Abstract
To determine the clinicopathologic characteristics and prognosis of gastric cancer in young patients, a total of 1985 gastric cancer patients who had undergone gastrectomy at our hospital were reviewed. The male-to-female ratio was significantly lower in the young patients than in either the middle-aged (P < 0.0001) or elderly patients (P < 0.0001). Undifferentiated carcinoma was observed more frequently in the young patients compared with either the middle-aged (P < 0.0001) or elderly patients (P < 0.0001). Furthermore, peritoneal metastasis was observed more frequently in the young patients than in either the middle-aged (P < 0.005) or elderly patients (P < 0.005). Five-year survival rates were 61.0, 73.6 and 68.1% in the young, middle-aged and elderly patients, respectively. The prognosis of the middle-aged patients was significantly better than that of either the young or the elderly patients (P < 0.05). Multivariate analysis indicated that age was an independent prognostic factor. Peritoneal recurrence was more frequently observed in the young patients than either the middle-aged or the elderly patients (P < 0.05). Gastric cancer in young patients has unique characteristics, namely, a predominance of female patients and a high frequency of undifferentiated cancer and peritoneal metastasis and recurrence.
Keywords: age, gastric cancer, prognosis
Gastric cancer is frequent in middle-aged and elderly populations. Although gastric cancer is rare in young populations (Okamoto et al., 1988; Mitsudomi et al., 1989), it has been reported that gastric cancer in young patients has some unique characteristics compared with that in middle-aged and elderly patients. For instance, the male-to-female ratio shows a predominance of females among younger patients (Bloss et al., 1980; Mori et al., 1985; Sandler and Holland, 1987; Tso et al., 1987). Furthermore, a significantly higher frequency of both Borrmann type 4 and poorly differentiated adenocarcinoma with a scirrhus growth pattern has been noted as a characteristic of gastric cancer in young patients (Bloss et al., 1980; Sandler and Holland, 1987; Tso et al., 1987; Okamoto et al., 1988; Mitsudomi et al., 1989). On the other hand, the prognosis of gastric cancer in young patients remains unclear thus far. The aim of the present study was to clarify the clinicopathologic characteristics and prognosis of gastric cancer in young patients.
Materials and Methods
Patients
The present study was based on a retrospective analysis of 1985 patients with gastric adenocarcinoma who underwent gastrectomy at our institution between January 1975 and December 2000. The clinicopathologic findings were determined according to the Japanese Classification of Gastric Carcinoma (Japanese Gastric Carcinoma Association, 1998). Patients were periodically checked for early recurrence by diagnostic imaging (chest X-ray, double-contrast barium meal study, upper gastrointestinal fiberscopy, ultrasonography and computed tomography). All patients were monitored for at least 5 years. The causes of death and pattern of recurrence were determined by reviewing the medical records, including laboratory data, ultrasonography, computed tomography, scintigraphy, peritoneal puncture and laparotomy, or by direct inquiries addressed to bereaved families. In some cases, postmortems were performed to establish the cause of death.
Statistical analysis
Association among factors was evaluated by the chi-squared test, and the significance of differences among the means was determined by the Mann-Whitney U test. Survival curves were calculated according to the Kaplan-Meier method. Survival data shown in the present study were cancer specific. To this end, patients who died from another malignancy, another disease or an accident were treated as censored cases in the survival analysis. Differences between survival curves were examined with the log rank test. Multivariate analysis was performed using the Cox proportional hazards model and a stepwise procedure. The covariates included gender, age, histological classification, tumor size, depth of invasion, lymph node metastasis, lymphatic vessel invasion, blood vessel invasion, peritoneal metastasis, liver metastasis and lymph node dissection. The accepted level of significance was P < 0.05. Stat View software (Abacus Concepts, Berkeley, CA) was used for all statistical analyses.
Results
Clinicopathologic characteristics of young patients
Patient age ranged from 20 to 93 years with a mean of 62 years, and 1266 patients were male and 719, female. According to previous reports (Maeta et al., 1995; Saito et al., 2006), patients were divided into 3 groups as follows: young patients aged under 40 years; middle-aged patients aged 40 years and over and under 70 years; and elderly patients aged 70 years and over. The correlation between age and clinicopathologic factors is shown in Table 1. The male-to-female ratio was significantly lower in the young patients than in either the middle-aged (P < 0.0001) or elderly patients (P < 0.0001). Undifferentiated carcinoma was observed more frequently in the young patients than in either the middle-aged (P < 0.0001) or elderly patients (P < 0.0001). Tumor size in the young patients was significantly larger than that in the middle-aged patients (P < 0.05). The frequency of blood vessel invasion was significantly lower in the young patients than that in the elderly patients (P < 0.05). Furthermore, peritoneal metastasis was observed more frequently in the young than in either the middle-aged (P < 0.005) or elderly patients (P < 0.005).
Table 1. Correlation between age and clinicopathologic features.
| Variables | Age classification | |||
| Young [84] | Middle-aged [1314] | Elderly [587] | ||
| Gender | Male | 32 (38.1)***, ††† | 871 (66.3) | 363 (61.8) |
| Female | 52 (61.9) | 443 (33.7) | 224 (38.2) | |
| Histology§ | Differentiated | 10 (11.9)***, ††† | 589 (44.8) | 330 (56.2)‡‡‡ |
| Undifferentiated | 74 (88.1) | 725 (55.2) | 257 (43.8) | |
| Tumor size | < 8 cm | 53 (63.1)* | 962 (73.2) | 430 (73.3) |
| ≥ 8 cm | 31 (36.9) | 352 (26.8) | 157 (26.7) | |
| Serosal invasion | Absent | 49 (58.3) | 825 (62.8) | 377 (64.2) |
| Present | 35 (41.7) | 489 (37.2) | 210 (35.8) | |
| Lymph node metastasis | Absent | 46 (54.8) | 760 (57.8) | 336 (57.2) |
| Present | 38 (45.2) | 554 (42.2) | 251 (42.8) | |
| Lymphatic involvement | Absent | 39 (46.4) | 701 (53.3) | 295 (50.3) |
| Present | 45 (53.6) | 613 (46.7) | 292 (49.7) | |
| Vascular involvement | Absent | 61 (72.6)† | 873 (66.4) | 345 (58.8)‡‡ |
| Present | 23 (27.4) | 441 (33.6) | 242 (41.2) | |
| Peritoneal metastasis | Absent | 73 (86.9)†† | 1222 (93.0) | 558 (95.1)‡‡ |
| Present | 11 (13.1) | 92 (7.0) | 29 (4.9) | |
| Liver metastasis | Absent | 84 (100) | 1274 (97.0) | 562 (95.7) |
| Present | 0 (0) | 40 (3.0) | 25 (4.3) | |
| Stage | I/II | 52 (61.9) | 852 (64.8) | 377 (64.2) |
| III/IV | 32 (38.1) | 462 (35.2) | 210 (35.8) | |
| Lymph node dissection|| | D0/D1 | 8 (9.5)†† | 206 (15.7) | 156 (26.6)‡‡‡ |
| D2/D3 | 76 (90.5) | 1108 (84.3) | 431 (73.4) | |
| Multiple gastric cancer | Absent | 82 (97.6) | 1233 (93.8) | 539 (91.8) |
| Present | 2 (2.4) | 81 (6.2) | 48 (8.2) | |
| Curability | Curative | 74 (88.1) | 1137 (86.5) | 520 (88.6) |
| Non-curative | 10 (11.9) | 177 (13.5) | 67 (11.4) | |
[ ], number of patients; ( ), %.
§Differentiated, papillary or tubular adenocarcinoma; undifferentiated, poorly differentiated or undifferentiated adenocarcinoma, or signet ring cell carcinoma.
||D0, no dissection; D1, dissection of group-1 lymph nodes; D2, dissection of group-1 and -2 lymph nodes; D3, dissection of group-1 to -3 lymph nodes.
*P < 0.05: young versus middle-aged.
†P < 0.05: young versus elderly.
‡‡P < 0.005: middle-aged versus elderly.
††P < 0.005: young versus elderly.
***P < 0.0001: young versus middle-aged.
†††P < 0.0001: young versus elderly.
‡‡‡P < 0.0001: middle-aged versus elderly.
Age and survival
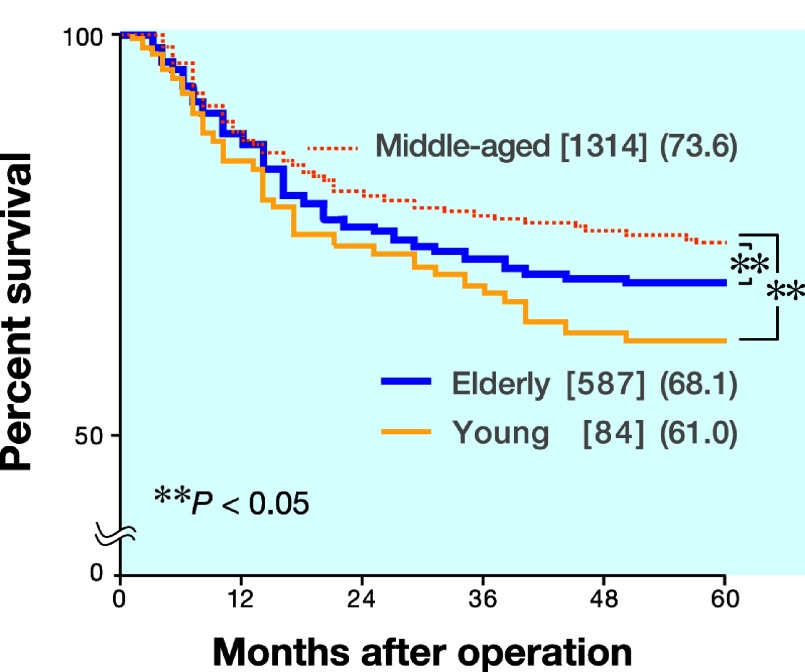
Among 1985 patients, 1731 underwent curative surgery and were included in the survival analysis. At the time of analysis, the median follow-up of 1069 surviving patients was 108 months. Of 662 deaths, 334 were related to recurrence of gastric cancer, 328 were due to either an unrelated malignancy, an unrelated disease or an accident. Five-year survival rates were 61.0, 73.6 and 68.1% in the young, middle-aged and elderly patients, respectively. The prognosis of the middle-aged patients was significantly better than that of either the young or the elderly patients (P < 0.05; Fig. 1). We employed Cox's proportional hazards model and a stepwise procedure to assess whether age represents an independent prognostic factor. The results showed that age, tumor size, depth of invasion, lymph node metastasis, peritoneal metastasis, liver metastasis, lymphatic or blood vessel invasion and curability were independent prognostic factors (Table 2).
Fig. 1.
Survival curves in age-classified patients with gastric cancer. Five-year survival rates are 61.0%, 73.6% and 68.1% in young, middle-aged and elderly patients, respectively. The prognosis in the middle-aged patients is significantly better than that in either the young or elderly patients (P < 0.05). [ ], number of patients; (), %.
Table 2. Multivariate analysis of various clinicopathologic factors in patients with gastric carcinoma.
| Variable | Hazard ratio | 95% confidence interval | P value |
| Tumor size [continuous variable] | 1.042 | 1.022–1.062 | < 0.0001 |
| Age: Young (versus middle-aged) | 1.577 | 1.079–2.304 | 0.019 |
| Age: Elderly (versus middle-aged) | 1.482 | 1.227–1.790 | < 0.0001 |
| Depth of invasion (T1–T4)* | 2.066 | 1.812–2.356 | < 0.0001 |
| Lymph node metastasis (N0–N3)† | 1.550 | 1.425–1.687 | < 0.0001 |
| Lymphatic vessel invasion (Ly0–Ly3)‡ | 1.207 | 1.108–1.314 | < 0.0001 |
| Blood vessel invasion (V0–V3)§ | 1.095 | 1.012–1.184 | 0.024 |
| Peritoneal metastasis (presence or absence) | 1.388 | 1.237–1.557 | < 0.0001 |
| Liver metastasis (presence or absence) | 1.449 | 1.270–1.652 | < 0.0001 |
| Curability | 2.532 | 1.972–3.257 | < 0.0001 |
*T1, tumor invasion of the lamina propria or submucosa; T2, invasion of the muscularis propria or the subserosa; T3, penetration of the serosa; T4, invasion of adjacent organs.
†N0, no regional lymph node metastasis; N1–N3, metastasis in group-1 to -3 lymph nodes.
‡Ly0–Ly3, grade of lymphatic invasion.
§V0–V3, grade of blood vessel invasion.
Age and recurrence pattern
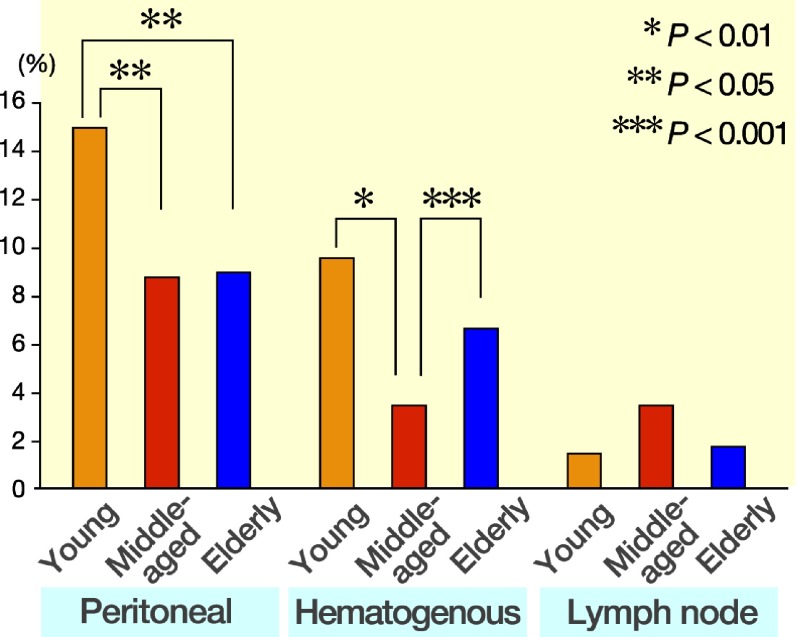
Figure 2 shows the correlation between age and mode of recurrence. Peritoneal recurrence was more frequently observed in the young patients than in either the middle-aged or elderly patients (P < 0.05). Moreover, hematogenous recurrence was more frequently observed in both the young (P < 0.01) and elderly patients (P < 0.001) than in the middle-aged patients.
Fig. 2.
The correlation between age and mode of recurrence. Peritoneal recurrence is more frequently observed in young patients than either middle-aged or elderly patients (P < 0.05). Moreover, hematogenous recurrence is more frequently observed in both young (P < 0.01) and elderly patients (P < 0.001) than middle-aged patients.
Discussion
We have previously demonstrated that gastric cancer in the elderly is characterized by high frequency of differentiated cancer, blood vessel invasion, hematogenous recurrence and poor prognosis (Saito et al., 2006). Therefore, age might have an impact on clinicopathologic characteristics, mode of recurrence and prognosis in patients with gastric cancer.
In the present study, the prognosis of gastric cancer patients in either the young age or old age group was significantly worse than that in the middle-aged population. On the other hand, there are conflicting results with regard to the prognosis of gastric cancer in young patients (Tso et al., 1987; Okamoto et al., 1988; Kim et al., 2003). We have previously reported that prognosis of gastric cancer in elderly patients is poor (Saito et al., 2006). Most previous studies have classified patients into 2 groups, either young or the rest, and have compared prognosis between the 2 groups. Therefore, the addition of elderly patients, whose prognosis was poor, to the middle-aged patients, whose prognosis was good, might lead to different results for prognosis. In fact, in the present study, there were no significant differences in the prognosis between patients aged ≤ 40 and those > 40 years (data not shown). Therefore, we recommend classifying patients into 3 groups, the young, middle-aged and elderly like we did in the present study, when the correlation between age and prognosis is determined.
As reported in previous papers (Bloss et al., 1980; Mori et al., 1985; Sandler and Holland, 1987; Tso et al., 1987), our results also demonstrated that the male-to-female ratio showed a predominance of female patients in the young age group. Undifferentiated carcinoma was also observed more frequently in the young age group. Moreover, the frequency of peritoneal metastasis was high in the young patients. With regard to mode of recurrence, peritoneal recurrence was observed more frequently in the young than in either the middle-aged or elderly patients (P < 0.05). It has been reported that undifferentiated gastric cancer preferentially metastasizes to the peritoneum (Moriguchi et al., 1991). Therefore, a high frequency of peritoneal recurrence might be correlated with a high frequency of undifferentiated gastric cancer in the young patients.
The Adjuvant Chemotherapy Trial of TS-1 for Gastric Cancer phase III trial has demonstrated that S-1 was effective as adjuvant chemotherapy for Japanese patients who had undergone curative D2 gastrectomy for gastric cancer and were diagnosed with pathological stage-2 or -3 disease (Sakuramoto et al., 2007). Therefore, a curative D2 dissection and adjuvant chemotherapy with S-1 are now the standard therapy for these patients in Japan (Sano and Aiko, 2011). On the other hand, majority of patients included in the present study did not take S-1 after operation as S-1 became commercially available since 1999. Therefore, S-1 may significantly improve the prognosis of the young patients since continuation of S-1 adjuvant chemotherapy is considered better than the elderly patients.
In conclusion, gastric cancer in young patients has unique characteristics, namely, a female predominance, a high frequency of undifferentiated cancer and peritoneal metastasis and recurrence. On account of the extremely poor prognosis in young patients, intensive chemotherapy should be considered even after curative operation.
References
- 1.Bloss RS, Miller TA, Copeland EM., 3rd Carcinoma of the stomach in the young adult. Surg Gynecol Obstet 1980;150:883–886 [PubMed] [Google Scholar]
- 2.Japanese Gastric Cancer Association Japanese Classification of Gastric Carcinoma: 2nd English edition. Gastric Cancer 1998;1:10–24 [DOI] [PubMed] [Google Scholar]
- 3.Kim DY, Ryu SY, Kim YJ, Kim SK. Clinicopathological characteristics of gastric carcinoma in young patients. Langenbecks Arch Surg 2003;388:245–249 [DOI] [PubMed] [Google Scholar]
- 4.Maeta M, Yamashiro H, Oka A, Tsujitani S, Ikeguchi M, Kaibara N. Gastric cancer in the young, with special reference to 14 pregnancy-associated cases: analysis based on 2,325 consecutive cases of gastric cancer. J Surg Oncol 1995;58:191–195 [DOI] [PubMed] [Google Scholar]
- 5.Mitsudomi T, Matsusaka T, Wakasugi K, Takenaka M, Kume K, Fujinaga Y.A clinicopathological study of gastric cancer with special reference to age of the patients: an analysis of 1,630 cases. World J Surg 1989;13:225–231; 225-230, discussion 230–231 [DOI] [PubMed] [Google Scholar]
- 6.Mori M, Sugimachi K, Ohiwa T, Okamura T, Tamura S, Inokuchi K. Early gastric carcinoma in Japanese patients under 30 years of age. Br J Surg 1985;72:289–291 [DOI] [PubMed] [Google Scholar]
- 7.Moriguchi S, Kamakura T, Odaka T, Nose Y, Maehara Y, Korenaga D.Clinical features of the differentiated and undifferentiated types of advanced gastric carcinoma: univariate and multivariate analyses. J Surg Oncol 1991;48:202–206 [DOI] [PubMed] [Google Scholar]
- 8.Okamoto T, Makino M, Kawasumi H, Kimura O, Nishidoi H, Kaibara N.Comparative study of gastric cancer in young and aged patients. Eur Surg Res 1988;20:149–155 [DOI] [PubMed] [Google Scholar]
- 9.Saito H, Osaki T, Murakami D, Sakamoto T, Kanaji S, Tatebe S.Effect of age on prognosis in patients with gastric cancer. ANZ J Surg 2006;76:458–461 [DOI] [PubMed] [Google Scholar]
- 10.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A.Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810–1820 [DOI] [PubMed] [Google Scholar]
- 11.Sander RS, Holland KL. Trends in gastric cancer sex ratio in the United States. Cancer 1987;59:1032–1035 [DOI] [PubMed] [Google Scholar]
- 12.Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer 2011;14:97–100 [DOI] [PubMed] [Google Scholar]
- 13.Tso PL, Bringaze WL, 3rd, Dauterive AH, Correa P, Cohn I., Jr Gastric carcinoma in the young. Cancer 1987;59:1362–1365 [DOI] [PubMed] [Google Scholar]