Abstract
Expertly collected, well-curated data sets consisting of comprehensive clinical characterization and raw structural, functional and diffusion-weighted DICOM images in schizophrenia patients and sex and age-matched controls are now accessible to the scientific community through an on-line data repository (coins.mrn.org). The Mental Illness and Neuroscience Discovery Institute, now the Mind Research Network (MRN, www.mrn.org), comprised of investigators at the University of New Mexico, the University of Minnesota, Massachusetts General Hospital, and the University of Iowa, conducted a cross-sectional study to identify quantitative neuroimaging biomarkers of schizophrenia. Data acquisition across multiple sites permitted the integration and cross-validation of clinical, cognitive, morphometric, and functional neuroimaging results gathered from unique samples of schizophrenia patients and controls using a common protocol across sites. Particular effort was made to recruit patients early in the course of their illness, at the onset of their symptoms. There is a relatively even sampling of illness duration in chronic patients. This data repository will be useful to 1) scientists who can study schizophrenia by further analysis of this cohort and/or by pooling with other data; 2) computer scientists and software algorithm developers for testing and validating novel registration, segmentation, and other analysis software; and 3) educators in the fields of neuroimaging, medical image analysis and medical imaging informatics who need exemplar data sets for courses and workshops. Sharing provides the opportunity for independent replication of already published results from this data set and novel exploration. This manuscript describes the inclusion/exclusion criteria, imaging parameters and other information that will assist those wishing to use this data repository.
Keywords: Medical Image Data repository, Schizophrenia, fMRI, DWI, mMRI, healthy controls
Introduction
Recent advances in methods for across site standardization and calibration of magnetic resonance image data acquisition by efforts such as the Morphometry and Function Biomedical Informatics Research Network (mBIRN and fBIRN) test beds and the Alzheimer’s Disease Neuroimaging Initiative (ADNI) have facilitated successful acquisition of robust, multi-site quantitative neuroimaging data that can be pooled for analysis (e.g. (Jovicich et al. 2006; Jovicich et al. 2009; Han et al. 2006; Friedman and Glover 2006; Potkin and Ford 2009; Potkin et al. 2009; Mueller et al. 2005b; Mueller et al. 2005a; Risacher et al. 2009; Shen et al. 2010); and (Desikan et al. 2010) and many more). The Mental Illness and Neuroscience Discovery Institute (MIND) Institute, now the Mind Research Network (MRN, www.mrn.org) formed the MIND Clinical Imaging Consortium (MCIC) in 2003 to conduct a multi-institutional, cross-sectional study of patients with schizophrenia and demographically matched, by sex and age, healthy controls to identify quantitative neuroimaging biomarkers for this devastating disease. The MCIC is comprised of investigators from academic departments located at four research sites: the University of New Mexico (UNM), the University of Minnesota (UMinn), Massachusetts General Hospital (MGH), and the University of Iowa (UIowa). The MCIC team collaborated with the mBIRN and fBIRN testbed projects to develop a calibrated, multi-modal imaging acquisition paradigm optimized for the study of schizophrenia. The MRN served as the coordinating site with investigators managing the overall execution and administration of the project, including data entry, assessment, analysis and storage. The explicit purpose of the multi-site effort was to permit the integration and cross-validation of clinical, cognitive, morphometric, and functional neuroimaging results gathered from unique samples of schizophrenia patients using a common investigational protocol across sites. Particular effort was made to recruit patients early in the course of their illness. Thus, this dataset includes a significant proportion of patients studied at the onset of their symptoms as well as a relatively even sampling of illness duration through chronic, well-established disease. This approach has supported the primary clinical aims of this multi-site project, investigation of neural substrates of the core cognitive deficits associated with schizophrenia and their links to clinical features of the disorder (for example, (C. C. Abbott et al. 2010; Demirci et al. 2008a; Demirci et al. 2008b; Ehrlich et al. 2011a; Ehrlich et al. 2011b; Kim et al. 2010; Sponheim et al. 2010; Walton et al. 2012; White et al. 2009; Ehrlich et al. 2010). In addition to conducting the initial data analyses, the MCIC leadership team explicitly envisioned the future possibility of sharing this data with the scientific community when the study was designed.
Towards the goal of data sharing, the MCIC investigators have created a publicly accessible, on-line data repository containing curated anatomical and functional MRI, diffusion weighted imaging (DWI), and associated clinical and neuropsychological metadata collected from individuals with and without a schizophrenia spectrum disorder. The rationale for sharing the data is that they will be valuable examples for use by educators in the fields of neuroimaging, medical image analysis and medical imaging informatics for courses and workshops; for testing and validating novel registration, segmentation, and other analysis software. These data may also continue to be valuable for the study of schizophrenia with further analysis of this cohort and/or by pooling with other similar data sets. With over 30 manuscripts already published and more in preparation, sharing provides the opportunity for independent replication of our results and novel exploration of this valuable data. While publications give a synopsis of the clinical assessments, imaging, and cognitive measures in these data, this data-sharing manuscript provides in greater detail the inclusion/exclusion criteria, imaging parameters and other information that will assist those wishing to use this data repository.
Methods
The MCIC conducted a preliminary across site standardization, calibration and validation study of the MR image acquisition sequences and fMRI experimental paradigms (Yendiki et al. 2010). The consortium members also performed cross-site calibration of study clinicians and raters with validated inter-rater reliability. All non-imaging data, including demographic, clinical rating scales, and summary scores from the neuropsychological battery for all patients and control subjects were double-entered, with independent verification of any discrepancies using a validated in house database software (Bockholt et al. 2010). Of the resulting data, we are providing the imaging and non-imaging data from three of the four sites. One site’s data is not being publically released due to IRB restrictions. In this paper we report on how the data were collected, how to access them, and selected summaries of the findings from these data intended to convey an accurate description of data quality and utility. The MCIC imaging and clinical data may be freely used with no restrictions. Users are encouraged to cite this paper and the data sharing web site. Anyone wishing to distribute derived data will be encouraged to include the original images. They will be required to accept the conditions of the MCIC Data Use Agreement before accessing the data.
Data will be made available through COINS (COllaborative Informatics Neuroimaging Suite), a neuroinformatics suite that offers tools to share existing data with other researchers, acquire data from other researchers, and manage studies from beginning to end. COINS currently manages over 400 studies with more than 232,000 assessments and 23,000 scans collected for 16,000 participants at the Mind Research Network, the Nathan Kline Institute, University of Colorado – Boulder, the Olin Neuropsychiatry Research Center, and other institutions.
Users will access the data through a COINS tool called the Data Exchange. The Data Exchange allows users to search, request and download data. The scan data will be separated by series (task) name. To download the data, the user will select which series are of interest, add them to the shopping cart and “Request the Data”. At the time of the request the user will be required to accept the conditions of the MCIC Data Use Agreement before accessing the data. The data downloads will then be enabled for that user. When the user selects the download link, the files will be zipped and an email will be sent to the requester when the zip file is available. The DICOM header and scan behavioral data files are scrubbed using the dcmdump and dcmodify utilities from http://dcmtk.org/ before they are downloaded. All identifying information (e.g., site information, birth date, scan date, weight, height, etc.) is removed and the anonymized ID is assigned. A separate file containing all the clinical and neuropsychological variables for each subject with the matching anonymous ID is also available for download.
Subject Characterization
Across the four research sites— UNM, UMinn, MGH and UIowa —188 patients with schizophrenia and 190 demographic, age and sex- matched healthy control subjects were recruited for this study between July 2004 and July 2006 (Table 1). To protect subject identity, testing sites are referred to by letter in this manuscript and online. The final cohort for whom data are available includes 162 patients and 169 controls with site distribution as follows; site A 46 patients/44 controls; site B 44/73; site C 33/27; site D 38/25, respectively. The remaining 47 subjects were not included in final analyses for reasons such as excessive motion during image acquisition, refusal to be scanned, claustrophobia or discomfort during MRI scanning, refusal to schedule or show up to appointments, or a determination of ineligibility based on screening exams and tests.
Table 1.
Number of datasets per data type for both patients and controls from each of the 4 sites
| Demographic | Diagnostic Information |
Neurocognitive | fMRI- SIRP |
fMRI- SM |
fMRI- AOD |
Breathhold | sMRI | DWI | |
|---|---|---|---|---|---|---|---|---|---|
| Site A Controls | 44 | 44 | 44 | 44 | 43 | 43 | 43 | 44 | 44 |
| Site A Patients | 48 | 43 | 45 | 44 | 45 | 45 | 43 | 45 | 42 |
| Site B Controls | 73 | 73 | 73 | 72 | 72 | 72 | 72 | 73 | 59 |
| Site B Patients | 44 | 44 | 44 | 40 | 39 | 39 | 39 | 42 | 26 |
| Site C Controls | 27 | 27 | 26 | 27 | 27 | 27 | 27 | 27 | 25 |
| Site C Patients | 32 | 32 | 31 | 32 | 32 | 33 | 32 | 33 | 31 |
| Site D Controls | 25 | 25 | 25 | 25 | 18 | 25 | 25 | 25 | 25 |
| Site D Patients | 38 | 34 | 36 | 37 | 28 | 37 | 37 | 38 | 37 |
| Total Controls | 169 | 169 | 168 | 168 | 160 | 167 | 167 | 169 | 153 |
| Total Patients | 162 | 153 | 156 | 153 | 144 | 154 | 151 | 158 | 136 |
|
| |||||||||
| Total Subjects | 331 | 322 | 324 | 321 | 304 | 321 | 318 | 327 | 289 |
fMRI-SIRP: Sternberg item recognition paradigm task data, fMRI-SM: sensory motor pardigm task data, fMRI-AOD: Auditory Oddball paradigm task data, sMRI: structural MRI scans, DWI: Diffusion weighted imaging scan
All subjects were between the ages of 18 and 60 and spoke English as their native language. To be included in the schizophrenia cohort, patients had to meet DSM-IV diagnostic criteria for schizophrenia, schizoaffective disorder or schizophreniform disorder. Concerted effort was made to recruit patients early in the course of their illness and especially those who were antipsychotic drug naïve.
The healthy control subjects with no current or past history of psychiatric illness including substance abuse or dependence were matched within site to the patient cohort for age, sex, and parental education (Table 2). Control subjects who had not been diagnosed with any psychiatric disorders, but had been medicated with antidepressants, anti-anxiety medication or medication for sleep disturbance were included in the study provided that the duration of their medication did not exceed 2 months of lifetime use and no medication was used within the 6 months preceding the baseline MRI scan.
Table 2.
Demographic Information for Subjects.
| Site | Subject Type |
N | Hand (R/L/Both) |
Sex | Race | Age | WRAT3 | Parental SES |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | White | Mean | SD | Mean | SD | Mean | SD | ||||||
| N | % | N | % | ||||||||||
| A | C | 44 | 39/0/5 | 9 | 45 | 25 | 41.7 | 28.7 | 11.5 | 50 | 5.7 | 2.4 | 0.9 |
| P | 46 | 39/2/4 | 11 | 55 | 35 | 58.3 | 32.5 | 12.2 | 47.5 | 5.3 | 3.1 | 1.3 | |
| B | C | 73 | 67/3/3 | 32 | 74.4 | 60 | 65.2 | 29.8 | 9.4 | 50.6 | 4 | 2.9 | 0.5 |
| P | 44 | 41/3/0 | 11 | 25.6 | 32 | 34.8 | 32.3 | 9.7 | 48.4 | 6.8 | 2.8 | 0.8 | |
| C | C | 27 | 25/1/0 | 12 | 54.5 | 20 | 48.8 | 32.1 | 12.6 | 50.9 | 3.2 | 2.3 | 0.8 |
| P | 33 | 27/3/1 | 10 | 45.5 | 21 | 51.2 | 31.8 | 10.2 | 46.6 | 5.5 | 2.5 | 0.7 | |
| D | C | 25 | 23/1/1 | 11 | 52.4 | 11 | 37.9 | 40.5 | 9.3 | 51.6 | 4.1 | 3.2 | 0.9 |
| P | 38 | 32/2/2 | 10 | 47.6 | 18 | 62.1 | 37.7 | 10 | 44.4 | 7.6 | 3.4 | 1.1 | |
| Total | C | 169 | 154/5/9 | 64 | 60.4 | 116 | 52.3 | 31.5 | 11.1 | 50.6 | 4.4 | 2.7 | 0.8 |
| P | 161 | 139/10/7 | 42 | 39.6 | 106 | 47.7 | 33.5 | 10.8 | 46.8 | 6.4 | 2.9 | 1 | |
Wide Range Achievement Test-3 (WRAT3), Social Economic Status (SES)
Control subjects who met criteria for current or past history of substance abuse or dependence were excluded from the study. Patients, however, were not excluded from the study unless criteria were met for current (i.e., within the past month) abuse or dependence except for 6 patients who were found to meet criteria for current abuse after the study data was collected. This information is clearly marked in the shared clinical data.
Both patients and controls were excluded if they had 1) an IQ less than 70 based on a standardized IQ test, 2) history of a head injury resulting in prolonged loss of consciousness, neurosurgical procedure, neurological disease, history of skull fracture, severe or disabling medical conditions, or 3) a contraindication for MRI scanning such as pregnancy, metal in body or head including implanted pacemaker, medication pump, vagal stimulator, deep brain stimulator, implanted TENS unit, or ventriculo-peritoneal shunt.
Clinical teams at each of the sites recruited the patients. Primary treating clinicians, not necessarily involved with the study, identified potential patients who met inclusion/exclusion criteria and were competent to give informed consent. Healthy control subjects were recruited from the community at each of the sites through posters, brochures, newspapers, website advertisements, and institutional subject recruitment resources. All subjects provided informed consent to participate in the study that was approved by the human research committees at each of the sites (UNM HRRC #03-429; UMinn IRB #0404M59124; MGH IRB# 2004P001360; UIowa IRB #1998010017). In addition to the informed consent, all patients successfully completed a questionnaire verifying that they understood the study procedures.
Clinical Assessments
The comprehensive battery of clinical assessments used in this study is detailed in Supplemental Table 1. Experienced clinicians from all sites who performed the diagnostic interviews completed two days of in-person training and testing using videotaped training materials with “gold standard ratings” to establish cross-site, inter-rater reliability. All staff obtained a greater than 85% match with the gold-standard on a minimum of 3 videos.
Core demographic information collected includes age, handedness, height, weight, ethnicity, race, educational achievement, parental education, and socioeconomic status (Table 2). A Structured Clinical Interview for DSM-IV (SCID/SCID-NP for controls)(First) or the Comprehensive Assessment of Symptoms and History (CASH) were used to diagnose primary and co-morbid psychiatric disorders in controls and patients (First); (Andreasen et al. 1992). Additionally, patients were interviewed with the Scale for Assessment of Negative Symptoms (SANS) (Andreasen 1983), Scale for Assessment of Positive Symptoms (SAPS) (Andreasen 1984) and the Calgary Depression Scale for Schizophrenia (CDSS) (Addington et al. 1992) to record symptoms and their current severity.
Extrapyramidal side effects caused by medication were evaluated using the Simpson-Angus Scale (SAS) (Simpson and Angus 1970), Barnes Akathisia Scale (BAS) (Barnes 1989) and Abnormal Involuntary Movement Scale (AIMS) (Health 1976). The Psychiatric Symptoms You Currently Have (PSYCH) (Andreasen 1987) assessment and CASH were used to capture additional information about lifetime diagnoses and durations, treatment (including medications) and the course of symptoms. These two assessments were instrumental in determining illness duration (ID) discussed below. Every effort was made to collect all clinical data within a 14-day window around the time of image acquisition.
Calculation of antipsychotic drug exposure
During the clinical interview, study clinicians collected information from patients on their current and past history of antipsychotic and other drug usage; including the names of the drugs taken, the dosage of each drug, and the timeline of usage. All antipsychotic dosages were converted into Chlorpromazine (CPZ) units using drug equivalency conversion factors (Andreasen et al. 2010) (Table S2). To calculate cumulative dose years, the following formulas were applied:
For current antipsychotic exposure, we converted all current doses into chlorpromazine units, using the formula below:
When calculating the number of days on the dose, any gap time in which the subject reported that they discontinued taking that drug or changed to a different dose of the drug was not included in the final number. 365.25 days/year is used to account for leap-years. Finally, all antipsychotics taken by each patient were then quantified separately for each of three categories: Typical, Atypical, and Clozapine. This categorization was necessary because different types of antipsychotic medication (atypical versus typical) have been reported to affect brain structure and function differently and estimating the contribution for each type may help tease apart these effects (Ehrlich et al. 2011a; Navari and Dazzan 2009; Smieskova et al. 2009). The number of dose years for each drug taken within each category was then summated to calculate the total dose years:
Calculation of Illness Duration measure
Special attention was paid to developing a useful metric for illness duration because our cohort included a large number of patients who were very early in the course of their schizophrenic disorder. It is well known that illness onset is not abrupt, but rather has a variable course that can be precipitous or indolent. Additional complexity is introduced by the highly variable delay between symptom onset and treatment seeking. For this data set, the illness duration (ID) measure was calculated using the following heuristic: For patients who had available diagnostic dates (n=144), the ID measure represents the length in years between the date of diagnosis and the baseline MRI scan date. For patients who only had treatment dates available (n=6), the measure represents the length in years between the date they were first treated for psychosis and the date of their baseline MRI scan. Finally, for patients who were not yet diagnosed and/or never treated, for whom we only had the dates when they first experienced psychotic symptoms (n=9), the measure represents the length in years between the date when they first experienced symptoms and their baseline MRI scan. For the patients in this final category, only psychotic symptoms with a severity greater than 3 (moderate severity) as determined by the rating scale on the PSYCH instrument were used. The ID measure is not available for 3 patients due to insufficient data.
Neuropsychological Metrics
The cognitive assessments selected for this study were chosen based on: ease of administration and scoring, good reliability of scoring, balancing the needs for completeness and brevity, and repeatability (Table S1). Trained psychometrists, supervised by neuropsychologists, conducted the assessments. For additional reliability, the neuropsychologists from each of the sites participated in standardization training in-person. Cognitive measurements were collected at the baseline visit, along with the other demographic and clinical data. For schizophrenia patients early in the course of their disease, the administration of these assessments was delayed until the individual was stabilized on medication in an effort to evaluate the best possible performance.
General cognitive abilities and achievement were measured using Block Design, Vocabulary, and Similarities subtests of the Wechsler Adult Intelligence Scale – Third Edition 16 (WAIS-III) (Wechsler 1997a) and the Reading subtest of the Wide Range Achievement Test – Third Edition (WRAT-3) (Wilkinson 1993). The Delis-Kaplan Executive Function System 18 (D-KEFS) (Delis 2001) assessed fluency using verbal and category fluency tests. The Benton Visual Retention Test (BVRT) (Sivan 1992) and the Face Recognition subtest of the Wechsler Memory Scale-III (WMS-III) (Wechsler 1997b) both measured visual memory. Verbal memory and learning were identified using the Logical Memory Test of the WMS-III (Wechsler 1997b) and the Hopkins Verbal Learning Test-Revised (HVLT) (Brandt 1991). The Letter-Number Sequencing subtest of the WAIS-III measured working memory. A computerized version of the Tower of London test (TOL) (Shallice 1982) was employed to measure planning and problem solving. The Grooved Pegboard Test (Ruff and Parker 1993)identified fine motor dexterity and speed. The Annett Scale of Hand Preference (Annett 1970) provided a numeric measure of handedness. The California Computerized Assessment Package (CalCap) (Miller 1990) a computerized reaction time test and trails A and B of the Trail Making Test (TMT) (Reitan 1958) measured processing speed during attention and working memory tasks.
Genetics
Blood samples were obtained from each participant and sent to the Harvard Partners Center for Genetics and Genomics (HPCGG) for DNA extraction. All DNA extraction and genotyping was done blinded to group assignment. Genotyping on the blinded DNA was performed at the MRN (Mind Research Network) Neurogenetics Core Lab using the Illumina HumanOmni-Quad BeadChip. Approximately 200 ng of DNA was used to genotype each subject sample according to the manufacturer’s protocol (Illumina, San Diego, CA). After amplification, fragmentation and hybridization, the specifically hybridized DNA was fluorescently labeled by a single base extension reaction and detected using an iScan scanner. Non-specifically hybridized fragments were removed by washing while remaining specifically hybridized DNA were processed for the single base extension reaction, stained and imaged on an Illumina iScan Reader. Normalized bead intensity data obtained for each sample were loaded into the GenomeStudio2010.1 software that generated SNP genotypes from fluorescent intensities using the manufacturer’s default cluster settings. The raw genotypic data were imported in a genome-wide data management system (Laboratory Information Management System, LIMS) to allow the tracking of individual samples, quality control and the export of user defined formats compatible with the genetic programs used for the statistical analysis.
Genetic quality assurance
Quality control procedures for SNPs included removal of SNPs that had a genotyping rate of less than 90% or failed the Hardy-Weinberg test with P < 1×10−6, which often indicates genotyping errors. No SNPs failed these tests and total genotyping rate was 99.7%. We did not set a minor allele frequency threshold commonly used to limit false positives and prevent a loss of power (Tabangin et al. 2009), because we did not analyze the effect of individuals SNPs but the combined effect represented by a genetic risk score.
Quality control for subjects included removal of subjects that had a genotyping rate of less than 90%. Two subjects were therefore excluded from further analysis. In two other cases, where the genotyping rate was above 90%, but less than 100%, missing values were replaced with the group mean. These genetic data will not be publically available due to consortium agreements and IRB restrictions; however, now that the MCIC data is released, researchers may contact the consortium with a proposal requesting collaborative use of the genetic data.
Cross-site Standardization of Imaging Procedures
The principal investigators and several collaborators at each of the participating sites are also members of the BIRN consortium and were part of multi-site MRI calibration studies conducted by the Morphometry BIRN (mBIRN) and Function BIRN (fBIRN) in the years immediately preceding and during data collection (see for example (Friedman and Glover 2006; L. Friedman et al. 2008; Han et al. 2006; Jovicich et al. 2006; Glover et al., in press). Lessons learned from those studies were applied to reduce disparities in the experimental set-up, data acquisition methods and scan sequences used for the study presented here. In particular, all sites had matched button press devices for collecting behavioral data, followed common audiovisual set-up calibration methods and paid particular attention to centering each subject’s head in the scanner bore to minimize gradient distortion effects. Sequence parameters such as bandwidth and echo spacing were optimized at each site for the best quality images. For the fMRI acquisitions, synchronization of the stimulus onset with the scan start was improved. In addition, the sites followed the quality assurance procedures recommended by the BIRN to ensure scanner stability (Friedman and Glover 2006; L. Friedman et al. 2008). Adopting these procedures effectively minimized the variability between the participating sites (Yendiki et al. 2010). Specifically for the fMRI data collected in the preceding calibration study using the same SIRP task paradigm as this study, in which a cohort of healthy human subjects (n = 10) traveled to the four sites for test-retest scan sessions, the variance between sites was found to be an order of magnitude lower than between subjects when using the average percent signal change within a defined ROI as an index (Yendiki et al. 2010).
Scanner Acquisition Parameters
For each subject (patients and healthy controls), structural, DWI, and functional MR data were collected. In most cases, two scan sessions were required to collect the complete imaging dataset.
All imaging data were collected using scanners with field strengths of 1.5T or 3.0T and using closely matched acquisition sequences. Site A utilized a 1.5T Siemens Sonata and site C used a 3T Siemens Trio. At site D, all structural and DWI data were acquired using a 1.5T Siemens, while all fMRI data were acquired on a 3T Siemens Trio. Site B acquired all structural data using a 1.5T GE Signa and all functional and DWI data using a 3T Siemens Trio.
Subjects were instructed to get a typical night’s sleep before each scan, not to drink more than one alcoholic beverage, and to abstain from drinking coffee within 2 hours preceding each scan.
Structural Scans
During each structural imaging session, coronal T1 and T2 scans were collected. Imaging parameters for the T1 scans were: TR=2530 ms for 3T, TR=12 ms for 1.5T; TE=3.79 ms for 3T, TE=4.76 ms for 1.5T; FA=7 for 3T, FA=20 for 1.5T; TI=1100 for 3T; Bandwidth=181 for 3T, Bandwidth=110 for 1.5T; 0.625×0.625 mm voxel size; slice thickness 1.5 mm; FOV 256×256×128 cm matrix; FOV=16 cm (could be increased to 18cm when needed for full brain coverage). T1- weighted scans took approximately 13 minutes each and 2-3 volumes were acquired. The instructions to the MR tech were to collect two good quality scans, so if they observed that one of the scans was of poor quality due to subject motion or a scan artifact, they repeated it. An additional T1-weighted with isotropic voxels was collected at site D only. All T1 MRI data were collected using an 8 channel head coil, except site A used a CP head coil. All scans for each subject are included in the shared data set. No notation is provided regarding data quality, as each user will have his/her own definition.
T2-weighted whole brain scans were acquired using a coronal TSE sequence with the following parameters: TR=10000 ms for 3T, TR=9000 ms for 1.5T; TE=14 ms for 3T, TE=64 ms for 1.5T; FA=149 for 3T, FA=180 for 1.5T; Bandwidth=193 for 3T, Bandwidth=149 for 1.5T; voxel size same as for T1 scans; NEX=1 for the 3T, NEX=2 for the 1.5T. Acquisition of each volume took approximately 14.5 minutes. Each site acquired two volumes.
DWI Scans
All DWI data were acquired with a slice thickness of 2 mm with a 2 mm isotropic resolution. Imaging parameters for the DWI scans at site A were as follows: TR = 9800 ms, TE = 86 ms, B values of 0 and 1000, NEX = 4, bandwidth=1502, 64 slices, and 12 directions. DWI scans at site B had the following parameters: TR = 9500 ms, TE = 90 ms, B values of 0 and 1000, NEX = 4, bandwidth=1954, 64 slices, and 6 directions. Site C used the following parameters: TR = 10500 ms, TE = 98 ms, B values of 0 and 1000, NEX = 2, bandwidth=1342, 64 slices, and 12 directions. Scanning parameters at site D were as follows: TR = 8900 ms, TE = 80 ms, B values of 0 and 700, NEX = 1, bandwidth=1860Hz/pixel, 60 slices, and 60 directions. The time of acquisition at sites A, B, and C was approximately 5 minutes. The acquisition time at site D was approximately 8 minutes.
Acquisition parameters for fMRI data
For all sites prospective acquisition correction (PACE) corrected (van der Kouwe et al. 2006), whole-brain, single-shot EPI data parallel to the AC-PC line (in-plane resolution 3.4mm, 27 slices, slice thickness=4 mm, 1mm skip, slice order interleaved, TE=30msec for 3T, TE=40msec for 1.5T, TR=2sec, FA=90, FOV=22cm, 3DDAs (dummy data acquisition), bandwidth=3126Hz/pixel) were acquired. Siemens auto align was used during the scanning session at site D.
Functional Imaging Procedures
Prior to fMRI scanning, all subjects participated in a “mock scanner” session during which time they were acclimated to the scanner environment, and trained to perform four fMRI tasks: Sternberg item recognition paradigm (SIRP) (Sternberg 1966), a sensory motor task (SM), an auditory oddball (AOD) and a breath hold task. Subjects were asked to practice these tasks until they were comfortable with the tasks and the scanning environment. For the SIRP, subjects were trained to perform the task on a computer prior to the first scan session to verify that they achieved a greater than chance performance.
Each site used an identical custom-manufactured magnet compatible input device for button presses (http://www.mrn.org/collaborate/imaging-equipment/) with custom cables and amplifiers to record subjects’ responses. During the fMRI tasks, subjects were instructed to press buttons under the appropriate digits to make a response. The input device was connected to a laptop running E-Prime software (EPrime v1.1, Pittsburg, PA), which was used to present visual stimuli to subjects and record their responses.
Following the recommendations of the fBIRN working group, the luminance of the projector systems and the intensity of the audio systems were calibrated to ensure that conditions remained consistent across scan sessions and sites. The intensity and left-right balance of the auditory stimuli were also calibrated to ensure that all subjects could adequately perceive the tones presented during the AOD and SM tasks.
Three runs of the SIRP working memory task each comprised of 177 time frames (5 minutes 54 seconds per run) were collected. Four runs of the AOD each comprised of 96 time frames (3 minutes 12 seconds per run) were collected. One breath hold scan and 2 SM runs, each comprised of 120 time frames were also collected each for 4 minutes per run.
Sternberg Item Recognition Paradigm (SIRP)
The SIRP elicits activation in a network of brain areas associated with working memory and has been used by many investigators to characterize working memory deficits in schizophrenia patients (e.g., (Manoach et al. 2000; Manoach et al. 1999; Ragland et al. 2007)). Variants of the SIRP paradigm were also used in many of the fBIRN studies (e.g., (Brown et al. 2009; Potkin and Ford 2009; Potkin et al. 2009)).
In this version of the SIRP task, during each scan the subject had to retain in memory a set of 1, 3 or 5 digits providing a parametrically varying range of task difficulty. First the subject was prompted by the word “Learn” for 1.5 seconds (prompt condition), followed by a blank screen for 0.5 seconds. Then all the targets (digits to be retained in working memory) were presented together in red font for 6 seconds (encode condition). The subject was then shown a sequence of probe digits in green font and had to indicate whether each probe digit was a target or a foil, i.e., whether it was a member of the memorized set or not (probe condition). The red and green font colors were used as supporting cues to facilitate conceptual understanding of the task. The probe condition lasted 38 seconds. Each probe digit was presented for up to 1.1 seconds in a pseudo-randomly jittered fashion within a 2.7 second interval. 14 probe digits in each block were presented, of which 7 were targets and 7 were foils, for a total of 84 probes per scan. Subjects were instructed to respond with a right-thumb button press on the input device if the probe digit was a target and a left-thumb button press if it was a foil. Subjects were instructed to respond as quickly and as accurately as they could. They were told that they would receive a bonus of $0.05 for every correct response.
Six 46 second working-memory (WM) blocks consisting of the prompt-encode-probe conditions were run in each scan, two at each set size (i.e., Probe 1, Probe 3, Probe 5) in a pseudorandom order. WM blocks alternated with blocks of fixation. The durations of the fixation blocks were random integer multiples of 2 seconds, chosen so that the total duration of all fixation blocks within a scan was 78 seconds. The digits that comprised the memory sets for each of the 3 scans were varied to eliminate learning effects. The target digits presented in each block were randomly chosen integers between 0 and 9, with no digit repeated within a single set. The order of targets and foils within a probe epoch was random, but no more than 3 consecutive digits could be targets. Each of the target digits presented during the encode epoch had to be presented at least once during the probe epoch. When the set presented during the encode epoch consisted of 3 target digits, each target digit had to be presented at least twice during the probe epoch.
Sensory Motor Task (SM)
The MCIC investigators developed this paradigm to robustly activate the auditory and somatosensory cortices. To accomplish this, subjects, keeping their eyes closed for the duration of the scan, were presented with a series of bi-aural audio tones of varying frequencies at irregular intervals and were asked to press a button in response to each of the stimuli as quickly as they could.
Sound stimuli were incorporated into E-Prime scripts for stimulus presentation. Auditory stimuli were delivered using sound insulated earphones (Avotec, Stuart, FL) to present stimuli to the subject and shield them from noise due to MRI gradient coil vibration. Prior to the SM scans, a test scan was performed to calibrate the volume of the auditory stimuli, ensuring that all test subjects were able to hear the tones comfortably over the background noise of the scanner. Consequently, the volume of the tones varied depending on the subject’s degree of hearing during the audio setup for the task, thus minimizing any auditory signal differences among groups with different hearing capabilities.
Subjects were presented with a sequence of auditory stimuli consisting of 16 different tones, ranging in frequency from 236 Hz to 1318 Hz and each lasting 200 msec. The first tone presented was set at the lowest pitch. Each tone that followed was at a higher pitch than the previous, creating a stair-step pattern of tones that rose to a peak, followed by a symmetric descent. Tones were presented with an approximately 500 msec inter-stimulus interval. The subject was instructed to press the right thumb button of the input device after hearing each individual tone. This pattern of ascending and descending scales continued for a duration of 16 seconds, comprising one auditory block. We alternated 15 auditory blocks with 15 fixation blocks, each also 16 seconds in duration. The first block of each scan was a fixation block.
Auditory Oddball (AOD)
Sound stimuli were identical to those used in Kiehl & Liddle (Kiehl and Liddle 2001), which found robust differences between patients with schizophrenia and healthy control subjects (Kiehl et al. 2005a), but with different presentation times and sequences to optimize the sensitivity of BOLD fMRI analysis.
Sound stimuli were incorporated into E-Prime scripts for stimulus presentation. A randomized event-related design was used. Standard stimuli were 1 kHz tones and occurred with a probability of p=0.82. Target and novel stimuli were infrequent and each occurred with a probability of p=0.09. Target stimuli were 1.2 kHz tones and novel stimuli were computer generated, complex sounds. The sequences for target and novel stimuli were exchanged between runs to balance their presentation. This ensured that responses were not influenced by different sequences used for different stimuli. Subjects were asked to respond by pressing a button with their right index finger every time they heard a target stimulus and not to respond to either standard tones or novel computer generated sounds. Each run was comprised of 90 stimuli and each stimulus was presented with a pseudorandom order with 200 msec duration. The inter-stimulus interval changed randomly in the interval 550–2050 msec with a mean of 1200 ms.
Presentation sequences were devised in order to produce orthogonal BOLD responses among the three stimulus types, using the method of Clark (Clark 2012; Clark et al. 2000). Briefly, a large number of random stimulus sequences were generated. Sequences with a low correlation of predicted BOLD responses among the three stimulus types (∣r∣ < 0.2) were used. This allows the BOLD response evoked by each stimulus type to be tested independently using multiple regression analysis.
Breath Hold Task
The breath hold task paradigm was designed to elicit a metabolic “activation” of all cortical gray matter by transiently elevating the partial pressure of CO2 thereby stimulating cerebral blood flow and thus mimicking the effects of neuronal activation as detected in the BOLD signal (Huettel 2009). The task consists of a block design with 15 alternating on/off blocks of 16 seconds periods of breath holding and normal breathing. During the off-block, the subject was shown a green screen during which they were instructed to breathe normally. During the last 2 seconds of the off-block, the screen became yellow, signifying to the subject that they should take a deep breath in and prepare for the breath hold. During the on-block, the subject was shown a red screen, during which time they were instructed to hold their breath. The subject resumed breathing when they saw the screen turn green again. Colored screens that provide these cues were presented with E-Prime programming software.
Database creation and Data Entry
At enrollment all MCIC subjects were assigned a randomly generated unique 9 digit subject identification number that included a site identifier. This ID was used to track subjects throughout the data collection and data entry processes. However, for purposes of sharing the MCIC dataset, each subject ID has been converted into a number (1 – 331) and each site has been converted into a letter to reduce the risk of privacy violation for the subjects.
All clinical data (diagnostic and symptom assessments, neuropsychological testing and fMRI paradigm results) were double entered into COINS by two separate operators. Any data entry conflicts between the two records were flagged by the database and resolved by a third individual (Bockholt et al. 2010).
Results and Significance
Different analysis methods can be more or less rigorous regarding the data quality requirements, so here we provide examples of the quality assurance (QA) metrics from published or in-house MCIC analyses of the data that have been completed. We also provide a brief summary of the characteristics of and findings available to date from the data for reference and to guide interested users in what to expect from the data.
Clinical Data
The SCID and CASH were used to determine schizophrenia spectrum diagnoses in patients, as well as past and current co-morbid disorders, such as cannabis abuse/dependence, alcohol abuse/dependence, anxiety and mood disorders. The most prevalent current and past co-morbid diagnoses were substance abuse/dependence (n= 54) and mood disorders (n= 30). While recruitment aimed at enrolling patients with schizophrenia, patients were also enrolled with schizoaffective disorder (n= 6) and schizophreniform disorder (n= 7) diagnoses. Although there are three patients with diagnostic codes that indicate schizophreniform disorder, they all later converted to schizophrenia. Five participants, classified as patients, do not have a formal schizophrenia spectrum diagnosis due to loss of contact before the SCID or CASH were administered.
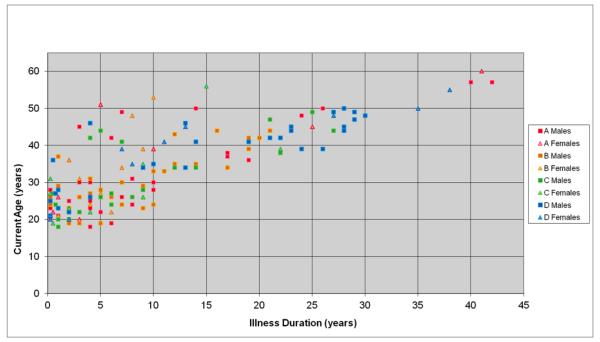
The SANS and SAPS instruments were used to determine the severity of symptoms in patients. See Table 3 for the mean and standard deviation for total negative global symptoms (affect, alogia, avolition, and anhedonia), positive global symptoms (delusions and hallucinations), and disorganized global symptoms (positive formal thought disorder and bizarre behavior) for each site. The table also displays the mean and standard deviation for the Calgary Depression Scale and for the Illness Duration (ID) for each site. The ID range was 0.25 to 42 years. The majority of the patients’ ID data came from their years since diagnosis (90.57%) and far fewer had only first symptom information (5.03%) and treatment information (3.77%). See Figure 1 for ID distribution and Methods for detailed explanation.
Table 3.
Mean and standard deviation for clinical data
| Illness Duration (ID in years) |
Total Positive Symptoms |
Total Negative Symptoms |
Total Disorganized Symptoms |
Calgary Depression |
|
|---|---|---|---|---|---|
|
All Sites
m ± sd |
10.67 ± 10.03 | 4.96± 2.77 | 8 ± 3.91 | 1.78± 1.89 | 3.88± 4.68 |
|
Site A
m ± sd |
9.35± 11 | 5.06 ± 2.66 | 7.92 ± 3.49 | 1.56 ± 1.7 | 5.89± 5.61 |
|
Site B
m ± sd |
9.69± 7.32 | 4.98 ± 2.89 | 9.59± 4.16 | 2.41 ± 1.88 | 2.55 ± 4.14 |
|
Site C
m ± sd |
9.26± 8.73 | 5.09 ± 2.26 | 7.44± 2.9 | 1.75 ± 2.08 | 3 ± 2.48 |
|
Site D
m ± sd |
14.71 ± 11.88 | 4.71 ± 3.24 | 6.74± 4.4 | 1.34 ± 1.86 | 3.68 ± 4.79 |
Figure 1.
Relation between age and Illness Duration (ID) by site and sex. As would be expected, duration of illness and age are closely related. The scatterplot graphically illustrates that the cohorts enrolled at each of the four centers had highly overlapping age, sex and illness duration distributions.
The heterogeneity of symptoms in psychotic disorders always presents a challenge in clinical work as well as clinical based research. It was important to capture, with as many measures as possible, the severity as well as duration of symptoms experienced. This study attempted to create a quality clinical framework from which the imaging and neuropsychological data could build. Interactions between clinical data and the other modalities have been included in most of the publications that have come from this dataset (see References marked with an *).
As with all clinical imaging studies, this dataset is not without limitations. Schizophrenia is not a static disorder and the presentation of the illness, both in terms of expressed symptoms and severity, can vary over time. This cross sectional dataset is from a single time point. It is reasonable to expect that some of the findings would change (in particular in patients with a shorter ID) if the study were longitudinal. First episode patients were included in the patient group with a diagnosis of schizophreniform disorder because it was too soon in the course of their illness to make a schizophrenia diagnosis.
In part because this was a multi-site study, incomplete and missing data were hard to reconcile at time of data collection requiring sophisticated methods to generate key variables for every subject. For instance, classifying the ID proved to be a challenge because diagnostic information gathered from the patients was not always consistent or easy to obtain, especially in the early onset subjects. Although creating the ID heuristic was complicated, the ability to derive the same information through multiple methods resulted in a value with high reliability. The ID has been useful in several publications. For example, it was used to help parse out the differences in fractional anisotropy between first-episode and chronic patients with schizophrenia in White et al. (White et al. 2009). Since chronic patients showed lower fractional anisotropy compared to the first-episode patients, it was unclear if this was related to medication exposure or ID, two measures that are highly correlated (r = 0.69). Thus, a group was randomly selected that had a narrow range of ID, but a broader range of medication exposure. In turn, a group was randomly selected that had a narrow range of medication exposure, but a larger range in ID. These analyses, in combination with partial correlations, gave support to the hypothesis that dose years of medication played a greater role in the fractional anisotropy differences as compared to ID.
Neuropsychological Metrics
Our recently published comprehensive analyses of the neuropsychological indices arbitrarily separated schizophrenia patients into first-episode and chronic groups, and the control subjects into two groups comparable to the patient groups to investigate course of cognitive dysfunction from onset to the chronic phase of schizophrenia (Sponheim et al. 2010). All groups had similar parental socioeconomic status. The controls for chronic schizophrenia patients had a higher percentage of females, thus sex was specified as a factor in analyses of neuropsychology indices. Both schizophrenia groups had significantly lower reading achievement (regarded as an estimate of premorbid intelligence), suggesting that schizophrenia patients may have had some cognitive impairment prior to illness onset. Not surprisingly, chronic schizophrenia patients had fewer years of education than their control subjects, and both schizophrenia groups had lower socio-economic status than their respective control groups. There were interactions of diagnosis and cohort for socio-economic status, F1,281 = 6.35, p = .01, and years of education, F1,276 = 4.30, p = .04, indicating that socio-economic and educational disadvantages were greater in chronic schizophrenia than among those with recent-onset schizophrenia. ANOVA’s failed to reveal any differences in symptom dimensions between recent-onset and chronic schizophrenia samples. An interaction of sex and cohort, F1,143 = 4.33, p = .04, for negative symptomology resulted from recent-onset male patients exhibiting more negative symptoms than male chronic patients, while female patients showed the opposite pattern across recent-onset and chronic cohorts.
To examine whether ID was related to cognitive functions in schizophrenia partial correlations were computed between ID and cognitive variables with age as a covariate (to address effects of normal aging). The analysis included 147 schizophrenia patients and revealed several significant but small associations. After removing variance related to age, ID was associated with several measures of episodic memory. Longer duration of disorder was associated with worse scores (r=−.21, p<.01) and more errors (r=.21, p<.01) on the Benton Visual Retention Test, worse retention of verbal material on Logical Memory (r=−.26, p<.01), and lower total recall of words on the Hopkins Verbal Learning Test in immediate (r=−.20, p<.05) and delayed (r=−.23, p<.01) conditions. Longer ID was also associated with slower completion of the grooved pegboard test of fine motor movements with the dominant (r=.28, p<.01) and nondominant (r=.26, p<.01) hands, worse performance on indices of executive functioning (Letter-Number Sequencing [r=−.19, p<.05], excess moves on the Tower of London test [r=.20, p<.05]), and worse performance on the Vocabulary subtest of the WAIS-III (r=−.21, p<.01). The associations with ID are consistent with the greater motor and problem solving deficits noted in chronic patients as compared to recent-onset patients, but also point to the possibility of slight progression of episodic memory deficits in schizophrenia. When antipsychotic dosage and age were entered as covariates, no associations with ID showed a change in statistical significance and the values of the Pearson correlations were essentially unchanged. Complete results of analyses of neuropsychological indices are reported in Sponheim et al., Journal of Psychiatric Research, (Sponheim et al. 2010).
Our data demonstrates that schizophrenia patients have deficits across a broad set of cognitive measures that were not attributable to limited premorbid functioning. Deficits were largely independent of sex and site of data collection. Length of illness in the schizophrenia sample showed small but significant associations with worse performance on several indices of episodic memory, but the most variance accounted for by any index was only 6.7. We found marginal evidence of progressive declines in the efficiency of problem solving, fine motor dexterity, and episodic memory in advanced phases of the disorder. For problem solving and memory indices, the greater dysfunction observed with longer disorder duration appeared unrelated to medication status, symptomatology, and clinical status, and therefore may reflect changes in prefrontal cortex over the course of the disorder.
Because longitudinal data were not available, we could not directly examine the course of cognitive deficits in schizophrenia; however, the inclusion of schizophrenia patients with a wide range of length of illness and similarly aged controls allowed for testing the association of cognitive deficits with duration of disorder. Although longitudinal studies do provide a direct test for change in cognitive functions over the course of the disorder, they too have limitations such as variability due to reliance on small control samples, patients being lost to follow up, short observational periods of only one to two years, poorly controlled practice effects, and variation in use of first and second generation antipsychotic medication. Thus, this data set provides a valuable opportunity to further investigate the functional and structural neuroimaging correlates of the cognitive deficits evident across a broad set of domains in schizophrenia that are largely unrelated to duration of illness.
Antipsychotic Drug Exposure
Current and lifetime antipsychotic drug treatment status and antipsychotic class taken were characterized by diagnosis and site. The information is summarized for 161 of the 162 patients, including 7 patients for whom no data could be obtained. Table S3 shows how many patients used each type of atypical or typical therapy by site. In this study, most patients were being treated with a single antipsychotic drug and at all sites more patients were on atypical monotherapy (109/161 patients). There is an even distribution of atypical usage across sites, with very few patients on combination therapy (12/161 patients). Only 13 patients were on clozapine monotherapy. Nine of the patients were not currently on medication, but 2 of those have a prior pharmacotherapy history. The cumulative dose year exposure to typical, atypical, clozapine or total antipsychotic drug exposure didn’t differ by site at the p < 0.05 level on the basis of multiple regression analysis, controlling for either age or length of illness.
Structural Imaging Data
The brain volumes from the structural imaging data have been successfully segmented and analyzed using a variety of techniques, as detailed in Segall et al., 2009 and Ehrlich et al. 2009, 2011a, and 2011b. Additionally, the anatomic segmentations have been used to generate individually specific regions of interest for our fMRI studies (e.g. (Roffman et al. 2008)). Using voxel-based morphometry methods as in Segall et al., a small number of outliers were detected using a Pearson correlation with the average smoothed gray matter map, and removed if they could not be corrected. The occasional dataset required reorientation in order to segment properly. The effect of site was included as a factor in the regression model (Segall et al. 2009). The results showed similar gray matter concentration loss across frontal, temporal, and insular lobes as has been previously reported in the literature (Goldman et al. 2009; Kuperberg et al. 2003; Narr et al. 2005; Nesvag et al. 2008; Rimol et al. 2012; Schultz et al. 2010) and importantly we found there was no interaction between site and diagnosis. While site effects are significant as would be expected due to pooling data from both 1.5T and 3T scanners (Jovicich et al. 2009), the trend for subjects with schizophrenia to have reduced gray matter measures is maintained across sites.
Structural MRI data from the T1 weighted volumes were also co-registered, motion corrected, averaged and analyzed in an automated manner with atlas-based FreeSurfer software (http://surfer.nmr.mgh.harvard.edu, Version 4.0.1) (Dale et al. 1999; Desikan et al. 2006; Fischl and Dale 2000; Fischl et al. 2002; Fischl et al. 1999a; Fischl et al. 1999b; Segonne et al. 2005). Segmentation and surface reconstruction quality were assured by manual inspection of all raw MRI volumes, segmented volumes in three planes and pial as well as inflated volumes. The automated segmentation procedure failed in 5 subjects due to excessive motion artifacts. Furthermore, 11 participants’ MRI data failed the manual inspection. The data of 9 of the 11 subjects were then recovered with minor manual intervention following the FreeSurfer user guidelines. Comparison of grey matter volumes of pre-specified regions of interest from the subjects that passed quality assurance were performed using multiple regression models controlling for estimated intracranial volume (ICV) and acquisition site(Buckner et al. 2004; Fennema-Notestine et al. 2007; Reuter et al. 2010). The resulting a rater-independent segmentation volumes and cortical thickness measures have been used to test specific pathophysiological hypotheses (Brauns et al. 2011; Ehrlich et al. 2011a; Ehrlich et al. 2010; Ehrlich et al. 2011b) and more underway). We were also able to replicate previously published structural brain abnormalities in patients with schizophrenia.
For instance, we found that hippocampal volume in healthy controls was lateralized with a right greater than left asymmetry noted within each site (F=36.1, df=1, p<0.001, Figure 2). Schizophrenia patients had comparable hippocampal asymmetry (F=33.2, df=1, p<0.001), but, were noted to have consistently smaller hippocampal volumes bilaterally (left hippocampus: β=−107.1, df=315, p=0.008 and right hippocampus: β=−138.9, df=315, p=0.002) as has been previously reported (Adriano et al. 2012; Rimol et al. 2010; Watson et al. 2012). Overall we found a 4.17 % reduction in hippocampal volumes in patients with schizophrenia compared to healthy controls. The effects of acquisition site on hippocampal volumes were significant (left hippocampus: F=5.663, df=315, p=0.001 and right hippocampus: F=6.158, df=315, p<0.001). These results are consistent with a large body of previous work (Adriano et al. 2012; Honea et al. 2005; Nelson et al. 1998; Rimol et al. 2010; Watson et al. 2012; Wright et al. 2000) reporting decreased hippocampal volume in patients with schizophrenia. Furthermore our analyses confirmed the normative asymmetry between left and right hippocampus (R > L) for patients and controls. Age was negatively correlated with hippocampal volumes bilaterally (left hippocampus: β=−5.48, df=315, p=0.004 and right hippocampus: β=−7.62, df=315, p<0.001), also a finding that has been well established in the literature (Fennema-Notestine et al. 2007; Head et al. 2005)
Figure 2.
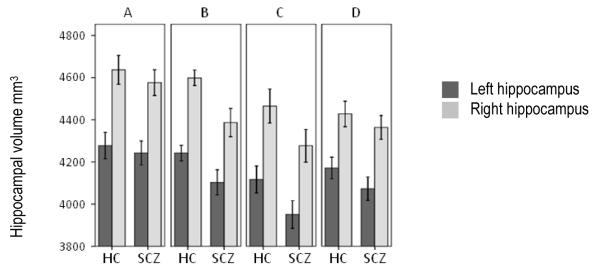
Freesurfer segmented right and left hippocampal volumes plotted by diagnosis for each acquisition site separately. The volumes are controlled for intracranial volume using the covariance method (unstandardized residuals added to sample mean) (O’Brien et al. 2006). HC, healthy controls; SCZ, schizophrenia patients.
Further, in line with previous research (and consistent with our published VBM results (Segall et al. 2009) we found substantially reduced cortical thickness in frontal, temporal, occipital, and parietal areas in patients with schizophrenia compared to healthy controls (Goldman et al. 2009; Kuperberg et al. 2003; Narr et al. 2005; Nesvag et al. 2008; Schultz et al. 2010).
Although we followed the best practice guidelines for multi-site MR acquisition by the Biomedical Informatics Research Network (http://www.nbirn.net) to minimize site differences, effects of acquisition site were found. This is not unexpected given the sensitivity to quantitative morphometric measures to differences in field strength (e.g. (Jovicich et al. 2009). However, previous multi-site studies have shown that scanner-related effects, regardless of magnitude, do not preclude pooling of the data, as long as these effects are adequately accounted for in the analyses (Segall et al. 2009; Stonnington et al. 2008).
Diffusion Weighted Imaging Data
Since DWI was relatively new at the start of the study, and sites varied widely in the DWI sequences available, the MCIC leadership decided to use the best available acquisition sequences at each site rather than calibrating to the “lowest common denominator”. This created challenges in approaches to combine data from different scanners with different field strengths and sequence parameters (White et al. 2009). While these differences in scanners, sequences, and field strength have been thought to be a limitation in the diffusion-weighted analyses, it could also be considered a strength of the study since strong and potentially clinically relevant findings should be platform independent. These differences thus provide a mechanism to assess abnormalities in patients with schizophrenia that are either (1) less dependent on these parameters or (2) when the dependencies on these parameters can be assessed and addressed allowing for the pooled data to be analyzed. In addition, the site-related differences in sequences allowed subsets of the data to be used for more intensive analyses than would otherwise have been possible (Yendiki et al. 2011).
There are known artifacts common to DWI sequences that can be partially corrected using image processing algorithms (Le Bihan et al. 2006). Examples of these include eddy current artifacts, susceptibility artifacts, ghosting, and motion. Whereas artifacts related to susceptibility, eddy currents, and minor motion can be partially corrected, excessive motion and spiking may render an image unusable. All DWI data from the MCIC study was evaluated for quality. Out of 331subjects participating in the MCIC study cohort, 42 individuals did not have a DWI scan collected. Of the 289 available DWI scans, up to 13% had artifacts, such as incomplete brain coverage (~ 2%), spiking or excessive movement (~ 6%), or difficulties registering the image with the high-resolution T1 image (~ 5%). A detailed description of data quality and site related differences are provided in White et al. (White et al. 2009; White et al. 2011).
The MCIC DWI data set is one of the largest sets currently collected in patients with schizophrenia (White et al. 2008). In addition, there was considerable effort to recruit patients early in the course of their illness, thus nearly 1/3 of the patients could be considered in their first-episode. The first analysis of the DWI data calculated scalar measures of fractional anisotropy and found lower WM fractional anisotropy in patients with a longer duration of illness (White et al. 2009). This finding replicated an earlier study with a smaller sample size (J. I. Friedman et al. 2008). In addition, we were able to map the age related trajectory of fractional anisotropy between patients and controls, which requires larger sample sizes to model accurate trajectories. We found that patients who smoke had lower levels of fractional anisotropy, although this appeared to be mostly mediated by a lower IQ in the patients who smoke (Cullen et al. 2012). In addition, a recent study evaluating the spatial characteristics of white matter abnormalities in schizophrenia found a characteristic pattern of WM abnormalities characterized by a generalized diffuse WM deficit coupled with localized ‘potholes.’ (White et al. 2012). Interestingly, the pothole approach (White et al. 2009) found that site D, which utilized 70 diffusion-weighted directions, had the best separation between patients and controls. Thus, scan quality for the ‘pothole’ approach may be important for better identifying the WM abnormalities in patients with schizophrenia. These studies, however, barely scratch the surface for the potential application of the DWI data.
For example, there has been little work in combining DWI data with fMRI and structural imaging data. The data set is well suited for these multimodal analyses as discussed below. In addition, there are clinical measures and a rich neuropsychological battery that could be coupled to DWI data to evaluate WM microstructure in relation to specific cognitive and clinical domains. We have to date only worked with the scalar fractional anisotropy measures, and thus the use of the data to evaluate tractography, small world networks, or probability based connectivity approaches have not yet been used to analyze the full MCIC diffusion-weighted dataset. Considering the profound heterogeneity in fractional anisotropy differences between patients with schizophrenia and controls (White, et al., 2008), the data could be used to evaluate the relationship between the heterogeneity of the clinical phenotype and the spatial characteristics of the WM microstructural abnormalities. Finally, because of the multiple sites and the inclusion of both patients and controls at all sites, the data set has great potential to help address methodological issues in DWI/DTI analyses and multimodal and multisite imaging.
We acknowledge that there are many challenges and specific limitations to the data set. For example, the data were collected at four different sites with essentially four different sequence protocols. Each site had ongoing DWI data collection and thus wanted to maintain their protocol at the time. While this may limit the ability to combine the data across sites, it offers a ‘real life’ situation to explore methodological issues in pooling data from different scanners. Interestingly, we did find considerable overlap in the fractional anisotropy differences between patients and control at three of the four sites. While we did perform a reliability study with 10 subjects who traveled to the four sites and scanned twice at each site, two sites changed their scanners between the reliability study and the multi-site study. This rendered the data less applicable to the multi-site study and there was not enough funding to repeat the reliability study. We believe that optimal multi-site DWI studies should include a reliability study similar to our original approach of traveling human phantoms. To evaluate differences with small effect sizes, combined genetic analyses, and/or select homogeneous groups, it would be optimal in multisite studies to utilize the same sequence parameters (i.e., directions, b-values, read-out directions, slice thickness, etc.) at each site.
fMRI data
The meticulous cross-site calibration methods proved adequate to analyze the multi-site fMRI data as a single set of combined patient and control cohorts. As will be detailed in the sections below, in each of the studies published from the fMRI data, analyses have included site as a factor when performing the analyses. For each of the experimental paradigms, the paradigm timing files and the raw image data as well as the EPrime behavioral response files for each subject are shared via the COINS site. At one site an error was made in the button press equipment set up that resulted in the time of trigger release rather the time of initial response being recorded. Thus, the EPrime reaction time (RT) data from that site could not be used for analysis.
Sternberg Item Recognition Paradigm (SIRP)
The SIRP fMRI and behavioral data have been analyzed using both general linear models as part of other analyses (e.g., (Brauns et al. 2011; Ehrlich et al. 2011b; Walton et al. 2012) and in conjunction with another multi-site dataset, using a multivariate, independent components analysis (ICA) (V. Calhoun, Adali T. in press; Kim et al. 2009b).
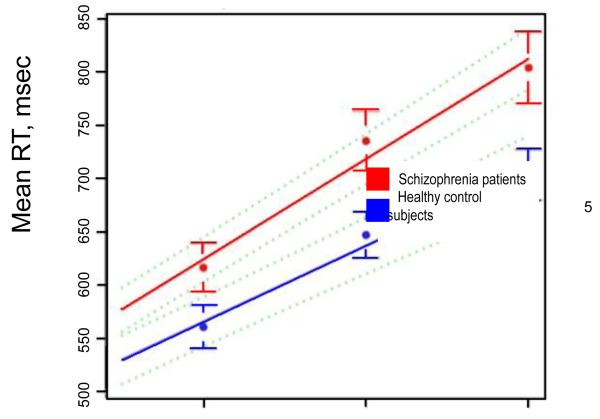
The behavioral results of the SIRP task were as expected from past studies using similar versions of this paradigm. Almost all of the schizophrenia patients and control subjects were able to easily learn the task and to meet the criteria for performance to allow participation in the study. Insufficient task performance on the SIRP was defined as a block completed with less than a 70% accuracy rate, and/or less than a 60% response rate. Four subjects (1 control, 3 patients) met these criteria for insufficient task performance for four or more of the 18 SIRP blocks and were excluded from the analyses in Brauns et al. 2011. As in previous studies, patients were slightly, but significantly less accurate than controls at all load levels (Probe 1: 97 ± 6% vs. 98 ± 3%, Probe 3: 95 ± 6% vs. 98 ± 3%, Probe 5: 94 ± 6% vs. 98 ± 3%, p<0.001) and for the three sites with useable data, patients had longer reaction times than controls for correct responses at each load level (Probe 1: 619 ± 101 ms vs. 559 ± 82 ms; Probe 3: 739 ± 122 ms vs. 647 ± 87 ms; Probe 5: 812 ± 151 ms vs. 701 ± 95 ms, overall p=0.001). Patients had significantly steeper slopes and higher intercepts than the controls, (all p< 001). There was no effect of site on accuracy or RT (Figure 3). (For both the accuracy data and the RT data, repeated measures ANOVA showed a significant main effect for load and diagnosis as well as their interaction (all p<0.001) reflecting the fact that patients were disproportionately affected by increases in WM load.
Figure 3.
The SIRP reaction time (RT) data showed a significant main effect for load and diagnosis as well as their interaction (all p<0.001) reflecting the fact that patients were disproportionately affected by increases in WM load. Red markers and lines indicate schizophrenia patients and the blue matched controls (mean ± SD), averaged across subjects from three of the four sites. No RT data were available from one site due to a technical error during acquisition. Green broken lines are pointwise 95% confidence intervals.
We found that the overall fMRI SIRP data quality was very good and was comparable for the patients and the controls. We performed quality assurance measures to identify and correct for artifacts in the SIRP fMRI scans from those subjects meeting performance criteria. Outlier time frames in each fMRI data time series detected using Artifact Detection Tools (http://www.nitrc.org/projects/artifact_detect) (Whitfield-Gabrieli et al. 2009) were defined by: (i) Global mean image intensity that differed by more than 3 standard deviations from the mean of the entire series of time frames in a scan, (ii) Displacement due to motion by more than 1 mm in the x, y or z direction relative to the previous time frame or (iii) Rotation due to motion by more than 0.1 rad around any of the three axes relative to the previous time frame. In Ehrlich et al. 2011, Brauns et al. 2011b and Walton et al. 2012, we removed the outlier time frames through the use of nuisance regressors in the linear model. In the case of runs where more than 15% of the time frames were flagged as outliers, the entire run was dropped from the analysis (3 patients and one healthy control of the initial sample) or the subject had to be excluded (1 patient with >15% outliers in 2 runs). The results of the ART QA are not provided as part of the download; however, these details are included to apprise potential users of the overall quality of the acquisitions and can be generalized to the other fMRI task data.
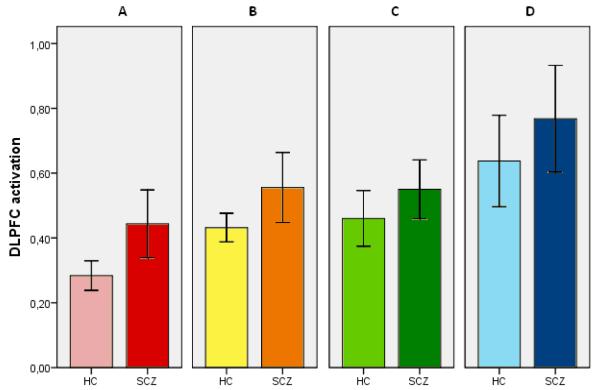
The overall group results from the fMRI analyses replicated the findings in the literature based on the same or similar task paradigms comparing patients with schizophrenia to matched control subjects (e.g. (Manoach et al. 2000; Manoach et al. 1999). Performance of the SIRP task was associated with load dependent activation in a broad network of brain regions including intraparietal sulcus, dorsolateral prefrontal cortex and anterior insula. Our team has focused our efforts on analyzing the quantitative activation metrics from a region that distinguishes the patients from the controls. We found increased activation in the left DLPFC in schizophrenia patients compared to healthy controls (F(1,302)=13.28; p<0.001) controlling for the effects of site, sex and age (Figure 4). There were also significant effects of site F(3,302)=11.83; p<0.001) and age F(1,302)=6.99; p=0.009). Patients and controls did not differ in their DLPFC activation in the right hemisphere.
Figure 4.
Left hemisphere DLPFC activation during performance of the SIRP task, indexed by maximum percentage signal change averaged across all loads, plotted by diagnosis and acquisition site demonstrates the slightly greater activity in patients over controls (F(1,302)=13.28; p<0.001) controlling for the effects of site, sex and age. There were also significant effects of site F(3,302)=11.83; p<0.001) and age F(1,302)=6.99; p=0.009). Error bars represent two standard errors. HC, healthy controls; SCZ, schizophrenia patients.
The SIRP fMRI datasets have already proved their value in identifying areas of increased BOLD signal response in subjects with schizophrenia (or those carrying genetic risk variants for schizophrenia) engaged in a working memory task (Brauns et al. 2011; Kim et al. 2009a; Walton et al. 2012) . These data allow the learning and retrieving phases, the “Encode” and “Probe” conditions, to be analyzed separately as a function of memory load. Moving beyond voxelwise comparisons of task-load and conditions we have also found interesting group differences in various small world network metrics evaluated on ICA timecourses extracted from the SIRP data (He, in press).
The fBIRN consortium used the same task and their analyses found similar “neural inefficiency” during the probe conditions at moderate demand levels (Potkin et al. 2009). As noted above, the task itself is somewhat more difficult for subjects with schizophrenia; the reaction times are slower, and the overall performance is not as high. However, even behaviorally matched subsets show the same over activation, as reported in Potkin et al. 2009.
Sensorimotor Task
Initially the sensory motor (SM) task was used for assessing and controlling between-site, within-site and within-subject variability (Michael et al. 2009a). However, the findings showed robust medication effects in the motor cortex(C. Abbott et al. 2011) and patient vs. control differences in the temporal lobe (Kim et al. 2010) as well as other task-related regions (Michael et al. 2009a; Sui et al. 2010).
Abbott et al. (2011) used the SM task and independent component analysis (ICA) to study the relationship of antipsychotic exposure on the fMRI BOLD signal in patients with schizophrenia. Consistent with earlier work (Braus et al. 1999; Muller et al. 2002; Tost et al. 2006) the results indicate that antipsychotic dose diminishes neural activation in motor (cortical and subcortical) and default mode networks in patients with schizophrenia. The current study also suggests that higher potency, typical antipsychotics further affect the BOLD signal. In 2010, Kim et al. used the general linear model (GLM) and independent component analysis (ICA) to extract fMRI features maps from the analysis of the AOD, SIRP and SM tasks. The feature maps were run through a coefficient-constrained independent component analysis (CCICA) to identify potential neurobiological markers. The results reveal spatial variability in the superior/middle temporal and frontal gyri, bilateral parietal lobules, and regions of the thalamus. When ranked on differences found in the amplitude of their feature signals, the top ten CCICA components all contained a frontal pole ICA component network from the SM task. The frontal pole network and the spatial variability indicate that these regions may play a significant role in the pathophysiology of schizophrenia.
Auditory Oddball Task
The auditory oddball data have been published in a variety of manuscripts (C. C. Abbott et al. 2010; Demirci et al. 2008a; Demirci et al. 2008b; Kim et al. 2010; Michael et al. 2009a). Auditory oddball task behavioral and BOLD fMRI data have been assessed for overall quality and summary statistics: Control subjects detected targets with an accuracy of 97% (SE 0.51%) and a mean reaction time of 607.5 ms (SE 14.17 ms). Patients detected targets with an accuracy of 95.9% (SE 0.67%) and mean reaction time of 598.9 ms (SE 15.56 ms). These differences were not significant (T=1.73 for accuracy and T=0.95 for reaction time). Analysis of the behavioral data indicated that accuracy was not different across sites. Site differences in reaction time data could not be interpreted due to confounds introduced by the instrumentation error for most of the data collected from site B as noted above. However, within each site, reaction time was not significantly different between patients and control subjects.
Auditory stimuli evoked a robust BOLD response in both healthy and chronic schizophrenia patients across a variety of brain regions, similar to those found by Kiehl et al. (Kiehl et al. 2005a), who computed difference in BOLD response amplitudes to target stimuli vs. standard stimuli to examine the effect of schizophrenia on the target response, independently from a generalized stimulus response effect. Using this same stimulus response comparison, differences between healthy controls and chronic schizophrenia patients revealed a number of effects across brain regions in the present study. On the average across sites, healthy subjects showed greater BOLD responses to target stimuli than patients in subcortical regions including right caudate, bilateral thalamus, and bilateral cerebellar regions, while chronic patients showed greater BOLD responses in cortical regions, including bilateral occipital, temporal and parietal cortex and premotor cortex greater on the left side. Interestingly, there were a number of differences found for data collected at the different sites.
The auditory oddball task has been used to study schizophrenia, in part because of its sensitivity to CNS function in general, and to schizophrenia in particular (Kiehl and Liddle 2001; Kiehl et al. 2005a; Kiehl et al. 2005b; Walton et al. 2012; White et al. 2009; Clark 2012; Clark et al. 2000). The results found in this study are different than previous studies in a number of respects. This includes the finding that patients were able to perform the task with similar accuracy and reaction time when compared with controls. This may have resulted from more rigorous matching of healthy subjects to the patient cohort than employed in previous studies. In addition, the fMRI data revealed that patients responded to target stimuli in cortical regions with a significantly larger and more positive BOLD response relative to controls. This also contradicts previous studies, which typically find reduced responses in cortical regions of patients (Kiehl and Liddle 2001; Kiehl et al. 2005a). Again, this may have resulted from more careful subject selection including less affected patients in this study. However, the differences found across sites, with data from site C showing this effect and data from site B showing the opposite effect (e.g. patients showing generally reduced activity relative to controls) suggests that there may have been some other experimental or environmental factors involved. Other, as yet unidentified sources of variance may yet be found to explain these differences across sites, and across studies. One promising approach that can capture and account for site differences is independent component analysis that we have used to analyze the fBIRN auditory oddball data that is a similar but not identical multi-site schizophrenia data set (Kim et al. 2009b).
Breath Hold Task
Even though the consortium has not analyzed the breath hold data, it is available for download with the rest of the image data.
Data Fusion
Because each imaging modality/task provides a different view of brain function or structure, combining multiple modalities/tasks may further help uncover previously hidden relationships that can unify disparate findings in brain imaging (V. D. Calhoun et al. 2006; V. Calhoun, Adali T. 2009). The MCIC datasets thus provide a valuable test bed for multi-modal/multi-task fusion. As a technique that capitalizes on the strength of each imaging modality/task and their inter-relationships in a joint analysis, data fusion is an important tool to help unravel the complex pathophysiological mechanisms underlying schizophrenia (V. D. Calhoun et al. 2006; Sui et al. 2012a; V. Calhoun, Adali T. 2009).
Recent advances in data-driven approaches and their use in integrating multiple (task) fMRI data sets from the same subject (Sui et al. 2010; Sui et al. 2009b) can specify common versus specific sources of activity to a greater degree than traditional general linear model-based approaches. This can increase confidence in conclusions about the functional significance of brain regions, and of activation changes therein in schizophrenia. In addition, by using a combination of extracted multi-task fMRI activation indices and summary scores from neuropsychological tests, (Kim et al. 2010; Sui et al. 2009a; Demirci et al. 2009) attempted to find the optimal group-discriminative feature combinations that might provide biological insight into schizophrenia disorder. In another case, using combined genetic and fMRI data achieved better group discrimination or classification power than using either alone (Yang et al. 2010; Liu et al. 2009), indicating that genetic and brain function representing different, but partially complementary aspects and these can be detected by multimodal fusion. A recent application of a parallel ICA model to the MCIC sensorimotor fMRI data and the SNP array data revealed group differences in precentral and postcentral gyri coupled with SNPs involved in four neurotransmitter pathways: GABA receptor signaling, dopamine receptor signaling, neuregulin signaling and glutamate receptor signaling (Chen et al. 2012). Finally, the combination of function and structure may provide more informative insights into both altered brain patterns and connectivity in schizophrenia. For example, a lower and different function–structure connection is often found in schizophrenia (Michael et al. 2010; Yu et al. 2011; Michael et al. 2009b; Michael et al. 2011), while varied brain patterns were also identified frequently (Xu et al. 2009; Sui et al. 2011; V.D. Calhoun et al. 2008). Other work has shown that evaluating the relationship between multiple neuropsychological markers and structural MRI measures have the potential to be predictive (Karageorgiou et al. 2011). In summary, via a multi-site analysis with more subjects, higher statistical power can be achieved in brain imaging data fusion, which has shown increasing utility in answering both scientifically interesting and clinically relevant questions (Sui et al. 2012b).
Conclusions
The combination of structural, functional, neuropsychological and DTI data on the same subjects has already led to an improved understanding of the relationship between brain structures and schizophrenia. There are still many questions to which these datasets can be applied regarding the relationships between cognitive functions, symptom severity, breath-hold driven BOLD changes, and the interplay of various functional and structural neural networks within the context of schizophrenia.
Most importantly, these data sets are being made available to the scientific community to facilitate the advancement of clinical neuroscience not just specifically for the understanding of the pathophysiology of schizophrenia, but more broadly as these data can be used as controls for other studies, for advanced image analysis algorithm development (e.g. Christodoulou, in press) and for training the next generation of clinical neuroscientists seeking to harness the tools of quantitative neuroimaging.
Supplementary Material
Information Sharing Statement.
The MCIC imaging and clinical data are available through the COINS (COllaborative Informatics Neuroimaging Suite) database (Scott et al. 2011) and may be freely used with no restrictions. Users are encouraged to cite this paper and the data sharing web site (http://coins.mrn.org/dataexchange). To download the data, use the groups and filters to select data of interest, click the “Send Request” button, attach a message with required information, and an MCIC study staff member will reply with a link to the MCIC Data Use Agreement. The user will be required to accept the conditions of this agreement before accessing the data. The data downloads will then be made available.
Acknowledgements
The authors wish to thank our many colleagues who served as mentors, advisors and supporters during the inception and conduct of the study including Donald Goff, Gina Kuperberg, Jill Goldstein, Martha Shenton, Robert McCarley, Stephan Heckers, Cynthia Wible, Raquelle Mesholam-Gately, and Mark Vangel.
We thank the study staff and clinicians at each site that were responsible for the data acquisition. These include: Stuart Wallace, Ann Cousins, Raquelle Mesholam-Gately, Steven Stufflebeam, Oliver Freudenreich, Daphne Holt, Laura Kunkel, Frank Fleming, George He, Hans Johnson, Ron Pierson, Arvind Caprihan, Phyllis Somers, Christine Portal, Kaila Norman, Diana South, Michael Doty and Haley Milner.
We would also like to acknowledge the expert guidance on image and other types of data acquisition we obtained from Lee Friedman, Stephan Posse, Jorge Jovicich, and Tom Wassink.
We would also like to acknowledge the many research assistants, students and colleagues who assisted in data curation over the years since data acquisition was completed. These include: Stuart Wallace, Carolyn Zyloney, Komal Sawlani, Esther Walton, Jill Fries, Adam Scott, Dylan Wood, Runtang Wang, William Courtney, Angie Guimaraes, Lisa Shenkman, Mustafa Kendi, Aysa Tuba Karagulle Kendi, Ryan Muetzel, Tara Biehl, and Marcus Schmidt.
Funding Acknowledgments: This work was supported primarily by the Department of Energy DE-FG02-99ER62764 through its support of the Mind Research Network (MRN, formerly known as the MIND Institute) and the consortium as well as by the National Association for Research in Schizophrenia and Affective Disorders (NARSAD) Young Investigator Award (to SE) as well as through the Blowitz-Ridgeway and Essel Foundations, and through NWO ZonMw TOP 91211021, the DFG research fellowship (to SE), the Mind Research Network, National Institutes of Health through NCRR 5MO1-RR001066 (MGH General Clinical Research Center), NIMH K08 MH068540, the Biomedical Informatics Research Network with NCRR Supplements to P41 RR14075 (MGH), M01 RR 01066 (MGH), NIBIB R01EB006841 (MRN), R01EB005846 (MRN), 2R01 EB000840 (MRN), 1RC1MH089257 (MRN), as well as grant U24 RR021992.
References
(* refers to published manuscripts that have used data from this shared MCIC repository)
- *.Abbott C, Juarez M, White T, Gollub RL, Pearlson GD, Bustillo J, et al. Antipsychotic dose and diminished neural modulation: a multi-site fMRI study. [Clinical Trial Multicenter Study Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] Progress in neuro-psychopharmacology & biological psychiatry. 2011;35(2):473–482. doi: 10.1016/j.pnpbp.2010.12.001. doi:10.1016/j.pnpbp.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott CC, Kim D, 2nd, Sponheim SR, Bustillo J, Calhoun VD. Decreased default mode neural modulation with age in schizophrenia. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2010;18(10):897–907. doi: 10.1097/JGP.0b013e3181e9b9d9. doi:10.1097/JGP.0b013e3181e9b9d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington D, Addington J, Maticka-Tyndale E, Joyce J. Reliability and validity of a depression rating scale for schizophrenics. [Comparative Study] Schizophrenia research. 1992;6(3):201–208. doi: 10.1016/0920-9964(92)90003-n. [DOI] [PubMed] [Google Scholar]
- Adriano F, Caltagirone C, Spalletta G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. [Meta-Analysis Research Support, Non-U.S. Gov’t Review] The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2012;18(2):180–200. doi: 10.1177/1073858410395147. doi:10.1177/1073858410395147. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; Iowa City: 1983. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa; Iowa City, IA: 1984. [Google Scholar]
- Andreasen NC. Psychiatric Symptoms You Currently Have—Baseline (PSYCH-BASE) The University of Iowa; Iowa City, Iowa: 1987. [Google Scholar]
- Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Archives of general psychiatry. 1992;49(8):615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. [Research Support, N.I.H., Extramural] Biological psychiatry. 2010;67(3):255–262. doi: 10.1016/j.biopsych.2009.08.040. doi:10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. British journal of psychology. 1970;61(3):303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Barnes TR. A rating scale for drug-induced akathisia. The British journal of psychiatry : the journal of mental science. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Bockholt HJ, Scully M, Courtney W, Rachakonda S, Scott A, Caprihan A, et al. Mining the mind research network: a novel framework for exploring large scale, heterogeneous translational neuroscience research data sources. Frontiers in neuroinformatics. 2010;3:36. doi: 10.3389/neuro.11.036.2009. doi:10.3389/neuro.11.036.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. 1991. pp. 5pp. 125–142.
- *.Brauns S, Gollub RL, Roffman JL, Yendiki A, Ho BC, Wassink TH, et al. DISC1 is associated with cortical thickness and neural efficiency. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] NeuroImage. 2011;57(4):1591–1600. doi: 10.1016/j.neuroimage.2011.05.058. doi:10.1016/j.neuroimage.2011.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braus DF, Ende G, Weber-Fahr W, Sartorius A, Krier A, Hubrich-Ungureanu P, et al. Antipsychotic drug effects on motor activation measured by functional magnetic resonance imaging in schizophrenic patients. [Clinical Trial Comparative Study Research Support, Non-U.S. Gov’t] Schizophrenia research. 1999;39(1):19–29. doi: 10.1016/s0920-9964(99)00032-8. [DOI] [PubMed] [Google Scholar]
- Brown GG, McCarthy G, Bischoff-Grethe A, Ozyurt B, Greve D, Potkin SG, et al. Brain-performance correlates of working memory retrieval in schizophrenia: a cognitive modeling approach. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] Schizophrenia bulletin. 2009;35(1):32–46. doi: 10.1093/schbul/sbn149. doi:10.1093/schbul/sbn149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] NeuroImage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. doi:10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Calhoun V, Adali T. Feature-based Fusion of Medical Imaging Data, IEEE Transactions on Information Technology in Biomedicine. IEEE transactions on information technology in biomedicine : a publication of the IEEE Engineering in Medicine and Biology Society. 2009;13:1–10. doi: 10.1109/TITB.2008.923773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Calhoun V, Adali T. Multi-subject Independent Component Analysis of fMRI: A Decade of Intrinsic Networks, Default Mode, and Neurodiagnostic Discovery. IEEE Reviews in Biomedical Engineering. 2012;vol. 5:60–73. doi: 10.1109/RBME.2012.2211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Giuliani NR, Pekar JJ, Kiehl KA, Pearlson GD. Method for multimodal analysis of independent source differences in schizophrenia: combining gray matter structural and auditory oddball functional data. Human brain mapping. 2006;27(1):47–62. doi: 10.1002/hbm.20166. doi:10.1002/hbm.20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA. Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum Brain Mapp. 2008;29(11):1265–1275. doi: 10.1002/hbm.20463. doi:10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Calhoun VD, Pearlson GD, Ehrlich S, Turner JA, Ho BC, et al. Multifaceted genomic risk for brain function in schizophrenia. [Research Support, N.I.H., Extramural] NeuroImage. 2012;61(4):866–875. doi: 10.1016/j.neuroimage.2012.03.022. doi:10.1016/j.neuroimage.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou AG, Bauer TE, Kiehl KA, Feldstein Ewing S, Bryan AD, Calhoun VD. A Quality Control Method for Detecting and Suppressing Uncorrected Residual Motion in fMRI Studies. Magnetic Resonance Imaging. doi: 10.1016/j.mri.2012.11.007. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP. A history of randomized task designs in fMRI. [Historical Article Review] NeuroImage. 2012;62(2):1190–1194. doi: 10.1016/j.neuroimage.2012.01.010. doi:10.1016/j.neuroimage.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Clark VP, Fannon S, Lai S, Benson R, Bauer L. Responses to rare visual target and distractor stimuli using event-related fMRI. [Clinical Trial] Journal of neurophysiology. 2000;83(5):3133–3139. doi: 10.1152/jn.2000.83.5.3133. [DOI] [PubMed] [Google Scholar]
- *.Cullen KR, Wallace S, Magnotta VA, Bockholt J, Erlich S, Gollub RL, et al. Cigarette smoking and white matter microstructure in schizophrenia. Psychiatry research. 2012 doi: 10.1016/j.pscychresns.2011.08.010. doi:10.1016/j.pscychresns.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. doi:10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J. Delis–Kaplan Executive Function System. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- *.Demirci O, Clark VP, Calhoun VD. A projection pursuit algorithm to classify individuals using fMRI data: Application to schizophrenia. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] NeuroImage. 2008a;39(4):1774–1782. doi: 10.1016/j.neuroimage.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Demirci O, Clark VP, Magnotta VA, Andreasen NC, Lauriello J, Kiehl KA, et al. A Review of Challenges in the Use of fMRI for Disease Classification / Characterization and A Projection Pursuit Application from Multi-site fMRI Schizophrenia Study. Brain imaging and behavior. 2008b;2(3):147–226. doi: 10.1007/s11682-008-9028-1. doi:10.1007/s11682-008-9028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Demirci O, Stevens MC, Andreasen NC, Michael A, Liu J, White T, et al. Investigation of relationships between fMRI brain networks in the spectral domain using ICA and Granger causality reveals distinct differences between schizophrenia patients and healthy controls. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] NeuroImage. 2009;46(2):419–431. doi: 10.1016/j.neuroimage.2009.02.014. doi:10.1016/j.neuroimage.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Cabral HJ, Settecase F, Hess CP, Dillon WP, Glastonbury CM, et al. Automated MRI measures predict progression to Alzheimer’s disease. [Comparative Study Multicenter Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Neurobiology of aging. 2010;31(8):1364–1374. doi: 10.1016/j.neurobiolaging.2010.04.023. doi:10.1016/j.neurobiolaging.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. doi:10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- *.Ehrlich S, Brauns S, Yendiki A, Ho BC, Calhoun V, Schulz SC, et al. Associations of Cortical Thickness and Cognition in Patients With Schizophrenia and Healthy Controls. Schizophrenia bulletin. 2011a doi: 10.1093/schbul/sbr018. doi:10.1093/schbul/sbr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Ehrlich S, Morrow EM, Roffman JL, Wallace SR, Naylor M, Bockholt HJ, et al. The COMT Val108/158Met polymorphism and medial temporal lobe volumetry in patients with schizophrenia and healthy adults. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] NeuroImage. 2010;53(3):992–1000. doi: 10.1016/j.neuroimage.2009.12.046. doi:10.1016/j.neuroimage.2009.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Ehrlich S, Yendiki A, Greve DN, Manoach DS, Ho BC, White T, et al. Striatal function in relation to negative symptoms in schizophrenia. Psychological medicine. 2011b:1–16. doi: 10.1017/S003329171100119X. doi:10.1017/S003329171100119X. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Gamst AC, Quinn BT, Pacheco J, Jernigan TL, Thal L, et al. Feasibility of multi-site clinical structural neuroimaging studies of aging using legacy data. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] Neuroinformatics. 2007;5(4):235–245. doi: 10.1007/s12021-007-9003-9. doi:10.1007/s12021-007-9003-9. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders -Patient Edition. Version 2.0, 8/98 revision ed. Biometrics Research Department New York State Psychiatric Institute; 722 West 168th Street New York, New York 10032: SCID-I/P. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. [Research Support, U.S. Gov’t, P.H.S.] Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. doi:10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. [Comparative Study Research Support, U.S. Gov’t, P.H.S.] Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999a;9(2):195–207. doi: 10.1006/nimg.1998.0396. doi:10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Human brain mapping. 1999b;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JI, Tang C, Carpenter D, Buchsbaum M, Schmeidler J, Flanagan L, et al. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. [Research Support, N.I.H., Extramural] The American journal of psychiatry. 2008;165(8):1024–1032. doi: 10.1176/appi.ajp.2008.07101640. doi:10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- Friedman L, Glover GH. Reducing interscanner variability of activation in a multicenter fMRI study: controlling for signal-to-fluctuation-noise-ratio (SFNR) differences. [Multicenter Study Research Support, N.I.H., Extramural] NeuroImage. 2006;33(2):471–481. doi: 10.1016/j.neuroimage.2006.07.012. doi:10.1016/j.neuroimage.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Friedman L, Stern H, Brown GG, Mathalon DH, Turner J, Glover GH, et al. Test-retest and between-site reliability in a multicenter fMRI study. [Comparative Study Research Support, N.I.H., Extramural Validation Studies] Human brain mapping. 2008;29(8):958–972. doi: 10.1002/hbm.20440. doi:10.1002/hbm.20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover G, Mueller B, Van Erp T, Liu T, Greve D, Voyvodic J, Rasmussen J, Turner J, Brown G, Keator D, Calhoun VD, Lee HJ, Ford J, Mathalon D, Diaz M, O’Leary D, Gadde S, Preda A, Wible C, Stern H, McCarthy G, Ozyurt B. Function Biomedical Informatics Research Network Recommendations for Prospective Multi-Center Functional Neuroimaging Studies. Journal of Magnetic Resonance Imaging. 2012;vol. 36:39–54. doi: 10.1002/jmri.23572. PMC Journal - In Process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Chen Q, et al. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t] Archives of general psychiatry. 2009;66(5):467–477. doi: 10.1001/archgenpsychiatry.2009.24. doi:10.1001/archgenpsychiatry.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] NeuroImage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. doi:10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- *.He H, Sui J, Yu Q, Turner JA, Sponheim SR, Manoach DS, Clark VP, Calhoun VD. Altered Small-World Brain Networks in Schizophrenia Patients during Working Memory Performance. PLoS ONE. doi: 10.1371/journal.pone.0038195. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Snyder AZ, Girton LE, Morris JC, Buckner RL. Frontal-hippocampal double dissociation between normal aging and Alzheimer’s disease. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Cerebral cortex. 2005;15(6):732–739. doi: 10.1093/cercor/bhh174. doi:10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- Health N. I. o. M. ECDEU Assessment Manual. U.S. Department of Health, Education, and Welfare; 1976. Abnormal Involuntary Movement Scale; p. 534. [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. [Comparative Study Meta-Analysis Research Support, Non-U.S. Gov’t Review] The American journal of psychiatry. 2005;162(12):2233–2245. doi: 10.1176/appi.ajp.162.12.2233. doi:10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Functional Magnetic Resonance Imaging. Second edition Sinauer Associates Publishers; Sunderland, Mass: 2009. [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. [Research Support, N.I.H., Extramural] NeuroImage. 2006;30(2):436–443. doi: 10.1016/j.neuroimage.2005.09.046. doi:10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] NeuroImage. 2009;46(1):177–192. doi: 10.1016/j.neuroimage.2009.02.010. doi:10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Karageorgiou E, Schulz SC, Gollub RL, Andreasen NC, Ho BC, Lauriello J, et al. Neuropsychological testing and structural magnetic resonance imaging as diagnostic biomarkers early in the course of schizophrenia and related psychoses. [Clinical Trial Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Validation Studies] Neuroinformatics. 2011;9(4):321–333. doi: 10.1007/s12021-010-9094-6. doi:10.1007/s12021-010-9094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF. An event-related functional magnetic resonance imaging study of an auditory oddball task in schizophrenia. Schizophrenia research. 2001;48(2-3):159–171. doi: 10.1016/s0920-9964(00)00117-1. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Stevens MC, Celone K, Kurtz M, Krystal JH. Abnormal hemodynamics in schizophrenia during an auditory oddball task. [Comparative Stud Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.] Biological psychiatry. 2005a;57(9):1029–1040. doi: 10.1016/j.biopsych.2005.01.035. doi:10.1016/j.biopsych.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Stevens MC, Laurens KR, Pearlson G, Calhoun VD, Liddle PF. An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale (n = 100) fMRI study of an auditory oddball task. [Research Support, Non-U.S. Gov’t] NeuroImage. 2005b;25(3):899–915. doi: 10.1016/j.neuroimage.2004.12.035. doi:10.1016/j.neuroimage.2004.12.035. [DOI] [PubMed] [Google Scholar]
- *.Kim DI, Manoach DS, Mathalon DH, Turner JA, Mannell M, Brown GG, et al. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] Human brain mapping. 2009a;30(11):3795–3811. doi: 10.1002/hbm.20807. doi:10.1002/hbm.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kim DI, Mathalon DH, Ford JM, Mannell M, Turner JA, Brown GG, et al. Auditory oddball deficits in schizophrenia: an independent component analysis of the fMRI multisite function BIRN study. [Research Support, N.I.H., Extramural] Schizophrenia bulletin. 2009b;35(1):67–81. doi: 10.1093/schbul/sbn133. doi:10.1093/schbul/sbn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kim DI, Sui J, Rachakonda S, White T, Manoach DS, Clark VP, et al. Identification of imaging biomarkers in schizophrenia: a coefficient-constrained independent component analysis of the mind multi-site schizophrenia study. [Multicenter Study Research Support, N.I.H., Extramural] Neuroinformatics. 2010;8(4):213–229. doi: 10.1007/s12021-010-9077-7. doi:10.1007/s12021-010-9077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. [Comparative Study Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Archives of general psychiatry. 2003;60(9):878–888. doi: 10.1001/archpsyc.60.9.878. doi:10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Poupon C, Amadon A, Lethimonnier F. Artifacts and pitfalls in diffusion MRI. [Review] Journal of magnetic resonance imaging : JMRI. 2006;24(3):478–488. doi: 10.1002/jmri.20683. doi:10.1002/jmri.20683. [DOI] [PubMed] [Google Scholar]
- Liu J, Pearlson G, Windemuth A, Ruano G, Perrone-Bizzozero NI, Calhoun V. Combining fMRI and SNP data to investigate connections between brain function and genetics using parallel ICA. Hum Brain Mapp. 2009;30(1):241–255. doi: 10.1002/hbm.20508. doi:10.1002/hbm.20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, et al. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. [Research Support, Non-U.S. Gov’t] Biological psychiatry. 2000;48(2):99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, et al. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. [Research Support, Non-U.S. Gov’t] Biological psychiatry. 1999;45(9):1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- *.Michael AM, Baum SA, Fries JF, Ho BC, Pierson RK, Andreasen NC, et al. A method to fuse fMRI tasks through spatial correlations: applied to schizophrenia. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] Human brain mapping. 2009a;30(8):2512–2529. doi: 10.1002/hbm.20691. doi:10.1002/hbm.20691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Michael AM, Baum SA, Fries JF, Ho BC, Pierson RK, Andreasen NC, et al. A method to fuse fMRI tasks through spatial correlations: applied to schizophrenia. Hum Brain Mapp. 2009b;30(8):2512–2529. doi: 10.1002/hbm.20691. doi:10.1002/hbm.20691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Michael AM, Baum SA, White T, Demirci O, Andreasen NC, Segall JM, et al. Does function follow form?: methods to fuse structural and functional brain images show decreased linkage in schizophrenia. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] NeuroImage. 2010;49(3):2626–2637. doi: 10.1016/j.neuroimage.2009.08.056. doi:10.1016/j.neuroimage.2009.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Michael AM, King MD, Ehrlich S, Pearlson G, White T, Holt DJ, et al. A Data-Driven Investigation of Gray Matter-Function Correlations in Schizophrenia during a Working Memory Task. Frontiers in human neuroscience. 2011;5:71. doi: 10.3389/fnhum.2011.00071. doi:10.3389/fnhum.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. California Computerized Assessment Battery (CalCAP) Norland Software. 1990.
- Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack C, Jagust W, et al. The Alzheimer’s disease neuroimaging initiative. [Research Support, N.I.H., Extramural Review] Neuroimaging clinics of North America. 2005a;15(4):869–877. xi–xii. doi: 10.1016/j.nic.2005.09.008. doi:10.1016/j.nic.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack CR, Jagust W, et al. Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2005b;1(1):55–66. doi: 10.1016/j.jalz.2005.06.003. doi:10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JL, Roder C, Schuierer G, Klein HE. Subcortical overactivation in untreated schizophrenic patients: a functional magnetic resonance image finger-tapping study. Psychiatry and clinical neurosciences. 2002;56(1):77–84. doi: 10.1046/j.1440-1819.2002.00932.x. doi:10.1046/j.1440-1819.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Cerebral cortex. 2005;15(6):708–719. doi: 10.1093/cercor/bhh172. doi:10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. [Comparative Study Review] Psychological medicine. 2009;39(11):1763–1777. doi: 10.1017/S0033291709005315. doi:10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. [Comparative Study Meta-Analysis Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Archives of general psychiatry. 1998;55(5):433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Nesvag R, Lawyer G, Varnas K, Fjell AM, Walhovd KB, Frigessi A, et al. Regional thinning of the cerebral cortex in schizophrenia: effects of diagnosis, age and antipsychotic medication. [Comparative Study Research Support, Non-U.S. Gov’t] Schizophrenia research. 2008;98(1-3):16–28. doi: 10.1016/j.schres.2007.09.015. doi:10.1016/j.schres.2007.09.015. [DOI] [PubMed] [Google Scholar]
- O’Brien LM, Ziegler DA, Deutsch CK, Kennedy DN, Goldstein JM, Seidman LJ, et al. Adjustment for whole brain and cranial size in volumetric brain studies: a review of common adjustment factors and statistical methods. Harv Rev Psychiatry. 2006;14(3):141–151. doi: 10.1080/10673220600784119. doi:L253867Q62801164 [pii] 10.1080/10673220600784119. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Ford JM. Widespread cortical dysfunction in schizophrenia: the fBIRN imaging consortium. [Review] Schizophrenia bulletin. 2009;35(1):15–18. doi: 10.1093/schbul/sbn159. doi:10.1093/schbul/sbn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkin SG, Turner JA, Brown GG, McCarthy G, Greve DN, Glover GH, et al. Working memory and DLPFC inefficiency in schizophrenia: the fBIRN study. [Multicenter Study Research Support, N.I.H., Extramural] Schizophrenia bulletin. 2009;35(1):19–31. doi: 10.1093/schbul/sbn162. doi:10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Yoon J, Minzenberg MJ, Carter CS. Neuroimaging of cognitive disability in schizophrenia: search for a pathophysiological mechanism. [Review] International review of psychiatry. 2007;19(4):417–427. doi: 10.1080/09540260701486365. doi:10.1080/09540260701486365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. Validity of the trail making test as indicator of organic brain damage. Perceptual and motor skills. 1958;(8):271–276. [Google Scholar]
- Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] NeuroImage. 2010;53(4):1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. doi:10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol LM, Hartberg CB, Nesvag R, Fennema-Notestine C, Hagler DJ, Jr., Pung CJ, et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. [Research Support, Non-U.S. Gov’t] Biological psychiatry. 2010;68(1):41–50. doi: 10.1016/j.biopsych.2010.03.036. doi:10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Nesvag R, Hagler DJ, Jr., Bergmann O, Fennema-Notestine C, Hartberg CB, et al. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. [Research Support, Non-U.S. Gov’t] Biological psychiatry. 2012;71(6):552–560. doi: 10.1016/j.biopsych.2011.11.026. doi:10.1016/j.biopsych.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Risacher SL, Saykin AJ, West JD, Shen L, Firpi HA, McDonald BC. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. [Multicenter Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Current Alzheimer research. 2009;6(4):347–361. doi: 10.2174/156720509788929273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Roffman JL, Gollub RL, Calhoun VD, Wassink TH, Weiss AP, Ho BC, et al. MTHFR 677C --> T genotype disrupts prefrontal function in schizophrenia through an interaction with COMT 158Val --> Met. [Comparative Study Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] Proceedings of the National Academy of Sciences of the United States of America. 2008;105(45):17573–17578. doi: 10.1073/pnas.0803727105. doi:10.1073/pnas.0803727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RM, Parker SB. Gender- and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the Finger Tapping and Grooved Pegboard Tests. Perceptual and motor skills. 1993;76(3 Pt 2):1219–1230. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- Schultz CC, Koch K, Wagner G, Roebel M, Schachtzabel C, Gaser C, et al. Reduced cortical thickness in first episode schizophrenia. [Research Support, Non-U.S. Gov’t] Schizophrenia research. 2010;116(2-3):204–209. doi: 10.1016/j.schres.2009.11.001. doi:10.1016/j.schres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Scott A, Courtney W, Wood D, de la Garza R, Lane S, King M, et al. COINS: An Innovative Informatics and Neuroimaging Tool Suite Built for Large Heterogeneous Datasets. Frontiers in neuroinformatics. 2011;5:33. doi: 10.3389/fninf.2011.00033. doi:10.3389/fninf.2011.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Segall JM, Turner JA, van Erp TG, White T, Bockholt HJ, Gollub RL, et al. Voxel-based morphometric multisite collaborative study on schizophrenia. [Multicenter Study Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] Schizophrenia bulletin. 2009;35(1):82–95. doi: 10.1093/schbul/sbn150. doi:10.1093/schbul/sbn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Grimson E, Fischl B. A genetic algorithm for the topology correction of cortical surfaces. [Evaluation Studies] Information processing in medical imaging : proceedings of the … conference. 2005;19:393–405. doi: 10.1007/11505730_33. [DOI] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Phil. Trans Roy. Soc. 1982:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Shen L, Qi Y, Kim S, Nho K, Wan J, Risacher SL, et al. Sparse bayesian learning for identifying imaging biomarkers in AD prediction. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Medical image computing and computer-assisted intervention : MICCAI … International Conference on Medical Image Computing and Computer-Assisted Intervention. 2010;13(Pt 3):611–618. doi: 10.1007/978-3-642-15711-0_76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. [Clinical Trial Comparative Study Controlled Clinical Trial] Acta psychiatrica Scandinavica. Supplementum. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Sivan AB. Benton Visual Retention Test. 5th ed The Psychological Corporation; San Antonio, TX: 1992. [Google Scholar]
- Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, et al. The effects of antipsychotics on the brain: what have we learnt from structural imaging of schizophrenia?--a systematic review. [Comparative Study Research Support, Non-U.S. Gov’t Review] Current pharmaceutical design. 2009;15(22):2535–2549. doi: 10.2174/138161209788957456. [DOI] [PubMed] [Google Scholar]
- *.Sponheim SR, Jung RE, Seidman LJ, Mesholam-Gately RI, Manoach DS, O’Leary DS, et al. Cognitive deficits in recent-onset and chronic schizophrenia. [Research Support, U.S. Gov’t, Non-P.H.S.] Journal of psychiatric research. 2010;44(7):421–428. doi: 10.1016/j.jpsychires.2009.09.010. doi:10.1016/j.jpsychires.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153(3736):652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Stonnington CM, Tan G, Kloppel S, Chu C, Draganski B, Jack CR, Jr., et al. Interpreting scan data acquired from multiple scanners: a study with Alzheimer’s disease. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] NeuroImage. 2008;39(3):1180–1185. doi: 10.1016/j.neuroimage.2007.09.066. doi:10.1016/j.neuroimage.2007.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sui J, Adali T, Pearlson G, Yang H, Sponheim SR, White T, et al. A CCA+ICA based model for multi-task brain imaging data fusion and its application to schizophrenia. [Comparative Study Research Support, N.I.H., Extramural] NeuroImage. 2010;51(1):123–134. doi: 10.1016/j.neuroimage.2010.01.069. doi:10.1016/j.neuroimage.2010.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sui J, Adali T, Pearlson GD, Calhoun VD. An ICA-based method for the identification of optimal FMRI features and components using combined group-discriminative techniques. Neuroimage. 2009a;46(1):73–86. doi: 10.1016/j.neuroimage.2009.01.026. doi:S1053-8119(09)00070-6 [pii] 10.1016/j.neuroimage.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sui J, Adali T, Pearlson GD, Clark VP, Calhoun VD. A method for accurate group difference detection by constraining the mixing coefficients in an ICA framework. Hum Brain Mapp. 2009b;30(9):2953–2970. doi: 10.1002/hbm.20721. doi:10.1002/hbm.20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sui J, Adali T, Yu Q, Chen J, Calhoun VD. A review of multivariate methods for multimodal fusion of brain imaging data. [Comparative Study Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S. Review] Journal of neuroscience methods. 2012a;204(1):68–81. doi: 10.1016/j.jneumeth.2011.10.031. doi:10.1016/j.jneumeth.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sui J, He H, Pearlson GD, Adali T, Kiehl KA, Yu Q, et al. Three-way (N-way) fusion of brain imaging data based on mCCA+jICA and its application to discriminating schizophrenia. NeuroImage. 2012b;66C:119–132. doi: 10.1016/j.neuroimage.2012.10.051. doi:10.1016/j.neuroimage.2012.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sui J, Pearlson G, Caprihan A, Adali T, Kiehl KA, Liu J, et al. Discriminating schizophrenia and bipolar disorder by fusing fMRI and DTI in a multimodal CCA+ joint ICA model. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] NeuroImage. 2011;57(3):839–855. doi: 10.1016/j.neuroimage.2011.05.055. doi:10.1016/j.neuroimage.2011.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabangin ME, Woo JG, Martin LJ. The effect of minor allele frequency on the likelihood of obtaining false positives. BMC proceedings. 2009;3(Suppl 7):S41. doi: 10.1186/1753-6561-3-S7-S41. doi:10.1186/1753-6561-3-S7-S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Meyer-Lindenberg A, Klein S, Schmitt A, Hohn F, Tenckhoff A, et al. D2 antidopaminergic modulation of frontal lobe function in healthy human subjects. [Clinical Trial] Biological psychiatry. 2006;60(11):1196–1205. doi: 10.1016/j.biopsych.2006.04.014. doi:10.1016/j.biopsych.2006.04.014. [DOI] [PubMed] [Google Scholar]
- van der Kouwe AJ, Benner T, Dale AM. Real-time rigid body motion correction and shimming using cloverleaf navigators. [Evaluation Studies Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2006;56(5):1019–1032. doi: 10.1002/mrm.21038. doi:10.1002/mrm.21038. [DOI] [PubMed] [Google Scholar]
- *.Walton E, Turner J, Gollub RL, Manoach DS, Yendiki A, Ho BC, et al. Cumulative Genetic Risk and Prefrontal Activity in Patients With Schizophrenia. Schizophrenia bulletin. 2012 doi: 10.1093/schbul/sbr190. doi:10.1093/schbul/sbr190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DR, Bai F, Barrett SL, Turkington A, Rushe TM, Mulholland CC, et al. Structural changes in the hippocampus and amygdala at first episode of psychosis. [Comparative Study Research Support, Non-U.S. Gov’t] Brain imaging and behavior. 2012;6(1):49–60. doi: 10.1007/s11682-011-9141-4. doi:10.1007/s11682-011-9141-4. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3 ed Psychological Corporation; San Antonio, TX: 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3rd ed Psychological Corporation; San Antonio, TX: 1997b. [Google Scholar]
- *.White T, Ehrlich S, Ho BC, Manoach DS, Caprihan A, Schulz SC, et al. Spatial Characteristics of White Matter Abnormalities in Schizophrenia. Schizophrenia bulletin. 2012 doi: 10.1093/schbul/sbs106. doi:10.1093/schbul/sbs106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.White T, Magnotta VA, Bockholt HJ, Williams S, Wallace S, Ehrlich S, et al. Global white matter abnormalities in schizophrenia: a multisite diffusion tensor imaging study. [Comparative Study Evaluation Studies Multicenter Study Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] Schizophrenia bulletin. 2011;37(1):222–232. doi: 10.1093/schbul/sbp088. doi:10.1093/schbul/sbp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Nelson M, Lim KO. Diffusion tensor imaging in psychiatric disorders. [Research Support, N.I.H., Extramural Review] Topics in magnetic resonance imaging : TMRI. 2008;19(2):97–109. doi: 10.1097/RMR.0b013e3181809f1e. doi:10.1097/RMR.0b013e3181809f1e. [DOI] [PubMed] [Google Scholar]
- White T, Schmidt M, Karatekin C. White matter ‘potholes’ in early-onset schizophrenia: a new approach to evaluate white matter microstructure using diffusion tensor imaging. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Psychiatry research. 2009;174(2):110–115. doi: 10.1016/j.pscychresns.2009.04.014. doi:10.1016/j.pscychresns.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Proceedings of the National Academy of Sciences of the United States of America. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. doi:10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G. Wide Range Achievement Test. 3rd ed Wide Range, Inc.; Wilmington, DE: 1993. [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. [Comparative Study Meta-Analysis Research Support, Non-U.S. Gov’t] The American journal of psychiatry. 2000;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Xu L, Pearlson G, Calhoun VD. Joint source based morphometry identifies linked gray and white matter group differences. Neuroimage. 2009;44(3):777–789. doi: 10.1016/j.neuroimage.2008.09.051. doi:S1053-8119(08)01069-0 [pii] 10.1016/j.neuroimage.2008.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Liu J, Sui J, Pearlson GD, Calhoun VD. A Hybrid Machine Learning Method for Fusing fMRI and Genetic Data: Combining both Improves Classification of Schizophrenia. Front Hum Neurosci. 2010;4:192. doi: 10.3389/fnhum.2010.00192. doi:10.3389/fnhum.2010.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Yendiki A, Greve DN, Wallace S, Vangel M, Bockholt J, Mueller BA, et al. Multi-site characterization of an fMRI working memory paradigm: reliability of activation indices. NeuroImage. 2010;53(1):119–131. doi: 10.1016/j.neuroimage.2010.02.084. doi:10.1016/j.neuroimage.2010.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Yendiki A, Panneck P, Srinivasan P, Stevens A, Zollei L, Augustinack J, et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Frontiers in neuroinformatics. 2011;5:23. doi: 10.3389/fninf.2011.00023. doi:10.3389/fninf.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Sui J, Rachakonda S, He H, Pearlson G, Calhoun VD. Altered small-world brain networks in temporal lobe in patients with schizophrenia performing an auditory oddball task. Front Syst Neurosci. 2011;5:7. doi: 10.3389/fnsys.2011.00007. doi:10.3389/fnsys.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.