 1 | 0.11 | > 100 |
| Sample 2 | 0.002 | > 100 |
Figure 5.
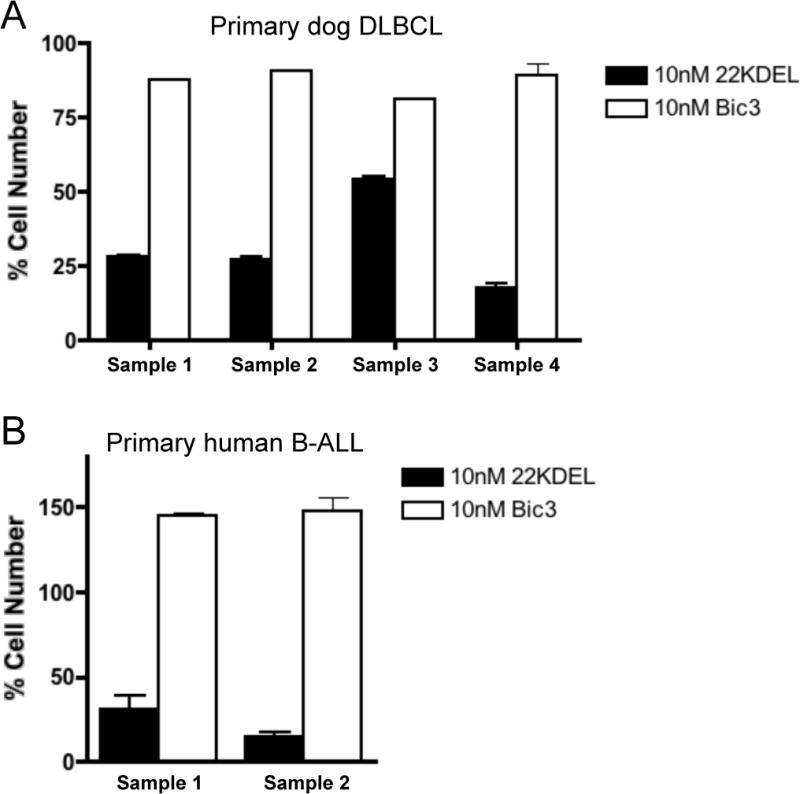
Comparable assessment of cytotoxicity using dog and human B-cell malignancies. (A) Four independent primary dog DLBCL samples; #1–3 are the same as in Figure 4, #4 is from a different dog, and (B) two human primary B-ALL samples from different patients than those shown in Figure 4, were cultured for 72 hours with a log serial dilutions (0.01–100 nM) of 22KDEL and Bic3 immunotoxins in the presence of shuCD40L. Experiments were performed in duplicate and cell numbers were determined using the MTS assay. Results with 10 nM immunotoxins are shown, and a summary result of the IC50 for 22KDEL and Bic3 is shown in Table I.
DISCUSSION
In addition to the biologic and behavioral similarities between canine and human cancers, recent advances in canine research have opened the door to perform high-standard comparative lymphoma studies. Nonetheless, limitations still exist; for example, there are few well-established canine B-cell lymphoma cell lines [29] and, as is true in humans, methods to maintain primary DLBCL cells to perform in vitro preclinical analysis have not been generally reliable. Here, we document a reproducible system to culture primary dog DLBCL cells using human CD40L-expressing feeder cells or recombinant shuCD40L. Multiple DLBCL cases/specimens maintained under these conditions for more than one month retained the phenotypes, clonality and the frequency of a disease-associated cytogenetic marker found in the original tumors. Furthermore, the culture system is sufficiently robust to perform in vitro preclinical cytotoxicity assays.
The KtCD40L feeder system has several advantages over NIH-3T3 transfectants for primary B-cell culture; cells are grown in suspension, making harvesting less cumbersome and requiring fewer re-stimulations to support long-term growth [21]. The KtCD40L feeder system, while exceptionally well suited to expand primary cells, has disadvantages in applications that require precise information about sensitivity of B-cells to potential therapeutic agents. Specifically, feeder cells can interfere with data from assays such as MTT reduction or flow cytometry. Feeder cells are especially problematic for reagents that can target the feeder cells themselves (i.e., kinase inhibitors). In those cases, the results would be compromised by this direct effect on feeder cells, which could alter the background and/or indirectly affect tumor cells by killing the feeder cells that provide CD40L support. In contrast, shuCD40L is more easily adapted to cytotoxicity assays, providing a robust complement to the feeder expansion for in vitro pre-clinical development.
Previous studies on CD40 stimulation of malignant B-cells in vitro have reported conflicting results [7–9], probably because of intrinsic differences in the tumor cells used and variation in their methods with regard to the type of CD40L and/or cytokine supplementation. A recent study showed that normal canine peripheral B-cells could be expanded effectively by culture with KtCD40L in the presence of canine recombinant IL-4 [21]. In our experiments, neither recombinant canine IL-4 nor recombinant human BAFF were necessary to support viability and expansion of canine B cell lymphoma cells in the presence of KtCD40L. Along with the observation that DLBCL cell growth was completely blocked by anti-CD40L neutralizing antibodies, the data show that CD40L is necessary and sufficient factor to primary DLBCL cells growth in vitro.
Several targeted therapies have been developed for B-cell malignancies. Among these is the immunotoxin 22KDEL, which consists of the Fv portion of an anti-human CD22 monoclonal antibody (clone RFB4) fused to a 38kDa portion of Pseudomonas exotoxin. The epitope recognized by RFB4 is conserved in dogs [22], thus providing the opportunity to use this compound for proof of principle testing for cytotoxicity using the CD40L system. Our data show that culture of DLBCL cells with multimeric shuCD40L is reliable, and will be readily adaptable to high throughput systems to evaluate biological therapies, small molecules, and other targeted compounds against diverse collections of DLBCL cells. Equally important, the system offers robust support for cells derived from primary human B-cell malignancies, including B-ALLs tested here.
The IC50 values for 22KDEL in primary dog DLBCL cells (IC50 = 1.95 – 39.2 nM), in DHL-4 cells (IC50 = 0.02 nM), and in primary human B-ALL (IC50 = 0.11 and 0.002 nM), which are not dissimilar to those previously reported for human Raji Burkitt lymphoma cells (~0.3 nM) [26], might reflect CD22 receptor density or intrinsic resistance by the cells, faithfully reflecting the likely range that would be encountered in patients. Indeed, the results are a good indicator of the relatively wide dynamic range that can be achieved by this assay (in this case, more than 5 logs, or ~0.002–40 nM) when testing samples are potentially highly heterogeneous both within each tumor and among multiple patients. This is unlike established cell lines that reflect only the characteristics of a select component of the original tumor; thus, tests that use primary tumor samples may provide precise and complementary data for clinical translation.
In summary, we established a CD40L-dependent culture system for malignant B-cells (DLBCL and B-ALL), which allows us to test candidate compounds for treatment in primary tumor samples from dogs and humans. While the feeder cell system can be efficiently used for long-term culture and expansion of the malignant cells, the simple feeder cell-free system provides additional benefits for high-throughput screening assays, overcoming potential analysis obstacles created by the presence of feeder cells.
Acknowledgments
The authors would like to thank all the dog owners who allowed their pets to participate in this study, as well as Drs. Antonella Borgatti and Michael Henson (University of Minnesota, Minneapolis/St. Paul, MN), Dr. Kristine Burgess (Tufts Cummings School of Veterinary Medicine, North Grafton, MA), Dr. Gretchen Gerber (Country Care Pet Care, Washburn, WI), and Dr. Janice Baserga (Scarborough Animal Hospital, Scarborough, ME) for assistance collecting biopsy samples. We wish to acknowledge the assistance of Dr. Aaron Sarver (University of Minnesota) for microarray data analysis and also thank Drs. Aaron Sarver, Jeffrey Miller, Michael Verneris, (University of Minnesota), and Douglas Thamm (Colorado State University, Fort Collins, CO) for critical review of the manuscript and helpful discussions.
This work was supported by MAF First Award Grant D12CA-302 (DI), AKC Canine Health Foundation grant 615 (JFM and MB), NIH grant R01CA112211 (MB), AKC Canine Health Foundation grant 1113 (TDOB and JFM), NIH grant P30CA077598 (NCI Core Support Grant for the Masonic Cancer Center), and by philanthropic funds from the University of Minnesota Animal Cancer Care and Research Program, the Starlight Fund, and the Land of PureGold Foundation, Inc (JFM).
Footnotes
POTENTIAL CONFLICT OF INTEREST
Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Kuwashima N, Kageyama S, Eto Y, Urashima M. CD40 ligand immunotherapy in cancer: an efficient approach. Leuk Lymphoma. 2001;42:1367–1377. doi: 10.3109/10428190109097765. [DOI] [PubMed] [Google Scholar]
- 2.van Kooten C, Banchereau J. CD40-CD40 ligand. Journal of leukocyte biology. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 3.Challa A, Eliopoulos AG, Holder MJ, et al. Population depletion activates autonomous CD154-dependent survival in biopsylike Burkitt lymphoma cells. Blood. 2002;99:3411–3418. doi: 10.1182/blood.v99.9.3411. [DOI] [PubMed] [Google Scholar]
- 4.Pham LV, Tamayo AT, Yoshimura LC, et al. A CD40 Signalosome anchored in lipid rafts leads to constitutive activation of NF-kappaB and autonomous cell growth in B cell lymphomas. Immunity. 2002;16:37–50. doi: 10.1016/s1074-7613(01)00258-8. [DOI] [PubMed] [Google Scholar]
- 5.Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. The Journal of experimental medicine. 2001;194:1861–1874. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hömig-Hölzel C, Hojer C, Rastelli J, et al. Constitutive CD40 signaling in B cells selectively activates the noncanonical NF-kappaB pathway and promotes lymphomagenesis. The Journal of experimental medicine. 2008;205:1317–1329. doi: 10.1084/jem.20080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Planken EV, Dijkstra NH, Willemze R, Kluin-Nelemans JC. Proliferation of B cell malignancies in all stages of differentiation upon stimulation in the ‘CD40 system’. Leukemia. 1996;10:488–493. [PubMed] [Google Scholar]
- 8.Visser HP, Tewis M, Willemze R, Kluin-Nelemans JC. Mantle cell lymphoma proliferates upon IL-10 in the CD40 system. Leukemia. 2000;14:1483–1489. doi: 10.1038/sj.leu.2401829. [DOI] [PubMed] [Google Scholar]
- 9.Andersen NS, Larsen JK, Christiansen J, et al. Soluble CD40 ligand induces selective proliferation of lymphoma cells in primary mantle cell lymphoma cell cultures. Blood. 2000;96:2219–2225. [PubMed] [Google Scholar]
- 10.Spriggs MK, Armitage RJ, Strockbine L, et al. Recombinant human CD40 ligand stimulates B cell proliferation and immunoglobulin E secretion. J Exp Med. 1992;176:1543–1550. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funakoshi S, Longo DL, Beckwith M, et al. Inhibition of human B-cell lymphoma growth by CD40 stimulation. Blood. 1994;83:2787–2794. [PubMed] [Google Scholar]
- 12.Henriquez NV, Floettmann E, Salmon M, Rowe M, Rickinson AB. Differential responses to CD40 ligation among Burkitt lymphoma lines that are uniformly responsive to Epstein-Barr virus latent membrane protein 1. J Immunol. 1999;162:3298–3307. [PubMed] [Google Scholar]
- 13.French LE, Huard B, Wysocka M, et al. Impaired CD40L signaling is a cause of defective IL-12 and TNF-alpha production in Sézary syndrome: circumvention by hexameric soluble CD40L. Blood. 2005;105:219–225. doi: 10.1182/blood-2004-03-1055. [DOI] [PubMed] [Google Scholar]
- 14.Wyzgol A, Müller N, Fick A, et al. Trimer stabilization, oligomerization, and antibody-mediated cell surface immobilization improve the activity of soluble trimers of CD27L, CD40L, 41BBL, and glucocorticoid-induced TNF receptor ligand. J Immunol. 2009;183:1851–1861. doi: 10.4049/jimmunol.0802597. [DOI] [PubMed] [Google Scholar]
- 15.Lindblad-Toh K, Wade CM, Mikkelsen TS, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 16.Modiano J, Breen M, Valli V, Wojcieszyn J, Cutter G. Predictive value of p16 or Rb inactivation in a model of naturally occurring canine non-Hodgkin’s lymphoma. Leukemia. 2007;21:184–187. doi: 10.1038/sj.leu.2404392. [DOI] [PubMed] [Google Scholar]
- 17.Breen M, Modiano JF. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans–man and his best friend share more than companionship. Chromosome research: an international journal on the molecular, supramolecular and evolutionary aspects of chromosome biology. 2008;16:145–154. doi: 10.1007/s10577-007-1212-4. [DOI] [PubMed] [Google Scholar]
- 18.Hansen K, Khanna C. Spontaneous and genetically engineered animal models; use in preclinical cancer drug development. Eur J Cancer. 2004;40:858–880. doi: 10.1016/j.ejca.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 19.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–156. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 20.Kelsey JL, Moore AS, Glickman LT. Epidemiologic studies of risk factors for cancer in pet dogs. Epidemiologic reviews. 1998;20:204–217. doi: 10.1093/oxfordjournals.epirev.a017981. [DOI] [PubMed] [Google Scholar]
- 21.Mason N, Coughlin C, Overley B, et al. RNA-loaded CD40-activated B cells stimulate antigen-specific T-cell responses in dogs with spontaneous lymphoma. Gene therapy. 2008;15:955–965. doi: 10.1038/gt.2008.22. [DOI] [PubMed] [Google Scholar]
- 22.Ito D, Endicott MM, Jubala CM, et al. A tumor-related lymphoid progenitor population supports hierarchical tumor organization in canine B-cell lymphoma. J Vet Intern Med. 2011;25:890–896. doi: 10.1111/j.1939-1676.2011.0756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas R, Seiser EL, Motsinger-Reif A, et al. Refining tumor-associated aneuploidy through ‘genomic recoding’ of recurrent DNA copy number aberrations in 150 canine non-Hodgkin lymphomas. Leuk Lymphoma. 2011;52:1321–1335. doi: 10.3109/10428194.2011.559802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas R, Duke SE, Karlsson EK, et al. A genome assembly-integrated dog 1 Mb BAC microarray: a cytogenetic resource for canine cancer studies and comparative genomic analysis. Cytogenet Genome Res. 2008;122:110–121. doi: 10.1159/000163088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breen M, Hitte C, Lorentzen TD, et al. An integrated 4249 marker FISH/RH map of the canine genome. BMC Genomics. 2004;5:65. doi: 10.1186/1471-2164-5-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vallera DA, Oh S, Chen H, Shu Y, Frankel AE. Bioengineering a unique deimmunized bispecific targeted toxin that simultaneously recognizes human CD22 and CD19 receptors in a mouse model of B-cell metastases. Mol Cancer Ther. 2010;9:1872–1883. doi: 10.1158/1535-7163.MCT-10-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vallera DA, Chen H, Sicheneder AR, Panoskaltsis-Mortari A, Taras EP. Genetic alteration of a bispecific ligand-directed toxin targeting human CD19 and CD22 receptors resulting in improved efficacy against systemic B cell malignancy. Leuk Res. 2009;33:1233–1242. doi: 10.1016/j.leukres.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott MC, Sarver AL, Gavin KJ, et al. Molecular subtypes of osteosarcoma identified by reducing tumor heterogeneity through an interspecies comparative approach. Bone. 2011;49:356–367. doi: 10.1016/j.bone.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rütgen BC, Hammer SE, Gerner W, et al. Establishment and characterization of a novel canine B-cell line derived from a spontaneously occurring diffuse large cell lymphoma. Leuk Res. 2010;34:932–938. doi: 10.1016/j.leukres.2010.01.021. [DOI] [PubMed] [Google Scholar]


