Abstract
There is enormous worldwide demand for therapies to promote the efficient resolution of hard-to-heal wounds with minimal appearance of scarring. Recent in vitro studies with mesenchymal stem cells (MSCs) have identified numerous mechanisms by which these cells can promote the process of wound healing, and there is significant interest in the clinical translation of an MSC-based therapy to promote dermal regeneration. This review provides a systematic analysis of recent preclinical and clinical research to evaluate the use of MSCs in wound healing applications. These in vivo studies provide overwhelming evidence that MSCs can accelerate wound closure by modulating the inflammatory environment, promoting the formation of a well-vascularized granulation matrix, encouraging the migration of keratinocytes, and inhibiting apoptosis of wound healing cells. The trophic effects of MSC therapy also appear to augment wound healing in diabetic tissues, thereby preventing the formation of nonhealing ulcers. Finally, a number of delivery systems have been evaluated and indicate that MSCs could be the basis of a versatile therapy to fulfill the clinical needs for dermal regeneration. However, despite the apparent advantages of MSC-based therapies, there have been only limited clinical investigations of this type of therapy in humans. Thus, our review concludes with a discussion of the translational barriers that are limiting the widespread clinical use of MSCs to enhance wound healing.
Keywords: Regenerative medicine, Dermal regeneration, Clinical translation, Diabetic ulcer, Tissue engineering, Preclinical research, Immunomodulation, Angiogenesis
Introduction
Wound healing is usually a highly successful biological process to restore the integrity of the skin following injury. The mechanisms of wound healing typically fall along a spectrum between tissue regeneration, the end result of which would be functionally equivalent to the uninjured skin, and tissue repair, during which the skin's functional characteristics are sacrificed in favor of rapidly closing the wound with fibrotic scar tissue. Scars are an undesirable consequence of cutaneous wound healing not only because of their appearance but because of their poor mechanical strength relative to the surrounding tissue [1]. Substantial dysregulation of wound healing can result in a nonhealing wound or ulcer, as frequently observed in diabetic individuals because of their microvascular deficiencies [2]. As a result, the annual worldwide market for advanced wound care products to promote healing in hard-to-heal wounds and to reduce the appearance of scars exceeds $5 billion.
Mesenchymal stem cells (MSCs) have been proposed as a potential therapy to enhance cutaneous wound healing. MSCs are readily available from commercial, allogeneic sources or as autologous cells that can be harvested at the point of care from various tissues, which has lowered some of the barriers to the clinical translation of these cells for a variety of therapeutic applications [3]. As with many cell-based therapies, MSCs act through complex interactions with the endogenous cells and tissues, and they may function in the tissue via multiple mechanisms of action. MSCs are also responsive to their environment, and they can modify their activities and functions depending on the biomolecular context. Therefore, MSCs offer several advantages that make them an attractive treatment option during wound healing, including the fact that they appear to be a native constituent of the wound bed [4], where they can regulate the wound healing process by mechanisms that have been extensively studied (reviewed in Jackson et al. [5]). This review provides a systematic evaluation of recent preclinical research to evaluate the use of MSCs in wound healing applications. It will conclude by highlighting the clinical research efforts currently under way to apply MSCs to enhance the process of cutaneous wound healing.
MSCs in Wound Healing Biology
Dermal wound healing is usually a highly predictable process that can be divided into four overlapping phases: (a) coagulation and hemostasis, (b) inflammation, (c) proliferation, and (d) remodeling (reviewed in Martin [6] and Velnar et al. [7]). Hemostasis begins coincident with injury once the integrity of blood vessels below the dermal tissue layer has been compromised and escaping platelets come into contact with collagen and other extracellular matrix (ECM) molecules [8]. The stimulated platelets initiate the clotting cascade by undergoing degranulation and releasing intracellular stores of clotting factors and inflammatory cytokines [6, 9]. The inflammatory phase begins within hours of injury as neutrophils respond to these cytokines by entering the fibrin clot, followed by macrophages and leukocytes, which participate in a coordinated response to neutralize foreign antigens and achieve sterility in the wound. These immune cells then recruit reparative cells from the surrounding dermal tissue [10] and circulation [11, 12] to form a well-vascularized granulation tissue during the proliferation stage [6], and this serves as a scaffold for tissue regeneration and enables re-epithelialization of the wound to close off the wound bed. In the final phase of wound healing, endogenous dermal fibroblasts reorganize the ECM to reinforce the provisional granulation tissue matrix and produce additional ECM proteins to regenerate the dermal tissues [7].
MSCs can be found in a variety of adult tissues throughout the body, including bone marrow [13], adipose tissue [14, 15], synovial fluid [16–18], and traumatized muscle [19, 20]. They have also been derived from fetal tissues that can be collected harmlessly during or after pregnancy, such as amniotic fluid [21] and umbilical cord [22], and these have been proposed as potential sources of MSCs for cell banking applications. MSCs exhibit specific characteristics when cultured in vitro, regardless of their anatomical origin [23], and there is emerging evidence that most if not all MSCs may be derived from a common perivascular niche [24]. In vivo, MSCs appear to enhance the regenerative potential of multiple tissues types as a result of various trophic mechanisms that become activated when exposed to the biochemical factors that are characteristic of an injury environment [25]. Endogenous MSCs or a related population of endogenous cells (i.e., vascular pericytes [24, 26]) likely participate in the wound healing response by migrating to the site of cutaneous tissue damage. The inflammatory mediators interferon-γ (IFNγ) and tumor necrosis factor-α (TNFα) can regulate homing and migration of MSCs through the ECM [27], and MSCs have demonstrated chemotaxis toward a variety of wound healing cytokines in vitro [27, 28]. Engraftment of mouse [29, 30] and human [31] MSCs to regions of inflammation and tissue damage has also been observed in murine models of injury and disease. Once they are localized to the wound bed, there is evidence that MSCs can enhance dermal regeneration during multiple phases of the wound healing process.
During the inflammation phase, proinflammatory mediators, such as IFNγ, TNFα, and interleukin-1β (IL1β), can activate regulatory functions in MSCs that enable them to modulate the immune response [32]. In this state, the MSCs can inhibit the recruitment, proliferation, and biological activity of mast cells [33], T cells [32, 34], B cells [35], and natural killer cells [36], thereby attenuating the acute immune response to injury. The inflammatory wound environment of the wound also stimulates COX2 activity in MSCs, leading to upregulation of prostaglandin E2 (PGE2) [37] and a transition in wound function in favor of dermal regeneration. In addition to attenuating T-cell proliferation [38], PGE2 can alter the behavior of leukocytes resident in the wound, corresponding to decreased expression of IL2 [39] and IFNγ [40] and increased expression of IL4 [40] and IL10 [37, 41]. This shift in favor of anti-inflammatory cytokine expression encourages wound fibroblasts to upregulate their expression of matrix metalloproteinases (MMPs) and downregulate the expression of various collagen types [42, 43], resulting in the formation of a less dense, fibrotic granulation tissue in the wound bed. As a result, the activity of MSCs in the wound appears to favor wound healing over inflammation and promotes functional regeneration of the tissues by mesenchymal cells during the proliferation phase [43, 44].
There is also substantial evidence that MSCs continue to support tissue regeneration during the proliferation phase. Angiogenesis is necessary at this step of wound healing to ensure sufficient nutrient delivery to fibroblasts responsible for forming a provisional granulation matrix [33]. Inadequate microvasculature in the wound bed may inhibit wound closure and lead to a chronic nonhealing wound, as occurs frequently in diabetic patients. MSCs express several factors, including basic fibroblast growth factor (bFGF), vascular endothelial growth factor-A (VEGF-A), and adrenomedullin [45], that promote the proliferation of microvascular endothelial cells [46, 47], vascular stability [48, 49], and the development of a long-lasting functional vascular network [50]. MSCs also secrete a variety of cytokines and growth factors that have antifibrotic properties, including hepatic growth factor (HGF), IL10, adrenomedullin [45, 51, 52], and MMP-9 [53], that promote turnover of the ECM [54–56], keratinocyte proliferation [57], and inhibition of myofibroblast differentiation [58]. Therefore, MSCs in the wound bed contribute to the generation of a high-quality, well-vascularized granulation tissue, enhance re-epithelialization of the wound, and attenuate the formation of fibrotic scar tissue.
In the final stage of wound healing, dermal fibroblasts begin to remodel the wound matrix to generate new dermal tissue. ECM-generating cells from other sources may also be recruited to the tissue as it is remodeled, such as endothelial cells undergoing endothelial to mesenchymal transdifferentiation (EndoMT) [59] and circulating fibrocytes [60]. After exposure to transforming growth factor-β1 (TGF-β1) in the wound, these cells rapidly differentiate into myofibroblasts in the presence of TGF-β1 and begin expressing high levels of ECM proteins [11, 59]. This generic wound-healing response is sufficient to restore tissue integrity, but the myofibroblastic cell types produce excessive amounts of ECM compared with dermal fibroblasts, resulting in scar tissue and poor functional regeneration [61]. MSCs express HGF [62] and PGE2 [63] in the wound, which can inhibit EndoMT and myofibroblastic differentiation of fibroblasts, thus promoting wound healing functions that are specific to dermal fibroblasts [64]. Finally, MSCs can transdifferentiate into epidermal cells, keratinoctes, and microvascular endothelial in vitro under defined induction conditions [65–67] or in coculture with native epidermal cells [68]. Therefore, MSCs appear to promote the production of an ECM that more closely resembles uninjured dermal tissue.
Preclinical Validation of MSCs to Promote Wound Healing
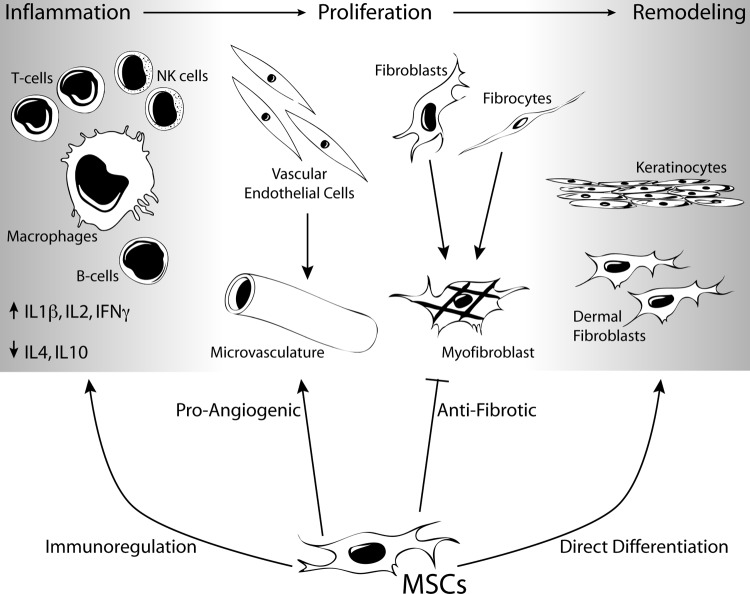
As described in the previous section, MSCs appear to be an attractive cell type for a cell-based therapy to promote dermal regeneration. In addition to their differentiation potential, MSCs exhibit substantial trophic support to regenerating tissues. Autologous cells are readily available from bone marrow [3] or adipose tissue [69] using well-established procedures. Given the array of mechanisms by which the MSCs can promote wound healing (Fig. 1), there have been a number of studies to evaluate their function in vivo using a variety of cutaneous wound healing models.
Figure 1.
MSCs can enhance dermal regeneration by acting on multiple cell types throughout the phases of wound healing. Abbreviations: IFN, interferon; IL, interleukin; MSC, mesenchymal stem cell; NK, natural killer.
Several recent studies have demonstrated the efficacy of MSCs in improving dermal wounding by direct application of the cells to the wound. Chen et al. demonstrated that the cytokines and growth factors secreted by murine MSCs are sufficient for improved wound healing by applying concentrated MSC-conditioned medium directly to a full-thickness excisional wound in mice via injection and topical administration [52]. The conditioned medium encouraged the migration of macrophages and endothelial cells into the wound space and promoted neovascularization in the regenerating tissues, thereby accelerating the rate of wound closure. In a similar study, murine MSCs were applied to excisional wounds in mice, and syngeneic and allogeneic cells demonstrated an equivalent ability to migrate through the tissue and to attenuate the local inflammatory response, as compared with the control allo-fibroblasts, which were restricted to the sites of injection [30]. This study corroborated previous findings that xenograft MSCs elicit little immunogenicity from their host [70, 71] and indicate that MSCs are immunoprivileged during cutaneous wound healing.
Following direct injection into a wound, the MSCs appear to contribute to wound healing by multiple mechanisms that could result in better dermal regeneration with less visual appearance of a scar. Nie et al. demonstrated that adipose-derived stem cells (ASCs) express VEGF-A, HGF, and fibroblast growth factor-2, and in a full-thickness excisional injury, ASCs from rats significantly enhanced neovasculogenesis and accelerated the time to wound closure [72]. In this study, there was also evidence that the ASCs differentiated directly into endothelial and epithelial cell types and were integrated directly into the regenerated tissue. Similar results have been observed in a murine excisional injury model, such that mouse bone marrow-derived MSCs accelerated wound closure by increasing re-epithelialization, cellularity, and angiogenesis and by differentiating directly into epithelial cells expressing keratin, a keratinocyte-specific protein, that were localized to appendage-like structures [68]. Finally, in a rabbit model, human bone marrow-derived MSCs were injected into an incisional wound, and the cellular therapy resulted in improved wound closure with better wound tensile strength and with a significant reduction in the appearance of a scar [73].
The trophic functions of MSCs in the wound healing environment have been further elucidated using animal models of diabetes to evaluate their function in the context of impaired cellular metabolism and its consequences on microvascular function (reviewed in Groop et al. [2]) Using the db/db diabetic mouse model of impaired wound healing, murine bone marrow-derived MSCs were applied directly to a full-thickness excisional wound, which promoted the formation of a well-vascularized granulation tissue, more rapid re-epithelialization, and better gap closure, thereby preventing the development of a chronic nonhealing wound [74]. Similarly, in a streptozotocin-induced model of diabetes in rats, local injection of ASCs accelerated closure of full-thickness excisional wounds through mechanisms that did not require a significant increase in the expression of ECM proteins or vascular density [75]. This experiment demonstrated the importance of MSCs in inhibiting inflammation and apoptosis of wound-healing cells, and thus, MSCs may substantially improve the wound healing in the absence of any observable effects on the wound healing fibroblasts.
MSCs have also been incorporated into three-dimensional scaffolds as a strategy to improve skin substitutes that are used to repair full-thickness wounds resulting from trauma or burns [76]. One substantial benefit of including MSCs in these tissue-engineered constructs is to promote angiogenesis throughout the scaffold, which allows for the transport of nutrients and reparative cell types that can remodel the scaffold. For example, human MSCs from the V54/2 cell line were seeded into a collagen scaffold and implanted into a full-thickness defect in mice, and the MSCs significantly improved the vascularity of the constructs during dermal regeneration [77]. There was also evidence that the MSCs were integrated into the structure of the regenerated tissue. In a similar study, allogeneic bone marrow MSCs seeded onto a nanofiber matrix and applied to a full-thickness excisional wound model in rats significantly increased the rate of wound closure [78]. The MSCs were labeled with fluorescent quantum dots prior to seeding, and quantum dots colocalized with cells expressing the epithelial cell-specific markers keratin-10 and filaggrin after wound closure, suggesting that some of the MSCs had undergone epidermal differentiation.
Alternative methods of delivering MSCs have also been evaluated as a means of facilitating the translation of a cell-based wound healing therapy into clinical practice. For example, human bone marrow-derived MSCs have been incorporated into a fibrin spray and used as a regenerative dressing for full-thickness wounds. This biologic wound dressing was sufficient to stimulate complete closure of full-thickness excisional wounds in diabetic mice, whereas controls remained in a chronic, nonhealing state [79]. Fibrin sealant has also been enriched with autograft bone marrow-derived swine MSCs and combined with either bFGF or epidermal growth factor. These therapies were sufficient to enhance the repair of excisional wounds by decreasing the time required for re-epithelialization and by increasing the thickness of the regenerated skin relative to the controls containing no cells [65]. Furthermore, the MSCs appeared to promote functional regeneration in the tissues, as there was evidence of MSC differentiation into endothelial and epidermal cell types and the formation of rete ridges extending into the dermis.
Finally, there is accumulating evidence that MSCs can be administered systemically during dermal wound healing, and they will home to the location of injury to impart their regenerative effects. In a bleomycin model of injury to lung epithelial tissues in mice, it has been established that MSCs will home to the site of inflammation, attenuate the acute inflammatory response, and decrease ECM deposition, thereby minimizing the extent of pulmonary fibrosis [29]. These findings have been extended to dermal wound healing as systemically injected murine MSCs engrafted at the site of an excisional wound model in mice and appeared to transdifferentiate into keratinocytes, endothelial cells, and pericytes and to accelerate the rate of wound healing [66]. In this study, the engraftment of MSCs was enhanced by local injection of the chemokine CCL21 into the dermal wound margin, thereby identifying a likely chemokine that might be used to promote homing of endogenous MSCs to the site of injury. An additional study has demonstrated that systemically administered allogeneic and syngeneic bone marrow-derived MSCs were effective in improving the rate and quality of ECM deposition and increasing the tensile strength of incisional wounds [80]. In the MSC-treated wounds, there appeared to be rapid resolution of the acute inflammatory phase of the injury, followed by early formation of the granulation tissue, and as a result, the wounds could readily transition into the remodeling phase to minimize the appearance of a scar.
The Future of MSC Therapy for Wound Healing
In light of the extensive data demonstrating the promise of MSCs for dermal wound healing, the clinical translation of these cells remains somewhat limited. In one study, autologous bone marrow-derived MSCs were delivered via a fibrin spray to patients who suffered from acute and chronic wounds. By obtaining sequential biopsy specimens of the wound bed after MSC application, it was possible to observe the migration of the MSCs into the wound, and the MSCs appeared to stimulate elastin expression in the wound bed, which contributed to the synthesis of a dermal matrix with improved ECM composition [79]. Furthermore, there was an inverse correlation between the concentration of injected cells and size of the chronic wounds. This study demonstrated that the use of cultured MSCs was a safe therapy for dermal wounds without treatment-related adverse events. In a second study, autologous bone marrow-derived MSCs seeded on a collagen sponge were effective to facilitate the closure of ulcerated wounds [81]. Despite the success of these studies, there are currently only four active clinical trials registered at ClinicalTrials.gov pertaining to the treatment of dermal wounds with MSCs [82].
This review has highlighted the exciting preclinical research that has validated MSCs as biologic therapy to enhance dermal wound healing (Table 1). However, prior to the widespread clinical use of MSCs therapy, it will be necessary to identify and overcome the barriers to clinical translation. One limitation to MSC therapy is the method of delivering the cells. Although systemic delivery is an attractive option, the engraftment efficiency can be difficult to predict without improved methods to ensure that the cells will home to the site of injury. Local administration of the MSCs may require an appropriate carrier and/or scaffold to ensure that the cells remain viable and can efficiently migrate into the wound bed (reviewed in Sorrell and Caplan. [83]). Products are currently in development that could be adapted as a suitable carrier for MSCs, such as a novel skin substitute produced by neonatal immortalized keratinocytes (StrataGraft; StrataTech, Madison, WI, http://www.stratatechcorp.com/) [84] or a bilayered graft produced by fibroblasts and keratinocytes (VCT-01; Organogenesis, Canton, MA, http://www.organogenesis.com/). There is also evidence that committed progenitor cells derived from split thickness cutaneous biopsies can be effectively applied directly to the wounds using spray deposition [85], and commercial systems designed for spray deposition of a cellular suspension (e.g., ReCell [86]; Avita Medical, Cambridge, U.K., http://www.avitamedical.com/) might also facilitate the clinical translation of MSC-based therapies.
Table 1.
Preclinical studies using MSC therapies to enhance cutaneous wound healing
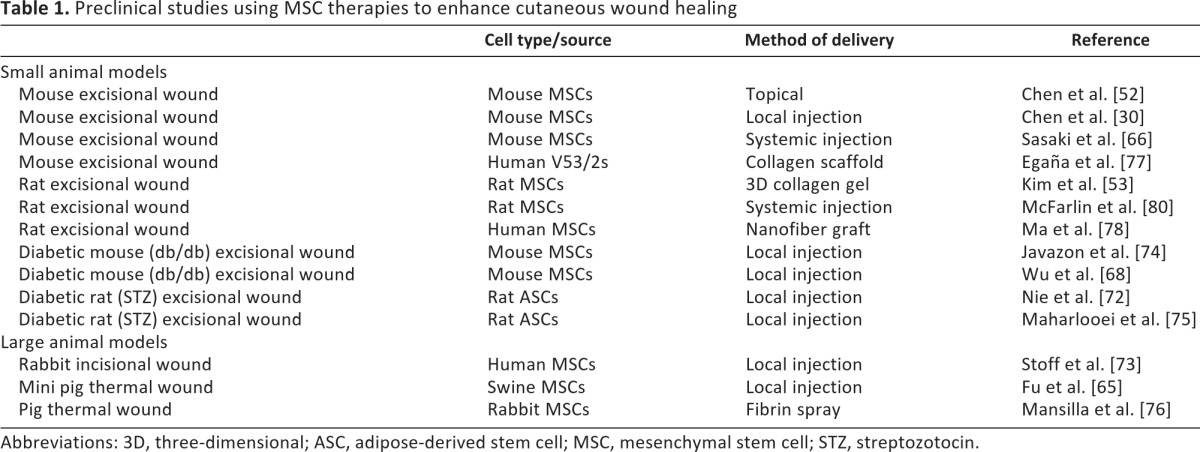
Abbreviations: 3D, three-dimensional; ASC, adipose-derived stem cell; MSC, mesenchymal stem cell; STZ, streptozotocin.
Further investigation is also needed to elucidate the interactions between MSCs and the various immune and wound healing cell types that are present in the wound bed, as these studies would be useful to optimize the timing and dosage of MSC delivery to the wound for maximal efficacy [3]. The use of MSCs as a therapy presents advantages over pharmaceuticals, protein/growth factors, and committed progenitor cells, as MSCs are able to interact with the surrounding cell types and biochemical environment to express in a regulated manner the appropriate trophic factors for enhanced dermal wound healing. Therefore, MSCs will likely emerge as an important therapy to reduce the formation of fibrotic tissue and the appearance of scars following cutaneous injury.
Acknowledgments
This work was supported in part by the NIH Intramural Research Program (1ZIAAR041191), and by grants from the Department of Defense Military Amputee Research Program at Walter Reed Army Medical Center (PO5-A011), Defense Medical Research and Development Program (D10_I_AR_J8_981), and Peer-Reviewed Orthopaedic Research Program (W81XWH-10-2-0084) and from the Commonwealth of Pennsylvania Department of Health. Figure 1 was produced using Servier Medical Art.
Author Contributions
W.M.J.: conception/design, analysis/interpretation, manuscript writing; L.J.N.: financial support, analysis/interpretation, manuscript writing; R.S.T.: conception/design, financial support, manuscript writing, final approval.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Clark R. Wound repair overview and general considerations. In: Clark R, editor. The Molecular and Cellular Biology of Wound Repair. New York, NY: Plenum Press; 1996. pp. 3–50. [Google Scholar]
- 2.Groop PH, Forsblom C, Thomas MC. Mechanisms of disease: Pathway-selective insulin resistance and microvascular complications of diabetes. Nat Clin Pract Endocrinol Metab. 2005;1:100–110. doi: 10.1038/ncpendmet0046. [DOI] [PubMed] [Google Scholar]
- 3.Salem HK, Thiemermann C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badiavas EV, Abedi M, Butmarc J, et al. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol. 2003;196:245–250. doi: 10.1002/jcp.10260. [DOI] [PubMed] [Google Scholar]
- 5.Jackson WM, Nesti LJ, Tuan RS. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res Ther. 2012 doi: 10.1186/scrt111. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin P. Wound healing: Aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 7.Velnar T, Bailey T, Smrkolj V. The wound healing process: An overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–1542. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- 8.Arnout J, Hoylaerts MF, Lijnen HR. Haemostasis. Handb Exp Pharmacol. 2006;(176):1–41. doi: 10.1007/3-540-36028-x_1. [DOI] [PubMed] [Google Scholar]
- 9.Barrientos S, Stojadinovic O, Golinko MS, et al. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 10.Diegelmann RF, Evans MC. Wound healing: An overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 11.Abe R, Donnelly SC, Peng T, et al. Peripheral blood fibrocytes: Differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 12.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 13.Friedenstein AJ, Deriglasova UF, Kulagina NN, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- 14.Gimble JM, Guilak F. Adipose-derived adult stem cells: Isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 15.Meliga E, Strem BM, Duckers HJ, et al. Adipose-derived cells. Cell Transplant. 2007;16:963–970. doi: 10.3727/096368907783338190. [DOI] [PubMed] [Google Scholar]
- 16.Jones EA, English A, Henshaw K, et al. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum. 2004;50:817–827. doi: 10.1002/art.20203. [DOI] [PubMed] [Google Scholar]
- 17.Vandenabeele F, De Bari C, Moreels M, et al. Morphological and immunocytochemical characterization of cultured fibroblast-like cells derived from adult human synovial membrane. Arch Histol Cytol. 2003;66:145–153. doi: 10.1679/aohc.66.145. [DOI] [PubMed] [Google Scholar]
- 18.Fan J, Varshney RR, Ren L, et al. Synovium-derived mesenchymal stem cells: A new cell source for musculoskeletal regeneration. Tissue Eng Part B. 2009;15:75–86. doi: 10.1089/ten.teb.2008.0586. [DOI] [PubMed] [Google Scholar]
- 19.Nesti LJ, Jackson WM, Shanti RM, et al. Differentiation potential of multipotent progenitor cells derived from war-traumatized muscle tissue. J Bone Joint Surg Am. 2008;90:2390–2398. doi: 10.2106/JBJS.H.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson WM, Aragon AB, Djouad F, et al. Mesenchymal progenitor cells derived from traumatized human muscle. J Tissue Eng Regen Med. 2009;3:129–138. doi: 10.1002/term.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Coppi P, Bartsch G, Jr., Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 22.Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2:155–162. doi: 10.1007/s12015-006-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 24.Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Caplan AI. Why are MSCs therapeutic? New data: New insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Hemeda H, Jakob M, Ludwig AK, et al. Interferon-γ and tumor necrosis factor-α differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev. 2010;19:693–706. doi: 10.1089/scd.2009.0365. [DOI] [PubMed] [Google Scholar]
- 28.Mishima Y, Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res. 2008;26:1407–1412. doi: 10.1002/jor.20668. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Tredget EE, Liu C, et al. Analysis of allogenicity of mesenchymal stem cells in engraftment and wound healing in mice. PLoS One. 2009;4:e7119. doi: 10.1371/journal.pone.0007119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 32.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Brown JM, Nemeth K, Kushnir-Sukhov NM, et al. Bone marrow stromal cells inhibit mast cell function via a COX2-dependent mechanism. Clin Exp Allergy. 2011;41:526–534. doi: 10.1111/j.1365-2222.2010.03685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Djouad F, Bouffi C, Ghannam S, et al. Mesenchymal stem cells: Innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol. 2009;5:392–399. doi: 10.1038/nrrheum.2009.104. [DOI] [PubMed] [Google Scholar]
- 35.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 36.Sotiropoulou P, Perez S, Gritzapis A, et al. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 37.Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Djouad F, Charbonnier LM, Bouffi C, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 39.Jarvinen L, Badri L, Wettlaufer S, et al. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol. 2008;181:4389–4396. doi: 10.4049/jimmunol.181.6.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aggarwal S, Pittenger M. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 41.Benbernou N, Esnault S, Shin HC, et al. Differential regulation of IFN-γ, IL-10 and inducible nitric oxide synthase in human T cells by cyclic AMP-dependent signal transduction pathway. Immunology. 1997;91:361–368. doi: 10.1046/j.1365-2567.1997.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reitamo S, Remitz A, Tamai K, et al. Interleukin-10 modulates type I collagen and matrix metalloprotease gene expression in cultured human skin fibroblasts. J Clin Invest. 1994;94:2489–2492. doi: 10.1172/JCI117618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeon YK, Jang YH, Yoo DR, et al. Mesenchymal stem cells' interaction with skin: Wound-healing effect on fibroblast cells and skin tissue. Wound Repair Regen. 2010;18:655–661. doi: 10.1111/j.1524-475X.2010.00636.x. [DOI] [PubMed] [Google Scholar]
- 44.Peranteau WH, Zhang L, Muvarak N, et al. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol. 2008;128:1852–1860. doi: 10.1038/sj.jid.5701232. [DOI] [PubMed] [Google Scholar]
- 45.Renault MA, Roncalli J, Tongers J, et al. The Hedgehog transcription factor Gli3 modulates angiogenesis. Circ Res. 2009;105:818–826. doi: 10.1161/CIRCRESAHA.109.206706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gruber R, Kandler B, Holzmann P, et al. Bone marrow stromal cells can provide a local environment that favors migration and formation of tubular structures of endothelial cells. Tissue Eng. 2005;11:896–903. doi: 10.1089/ten.2005.11.896. [DOI] [PubMed] [Google Scholar]
- 47.Kaigler D, Krebsbach PH, Polverini PJ, et al. Role of vascular endothelial growth factor in bone marrow stromal cell modulation of endothelial cells. Tissue Eng. 2003;9:95–103. doi: 10.1089/107632703762687573. [DOI] [PubMed] [Google Scholar]
- 48.Lozito TP, Taboas JM, Kuo CK, et al. Mesenchymal stem cell modification of endothelial matrix regulates their vascular differentiation. J Cell Biochem. 2009;107:706–713. doi: 10.1002/jcb.22166. [DOI] [PubMed] [Google Scholar]
- 49.Kato J, Tsuruda T, Kita T, et al. Adrenomedullin: A protective factor for blood vessels. Arterioscler Thromb Vasc Biol. 2005;25:2480–2487. doi: 10.1161/01.ATV.0000184759.91369.f8. [DOI] [PubMed] [Google Scholar]
- 50.Au P, Tam J, Fukumura D, et al. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L, Zhang Y, Li Y, et al. Mesenchymal stem cell transplantation attenuates cardiac fibrosis associated with isoproterenol-induced global heart failure. Transpl Int. 2008;21:1181–1189. doi: 10.1111/j.1432-2277.2008.00742.x. [DOI] [PubMed] [Google Scholar]
- 52.Chen L, Tredget EE, Wu PY, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim CH, Lee JH, Won JH, et al. Mesenchymal stem cells improve wound healing in vivo via early activation of matrix metalloproteinase-9 and vascular endothelial growth factor. J Korean Med Sci. 2011;26:726–733. doi: 10.3346/jkms.2011.26.6.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schievenbusch S, Strack I, Scheffler M, et al. Profiling of anti-fibrotic signaling by hepatocyte growth factor in renal fibroblasts. Biochem Biophys Res Commun. 2009;385:55–61. doi: 10.1016/j.bbrc.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 55.Mou S, Wang Q, Shi B, et al. Hepatocyte growth factor suppresses transforming growth factor-β-1 and type III collagen in human primary renal fibroblasts. Kaohsiung J Med Sci. 2009;25:577–587. doi: 10.1016/S1607-551X(09)70560-1. [DOI] [PubMed] [Google Scholar]
- 56.Kanemura H, Iimuro Y, Takeuchi M, et al. Hepatocyte growth factor gene transfer with naked plasmid DNA ameliorates dimethylnitrosamine-induced liver fibrosis in rats. Hepatol Res. 2008;38:930–939. doi: 10.1111/j.1872-034X.2008.00340.x. [DOI] [PubMed] [Google Scholar]
- 57.Bevan D, Gherardi E, Fan TP, et al. Diverse and potent activities of HGF/SF in skin wound repair. J Pathol. 2004;203:831–838. doi: 10.1002/path.1578. [DOI] [PubMed] [Google Scholar]
- 58.Shukla MN, Rose JL, Ray R, et al. Hepatocyte growth factor inhibits epithelial to myofibroblast transition in lung cells via Smad7. Am J Respir Cell Mol Biol. 2009;40:643–653. doi: 10.1165/rcmb.2008-0217OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McAnulty RJ. Fibroblasts and myofibroblasts: Their source, function and role in disease. Int J Biochem Cell Biol. 2007;39:666–671. doi: 10.1016/j.biocel.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Opalenik SR, Davidson JM. Fibroblast differentiation of bone marrow-derived cells during wound repair. FASEB J. 2005;19:1561–1563. doi: 10.1096/fj.04-2978fje. [DOI] [PubMed] [Google Scholar]
- 61.Bucala R, Spiegel LA, Chesney J, et al. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 62.Yang J, Dai C, Liu Y. A novel mechanism by which hepatocyte growth factor blocks tubular epithelial to mesenchymal transition. J Am Soc Nephrol. 2005;16:68–78. doi: 10.1681/ASN.2003090795. [DOI] [PubMed] [Google Scholar]
- 63.Zhang A, Wang MH, Dong Z, et al. Prostaglandin E2 is a potent inhibitor of epithelial-to-mesenchymal transition: Interaction with hepatocyte growth factor. Am J Physiol Renal Physiol. 2006;291:F1323–F1331. doi: 10.1152/ajprenal.00480.2005. [DOI] [PubMed] [Google Scholar]
- 64.Smith AN, Willis E, Chan VT, et al. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res. 2010;316:48–54. doi: 10.1016/j.yexcr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu X, Fang L, Li X, et al. Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Repair Regen. 2006;14:325–335. doi: 10.1111/j.1743-6109.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 66.Sasaki M, Abe R, Fujita Y, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 67.Jin G, Prabhakaran MP, Ramakrishna S. Stem cell differentiation to epidermal lineages on electrospun nanofibrous substrates for skin tissue engineering. Acta Biomater. 2011;7:3113–3122. doi: 10.1016/j.actbio.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 68.Wu Y, Chen L, Scott PG, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 69.Yang JA, Chung HM, Won CH, et al. Potential application of adipose-derived stem cells and their secretory factors to skin: Discussion from both clinical and industrial viewpoints. Expert Opin Biol Ther. 2010;10:495–503. doi: 10.1517/14712591003610598. [DOI] [PubMed] [Google Scholar]
- 70.Mansilla E, Marin GH, Sturla F, et al. Human mesenchymal stem cells are tolerized by mice and improve skin and spinal cord injuries. Transplant Proc. 2005;37:292–294. doi: 10.1016/j.transproceed.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 71.Ryan JM, Barry FP, Murphy JM, et al. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nie C, Yang D, Xu J, et al. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011;20:205–216. doi: 10.3727/096368910X520065. [DOI] [PubMed] [Google Scholar]
- 73.Stoff A, Rivera AA, Banerjee NS, et al. Promotion of incisional wound repair by human mesenchymal stem cell transplantation. Exp Dermatol. 2009;18:362–369. doi: 10.1111/j.1600-0625.2008.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Javazon EH, Keswani SG, Badillo AT, et al. Enhanced epithelial gap closure and increased angiogenesis in wounds of diabetic mice treated with adult murine bone marrow stromal progenitor cells. Wound Repair Regen. 2007;15:350–359. doi: 10.1111/j.1524-475X.2007.00237.x. [DOI] [PubMed] [Google Scholar]
- 75.Maharlooei MK, Bagheri M, Solhjou Z, et al. Adipose tissue derived mesenchymal stem cell (AD-MSC) promotes skin wound healing in diabetic rats. Diabetes Res Clin Pract. 2011;93:228–234. doi: 10.1016/j.diabres.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 76.Mansilla E, Spretz R, Larsen G, et al. Outstanding survival and regeneration process by the use of intelligent acellular dermal matrices and mesenchymal stem cells in a burn pig model. Transplant Proc. 2010;42:4275–4278. doi: 10.1016/j.transproceed.2010.09.132. [DOI] [PubMed] [Google Scholar]
- 77.Egaña JT, Fierro FA, Kruger S, et al. Use of human mesenchymal cells to improve vascularization in a mouse model for scaffold-based dermal regeneration. Tissue Eng Part A. 2009;15:1191–1200. doi: 10.1089/ten.tea.2008.0097. [DOI] [PubMed] [Google Scholar]
- 78.Ma K, Liao S, He L, et al. Effects of nanofiber/stem cell composite on wound healing in acute full-thickness skin wounds. Tissue Eng Part A. 2011;17:1413–1424. doi: 10.1089/ten.TEA.2010.0373. [DOI] [PubMed] [Google Scholar]
- 79.Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–1312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 80.McFarlin K, Gao X, Liu YB, et al. Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen. 2006;14:471–478. doi: 10.1111/j.1743-6109.2006.00153.x. [DOI] [PubMed] [Google Scholar]
- 81.Yoshikawa T, Mitsuno H, Nonaka I, et al. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg. 2008;121:860–877. doi: 10.1097/01.prs.0000299922.96006.24. [DOI] [PubMed] [Google Scholar]
- 82.Clinical Trials.gov. [Accessed August 29, 2011]. Available at http://www.clinicaltrials.gov.
- 83.Sorrell JM, Caplan AI. Topical delivery of mesenchymal stem cells and their function in wounds. Stem Cell Res Ther. 2010;1:30. doi: 10.1186/scrt30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schurr MJ, Foster KN, Centanni JM, et al. Phase I/II clinical evaluation of StrataGraft: A consistent, pathogen-free human skin substitute. J Trauma. 2009;66:866–873. doi: 10.1097/TA.0b013e31819849d6. discussion 873–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gerlach JC, Johnen C, McCoy E, et al. Autologous skin cell spray-transplantation for a deep dermal burn patient in an ambulant treatment room setting. Burns. 2011;37:e19–e23. doi: 10.1016/j.burns.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 86.Gravante G, Di Fede MC, Araco A, et al. A randomized trial comparing ReCell system of epidermal cells delivery versus classic skin grafts for the treatment of deep partial thickness burns. Burns. 2007;33:966–972. doi: 10.1016/j.burns.2007.04.011. [DOI] [PubMed] [Google Scholar]