Abstract
We have realized that N-formylations of free amines of some drug leads can improve PK/PD property of parent molecules without decreasing their biological activities. In order to selectively formylate primary amines of polyfunctional molecules, we have sought a mild and convenient formylation reaction. In our screening of N-formylation of an α-amino acid, L-phenylalanine, none of formylation conditions reported to date yielded the desired HCO-L-Phe-OH with satisfactory yield. N-Formylations of amino acids with HCO2H require the reactions in a water-containing media and suppress polymerization reactions due to the competitive reactions among carboxylic acids. We found that N-formylations of α-amino acids could be achieved with a water-soluble peptide coupling additive, an oxyma derivative, (2,2-dimethyl-1,3-dioxolan-4-yl)methyl-2-cyano-2-(hydroxyimino)acetate (2), EDCI, and NaHCO3 in water or a mixture of water and DMF system, yielding N-formylated α-amino acids with excellent yields. Moreover, these conditions could selectively formylate primary amines over secondary amines at a controlled temperature. A usefulness of these conditions was demonstrated by selective formylation of daptomycin antibiotic which contains three different amino groups.
Keywords: N-formylation, Reactions in water media, Water-soluble oxyma, Glyceroacetonide-oxyma, Amino acids, Kanamycin, Spectinomycin, Daptomycin
1. Introduction
In our SAR studies of antibacterial agents, we have realized that N-formylations of free amines of some antibiotics do not significantly decrease their bioactivities and can be applied to improve PK/PD property of parental molecules. Because of necessity of selective formylation reactions of antibiotics and antibacterial agents in our ongoing programs, we have sought a mild and convenient N-formylation reaction condition that can be applied to a wide range of complex natural products, oligo- to poly-peptides, and amino acids. To date, the numerous formylating agents and conditions have been reported.1 Although several formylating agents can be applicable for the formylations of C-protected amino acids, it is not possible to achieve effective formylation reactions for non-protected amino acids with reported reagents and conditions.2 In addition, many formylating agents are hygroscopic and are not tolerated in appropriate solvents for the reactions for amino acids and oligo-peptides (e.g. water-containing solvents). In our recent finding of amide-forming reactions with the ethyl 2-cyano-2-(hydroxyimino)acetate (Oxyma, 1) derivative, glyceroacetonide-Oxyma 2 in water media (Figure 1),3 it was observed that formylation of H-L-Phe-OH could be achieved with HCO2H (5 eq.), 2 (2 eq.), EDCI (2 eq.) and NaHCO3 (10 eq.) in water (0.2–0.3 M) to yield the corresponding HCO-L-Phe-OH in greater than 90% yield. On the other hand, the same reaction in the absence of glyceroacetonide-Oxyma 2 did not furnish the desired HCO-L-Phe-OH. Thus, effectiveness of glyceroacetonide-Oxyma 2 in the formylation of amino acid in water was unambiguously determined. Herein, we report mild and convenient N-formylations in water or water-containing solvent systems, and selective N-formylations of primary amines.
Figure 1.
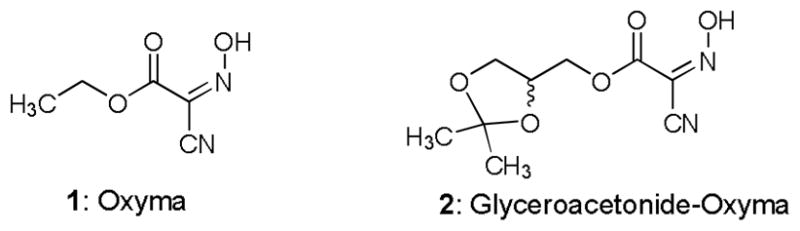
Structures of Oxyma 1 and glyceroacetonide-Oxyma 2.
2. Results and discussion
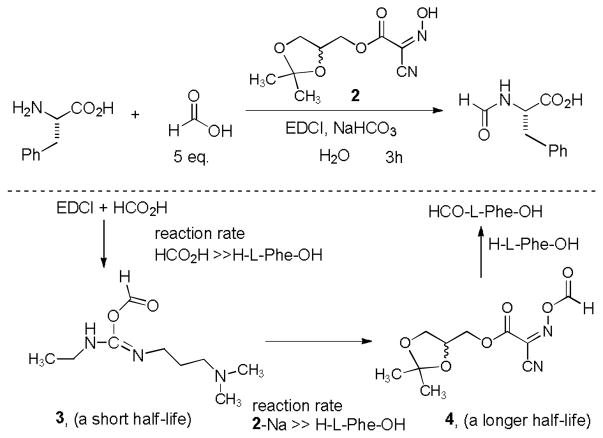
Formylation of H-L-Phe-OH with HCO2H, glyceroacetonide-Oxyma 2, EDCI, NaHCO3 in water seems to undergo through the well-known reaction mechanism with EDCI,4 however, in this reaction several interesting chemical observations are worth mentioning. HCO2H reacts with EDCI faster than H-L-Phe-OH; 5 equivalent of HCO2H could completely suppress the undesired competitive reaction with H-L-Phe-OH. Due to the fact that formylation of H-L-Phe-OH with EDCI in water did not proceed in the absence of 2, the initial intermediate, carbamimidic formic anhydride 3 may have a relatively short half-life or not be a good electrophile as a formylating agent in water. However, the intermediate 3 reacts with the glyceroacetonide-Oxyma 2-sodium salt5 to furnish the active ester 4 which has a relatively long half-life and serves as N-formylating agent in water. It is important to note that formylation of H-L-Phe-OH with Oxyma 1 in water furnished the desired product in very low yield (<10%). As observed in peptide-forming reactions, formylation using 1 could be improved dramatically when the reaction was performed in a mixture of DMF-H2O (9/1).3b Thus, 1 and 2 can efficiently be utilized for formylation of H-L-Phe-OH by using water or a mixture of water and DMF. However, glyceroacetonide-Oxyma 2 has a significant advantage over 1 in that 2 can be removed completely after the reactions via an acidic water work-up, thus, the only formylated-products can be extracted from reaction mixtures after a simple work-up.
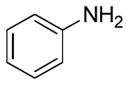
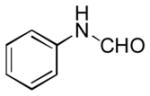
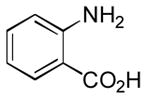
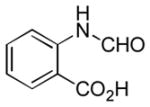
In order to examine scope and limitations of N-formylation reactions with HCO2H, 2 (or 1), EDCI, and NaHCO3 in H2O (condition A) or in DMF-H2O (9/1, condition B), we have applied these conditions to a wide variety of primary and secondary amines, and α-amino acids. As observed for H-L-Phe-OH, formylations of all α-amino acids tested in this program provided the corresponding N-formylated products in H2O. Representative data are summarized in Table 1 (entries 15–18). In all cases N-formylations of α-amino acids with condition A furnished the desired products in better yield than those with condition B (85–95 vs 30–60% yield). We have demonstrated N-formylation of an oligopeptide in water; N-formylation of the pentapeptide with condition A yielded the corresponding formylation product in 90% (entry 19). N-Formylation of C-protected α-amino acids could be achieved efficiently either with condition A or B without noticeable difference in yield of the products (entries 8–10). Thus, formylations of aliphatic and aromatic amines were performed with Oxyma 1 in DMF-H2O (condition B); N-formylations of benzylamine, octylamine, and aniline provided the corresponding products in quantitative yield (entries 1, 2, and 5). N-Formylation reactions of a monoprotected 1,3-diamine and an amino-alcohol provided the N-formylated products in excellent yields (entries 3 and 4). On the other hand, N-formylations of 2-aminobenzoic acid and 2-aminophenol gave rise to the desired products in 30% and 25% yield, respectively (entries 6 and 7).6 Formylations of secondary amines, piperidine, morpholine, L-Pro-OMe, and N-Me-L-Val-OMe were completed within 3h to yield the corresponding products in good yields (entries 11, 12, 13, and 14). Interestingly, formylation of a secondary amine, N-Me-L-Val-OMe provided the formylated-product in less than 5% yield at 0 °C, whereas a primary amine H-L-Val-OCH3 was formylated at 0 °C-rt. The rate of the reaction progress of formylations of N-Me-L-Val-OMe and H-L-Val-OCH3 in H2O (condition A) was monitored over time and their reaction kinetic curves are shown in Figure 2. The striking difference in reaction rate for formylations of primary and secondary amines was observed when the reactions were performed in water or in water-containing solvents.
Table 1.
N -Formylations of primary and secondary amines.11
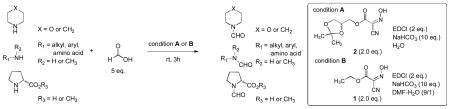
| ||||
|---|---|---|---|---|
| entry | starting material | product | condition | yield (%) |
| 1 |
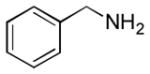
|
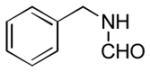
|
Ba | quant. |
| 2 |
|
|
Ba | quant. |
| 3 |
|
|
Ba | quant. |
| 4 |
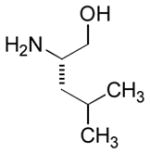
|
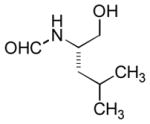
|
Ba | quant. |
| 5 |
|
|
Ba | quant. |
| 6 |
|
|
Ba | 30 |
| 7 |
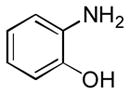
|
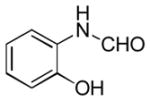
|
Ba | 25 |
| 8 |
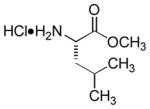
|
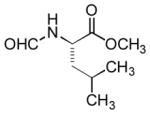
|
A or B | 95 |
| 9 |
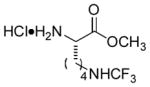
|
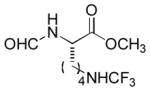
|
A or B | 95 |
| 10 |
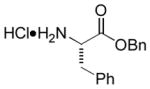
|
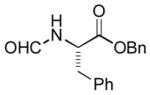
|
A or B | 95 |
| 11 |
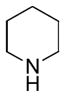
|
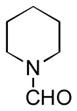
|
A or B | quant. |
| 12 |
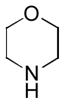
|
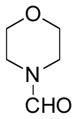
|
A or B | quant. |
| 13 |
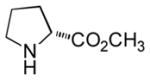
|
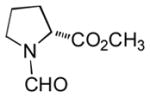
|
A or B | 95 |
| 14 |
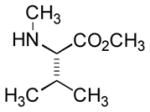
|
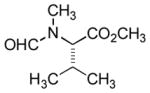
|
A or B | 95 |
| 15 |
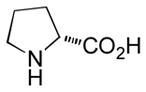
|
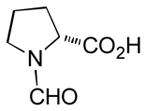
|
Ab | 90 |
| 16 |
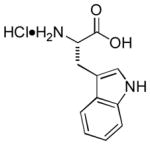
|
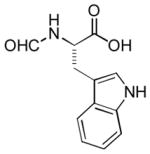
|
Ab | 85 |
| 17 |
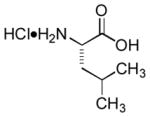
|
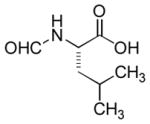
|
Ab | 95 |
| 18 |
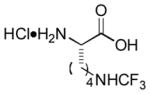
|
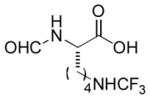
|
Ab | 90 |
| 19 |
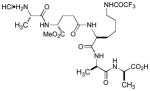
|
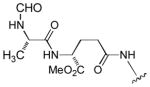
|
A | 90 |
| 20 |
|
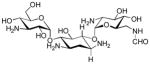
|
A | 30 |
| 21 |
|
|
A | 50 |
The condition A was also effective.
The same reaction under the condition B yielded the product in 30–60% yield.
Figure 2.
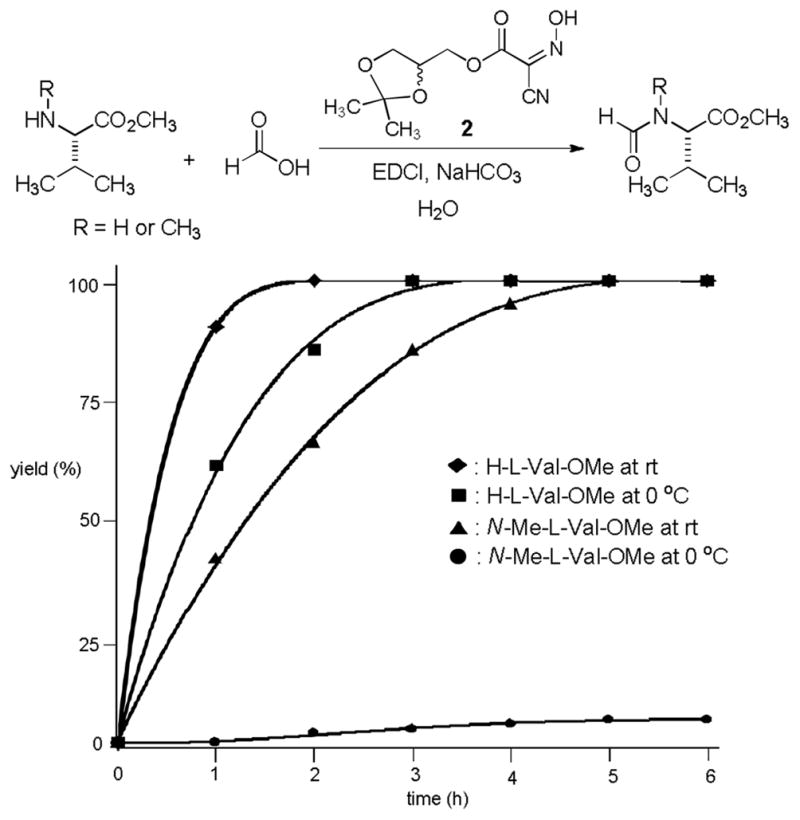
The reaction kinetic curves of formylations of H-LVal-OMe and N-Me-L-Val-OMe in H2O at rt and 0 °C.
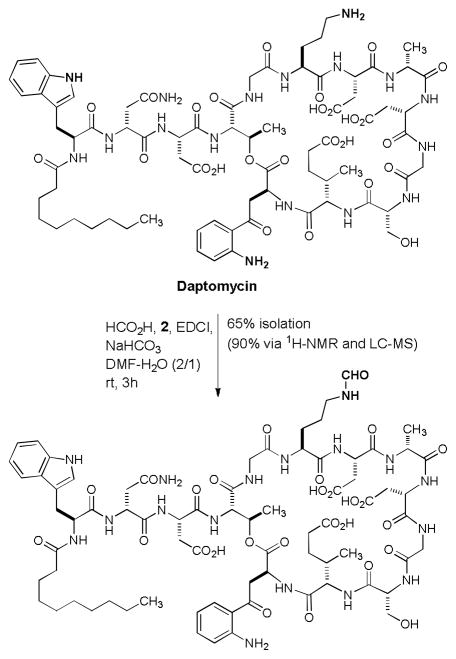
We have applied these formylation reaction conditions to several antibacterial natural products. Selective N-formylation of kanamycin A could be achieved at the primary amine, yielding the 6′-formylated kanamycin A in 30% isolation yield (65% yield based on LC-MS) (entry 20 in Table 1).7 Formylation of spectinomycin in H2O at rt furnished the mono-formylated product in 50% yield (entry 21).8 Daptomycin is a cyclic lipopeptide antibiotic used in the treatment of certain community-associated methicillin resistant S. aureus (CA-MRSA) and healthcare-associated-MRSA (HA-MRSA) infections.9 Daptomycin possesses stereoelectronically different three free amines, four carboxylic acids, a free alcohol in the molecule, however, shows limited water solubility. Selective N-formylation of daptomycin was achieved at the primary amine of the lysine residue in DMF-H2O (2/1) to provide the expected N-formylation product in 65% isolation yield after a reverse HPLC purification (90% yield based on analysis of the crude product via 1H-NMR and LC-MS) (Scheme 2).10
Scheme 2.
Selective formylation of daptomycin.
In summary, we have demonstrated selective N-formylation reactions using HCO2H, Oxyma 1 or glyceroacetonide-Oxyma 2, EDCI, and NaHCO3 in DMF-H2O system or in H2O.11 The N-formylation reaction conditions described here do not require strict anhydrous conditions necessary for ordinal formylation reactions.1,2 To the best of our knowledge, N-formylation reactions of α-amino acids have never been achieved efficiently without a suitable C-protection. We demonstrated that high yielding N-formylations of α-amino acids could readily be accomplished with the described conditions. Glyceroacetonide-Oxyma 2 displays remarkable physico-chemical properties as an additive of N-formylation reactions with EDCI in water media. Importantly, simple aqueous work-up procedures can remove all reagents utilized in the reactions to afford N-formylation products in high yield with excellent purity.
Scheme 1.
Formylation of H-L-Phe-OH in water and a plausible reaction mechanism.
Acknowledgments
The authors thank the National Institutes of Health (NIAID grant AI084411-02) and The University of Tennessee for generous financial supports. NMR data were obtained on instruments supported by the NIH Shared Instrumentation Grant.
References and notes
- 1.(a) Blicke FF, Lu CJ. J Am Chem Soc. 1952;74:3933. [Google Scholar]; (b) Sheehan JC, Yang DDH. J Am Chem Soc. 1958;80:1154. [Google Scholar]; (c) Pettit GR, Thomas EG. J Org Chem. 1959;24:895. [Google Scholar]; (d) Staab HA, Polenski B. Liebigs Ann Chem. 1962;655:95. [Google Scholar]; (e) Yale HL. J Org Chem. 1971;36:3238. [Google Scholar]; (f) Kraus MA. Synthesis. 1973:361. [Google Scholar]; (f) Effenberger F, Muck AO, Bessey E. Chem Ber. 1980;113:2086. [Google Scholar]; (g) Waki M, Meienhofer J. J Org Chem. 1977;42:2019. doi: 10.1021/jo00431a046. [DOI] [PubMed] [Google Scholar]; (h) Effenberger F, Bessey E. Chem Ber. 1980;113:2100. [Google Scholar]; (i) Effenberger F, Keil M, Bessey E. Chem Ber. 1980;113:2110. [Google Scholar]; (j) Gramain JC, Rémuson R. Synthesis. 1982:264. [Google Scholar]; (k) Martinez J, Laur J. Synthesis. 1982:979. [Google Scholar]; (l) Yazawa H, Goto S. Tetrahedron Lett. 1985;26:3703. [Google Scholar]; (m) Kisfaludi L, Ötvös L., Jr Synthesis. 1987:510. [Google Scholar]; (n) Olah GA, Ohannesian L, Arvanaghi M. Chem Rev. 1987;87:671. [Google Scholar]; (o) Strazzolini P, Giumanini AG, Cauci S. Tetrahedron. 1990;46:1081. [Google Scholar]; (p) Neveux M, Bruneau C, Dixneuf PH. J Chem Soc, Perkin Trans 1. 1991:1197. [Google Scholar]; (q) Katritzky AR, Chang HX, Yang B. Synthesis. 1995:503. [Google Scholar]; (r) Duczek W, Deutsch J, Vieth S, Niclas HJ. Synthesis. 1996:37. [Google Scholar]; (s) Berry MB, Blagg J, Craig D, Willis MC. Synlett. 1992:659. [Google Scholar]; (t) Akikusa N, Mitsui K, Sakamoto T, Kikugawa Y. Synthesis. 1992:1058. [Google Scholar]; (u) Chancellor T, Morton C. Synthesis. 1994:1023. [Google Scholar]; (v) Giard T, Be’nard D, Plaquevent JC. Synthesis. 1998:297. [Google Scholar]; (w) Meinnel T, Patiny L, Ragusa S, Blanquet S. Biochemistry. 1999;38:4287. doi: 10.1021/bi982622r. [DOI] [PubMed] [Google Scholar]; (x) Reddy PG, Kumar GDK, Baskaran S. Tetrahedron Lett. 2000;41:9149. [Google Scholar]; (y) Hill DR, Hsiao CN, Kurukulasuriya R, Wittenberger SJ. Org Lett. 2002;4:111. doi: 10.1021/ol016976d. [DOI] [PubMed] [Google Scholar]; (z) Cochet T, Bellosta V, Greiner A, Roche D, Cossy J. Synlett. 2011:1920. see reference 2. [Google Scholar]
- 2.(a) Jung SH, Ahn JH, Park SK, Choi JK. Bull Korean Chem Soc. 2002;23:149. [Google Scholar]; (b) Mihara M, Ishino Y, Minakata S, Komatsu M. Synthesis. 2003:2317. [Google Scholar]; (c) De Luca L, Giacomelli G, Porcheddu A, Salaris M. Synlett. 2004:2570. [Google Scholar]; (d) Iranpoor N, Firouzabadi H, Jamalian A. Tetrahedron Lett. 2005;46:7963. [Google Scholar]; (e) Bose AK, Ganguly SN, Manhas MG, Guha A, Pombo-Villars E. Tetrahedron Lett. 2006;47:4605. [Google Scholar]; (f) Hosseini-Sarvari M, Shargi H. J Org Chem. 2006;71:6652. doi: 10.1021/jo060847z. [DOI] [PubMed] [Google Scholar]; (g) Das B, Krishnaiah M, Balasubramanyam P, Veeranjaneyulu B, Kumar DN. Tetrahedron Lett. 2008;49:2225. [Google Scholar]; (h) Chandra Shekhar A, Ravi Kumar A, Sathaiah G, Luke Paul V, Sridhar M, Shanthan Rao P. Tetrahedron Lett. 2009;50:7099. [Google Scholar]; (i) Saidi O, Bamford MJ, Blacker AJ, Lynch J, Marsden SP, Plucinski P, Watson RJ, Willimas JMJ. Tetrahedron Lett. 2010;51:5804. [Google Scholar]; (j) Kim J-G, Jang DO. Synlett. 2010:1231. [Google Scholar]; (k) Chen FMF, Benoiton NL. Synthesis. 1979:709. [Google Scholar]; (l) Brahmachari G, Laskar S. Tetrahedron Lett. 2010;51:2319. [Google Scholar]; (m) Lei M, Ma L, Hu L. Tetrahedron Lett. 2010;51:4186. [Google Scholar]; (n) Shastri LA, Shastri SL, Bathula CD, Basanagouda M, Kulkarni MV. Synth Commun. 2011;41:476. [Google Scholar]; (o) Krishnakumar B, Swaminathan M. J Mol Catal A: Chem. 2011;334:98. [Google Scholar]; (p) Suchý M, Elmehriki AAH, Hudson RHE. Org Lett. 2011;13:3952. doi: 10.1021/ol201475j. [DOI] [PubMed] [Google Scholar]
- 3.(a) Wang Y, Aleiwi BA, Wang Q, Kurosu M. Org Lett. 2012;14:4910. doi: 10.1021/ol3022337. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang Q, Wang Y, Kurosu M. Org Lett. 2012;14:3372. doi: 10.1021/ol3013556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Khattab SN. Bull Chem Soc Jpn. 2010;83:1374. [Google Scholar]; (b) El-Faham, Subiros-Funosas R, Albericio F. Chem Eur J. 2010;19:3641. doi: 10.1039/c003719b. [DOI] [PubMed] [Google Scholar]; (c) Subiros-Funosas R, Prohens R, Barbas R, El-Faham A, Albericio F. Chem Eur J. 2009;15:9394. doi: 10.1002/chem.200900614. [DOI] [PubMed] [Google Scholar]
- 5.We have demonstrated that Oxyma 1and glyceroacetonide-Oxyma 2exist as their Na salts in aq. NaHCO3 solution.
- 6.Poor reactivity of 2-aminobenzoic acid and 2-aminophenol in these formylations is probably due to the strong formation of intramolecular hydrogen bonding between the NH2 and COOH or OH groups.
- 7.Difference in reactivity of the nitrogen atoms in kanamycin and amikacin, see Hanessian S, Kornienkoa A, Swayze EE. Tetrahedron. 2003;59:995.Bera S, Zhanel GG, Schweizer F. J Med Chem. 2010;53:3626. doi: 10.1021/jm1000437.Kawaguchi H, Naito T, Nakagawa S, Fujisawa K. Antibiotics. 1972;25:695. doi: 10.7164/antibiotics.25.695.Mingeot-Leclercq MP, Glupczynski Y, Tulkens PM. Antimicrob Agents Chemother. 1999;43:727. doi: 10.1128/aac.43.4.727.Mingeot-Leclercq MP, Tulkens PM. Antimicrob Agents Chemother. 1999;43:1003. doi: 10.1128/aac.43.5.1003.
- 8.(a) Andre B. Antimicrob Agents. 2005:470. [Google Scholar]; (b) Peeters M. Antimicrob Agents Chemother. 1984;26:608. doi: 10.1128/aac.26.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sanson-Lepors M. Antimicrob Agents Chemother. 1986;30:512. doi: 10.1128/aac.30.3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debono M, Barnhart M, Carrell CB, Hoffmann JA, Occolowitz JL, Abbott BJ, Fukuda DS, Hamill RL, Biemann K, Herlihy WC. J Antibiot. 1987;40:761. doi: 10.7164/antibiotics.40.761. [DOI] [PubMed] [Google Scholar]
- 10.[α]D23 = +30 °(c 0.1, CHCl3); IR (neat) 3302, 3063, 2928, 2856, 1723, 1717, 1657, 1545, 1536, 1503, 1454, 1408, 1203, 1142, 1024, 828, 742 cm−1; 1H NMR (400 MHz, DMSO-d6) δ 12.31 (s, 4H), 10.80 (d, J = 2.4 Hz, 1H), 8.51–8.43 (m, 2H), 8.37 (d, J = 7.6 Hz, 3H), 8.26 (t, J = 6.1 Hz, 1H), 8.16 (d, J = 7.4 Hz, 3H), 8.07 (d, J = 5.7 Hz, 1H), 8.03 (d, J = 6.3 Hz, 1H), 8.02 (d, J = 1.7 Hz, 1H), 7.96–7.91 (m, 1H), 7.77 (t, J = 9.1 Hz, 2H), 7.69–7.57 (m, 2H), 7.34 (d, J = 8.0 Hz, 2H), 7.30–7.25 (m, 1H), 7.18 (dd, J = 26.7, 24.4 Hz, 2H), 7.10–7.05 (m, 1H), 7.01–6.97 (m, 1H), 6.92 (s, 1H), 6.77 (d, J = 7.9 Hz, 1H), 6.56 (t, J = 7.6 Hz, 1H), 5.12–5.04 (m, 1H), 4.92–4.84 (m, 1H), 4.70–4.47 (m, 8H), 4.46–4.40 (m, 1H), 4.31–4.24 (m, 1H), 4.18–4.08 (m, 2H), 3.87 (d, J = 13.9 Hz, 1H), 3.49–3.42 (m, 2H), 3.21 (s, 1H), 3.15–3.04 (m, 3H), 2.94 (dd, J = 14.8, 9.0 Hz, 1H), 2.80 (ddd, J = 27.9, 16.7, 5.6 Hz, 2H), 2.68–2.57 (m, 2H), 2.51–2.39 (m, 4H), 2.37 (p, J = 1.9 Hz, 1H), 2.35–2.26 (m, 2H), 2.06 (t, J = 7.3 Hz, 2H), 1.94 (dd, J = 15.6, 10.2 Hz, 1H), 1.78–1.66 (m, 1H), 1.61–1.44 (m, 3H), 1.43–1.34 (m, 2H), 1.30–1.21 (m, 10H), 1.21–1.14 (m, 5H), 1.11 (d, J = 6.4 Hz, 6H), 0.87 (q, J = 6.9 Hz, 6H); HRMS (EI) calcd for C73H102N17O27 (M + H+): 1648.7131, found: 1648.7135.
- 11.General procedure for N-formylations: To a solution of amine (1 eq.), formic acid (5 eq.), sodium bicarbonate (10 eq.), and glyceroacetonide-Oxyma 1 (2 eq.) in H2O (0.2–0.3M) solution was added EDCI (2 eq.) The reaction mixture was stirred for 3h and quenched with 1% aq. HCl. The aqueous phase was extracted with EtOAc (or CHCl3 or CHCl3-MeOH (10/1). The combined organic extracts were dried over Na2SO4 and evaporated in vacuo. Purification by a silica gel chromatography (or sephadex LH20) afforded the desired compound (yields were given in Table 1). Similarly, N-formylations were performed with Oxyma 1in DMF-H2O (9/1).