Abstract
The exocytotic release of neurotransmitters requires active transport into synaptic vesicles and other types of secretory vesicles. Members of the SLC18 family perform this function for acetylcholine (SLC18A3, the vesicular acetylcholine transporter or VAChT) and monoamines such as dopamine and serotonin (SLC18A1 and 2, the vesicular monoamine transporters VMAT1 and 2, respectively). To date, no specific diseases have been attributed to a mutation in an SLC18 family member; however, polymorphisms in SLC18A1 and SLC18A2 may confer risk for some neuropsychiatric disorders. Additional members of this family include SLC18A4, expressed in insects, and SLC18B1, the function of which is not known. SLC18 is part of the Drug:H+ Antiporter-1 Family (DHA1, TCID 2.A.1.2) within the Major Facilitator Superfamily (MFS, TCID 2.A.1).
Keywords: SLC18, Monoamines, Serotonin, Dopamine, Noradrenaline, Acetylcholine, Vesicular transporter, Synaptic vesicle
1. Phylogeny and functional overview
The SLC18 group of the Major Facilitator Superfamily (MFS, Transporter Classification ID 2.A.1, see Transporter Classification Database, TCDB http://www.tcdb.org/superfamily.php) is subclassified as part of the Drug:H+ Antiporter-1 Family (DHA1, TCID 2.A.1.2) and includes the vesicular monoamine and acetylcholine transporters: VMAT and VAChT, respectively (see Fig. 1B). In mammals, distinct genes encode VMAT1 (SLC18A1), expressed primarily in the periphery and VMAT2 (SLC18A2), the major neural isoform. Invertebrates including the model organisms Drosophila melanogaster and Caenorhabditis elegans encode a single VMAT isoform (Fei and Krantz, 2009).1 A single VAChT isoform, SLC18A3 is expressed in both mammals and invertebrates. An additional gene, SLC18A4, related to both VMATs and VAChT has recently been identified in Drosophila and named portabella (prt) in reference to its expression in a well-known structure required for learning and memory in insects, the mushroom bodies (Brooks et al., 2011, see Fig. 1B and Section 10.3. C. elegans and Drosophila).
Fig. 1.
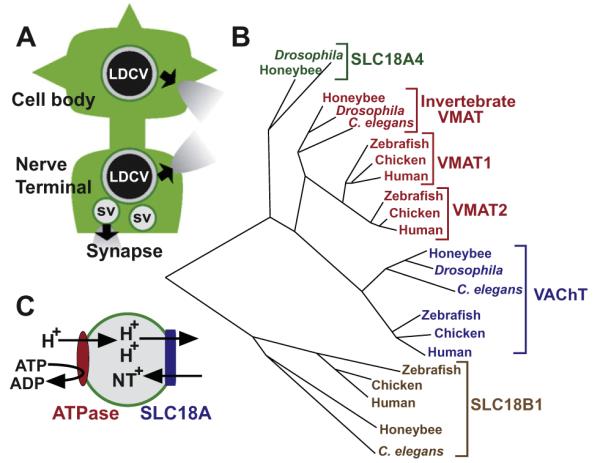
(A) Subcellular localization of SLC18A members. A canonical neuron showing a cell body and dendrites with an axonal process leading to the nerve terminal. Synaptic Vesicles (SVs) cluster at the nerve terminal and release neurotransmitter into the synapse when they fuse with the presynaptic plasma membrane. Large Dense Core Vesicles (LDCVs) localize more diffusely to sites throughout the cell body, dendrites and the nerve terminal and release classical as well as peptide neurotransmitters during exocytosis. SLC18A members can localize to one or both types of secretory vesicles. (B) Phylogenetic relationships. Phylogenetic tree with representative members of SLC18, including SLC18A1 (VMAT1), SLC18A2 (VMAT2), invertebrate VMAT, SLC18A3 (VAChT) and two newly defined subfamilies: SLC18A4, defined by the Drosophila gene portabella, and the novel subfamily SLC18B1. Note that portabella is present in additional insect species (not shown) but does not have an obvious vertebrate homolog. SLC18B1/C6orf192 homologs are present in multiple vertebrate and invertebrate species but not in Drosophila. Rat homologs of VMAT1, VMAT2 and VAChT overlap with Homo sapiens in this plot (not shown). Species represented in the diagram include Homo sapiens (Human), Gallus gallus (Chicken), Danio rerio (Zebrafish), Apis mellifera (Honeybee), Drosophila melanogaster (Drosophila) and Caenorhabditis elegans (C. elegans). Tree generated using the AllAll program of the Computational Biochemistry Research Group, Swiss Federal Institute of Technology, Zurich, Switzerland (http://www.cbrg.ethz.ch/services/AllAll) using protein sequences listed under the following accession numbers: NP_003046.2 (Human VAChT); NP_003045.2 (Human VMAT2); NP_001129163.1 (Human VMAT1); NP_439896.1 (Human C6orf192); NP_996865.1 (Chicken VAChT); XP_421782.2 (Chicken VMAT2); XP_428881.2 (Chicken VMAT1); XP_419737.3 (Chicken C6orf192); NP_001243154.1 (Zebrafish VMAT2); XP_002663262.2 (Zebrafish VMAT1), NP_001071018.1 (Zebrafish “probable vesicular acetylcholine transporter-A”); NP_001017841.1 (Zebrafish uncharacterized protein LOC550539 listed as C6orf192 in Jacobsson et al., 2010); AAF56164.2 (Drosophila CG10251/portabella); AAF55587.2 (Drosophila VAChT); AAX52708.1 (Drosophila VMAT, neural isoform DVMAT-A, see Greer et al., 2005), XP_001120960.2 (Honeybee VAChT); XP_392061.3 (Honey bee VMAT); XP_001122029.1, Honeybee portabella homolog annotated as “vesicular acetylcholine transporter unc-17-like”); XP_625161.2 (Honeybee C6orf192 homolog); CCA65520.1 (C. elegans VAChT, UNC-17 protein); NP_001024937.1 (C. elegans VMAT, CAT-1 protein); NP_741716.2 (hypothetical protein F55A4.8, listed as C. elegans C60orf192 in Jacobsson et al., 2010). Note that the sequence listed as “Drosophila C6orf192” in Jacobsson et al., 2010 is Drosophila VMAT (Greer et al., 2005) rather than a C6orf192 homolog. (C) Bioenergetics of SLC18A-dependent transport. Hydrolysis of ATP to ADP by a V-Type ATPase generates a proton gradient in the lumen of both SVs and LDCVs. The proton gradient (2 protons per cycle) drives transport of 1 molecule of positively charged neurotransmitter (NT) into the lumen of the vesicle via an antiport mechanism.
The SLC18 family is distantly related to the toxin extruding antiporters (TEXANS), bacterial transporters that have been proposed as a model for the function of the VMATs (Schuldiner et al., 1995). Their potential role in cellular detoxication aside, the major biological function for SLC18 family members is the storage and release of neurotransmitters (Fig. 1A). VAChT transports acetylcholine; VMAT1 and 2 transport several types of monoamine neurotransmitters including dopamine, serotonin and norepinephrine (see Section 3.2 (see Section 3.2. Transporter Substrates and Their Affinities and Table 1, Predominant substrates). The substrate(s) of SLC18A4 is not yet known, but structure–function analysis suggests that it is more similar to the monoamines than to acetylcholine (Brooks et al., 2011). All SLC18 family members localize to secretory vesicles responsible for neurotransmitter storage in both neurons and neuroendocrine cells (see Section 6, Tissue Expression and Subcellular Localization and Fig. 1A).
Table 1.
For detailed information about the SLC gene tables, please visit: http://www.bioparadigms.org.
| SLC gene name |
Protein | Aliases | Predominant substrates |
Transport type/ coupling ions |
Tissue distribution (selected) | Predominant subcellular distribution |
Link to disease |
Human gene locus |
Human sequence accession ID |
|---|---|---|---|---|---|---|---|---|---|
| SLC18A1 | VMAT1 | CGAT, VAT | 5-HT, DA, NE Epinephrine |
E/H+ | Sympathetic ganglia, Some GI neurons, Adrenal medulla |
LDCVs | (Unclear) | 8p21.3 | NM_003053 |
| SLC18A2 | VMAT2 | SVAT, SVMT, VAT2 | 5-HT, DA, NE Epinephrine, Histamine |
E/H+ | All CNS aminergic neurons, Mast cells, Platelets, Some GI neurons, Adrenal medulla |
LDCVs and Synaptic vesicles |
(Unclear) | 10q25 | NM_003054 |
| SLC18A3 | VAChT | Unc-17, Vesamicol binding protein |
Acetylcholine | E/H+ | Cholinergic neurons, Pancreatic stellate cells, Tracheal brush cells, |
Synaptic vesicles | (Unknown) | 10q11.2 | NM_003055 |
| SLC18A4 |
Drosophila portabella gene product |
CG10251 | Unknown | Unknown | Drosophila Mushroom bodies | LDCVs | – | –- | NM_001104417.1 |
| SLC18B1 | C6orf192 | Hypothetical human protein LOC116843, dJ55C23.6 gene product |
Unknown | Unknown | Broad, including brain, lung | Unknown | Unknown | 6q22.3 | NM_052831.2 |
Abbreviations: VMAT, vesicular monoamine transporter; VAChT, vesicular acetylcholine transporter; 5-HT, serotonin; DA, dopamine; NE norepinephrine; LDCVs, large dense core vesicles; GI, gastrointestinal tract; CNS, central nervous system.
SLC18A members share structural similarity with a newly identified group of predicted genes defined by human C6orf192, and classified here as SLC18B1 (Jacobsson et al., 2010, see Fig. 1B). SLC18B1 is structurally more distinct from the vesicular glutamate, aspartate and nucleotide transporter family (SLC17) and the vesicular GABA transporter (SLC16, see Chapters XX and YY).
2. History of identification and cloning
Active transport of monoamines into adrenal chromaffin granules was reported as early as 1962, and a wealth of information on the bioenergetics of transport followed over the next few decades (Eiden and Weihe, 2011; Schuldiner et al., 1995). Two groups used expression cloning to molecularly identify the VMATs and a third group used affinity purification to isolate VMAT2 (Schuldiner et al., 1995).
The electronic organ tissue of the Torpedo fish provided a critical tissue source for biochemical studies of acetylcholine storage (Parsons, 2000; Schuldiner et al., 1995). In parallel, the C. elegans unc-17 gene, found to be less sensitive to blockade of acetylcholine breakdown, was eventually identified as the worm ortholog of VAChT. Oligonucleotides representing unc-17 were used to identify vertebrate orthologs of VAChT and biochemical experiments confirmed that the VAChT cDNA conferred acetylcholine uptake activity in vitro (Parsons, 2000; Schuldiner et al., 1995).
3. Transport
3.1. Bioenergetics
The transport activities of VMATs and VAChT require the exchange of two lumenal protons for each molecule of cytosolic transmitter via an antiport mechanism, with the proton electrochemical gradient ΔμH+ generated by the vacuolar H+-ATPase (Fig. 1C, Schuldiner et al., 1995). Pharmacologic probes such as nigericin and valinomycin, used to selectively reduce either ΔpH or ΔΨ, respectively, demonstrate that both VMATs and VAChT primarily use the ΔpH component of ΔμH+ (Parsons, 2000). Ach is transported as a positively charged ion; since permanently charged species such as MPP+ are substrates of VMATs, endogenous substrates of VMATs are likely to undergo transport as positively charged species as well. Although the electrical properties of vesicular transport have not been measured directly, the exchange of two protons for one cationic neurotransmitter suggests an electrogenic process with a net flux of one positive charge per transport cycle.
3.2. Transporter substrates and their affinities
The transport cycles of VMATs and VAChT require binding and unbinding of substrate from at least two distinct conformations. Therefore, at least two substrate affinity constants (Kd) are involved in transport (Parsons, 2000). The actual Kds for binding of substrate to vesicular transporters can be measured directly, and rate constants have been derived for each step in the transport cycle (Parsons, 2000). For VAChT and VMATs, the apparent Kms derived from transport assays are 10–100 fold smaller than the Kd values; this difference may be caused by kinetic contributions to the apparent affinity (Parsons, 2000).
Both cell free and whole cell assays have been used to assay the relative apparent affinity of VMAT1 and VMAT2 for monoamine substrates in heterologously transfected cells (Chaudhry et al., 2008; Fei and Krantz, 2009; Weihe and Eiden, 2000; Wimalasena, 2010). The relative affinities across different amines are similar for both assays, with serotonin (5HT) > dopamine (DA), epinephrine, norepinephrine (NE) ⪢ histamine (see also Table 1). However, the absolute values are generally lower for the cell free versus whole cell assays (Chaudhry et al., 2008; Fei and Krantz, 2009; Wimalasena, 2010). The apparent affinity of VMAT2 for most monoamines is greater than VMAT1 (Chaudhry et al., 2008; Fei and Krantz, 2009; Wimalasena, 2010). Interestingly, the apparent affinity of histamine for both transporters is dramatically lower than for other amines, and histamine is not likely to be a physiological substrate for VMAT1, since only VMAT2 is expressed in histaminergic cells.
VAChT transports a number of structurally divergent organic molecules that are similar to acetylcholine only in that they carry a charge of +1 (Fei and Krantz, 2009). VAChT may also transport choline albeit with a 7-fold lower apparent affinity than Ach (Fei and Krantz, 2009); however, acetylcholine is thought to be the only endogenous substrate of VAChT (Parsons, 2000). The apparent Km of rat VAChT for acetylcholine is ~1 mM, three orders of magnitude lower than the apparent Km of VMAT for most amines (Fei and Krantz, 2009).
4. Pharmacology
4.1. VMAT inhibitors
The most commonly used VMAT inhibitors are reserpine and tetrabenazine. Reserpine binds with high affinity to both VMAT1 and 2 at, or very near, a substrate-binding site in VMATs on the cytoplasmic side of the transporter (Chaudhry et al., 2008; Fei and Krantz, 2009; Wimalasena, 2010). Tetrabenazine interacts with VMAT at a distinct site and differentially inhibits VMAT2 and VMAT1 (IC50s of 0.3 and 3 micromolar, respectively) (Chaudhry et al., 2008; Fei and Krantz, 2009; Wimalasena, 2010). Residues in transmembrane domains (TMs) 10 to 12 are particularly important for tetrabenazine binding to VMAT2 (Chaudhry et al., 2008; Fei and Krantz, 2009; Wimalasena, 2010). Interestingly, despite the apparent difference in binding sites, tetrabenazine inhibits reserpine binding, presumably via an allosteric mechanism (Chaudhry et al., 2008; Fei and Krantz, 2009; Wimalasena, 2010).
Two dopamine transporter inhibitors, GBR 12909 and 12935, also inhibit VMAT2 with high apparent affinity (34–45 nM) (Chaudhry et al., 2008; Fei and Krantz, 2009; Wimalasena, 2010). Additional VMAT inhibitors include ketanserin, amiodarone, some derivatives of 3-amino-2-phenylpropene (APP), lobeline, which also interact with plasma membrane monoamine transporters, and as a series of synthetic compounds structurally similar to lobeline (Chaudhry et al., 2008; Fei and Krantz, 2009; Wimalasena, 2010).
At least one derivative of lobeline has been shown to decrease the addictive effects of methamphetamine in rodent models, and members of this family of drugs have been proposed as potential treatments for methamphetamine dependence (Beckmann et al., 2011). At present, the only FDA approved drug that targets VMAT is tetrabenazine, used for the treatment of Huntington’s chorea (Wimalasena, 2010). Reserpine has been used in the past as an antihypertensive but is now rarely prescribed since it can induce a state resembling depression (Fei and Krantz, 2009).
4.2. Environmental toxins
Several environmental toxins and pesticides have been shown to inhibit VMAT activity. These include the organochlorine pesticide heptachlor and the structurally-related, polychlorinated biphenyls (PCBs) (Guillot and Miller, 2009). The inhibition of VMATs by heptachlor and PCBs may be relevant to Parkinson’s disease, since both PCBs and organochlorine pesticides have been shown to deplete dopamine stores in experimental animals, and organochlorines may be associated with an increased incidence of Parkinson’s disease (Guillot and Miller, 2009).
4.3. Efflux agents
Some drugs that interact with VMATs promote efflux of transmitter out of the vesicle lumen rather than inhibiting transport. Most, if not all of these are VMAT substrates, and are derivatives of amphetamines, including methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA, also known as Ecstasy) (Fleckenstein et al., 2007). Some formulations of methamphetamine are used to treat Attention Deficit Hyperactivity Disorder. The precise mechanisms by which amphetamines drive efflux out of the vesicle and subsequently out of the cell have received considerable attention but have not yet been fully resolved (Fleckenstein et al., 2007).
4.4. VAChT inhibitors
Vesamicol (l-trans-2-(4-phenyl[3,4-3H]piperidino)cyclohexanol) is the major pharmacologic inhibitor used for the study of VAChT (Parsons, 2000). Indeed, VAChT was originally known as the vesamicol receptor. Additional structural derivatives of vesamicol have been synthesized as alternative probes for VAChT (Fei and Krantz, 2009). Vesamicol binds to the cytosolic side of VAChT with an equilibrium dissociation constant Kd of ~5 nM (Parsons, 2000). Although the binding sites for vesamicol and acetylcholine are distinct, they are thought to be close to one another thus allowing some functional interactions (Fei and Krantz, 2009).
5. Models and structure/function studies
5.1. Models for structure and transport
An X-ray crystallographic structure is not yet available for any vesicular transporter, but the recent development of high yield heterologous expression systems may stimulate future structural studies (Elbaz et al., 2010). The feasiblilty of obtaining structural information is supported by recent progress in the crystallographic study of plasma membrane transporters. Meanwhile, it has been suggested that proteins related to vesicular transporters may be useful for modeling the transport mechanism (Khare et al., 2010a; Vardy et al., 2004). In the current working model of vesicular acetylcholine (and presumably amine) transport, the exchange of one lumenal proton is used to transport substrate into the vesicle lumen, and a second proton is used to reorient the substrate binding site back to the cytoplasm to allow the cycle to begin again (Parsons, 2000). Acetylcholine binding is likely to be at equilibrium; in contrast, the two proton dependent movements of the transporter are thought to be rate limiting, and the reorientation of the empty acetylcholine binding site is likely to be the slower of the two translocation events (Fei and Krantz, 2009; Parsons, 2000).
5.2. Membrane topology
For both VMATs and VACHT, most computer-based predictions suggest 12 TMs with the N and C termini facing the intracellular milieu and a larger lumenal loop between the first and second transmembrane domains (Fei and Krantz, 2009; Parsons, 2000; Wimalasena, 2010) (Fig. 2). Biochemical studies of the C-termini and the large extracellular loops between TMs 1 and 2 of both VMATs and VAChT support these predictions (Fei and Krantz, 2009).
Fig. 2.
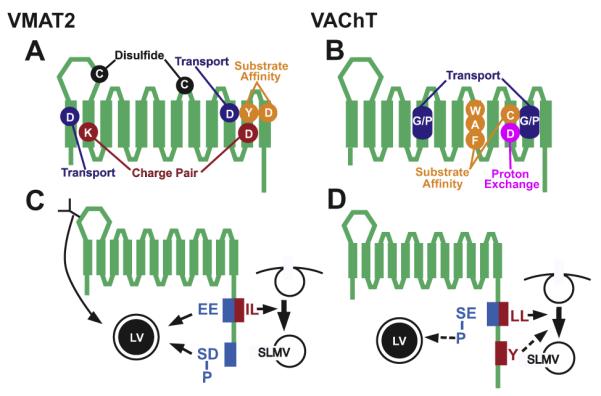
Structure Function Studies of Transport and Trafficking. VMAT2 (A and C) and VAChT (B and D) contain 12 transmembrane domains. (A and B) Site directed mutagenesis has revealed the role of several residues for transport activity in VMAT2 (A) and VAChT (B). For VMAT2 these include aspartates (D) in the first and tenth membrane-bound domains required for transport activity, a charge-pair between residues in TM2 (K) and TM11 (D), and a proposed disulfide bond between cysteines (C) in two lumenal loops. Residues important for substrate affinity include a tyrosine (Y) in TM11 and an aspartate (D) in TM12. For VAChT, tryptophan (W) alanine (A) and phenyalanine (F) residues in TM8 and a cysteine (C) in TM10 are important for affinity to ACh. Conserved regions that contain glycine and/or proline (G/P) are required for transport activity and an aspartate (D) in TM10 is likely to be involved in proton exchange. Note that location of the residues in the cartoon is approximate and does not accurately reflect their precise position within each domain. (C and D) Membrane trafficking signals in VMAT2 (C) and VAChT (D). Glycosylation (“Y”) facilitates sorting of VMAT2 to Large Dense Core Vesicles (LVs). All other known sorting signals for vesicular transporters are encoded in their C-terminal, cytoplasmic domains. For VMAT2, a dileucine motif (IL) is required for endocytosis and sorting to Synaptic Like Microvesicles (SLMVs) in neuroendocrine cells. The VMAT2 endocytosis signal is part of a larger motif, which includes upstream glutamate residues (EE). The upstream glutamates are required for localizing VMAT2 to LVs. A phosphorylated “acidic cluster” at the extreme C-terminus of VMAT2 (DDEE[P]SE[P]SD, shown as [P]SD in panel (A)) also helps sort VMAT2 to LVs. (B) VAChT contains a dileucine motif (LL) required for endocytosis and sorting to SLMVs. However, a tyrosine-based motif (Y) may direct these trafficking events under some circumstances. A serine upstream of the dileucine motif in VAChT (SE) undergoes phosphorylation by PKC. Phosphorylation of the serine ([P]SE) may drive a portion of VAChT onto LVs.
5.3. Structure/function studies of transport activity
The twelve TMs and the intervening loops of VMAT and VAChT include most if not all of the residues required for transport activity and substrate recognition (Fei and Krantz, 2009; Wimalasena, 2010) (Fig. 2A and B). These include several charged residues embedded in predicted transmembrane domains: For both rat VMAT2 and VAChT, mutation of an aspartate in the tenth transmembrane domain (TM10) abolishes transport (Fei and Krantz, 2009; Wimalasena, 2010). Glutamate can substitute for aspartate, indicating the importance of an acidic residue at this site (Fei and Krantz, 2009). Mutation of an aspartate in TM1 of VMAT2 but not VAChT also inhibits transport (Fei and Krantz, 2009). Additional charged residues in TM2 and TM11 of VMAT2 are thought to form a charge pair (Fei and Krantz, 2009) (Fig. 2A). The charges in VMAT2 can be exchanged without disrupting transport, underscoring the likelihood that they interact, and that TM2 and TM11 may be in direct contact. However, the analogous aspartate in TM11 of VAChT does not tolerate exchange with its proposed basic partner, suggesting that either VAChT has a more stringent structural requirement, or that this residue is more directly involved in substrate translocation (Fei and Krantz, 2009). Additional residues that are likely to have structural importance in VMATs include cysteines in the large lumenal loop between TMs 1 and 2 and the loop between TMs 7 and 8; these two residues are thought to form a disulfide bond (Fei and Krantz, 2009) (Fig. 2A).
For VMAT1 and 2, TMs 5–8 and 9–12 show the greatest contribution to substrate and drug affinity (Fei and Krantz, 2009; Wimalasena, 2010). A tyrosine in TM11 (Y434 in VMAT2) and an aspartate in TM12 (D461) are particularly important for the higher affinity of VMAT2 for serotonin and possibly histamine, as well the drug tetrabenazine (Fei and Krantz, 2009) (Fig. 2A). Y434 in VMAT2 is also responsible for the relatively low affinity of VMAT2 for tryptamine (Fei and Krantz, 2009). Mutations in TMs 5–8 of VMATs can reverse the effects of mutations in TM9-12 (Fei and Krantz, 2009) suggesting that the two regions may interact.
Site directed mutagenesis studies on VAChT have taken advantage of crystal structures available for other proteins that bind ACh and it has been hypothesized that the π elections of specific aromatic residues may interact with the positive charge in acetylcholine (Fei and Krantz, 2009). In particular, W331 in TM8 of VAChT may help form the substrate-binding pocket in VAChT via π electron solvation (Fei and Krantz, 2009) (Fig. 2B). Additional residues likely to contribute to substrate binding include A334 and F335 in TM8, and C391 in TM10 (Khare et al., 2010a). Residues contained within conserved proline- and glycine-rich regions in VAChT may be involved in transport (Fei and Krantz, 2009).
Some studies have attempted to map the site of proton translocation in VAChT and VMAT, based in part on the idea that conserved histidines or acidic residues may play important roles (Fei and Krantz, 2009; Parsons, 2000). Although the identity of the residues that accept and release protons during the transport cycle remains unresolved, one possible site in VAChT is D398 (TM10) (Khare et al., 2010b). Several other charged residues have been determined to be nonessential for this process (Wimalasena, 2010).
6. Tissue expression and subcellular localization
6.1. Subcellular localization
SLC18 family members localize primarily if not exclusively to secretory vesicles responsible for the storage and release of neurotransmitter. In neurons, these include synaptic vesicles (SVs) responsible for rapid neurotransmitter release at the synapse, and large dense core vesicles (LDCVs), which are released from sites throughout the neuron and also contain neuromodulatory peptides (Fig. 1A). VAChT localizes primarily to SVs, whereas VMAT2 is found on both SVs and LDCVs. VMAT1 primarily localizes to large dense core granules (LDCGs), the neuroendcorine analog of neuronal LDCVs. The portabella gene product also appears to localize primarily to dense core vesicles (Brooks et al., 2011).
6.2. Expression of VMATs
In the mature mammalian CNS, VMAT2 is expressed in dopaminergic, serotonergic and noradrenergic cells in the brainstem and all of their abundant projections; thus, VMAT2 is thought to be responsible for the storage of all monoamines in the CNS (Eiden and Weihe, 2011; Fei and Krantz, 2009). It is also expressed in most noradrenergic neurons of the sympathetic ganglia, where it allows the storage of norepinephrine and in enterochromaffin-like cells of the gastric mucosa, which release histamine in response to gastrin, in mast cells for the storage of both histamine and serotonin, and in platelets, presumably for the storage of serotonin (Fei and Krantz, 2009).
Interestingly, some additional neurons that synthesize amines do not express detectable levels of VMAT2 (Eiden and Weihe, 2011). These include aminergic neurons in the olfactory bulb and nucleus tractus solitarius (Eiden and Weihe, 2011). It remains unclear whether dopamine is released from these cells. Conversely, some cells that do not synthesize monoamines express VMAT. In the rodent and primate brain thalamocortical neurons that project to the barrel cortex are glutamatergic in the adult, but during development express VMAT2 and the plasma membrane serotonin transporter (SERT) (Eiden and Weihe, 2011). These cells also do not express the enzymes required for serotonin biosynthesis, and it has been proposed that they take up serotonin from the extracellular milieu rather than synthesizing it themselves (Eiden and Weihe, 2011).
VMAT1 is expressed in enterochromaffin cells in the small and large intestine for the storage of serotonin (Weihe and Eiden, 2000). It is also expressed in pinealocytes where it has been proposed to store serotonin and in dopaminergic cells in the kidney proximal tubule (Fei and Krantz, 2009). Both VMAT 1 and 2 are expressed in the adrenal medulla; the expression levels of each isoform vary across species (Wimalasena, 2010).
6.3. Expression of VAChT
VAChT has been shown to be expressed in all of the major, previously identified cholinergic cells in the CNS including cholinergic neurons in the basal forebrain and striatum (Fei and Krantz, 2009). VAChT may also be expressed in the median eminence and hypothalamus (Fei and Krantz, 2009).
In the periphery, VAChT and the machinery for Ach synthesis are expressed in cholinergic motoneurons and cholinergic cells of the autonomic nervous system. In addition, recent data suggest that VAChT is expressed in several unexpected, non-neuronal sites, including stellate and alpha cells in the pancreas where non-neuronal Ach may directly or indirectly regulate secretion of CCK and insulin (Phillips et al., 2010; Rodriguez-Diaz et al., 2011). Airway epithelial cells also express VAChT and release Ach to regulate breathing frequency (Krasteva et al., 2011).
6.4. Vesicular transporter co-expression
A developmental change in expression from VMAT1 to VMAT2 occurs in the dopaminergic glomus cells of the carotid body (Weihe and Eiden, 2000). These cells function as sensors for oxygen and thus help to regulate respiration. In the adult, they express VMAT1, but early in development they also express high levels of VMAT2 (Weihe and Eiden, 2000). Co-expression of VMAT1 and 2 occur in mature aminergic cells in some species (Fei and Krantz, 2009). In C. elegans, several motoneurons express VAChT and VMAT, and in the primate autonomic nervous system, VAChT and VMAT2 are co-expressed during development in several parasympathetic ganglia and constitutively in sweat glands (Eiden and Weihe, 2011; Fei and Krantz, 2009). Some parasympathetic cells do not express the biosynthetic enzymes for catecholamine synthesis, suggesting that VMAT2 may be used here for purposes other than transmitter release.
A number of recent studies have demonstrated co-expression of VMAT2 or VAChT with VGLUTs (El Mestikawy et al., 2011; Hnasko and Edwards, 2011). Although in some cases co-expression of VGLUT with VMAT or VAChT may function to allow co-release of neurotransmitters, other possible roles have received increasing attention. These include the potential for VGLUT activity to facilitate amine or Ach storage and release, or vice versa (El Mestikawy et al., 2011; Guzman et al., 2011; Hnasko and Edwards, 2011).
6.5. SLC18A4
Homologs of SLC18A4 are present in multiple insect species but there does not appear to be a mammalian ortholog of this gene (see Fig. 1B). The Drosophila SLC18A4 protein (the portabella gene product) is highly expressed in the mushroom bodies, a structure required for learning and memory in the fly, and some fifty additional neurons in the larval and the adult nervous system (Brooks et al., 2011).
6.6. SLC18B1
Rat C6orf192 is broadly expressed in a number of tissues including the brain with the highest level of expression in the lung (Jacobsson et al., 2010). Its function in these tissues is not known.
7. Biosynthesis and trafficking
As is the case for other integral membrane proteins, vesicular transporters are synthesized in the endoplasmic reticulum (ER), and post-translationally modified in the ER and Golgi. Based on their sensitivity to glycosidase and the results of mutagenesis studies, mammalian VMATs and VAChT undergo N-linked glycosylation (Fei and Krantz, 2009). The mutation of three predicted glycosylation sites in VMAT1 decreases transport activity but does not affect substrate affinity (Fei and Krantz, 2009). Decreased glycosylation does not disrupt the subcellular localization of VMAT1 in non-neuronal cells but may help mediate the localization of VMATs to LDCVs (Fei and Krantz, 2009) (see Section 7.2 below).
7.1. VMAT trafficking
Both the secretory and endocytic pathways are required for vesicular transporter trafficking to secretory vesicles: the biogenesis of synaptic vesicles is thought to involve an endocytic step at the plasma membrane of the nerve terminal, and SV recycling also requires endocytosis (Jahn and Rizzoli, 2007). Coat proteins such as clathrin are critical for these processes and adaptor proteins link cargo such as the vesicular transporters to the coats. VAChT has been suggested to bind AP1 and AP2, the adaptor complexes required for clathrin mediated Golgi exit and cell surface endocytosis respectively (Fei et al., 2008). Exit of VMAT2 from the Golgi may depend on the adaptor complex AP3 (Asensio et al., 2010). VMAT2 also binds the novel adaptor PACS-1, possibly regulating exit from immature LDCVs (Fei et al., 2008).
For VMAT2, a number of studies indicate that the cytoplasmic C-terminal domain is responsible for sorting to both SVs and LDCVs (Fig. 2C, Fei et al., 2008). A dileucine motif is required for the efficient endocytosis of VMAT2 in PC12 cells as well as in hippocampal neurons and is likely to help sort VMAT to SLMVs in PC12 cells (Fei et al., 2008). However, since multiple forms of endocytosis may occur in neurons (Jahn and Rizzoli, 2007) and proteins may sort to neuronal SVs via alternate mechanisms, it is possible that other motifs could participate in the sorting of VMAT2 to SVs in vivo.
Additional motifs are required for the localization of VMAT2 to LDCVs (Fig. 2C). These include acidic glutamate (E) residues upstream of the dileucine motif (EEXXXIL) (Fei et al., 2008). In addition, an acidic cluster or patch at the end of the C-terminal (DDEESESD) domain helps traffic VMAT2 to LDCVs (Fei et al., 2008). This motif was originally identified in the protease furin and determines whether proteins are retained in LDCVs as they mature (Fei et al., 2008). Phosphorylation of serines contained within the acid patch regulates these trafficking events (Fei et al., 2008). These sites are phosphoryated by CKII under baseline conditions and it is possible that dephosphorylation could shunt VMAT2 to other compartments. N-linked glycosylation may provide an additional signal for localizing VMAT2 to LDCVs in PC12 cells (Fei et al., 2008).
7.2. VAChT trafficking
For VAChT, endocytosis may depend on a dileucine motif similar to a site in VMAT2 or a distinct, downstream tyrosine-based motif (Fei et al., 2008) (Fig. 2D). Consistent with the predicted role for endocytosis in SV biogenesis, mutation of the dileucine motif blocks the localization of VAChT to SLMVs in PC12 cells (Fei et al., 2008).
VAChT also contains an upstream acidic residue and a PKC phosphorylation site that can mimic the acidic residues in the extended dileucine motif of VMAT2 ([Phospho-S]EXXXLL) (Fei et al., 2008) (Fig. 2D). The substitution of an acidic amino acid at this site drives VAChT to LDCVs in PC12 cells, mimicking the trafficking pattern of VMAT2 (Fei et al., 2008).
8. Regulation of expression and activity
8.1. VMAT transcription
Regulation of VMAT2 transcription has been demonstrated in cultured cell lines including the gastric epithelial cell line AGS-GR (Watson et al., 2000). These cells express the gastrin-CCK beta receptor and gastrin increases transcription of VMAT2 mRNA (Fei and Krantz, 2009). Gastrin also increases expression of HDC in enterochromaffin cells (Fei and Krantz, 2009). An increase in mRNA for both HDC and VMAT2 has been observed in animal models of hypergastrinemia, suggesting that transcriptional activity of these genes may be regulated by gastrin in vivo (Fei and Krantz, 2009).
The mechanism by which VMAT2 expression is regulated by gastrin may involve a novel function for the proteasome (Fei and Krantz, 2009). A proteasomal subunit was shown to bind a 10 base-pair segment in the VMAT2 promoter and thereby enhance gastrin-induced secretion (Fei and Krantz, 2009). Importantly, this activity is independent of the degradative functions of the proteasome elsewhere in the cell (Fei and Krantz, 2009).
Early studies using primary cultures of chromaffin cells and sympathetic ganglia suggested that increased stimulation and calcium influx might up-regulate VMAT transcription (Fei and Krantz, 2009). Conversely, minimal changes in expression are seen in vivo in response to VMAT inhibition using reserpine (Fei and Krantz, 2009). In rats, chronic administration of either the antipsychotic clozapine or the mood stabilizer lithium VMAT2 expression, presumably via increased transcription (Fei and Krantz, 2009). Up-regulation of VMAT2 in chromaffin cells has also been reported following immobilization stress in rodents (Eiden and Weihe, 2011).
8.2. VAChT transcription
VAChT is encoded in the first intron of the biosynthetic enzyme for ACh, ChAT, and is in the same transcriptional orientation. This peculiar relationship is evolutionarily conserved from C. elegans to humans suggesting an important functional role and the likelihood of co-regulated gene transcription. Indeed, studies in cultured neurons indicate that expression of ChAT and VAChT are coordinately up-regulated by retinoic acid and leukemia inhibitory factor/ciliary neurotrophic factor (LIF/CNTF) (Fei and Krantz, 2009). An upstream neuron-restrictive silencer element (NRSE) may function to coordinately regulate the expression of both genes (Fei and Krantz, 2009). However, under some circumstances VAChT and ChAT may be differentially expressed (Fei and Krantz, 2009). Some of the genomic regulatory regions responsible for differential expression of ChAT and VAChT have been mapped using transgenes containing varying amounts of the putative regulatory sequences; at least one segment of genomic DNA was shown to control expression of ChAT but not VAChT (Fei and Krantz, 2009).
8.3. Alternative mRNA splicing
Both VAChT and ChAT undergo alternative splicing of 5′ non-coding regions, and the splicing of ChAT and VAChT might be coordinated, although the functional significance of these splicing evens are not yet clear (Fei and Krantz, 2009). In Drosophila, dVMAT shows variable use of a 3′ splice site in the last exon of the gene, which leads to two divergent carboxy-terminal domains (Romero-Calderón et al., 2008). One variant is expressed in neurons, and the other in a defined subset of glia in the adult visual system (Romero-Calderón et al., 2008).
8.4. Trafficking and phosphorylation
Trafficking of both VAChT and VMAT2 may be directly regulated by phosphorylation of their cytoplasmic trafficking domains (see Section 7.2). Phosphorylation may also indirectly regulate transporter function, as suggested by a series of studies on cholinergic signaling at the NMJ showing that during periods of sustained SV release, PKC decreases and PKA increases quantal size (Fei and Krantz, 2009). In addition, although PKA does not directly phosphorylate VMATs, it is required for the localization of VMAT1 and 2 to LDCVs in PC12 cells (Eiden and Weihe, 2011; Fei and Krantz, 2009).
Trafficking of VMAT2 may also be regulated via DA-dependent signaling (Fleckenstein et al., 2007). Amphetamines decrease and cocaine increases localizaion of VMAT to SVs, and at least some of these effects appear to be dependent on DA-receptor activation by extracellular DA (Fleckenstein et al., 2007).
8.5. Heterotrimeric G proteins
VMAT transport activity is regulated by heterotrimeric G proteins (Brunk et al., 2006). This unexpected finding was first reported in 1998 and subsequent work has begun to elucidate the underlying mechanisms (Brunk et al., 2006). Interaction between VMATs and G proteins are likely to occur on secretory vesicles rather than the cell surface (Brunk et al., 2006). VMAT1 and 2 interact with either Gαo2 or Gαq depending on the cell type (Brunk et al., 2006). In each case, the downstream effect is inhibition of VMAT activity (Brunk et al., 2006). Current models suggest that a lumenal domain in the transporter itself may activate the G protein and that binding of substrate to VMAT is required for G protein inhibition of VMAT activity (Brunk et al., 2006). This in turn suggests that the function of this regulatory process may be to limit transport as the vesicle lumen fills with neurotransmitter.
9. Functional effects of altered expression and activity
9.1. Regulation of quantal size
Molecular genetic analyses of vesicular transporters have shown that presynaptic changes in their expression can regulate neurotransmitter release and post-synaptic signaling. Cholinergic signaling in vitro is increased by over-expression of VAChT, and amperometry has been used to show that over-expression of VMAT2 increases monoamine release (Edwards, 2007). Over-expression of Drosophila VMAT in vivo causes an increase in amine-dependent behaviors, consistent with an increase in a vesicular release (Fei and Krantz, 2009). In vitro studies using psychostimulants support the idea that reducing vesicular amines can decrease quantal size (Edwards, 2007).
9.2. Cytoplasmic clearance of dopamine and Parkinson’s disease
Based on their similarity to bacterial transporters and their sequestration of MPP+, it has been suggested that VMATs might help detoxify cells (Schuldiner et al., 1995). In addition to exogenous toxins, endogenous monoamines and in particular DA may cause intracellular toxicity (Guillot and Miller, 2009). In vitro overexpression of VMAT2 in PC12 cells decreases cytosolic DA, as shown indirectly by decreased neuromelanin formation, and more directly by amperometric measurements of cytoplasmic amines (Mosharov et al., 2009). Furthermore, over-expression of VMAT2 in PC12 cells lessens the toxic effects of cytosolic DA; reserpine has the opposite effect and potentiates DA’s toxicity (Fei and Krantz, 2009). Primary cultures from VMAT2 knockout mice are also more sensitive to the toxic effects of increased DA synthesis and decreased levels of VMAT2 can potentiate the neurotoxic effects of amphetamines (Guillot and Miller, 2009). Reduced expression of Drosophila VMAT potentiates the death of dopaminergic neurons in fly models of Parkinson’s disease (Lawal et al., 2010) and causes increased dopaminergic cell death in mice (Guillot and Miller, 2009). Conversely, over-expression of Drosophila VMAT exerts a neuro-protective effect on dopaminergic neurons in the fly (Lawal et al., 2010).
10. Behavioral genetics of animal models
10.1. VMAT mouse models
VMAT2 knockouts are lethal as homozygotes but important behavioral information has been obtained from studies of heterozygotes (Chaudhry et al., 2008; Eiden and Weihe, 2011; Fei and Krantz, 2009). VMAT2 heterozygotes show an increase in locomotor behavior in response to the aminergic drugs apomorphine, cocaine and amphetamine, suggesting that the synaptic response to amine release is elevated (Chaudhry et al., 2008; Fei and Krantz, 2009). However, the mice do not appear to undergo sensitization in response to chronic cocaine administration (Chaudhry et al., 2008; Fei and Krantz, 2009). VMAT2 heterozygotes also show a reduced preference for amphetamine in a place preference test (Chaudhry et al., 2008) and an increased locomotor response to ethanol (Chaudhry et al., 2008; Fei and Krantz, 2009). Moreover, males may show an increase in ethanol consumption (Chaudhry et al., 2008; Fei and Krantz, 2009). VMAT2 heterozygotes also perform worse than wild type littermates in rodent models of clinical depression (Fei and Krantz, 2009).
In addition to VMAT2 knockouts, VMAT2 knockdown animals have been generated (Fei and Krantz, 2009). VMAT2 knockdown mice show down-regulation of substance P expression and an upregulation of enkephalin expression, suggesting potential effects on signaling pathways mediated by endogenous opioids in the striatum (Fei and Krantz, 2009).
10.2. VAChT mouse models
VAChT knockdown mice have been generated and subjected to both biochemical and behavioral analyses (Fei and Krantz, 2009). In the central nervous system, extracellular acetylcholine is decreased by ~30% in the VAChT knockdown mice, and KCl-induced release of acetylcholine is also reduced. However, the total tissue levels of acetylcholine are increased in both heterozygotes and homozygotes relative to wild type (Fei and Krantz, 2009).
Homozygous VAChT knockdown mice have neuromuscular defects and their behavioral phenotype includes altered long-term habituation (Schmid et al., 2011). Neuromuscular function of the VAChT knockdown heterozygote is similar to wild type but behavioral deficits include a defect in remembering novel versus familiar objects and reduced habituation to an unfamiliar intruder (Fei and Krantz, 2009).
More recently, a VAChT knockout mouse has been generated (de Castro et al., 2009). As expected, the phenotype is more severe than that of the knockdown and the mice die soon after birth (de Castro et al., 2009). An additional line of mice with loxP sites flanking the VAChT locus has allowed the inducible deletion of VAChT in defined subsets of neurons (Guzman et al., 2011). The specific deletion of VAChT in striatal interneurons has helped to define the interaction of acetylcholine and DA in the basal ganglia, and the complexities of neurotransmitter release from cells that express both VAChT and VGLUT (Guzman et al., 2011).
10.3. C. elegans and Drosophila
The C. elegans ortholog of VAChT (unc-17) was originally identified as an uncoordinated mutant and showed resistance to cholinesterase inhibitors in some of the original behavioral screens performed in this system. The C. elegans ortholog of VMAT is encoded by cat-1 (Fei and Krantz, 2009). Mutants show a defect in their ability to regulate locomotor behavior in response to food (Fei and Krantz, 2009). This phenotype could result from decreased signaling in several aminergic pathways known to control the behavioral response to food in C. elegans.
Two mutant alleles of Drosophila VAChT have been generated including dVAChT1 which is embryonic lethal, and dVAChT2 (Fei and Krantz, 2009). VAChT2 larvae can survive through the second stage (instar) of development and locomote more slowly than wild type animals (Fei and Krantz, 2009). Similar to VAChT knock down mice, heterozygous dVAChT muant adults can survive, and although the behavior of adult heterozygotes has not been reported, electrophysiological assays suggest that during periods of sustained vesicle release, at least one circuit in the adult CNS fails to maintain normal levels of acetylcholine release (Fei and Krantz, 2009).
Mutation of dVMAT affects a number of amine-linked behaviors including courtship, locomotion and fertility (Simon et al., 2009). Overexpression of DVMAT results in an increase in motor and courtship behavior and a decreased behavioral response to cocaine (Fei and Krantz, 2009).
Mutation of Drosophila portabella causes a learning deficit consistent with its expression in the mushroom bodies, a critical structure for insect learning and memory (Brooks et al., 2011). The mutant also shows an unusual sexual phenotype characterized primarily by failure of the male to maintain position during mating (Brooks et al., 2011).
11. Human genetic studies and disease models
11.1. Genetics
The possibility that changes in VMAT function may be relevant to neuropsychiatric illness has prompted a search in humans for polymorphisms in both the coding and non-coding regions of the VMAT2 gene (Eiden and Weihe, 2011; Fei and Krantz, 2009; Wimalasena, 2010). In vitro transport assays indicate that only one coding polymorphism decreases the apparent affinity of VMAT2 for serotonin (Fei and Krantz, 2009). Polymorphisms in the coding region of VMAT2 have not been linked to Parkinson’s disease; however, polymorphisms in upstream regulatory domains have been shown to alter VMAT expression and may be protective for alcoholism (Eiden and Weihe, 2011; Fei and Krantz, 2009). In addition, VMAT2 promoter haplotypes that confer increased expression of VMAT2 in vitro may provide some protection against the development of Parkinson’s disease in women with high exposure to pesticides (Fei and Krantz, 2009). To date, the results of human genetic studies attempting to link VMAT2 polymorphisms with psychiatric illness have yielded mostly negative results ((Fei and Krantz, 2009) but see (Gutierrez et al., 2007)). Additional human genetic studies investigating a possible association of VMAT1 polymorphisms with schizophrenia or bipolar disorder have also yielded mixed results (Eiden and Weihe, 2011; Wimalasena, 2010). The lack of a clear-cut association of VMATs with human disease may reflect the conservation of their primary structure through evolution; radical changes may not be tolerated and many point mutants show minimal changes in activity. Polymorphisms associated with human disease have not yet been reported for VAChT.
11.2. Altered expression in human disease
In addition to genetic studies of VMAT polymorphisms, a number of labs have assessed VMAT expression levels in patients suffering from a variety of neuropsychiatric illnesses including schizophrenia, depression, Tourette’s syndrome and bipolar disorder (Eiden and Weihe, 2011; Fei and Krantz, 2009; Wimalasena, 2010). It is possible that altered VMAT2 expression represents a general risk factor for psychiatric disorders linked to aminergic circuits but additional studies will be needed to definitively establish this relationship.
The expression of VMAT2 has also been used to monitor the progression of neurodegenerative disorders such as PD, and a variety of radiolabeled ligands for VMAT2 have developed for use in Positron Emission Tomograpy (PET) (Eiden and Weihe, 2011; Wimalasena, 2010). Some of these ligands have also been used to follow the expression of VMAT2 in model systems and patients exposed to psychostimulants such as cocaine and amphetamines (Eiden and Weihe, 2011; Wimalasena, 2010). The relationship of psychostimulant use to VMAT expression is complex, and both increased and decreased expression has been reported (Eiden and Weihe, 2011; Wimalasena, 2010). Some variability may be due to competition of radiolabeled ligands with endogenous amines, and simultaneous changes in both VMAT expression and amine release (Eiden and Weihe, 2011).
12. Summary and future medical relevance
VAChT and VMATs have enormous potential relevance for human disease that as yet remains untapped. Molecular-genetic studies have identified specific domains and individual residues important for transport activity and localization to secretory vesicles, and shown that loss of transport activity has profound behavioral consequences. Anatomic studies demonstrating their widespread expression in both neurons and other sites underscores the potential relevance of these proteins to a variety of diagnostic and therapeutic issues; however, the relationship between vesicular transporter dysfunction and human disease remains vague. Increased clarity will likely require further advances in neuropsychiatric genetics and our understanding of complex illnesses caused by multiple interacting factors. The use of VAChT or VMAT as a marker for tracking neuronal function in vivo is also promising, but their advantage over other disease markers remains to be seen. Inhibition of VMAT activity may have some therapeutic use in addiction and further translational studies are needed. The effects of overexpression suggest that drugs which enhance the function of VMAT or VAChT could also have therapeutic value. Progress in high throughput screening strategies and the use of novel screening platforms will aid the development of these drugs. The eventual characterization of crystal structures for both VMAT and VAChT will also aid the development of new therapeutic strategies as well as to help elucidate the precise mechanisms by which these proteins transport neurotransmitters into secretory vesicles.
Acknowledgments
This work was supported by grants from the National Institute of Mental Health [MH076900], the National Institute of Environmental Health and Safety [ES015747] and NARSAD “The Brain and Behavior Research Foundation” (D.E.K.), additional funding from an NIEHS program project grant [ES016732, M.-F. Chesselet, PI) and a training fellowships from the National Institute of Environmental Health and Safety (H.O.L., ES015457).
Footnotes
Publication in part sponsored by the Swiss National Science Foundation through the National Center of Competence in Research (NCCR) TransCure, University of Bern, Switzerland; Director Matthias A. Hediger; Web: http://www.transcure.ch.
A large number of citations have been omitted due to space limitations; they are listed in Fei and Krantz, 2009.
References
- Asensio CS, Sirkis DW, Edwards RH. RNAi screen identifies a role for adaptor protein AP-3 in sorting to the regulated secretory pathway. J. Cell Biol. 2010;191:1173–1187. doi: 10.1083/jcb.201006131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Denehy ED, Zheng G, Crooks PA, Dwoskin LP, Bardo MT. The effect of a novel VMAT2 inhibitor, GZ-793A, on methamphetamine reward in rats. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2488-9. (in press, epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks ES, Greer CL, Romero-Calderon R, Serway CN, Grygoruk A, Haimovitz JM, Nguyen BT, Najibi R, Tabone CJ, Steven de Belle J, Krantz DE. A putative vesicular transporter expressed in drosophila mushroom bodies that mediates sexual behavior may define a neurotransmitter system. Neuron. 2011;72:316–329. doi: 10.1016/j.neuron.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk I, Holtje M, von Jagow B, Winter S, Sternberg J, Blex C, Pahner I, Ahnert-Hilger G. Regulation of vesicular monoamine and glutamate transporters by vesicle-associated trimeric G proteins: new jobs for long-known signal transduction molecules. Handb. Exp. Pharmacol. 2006;175:305–325. doi: 10.1007/3-540-29784-7_15. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Boulland JL, Jenstad M, Bredahl MK, Edwards RH. Pharmacology of neurotransmitter transport into secretory vesicles. Handb. Exp. Pharmacol. 2008;184:77–106. doi: 10.1007/978-3-540-74805-2_4. [DOI] [PubMed] [Google Scholar]
- de Castro BM, De Jaeger X, Martins-Silva C, Lima RD, Amaral E, Menezes C, Lima P, Neves CM, Pires RG, Gould TW, et al. The vesicular acetylcholine transporter is required for neuromuscular development and function. Mol. Cell. Biol. 2009;29:5238–5250. doi: 10.1128/MCB.00245-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RH. The neurotransmitter cycle and quantal size. Neuron. 2007;55:835–858. doi: 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Eiden LE, Weihe E. VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann. NY Acad. Sci. 2011;1216:86–98. doi: 10.1111/j.1749-6632.2010.05906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mestikawy S, Wallen-Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat. Rev. Neurosci. 2011;12:204–216. doi: 10.1038/nrn2969. [DOI] [PubMed] [Google Scholar]
- Elbaz Y, Danieli T, Kanner BI, Schuldiner S. Expression of neurotransmitter transporters for structural and biochemical studies. Protein Expr. Purif. 2010;73:152–160. doi: 10.1016/j.pep.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei H, Grygoruk A, Brooks ES, Chen A, Krantz DE. Trafficking of vesicular neurotransmitter transporters. Traffic. 2008;9:1425–1436. doi: 10.1111/j.1600-0854.2008.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei H, Krantz DE. Vesicular neurotransmitter transporters. In: Lajtha A, editor. Handbook of Neurochemisrty and Molecular Neurobiology: Neural Signaling Mechanisms. Springer; New York: 2009. pp. 89–122. [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu. Rev. Pharmacol. Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Greer CL, Grygoruk A, Patton DE, Ley B, Romero-Calderón R, Chang HY, Bainton RJ, DiAntonio A, Krantz DE. A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin and octopamine. J. Neurobiol. 2005;64:239–258. doi: 10.1002/neu.20146. [DOI] [PubMed] [Google Scholar]
- Guillot TS, Miller GW. Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol. Neurobiol. 2009;39:149–170. doi: 10.1007/s12035-009-8059-y. [DOI] [PubMed] [Google Scholar]
- Gutierrez B, Rosa A, Papiol S, Arrufat FJ, Catalan R, Salgado P, Peralta V, Cuesta MJ, Fananas L. Identification of two risk haplotypes for schizophrenia and bipolar disorder in the synaptic vesicle monoamine transporter gene (SVMT) Am. J. Med. Genet B. Neuropsychiatr. Genet. 2007;144:502–507. doi: 10.1002/ajmg.b.30499. [DOI] [PubMed] [Google Scholar]
- Guzman MS, De Jaeger X, Raulic S, Souza IA, Li AX, Schmid S, Menon RS, Gainetdinov RR, Caron MG, Bartha R, Prado VF, Prado MA. Elimination of the vesicular acetylcholine transporter in the striatum reveals regulation of behaviour by cholinergic-glutamatergic co-transmission. PLoS Biol. 2011;(9):e1001194. doi: 10.1371/journal.pbio.1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Edwards RH. Neurotransmitter corelease: mechanism and physiological role. Annu. Rev. Physiol. 2011;74:225–243. doi: 10.1146/annurev-physiol-020911-153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson JA, Stephansson O, Fredriksson R. C6ORF192 forms a unique evolutionary branch among solute carriers (SLC16, SLC17, and SLC18) and is abundantly expressed in several brain regions. J. Mol. Neurosci. 2010;41:230–242. doi: 10.1007/s12031-009-9222-7. [DOI] [PubMed] [Google Scholar]
- Jahn R, Rizzoli SO. Endocytosis in neurons: editorial introduction and overview. Traffic. 2007;8:1121–1122. doi: 10.1111/j.1600-0854.2007.00615.x. [DOI] [PubMed] [Google Scholar]
- Khare P, Mulakaluri A, Parsons SM. Search for the acetylcholine and vesamicol binding sites in vesicular acetylcholine transporter: the region around the lumenal end of the transport channel. J. Neurochem. 2010a;115:984–993. doi: 10.1111/j.1471-4159.2010.06990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare P, Ojeda AM, Chandrasekaran A, Parsons SM. Possible important pair of acidic residues in vesicular acetylcholine transporter. Biochemistry. 2010b;49:3049–3059. doi: 10.1021/bi901953j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Muhlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, et al. Cholinergic chemosensory cells in the trachea regulate breathing. Proc. Natl. Acad. Sci. USA. 2011;108:9478–9483. doi: 10.1073/pnas.1019418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal HO, Chang HY, Terrell AN, Brooks ES, Pulido D, Simon AF, Krantz DE. The Drosophila vesicular monoamine transporter reduces pesticide-induced loss of dopaminergic neurons. Neurobiol. Dis. 2010;40:102–112. doi: 10.1016/j.nbd.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, Sulzer D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons SM. Transport mechanisms in acetylcholine and monoamine storage. FASEB J. 2000;14:2423–2434. doi: 10.1096/fj.00-0203rev. [DOI] [PubMed] [Google Scholar]
- Phillips PA, Yang L, Shulkes A, Vonlaufen A, Poljak A, Bustamante S, Warren A, Xu Z, Guilhaus M, Pirola R, Apte MV, Wilson JS. Pancreatic stellate cells produce acetylcholine and may play a role in pancreatic exocrine secretion. Proc. Natl. Acad. Sci. USA. 2010;107:17397–17402. doi: 10.1073/pnas.1000359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Dando R, Jacques-Silva MC, Fachado A, Molina J, Abdulreda MH, Ricordi C, Roper SD, Berggren PO, Caicedo A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat. Med. 2011;17:888–892. doi: 10.1038/nm.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Calderón R, Uhlenbrock G, Boryz J, Simon AF, Grygoruk A, Yee SK, Shyer A, Ackerson LC, Maidment NT, Meinertzhagen IA, Hovemann BT, Krantz DE. A glial variant of the vesicular monoamine transporter is required to store histamine in the Drosophila visual system. PLoS Genet. 2008;4(11):e1000245. doi: 10.1371/journal.pgen.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S, Azzopardi E, De Jaeger X, Prado MA, Prado VF. VAChT knock-down mice show normal prepulse inhibition but disrupted long-term habituation. Genes Brain Behav. 2011;10:457–464. doi: 10.1111/j.1601-183X.2011.00686.x. [DOI] [PubMed] [Google Scholar]
- Schuldiner S, Shirvan A, Linial M. Vesicular neurotransmitter transporters: from bacteria to humans. Physiol. Rev. 1995;75:369–392. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- Simon AF, Daniels R, Romero-Calderón R, Grygoruk A, Chang HY, Najibi R, Shamouelian D, Salazar E, Solomon M, Ackerson LC, et al. Drosophila vesicular monoamine transporter mutants can adapt to reduced or eliminated vesicular stores of dopamine and serotonin. Genetics. 2009;181:525–541. doi: 10.1534/genetics.108.094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy E, Arkin IT, Gottschalk KE, Kaback HR, Schuldiner S. Structural conservation in the major facilitator superfamily as revealed by comparative modeling. Protein Sci. 2004;13:1832–1840. doi: 10.1110/ps.04657704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson F, Kiernan RS, Deavall DG, Varro A, Dimaline R. Transcriptional activation of the rat vesicular transporter 2 promoter in gastric epithelial cells: regulation by gastrin. J. Biol. Chem. 2000;276:7661–7671. doi: 10.1074/jbc.M006697200. [DOI] [PubMed] [Google Scholar]
- Weihe E, Eiden LE. Chemical neuroanatomy of the vesicular amine transporters. FASEB J. 2000;14:2435–2449. doi: 10.1096/fj.00-0202rev. [DOI] [PubMed] [Google Scholar]
- Wimalasena K. Vesicular monoamine transporters: structure-function, pharmacology, and medicinal chemistry. Med. Res. Rev. 2010;31:483–519. doi: 10.1002/med.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]


