Abstract
Mesenchymal stem cells (MSCs) are emerging as a promising therapeutic approach of cell-based therapy for a wide range of autoimmune disorders and degenerative diseases. In preclinical and clinical studies, MSCs have been shown to be highly efficient in treating graft-versus-host disease, systemic lupus erythematosus, multiple sclerosis, type 1 diabetes, myocardial infarction, liver cirrhosis, inflammatory bowel disease, and other disorders. The underlying therapeutic mechanisms of MSCs include their homing efficiency to the tissue injury sites, their differentiation potential, their capability to produce a large amount of trophic factors, and their immunomodulatory effect. Because tissue damage sites are complicated milieus with distinct types of inflammatory cells and factors, available data have demonstrated that the properties of MSCs could be fundamentally influenced by the inflammatory elements. Thus, an understanding of the interaction between MSCs and the inflammatory microenvironment will provide critical information in revealing the precise in vivo mechanisms of MSC-mediated therapeutic effects and designing more practical protocols for clinical use of these cells.
Keywords: Mesenchymal stem cells, Immunosuppression, Cell-based therapy, Translational medicine
Introduction
Stem cells are considered the master cells, capable of both self-renewal and multilineage differentiation. Recent investigations have identified them as a potentially novel cell therapy for regenerative medicine largely because of their ability to differentiate into many functional cell types. It is anticipated that stem cell therapy could solve many medical challenges facing humanity, increase our knowledge of pathogenesis, allow us to screen for new drugs for safety and effectiveness, and treat a variety of diseases. It is highly expected that detailed investigations of stem cell biology and clinical applicability will result in revolutions in medical technologies.
Most early work on stem cells was carried out with pluripotent embryonic stem (ES) cells derived from inner cell mass of blastocyst embryo, which, however, introduced a series of ethical problems in clinical applications. To avoid such ethical issues and create histocompatibility, new technologies have enabled tissue cells to become induced pluripotent stem (iPS) cells [1]. One characteristic of ES cells and iPS cells is their ability to form teratomas, which, in turn, is a major concern for future clinical application [2, 3]. Moreover, it is likely that iPS cells are more tumorigenic than ES cells [4]. The teratoma-forming property of stem cells is considered a major obstacle for biomedicine by the U.S. Food and Drug Administration (FDA) [5].
In almost all tissues, there are mesenchymal stem cells (MSCs) that are responsible for regeneration and cellular homeostasis. These cells were first isolated and characterized by Friedenstein et al. in 1974 [6]. MSCs are spindle-shaped, fibroblast-like multipotent stem cells. In the last decade, MSCs were successfully isolated from various tissues. The most intensely studied MSCs are those derived from bone marrow (BM). The BM-MSCs are nearly 10% of the hematopoietic stem cells (HSCs) in number, and they are always regarded as a component of the HSC niche. In addition to BM-MSCs, MSCs from other sources, such as umbilical cord and adipose tissue, are also able to be expanded in vitro rapidly with sustained stable phenotype and differentiation potential toward several mesenchymal lineages, such as fat, cartilage, and bone [7].
The physiological role of MSCs is elusive because of their low frequency in tissues and lack of specific surface markers for identification. In the last few years, several studies have demonstrated the in vivo characteristics of MSCs. Sacchetti et al. and Crisan et al. revealed that MSCs are likely linked to CD146+ CD45− perivascular pericytes, which are capable of producing angiopoietin-1, an important molecule in HSC microenvironment [8, 9]. Additionally, MSCs have also been identified as nestin+ cells in bone marrow, which play a critical role in constructing the HSC microenvironment [10].
MSCs hold great potential for the treatment of various degenerative diseases and immune disorders, largely because of their differentiation potential and immunoregulatory capacity. In vitro expanded MSCs have already been administered in vivo in both animals in preclinical models and patients in clinical settings, demonstrating promising clinical utilities. Unlike ES or iPS cells, MSCs have no ethical issues and have a low risk of forming teratomas; however, they are not completely free from malignancy potentials [11]. Nevertheless, pilot studies have demonstrated that MSCs are largely safe in vivo, and they are currently the most widely used stem cells in clinical settings.
Preclinical Studies with MSCs
A remarkable property of MSCs is their powerful capacity for regulating immune responses. As a result, current MSC-based therapy has mainly been applied to alleviating immune disorders. Various studies have evaluated the therapeutic effect of MSCs in preclinical animal models and demonstrated great clinical potential (Table 1). Some examples are discussed below.
Table 1.
Treatment of diseases by mesenchymal stem cells in animal models
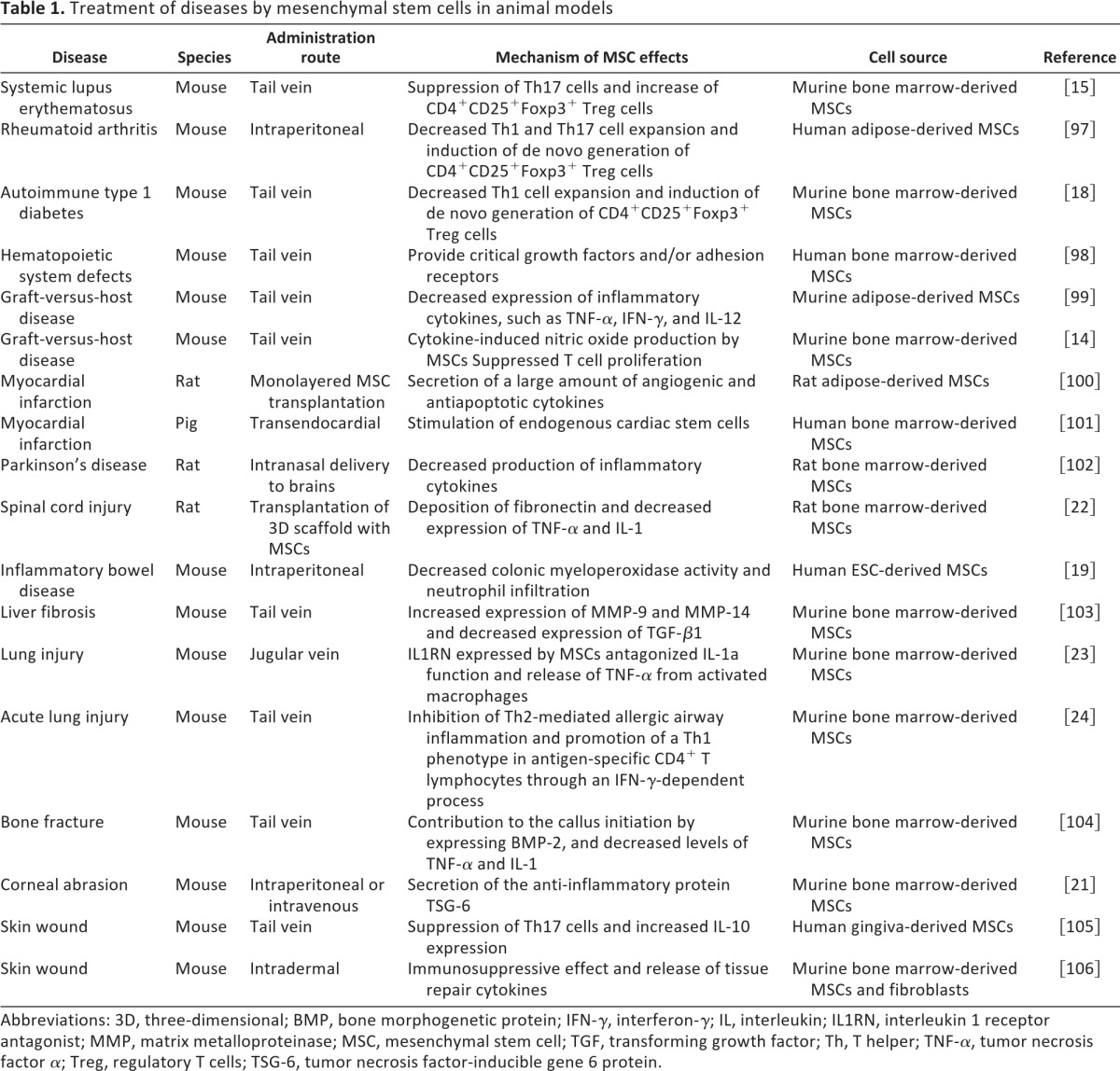
Abbreviations: 3D, three-dimensional; BMP, bone morphogenetic protein; IFN-γ, interferon-γ; IL, interleukin; IL1RN, interleukin 1 receptor antagonist; MMP, matrix metalloproteinase; MSC, mesenchymal stem cell; TGF, transforming growth factor; Th, T helper; TNF-α, tumor necrosis factor α; Treg, regulatory T cells; TSG-6, tumor necrosis factor-inducible gene 6 protein.
Graft-versus-host disease (GvHD) is a severe complication following bone marrow and HSC transplantation. It has been shown that more than 40% recipients with bone marrow transplantation develop GvHD, and many of them are hard to treat even with steroids [12]. In recent years, MSCs have been successfully applied to treating GvHD in mouse models. One or two infusions of MSCs after bone marrow transplantation greatly improve the survival rate of GvHD mice and dramatically reduce immune cell infiltration in various organs. These studies also demonstrated that proinflammatory cytokines are critical in MSC-mediated immunosuppression in vivo, as elimination of interferon-γ (IFNγ) signaling diminishes the therapeutic effect of MSCs [13, 14].
Systemic lupus erythematosus (SLE) is a generalized autoimmune disease that can be mimicked with mice lacking a functional fas gene. The therapeutic effect of MSCs has been validated in the mouse model of SLE [15]. MSCs derived from fas−/− mice were partially deficient in abundance and stemness, as revealed by plating assay and induced differentiation toward adipocytes. Allogenic MSC administration largely reduces the production of autoantibodies in the sera and ameliorate renal dysfunction in those mice with active SLE symptoms [15].
In experimental autoimmune encephalomyelitis (EAE), an established animal model of multiple sclerosis (MS), MSCs also exhibited beneficial effects [16, 17]. Decreased immune cell infiltration and demyelination in the central nerve system were observed after systemic MSC infusion. Along with less CD4+ T-cell migration, the plasma level of interleukin-17 (IL-17) was reduced. Notably, MSC injection at the peak of pathogenesis is more effective, whereas no improvement was observed in these studies if MSCs were injected at the disease-stable stages.
In the treatment of autoimmune type I diabetes, MSCs significantly delayed diabetes onset in non-obese diabetic (NOD) mice [18]. MSC infusion strikingly protected islets from destruction, as evidenced by insulin staining, lymphocyte infiltration, and islet morphology. In addition, hyperglycemia was reversed in 90% of diabetic mice receiving MSC treatment, and some of the mice remained normoglycemic for more than 2 months.
Recent studies have shown that human MSCs were effective in treating mouse inflammatory bowel disease (IBD) [19]. In this model, human ES cells were used to derive MSCs. These ES cell-derived MSCs possessed surface markers, morphology, and immunosuppressive capacity similar to those of BM-MSCs. After treatment with these MSCs, IBD animals exhibited much healthier appearance compared with control mice, as shown by significantly alleviated colon inflammation and body weight loss.
In addition to autoimmune diseases, MSCs are capable of producing trophic factors to help tissue repair. Investigations by the Prockop team have shown that human MSCs were effective in treating myocardial infarction [20] and cornea damage [21] through secretion of tumor necrosis factor-inducible gene 6 protein (TSG-6), which served to reduce inflammation and promote tissue reconstruction. A similar phenomenon has been reported with MSCs in treating other tissue injuries, such as spinal cord [22], lung [23, 24], and skin [25, 26]. Therefore, it is possible that MSC-derived soluble factors could replace MSCs in treating various diseases to evade the risk of cell-based therapies.
Clinical Applications of MSCs
The success of MSCs in modulating immune responses and promoting tissue repair in preclinical studies have prompted exploration of MSCs in clinical settings [14, 27–29]. Currently, there are 92 registered clinical trials evaluating the potential of MSC-based cell therapy worldwide (ClinicalTrials.gov, http://clinicaltrials.gov/). As shown in Table 2, 30 completed clinical trials have been announced, including phase III trials. Since 2008, several clinical trials have been carried out in the United States. With the advancement of preclinical research, more and more countries have recognized the good hope of MSC-based therapies and are participating in the expanding number of clinical trial registrations (35 in Europe, 22 in the United States, 18 in China, 5 in Korea, 4 in the Middle East, 3 in Canada, 2 in India, 1 in Africa, 1 in Japan, and 1 in Australia). It is anticipated that the completion of these trials will dramatically improve clinical applications of MSCs to treat various devastating diseases that affect human health.
Table 2.
Examples of published clinical trials of mesenchymal stem cell-based therapy registered on ClinicalTrials.gov
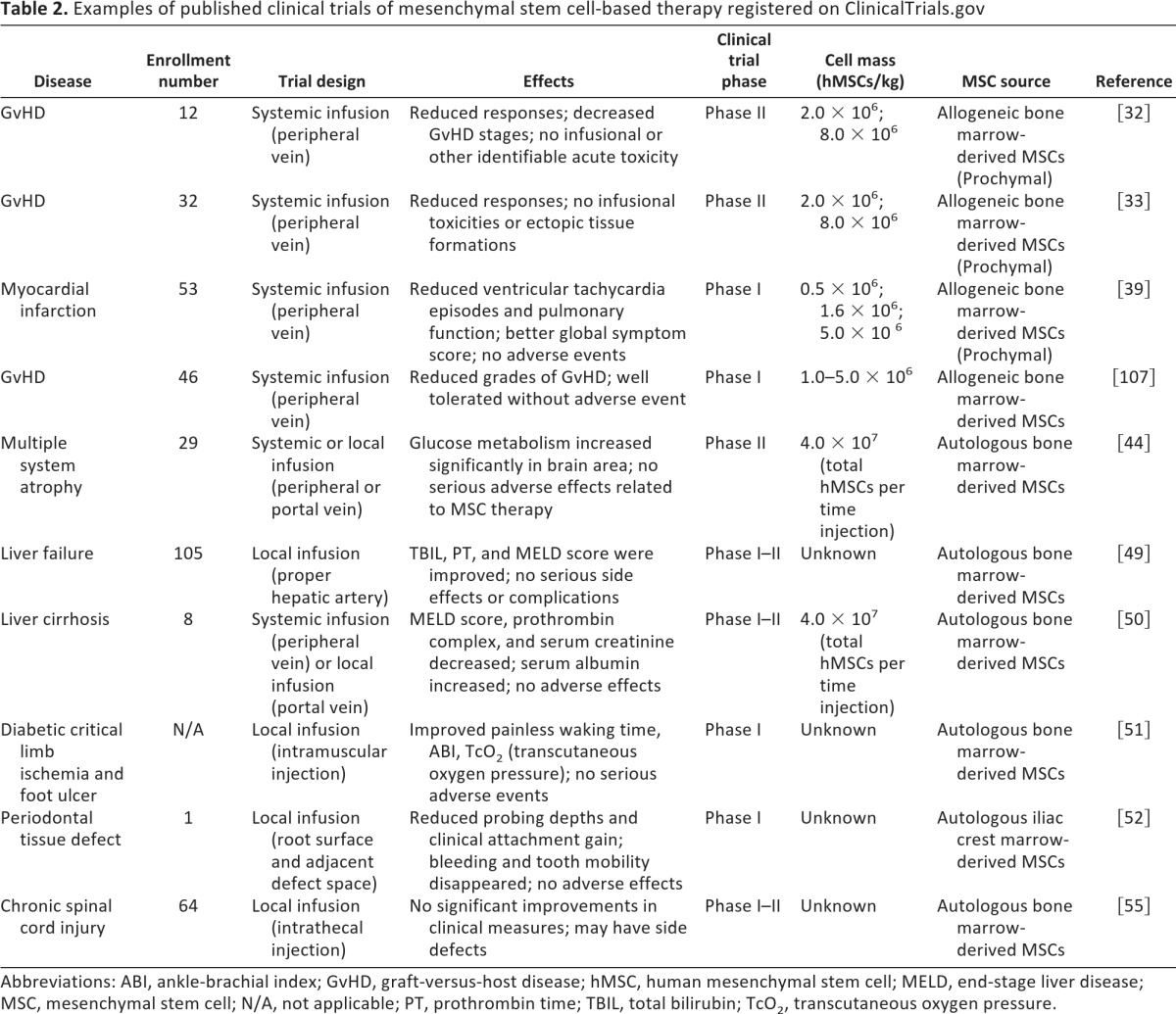
Abbreviations: ABI, ankle-brachial index; GvHD, graft-versus-host disease; hMSC, human mesenchymal stem cell; MELD, end-stage liver disease; MSC, mesenchymal stem cell; N/A, not applicable; PT, prothrombin time; TBIL, total bilirubin; TcO2, transcutaneous oxygen pressure.
Along with a better understanding of the molecular mechanisms underlying the therapeutic effects of MSCs, more and more therapeutic utilities of MSCs have been tried for various human diseases [30]. For instance, MSCs have been successfully applied to revert GvHD in patients receiving bone marrow transplantation [31, 32], especially in patients diagnosed with severe steroid resistance [33–35]. Similarly, in SLE and Crohn's disease patients, both autologous and allogeneic MSCs were able to suppress inflammation and reduce damage to kidneys and bowel, supposedly by induction of regulatory T cells in patients [25, 36–38]. In the cardiovascular system, allogeneic MSCs have been shown to reverse left ventricle acute myocardial infarction [39, 40]. In hematopoietic stem cell transplantation, MSCs provided support for the growth and differentiation of hematopoietic progenitor cells in the bone marrow microenvironment [41, 42]. In the endocrine system, placenta-derived MSCs increase the levels of insulin and C-peptide and improve the renal function and cardiac function after infusion in diabetic patients [43]. Various clinical studies have been carried out with patients suffering from neurological disorders. It has been reported that BM-MSCs improve multiple system atrophy [44], MS, amyotrophic lateral sclerosis [45–47], and stroke, likely through immediately immunomodulatory effects [48]. In the digestive system, autologous BM-MSCs improved clinical indices of liver function in liver cirrhosis patients and liver failure patients caused by hepatitis B [49, 50]. BM-MSCs can also exert strong therapeutic effects on tissue repair in muscle skeletal and skin diseases, including muscle remodeling, regeneration of periodontal tissue defects, diabetic critical limb ischemia, bone damage caused by osteonecrosis, and burn-induced skin defects [51–53]. It is noteworthy that Prochymal, an allogeneic human BM-MSCs-based stem cell product by Osiris Therapeutics (Baltimore, MD, http://www.osiristx.com) has passed Phase III clinical trials in treating GvHD and Crohn's disease and become the only stem cell-based drug approved by the FDA [33, 54]. Taken together, these clinical trials are providing critical information for the development of stem cell-based clinical application.
Although there is a long way to go before MSCs can be used as a regular clinical therapy, available clinical information is encouraging. It seems that MSC treatments are quite safe as long as the cells are administered properly. Under treatment for GvHD, SLE, and liver failure, a few patients developed fever, chill, liver damage, and other side effects [25, 33, 49, 55]. With the maturation of isolation and culture technologies, allogeneic MSCs are becoming more popular than autologous BM-MSCs [36, 38, 56]. Among the allogeneic MSCs, human umbilical cord- and placenta-derived MSCs are the most investigated. In last few years, adipose tissue-derived MSCs have attracted great attention as they are easy to be derived autologously and have been shown to be effective in treating Hurler syndrome, metachromatic leukodystrophy, GvHD, and SLE [25, 43, 57]. These clinical trial results demonstrated that MSCs derived from various sources exert similarly therapeutic effects on immune disorders [31, 33]. Notably, MSCs derived from different sources are also capable of supporting regeneration of damaged tissues in myocardial, kidney, bone, and skin [15, 39, 53]. Moreover, to increase the number of MSCs at the damaged sites and the efficiency of differentiation, studies on liver cirrhosis, osteonecrosis, skin defects, and spinal cord injury were using local injections instead of systemic administration of MSCs [53]. Nevertheless, extensive investigations are still needed to determine which cell sources are the best for the specific diseases.
Therapeutic Mechanisms of MSCs
As mentioned above, MSCs have displayed a great potential in treating a large number of immune and nonimmune diseases. However, there are still major questions concerning the optimal dosage of MSCs, routes of administration, and the fate of the cells after infusion [58]. Thus, it is critical to explore the mechanisms governing MSC-based therapies. Although a uniform mechanism may have never been discovered, the available data have revealed several working models for the beneficial effects of MSCs. On the basis of the current understanding, we sum up some key mechanisms that are significant to MSC-mediated therapies. It is noteworthy that for a given disease, multiple coordinated mechanisms likely contribute to the therapeutic effect of MSCs.
Homing Efficiency
MSCs have a tendency to home to damaged tissue sites. When MSCs are delivered exogenously and systemically administered into humans and animals, they are always found to migrate specifically to damaged tissue sites with inflammation [59, 60], although many of the intravenously administered MSCs are trapped in the lung [20, 61]. The inflammation-directed MSC homing has been demonstrated to involve several important cell trafficking-related molecules: chemokines, adhesion molecules, and matrix metalloproteinases (MMPs). Among the chemokines, the chemokine (C-X-C motif) ligand 12 (CCL-12)-chemokine (C-X-C motif) receptor 4 (CXCR4) and chemokine (C-C motif) ligand-2 (CCL-2)-chemokine (C-C motif) receptor 2 (CCR2) axes are most studied [62, 63]. Accordingly, CXCR4 has been transduced into MSCs to improve their in vivo engraftment and therapeutic efficacy in a rat myocardial infarction model [64]. Adhesion molecule P-selectin and vascular cell adhesion protein 1 (VCAM-1)-very late antigen-4 interaction have been shown as key mediators in MSC rolling and firm adherence to endothelial cells in vitro and in vivo [65]. Interestingly, in a recent report, VCAM-1 antibody-coated MSCs exhibited a higher efficiency in their engraftment into inflamed mesenteric lymph nodes and colon than uncoated MSCs in a mouse IBD model [66], suggesting that the modulations on the homing property of MSCs could be a viable approach in enhancing their therapeutic effectiveness. In addition to chemokines and adhesion molecules, several MMPs, such as MMP-2 and membrane type 1 MMP, have been shown to be essential in the invasiveness of MSCs [67, 68]. It is worth mentioning that all the homing-related molecules are able to be upregulated by inflammatory cytokines such as tumor necrosis factor-α (TNFα) and IL-1 [69, 70]. Therefore, different inflammation status (i.e., different levels of inflammatory cytokines) might lead to distinct MSC engraftment and therapeutic efficiency.
Tumors can be regarded as wounds that never heal and continuously generate various inflammatory cytokines [71]. Indeed, MSCs, either de novo mobilized or exogenously administered, have been found to migrate to the tumor and adjacent tissue sites [72]. In view of this property, approaches have been developed to engineer several tumor-killing agents, such as IFNα, IFNβ, IL-12, and TNF-related apoptosis-inducing ligand, in MSCs for tumor targeted therapy in animal models [73–77]. More recently, MSCs have also undergone development into vehicles for delivery of nanoparticles to enhance their tumoricidal effects [78, 79]. Further investigation in this direction may lead to novel therapeutic strategies for cancer.
Differentiation Potential and Tissue Engineering
As typical multipotent stem cells, MSCs have been shown to possess the capability to differentiate into a variety of cell types, including adipocytes, osteoblasts, chondrocytes, myoblasts, and neuron-like cells. Although it is currently believed that the therapeutic benefits of MSCs are due to more complicated mechanisms, they have been shown to be able to differentiate into osteoblasts, cardiomyocytes, and other tissue-specific cells after their in vivo systemic infusion in the treatment of osteogenesis imperfecta and myocardial infarction in both animals and humans [59, 80, 81].
Instead of the systemic delivery, MSCs can be delivered together with various natural and synthetic biomaterial scaffolds. Either undifferentiated or differentiated MSCs can be loaded onto the scaffolds before their implantation into the damaged tissue sites [82, 83]. Such technologies have been successfully applied in cartilage repair and long-bone repair with generation of well-integrated and functional hard tissues [84, 85]. The advantage of the tissue-engineered MSC delivery system lies in the ease of controlling and manipulating the implanted cells and tissues, with reduced side effects on other organs and tissues. The current improvement in delivery vehicles and compatibility between the scaffolds and MSCs will help develop a mature technology for clinical applications.
Production of Trophic Factors
Accumulating evidence has revealed that the therapeutic benefits from MSCs are largely dependent on their capacity to act as a trophic factor pool. After MSCs home to the damaged tissue sites for repair, they will closely interact with the local stimuli, such as inflammatory cytokines, ligands of Toll-like receptors, and the hypoxia condition, which would stimulate MSCs to produce a large amount of growth factors performing multiple functions for tissue regeneration [86, 87]. Many of these factors are critical mediators in angiogenesis and prevention of cell apoptosis, such as vascular endothelial growth factor, insulin-like growth factor 1, basic fibroblast growth factors, hepatocyte growth factor, IL-6, and CCL-2 [87, 88]. Interestingly, a recent study found that the therapeutic effect of neuronal progenitors on EAE was solely dependent on leukemia inhibitory factor, revealing a similar trophic function of other tissue progenitors/stem cells [89]. Moreover, many reports have demonstrated that pretreatment with growth factors or gene modification of MSCs enhances the therapeutic efficacy for myocardial infarction and other wound healing processes [90, 91]. Further understanding of the molecular pathways involved in growth factor production will be very helpful to develop better strategies for MSC-based therapies.
Immunomodulation
In the last few years, MSCs have been manifested to be very effective in treating various immune disorders in human and animal models. In both in vitro and in vivo studies, MSCs suppress the excessive immune responses from T cells, B cells, dendritic cells, macrophages, and natural killer cells [29]. It is believed that the underlying mechanisms are a combinational effect from many immunosuppressive mediators. Among the mediators, a majority of them are inducible by inflammatory stimuli, such as nitric oxide, indoleamine 2,3-dioxygenase, prostaglandin E2, TSG-6, CCL-2, and programmed death ligand 1 [14, 17, 62, 92–95]. These factors are minimally expressed in the inactivated MSCs unless they are stimulated by several inflammatory cytokines, IFNγ, TNFα, and IL-1 [14, 96]. Neutralization of either immunosuppressive effectors or inflammatory cytokines reverses MSC-mediated immunosuppression. The concept of inflammation-licensed immunosuppression will be in favor of a more rational design for clinical use of MSCs. First, an optimal administration time point should be carefully selected according to the levels and ratios of different cytokines in the body during the disease progression. Previous reports have demonstrated that administration of MSCs after disease onset should be better than at the same time of disease induction in a mouse GvHD model [13, 14]. Second, cytokine priming should be an attemptable maneuver to improve the therapeutic effect of MSCs. Polchert et al. reported that IFNγ-pretreated MSCs protect 100% of mice from GvHD-induced death [13]. Third, the therapeutic efficacy of MSCs probably depends on the nature of different diseases because of the distinct inflammatory environments existed. Even for one specific disease, the diversity of microenvironments in different tissues may also produce curative effects different from those of MSCs. Therefore, the precise in vivo mechanism of MSCs should be more complex than what we observed in vitro. Further defining such mechanisms will help us develop more trustworthy strategies for clinical use of MSCs.
Conclusion
Although MSCs have been widely applied in preclinical studies and clinical trials, with many of them displaying effective outcomes in prevention and control of a diversity of diseases, the underlying mechanisms, especially in the in vivo models, still remain largely undefined. Available data have suggested that MSCs have a close interaction with the inflammatory milieu in the tissue damage sites. The therapeutic effects of MSCs should not be simple actions from themselves but a coordinated process with the local microenvironment. Understanding such interactions will be helpful in choosing an optimal dose and time points for MSC administration and in predicting the range of diseases for which MSCs should be effective. Another major question is how to translate the in vitro readouts into clinical applications; this translation will probably also require a deep understanding of the intricate link between MSCs and the inflammatory conditions. Moreover, the above studies on MSC-based therapies will also have important implications for recognizing the physiological roles of stromal cells.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology of China (2010CB945600, 2008GR0606, 1027J11291, 2009ZX09503-024), Scientific Innovation Project of the Chinese Academy of Science (KSCX1-YW-22, KSCX2-YW-R-245, XDA01040000-Y11HJ11291), the National Science and Technology Project of China (31010103908), the National Institutes of Health (USA) (DE014913, DE019932, and GM866889), and the Robert Wood Johnson Foundation (67038).
Author Contributions
G.R., X.C., F.D., W.L., X.R., and Y.Z.: conception and design, manuscript writing; Y.S.: conception and design, manuscript writing, financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 2.Wong DJ, Liu H, Ridky TW, et al. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2:333–344. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miura K, Okada Y, Aoi T, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, et al. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells. 2010;28:1568–1570. doi: 10.1002/stem.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fink DW., Jr FDA regulation of stem cell-based products. Science. 2009;324:1662–1663. doi: 10.1126/science.1173712. [DOI] [PubMed] [Google Scholar]
- 6.Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Barry FP, Murphy JM. Mesenchymal stem cells: Clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 9.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prockop DJ, Brenner M, Fibbe WE, et al. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy. 2010;12:576–578. doi: 10.3109/14653249.2010.507330. [DOI] [PubMed] [Google Scholar]
- 12.Kernan NA, Bartsch G, Ash RC, et al. Analysis of 462 transplantations from unrelated donors facilitated by the National Marrow Donor Program. N Engl J Med. 1993;328:593–602. doi: 10.1056/NEJM199303043280901. [DOI] [PubMed] [Google Scholar]
- 13.Polchert D, Sobinsky J, Douglas G, et al. IFN-γ activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Sun L, Akiyama K, Zhang H, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 17.Rafei M, Campeau PM, Aguilar-Mahecha A, et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182:5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- 18.Fiorina P, Jurewicz M, Augello A, et al. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol. 2009;183:993–1004. doi: 10.4049/jimmunol.0900803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sánchez L, Gutierrez-Aranda I, Ligero G, et al. Enrichment of human ESC-derived multipotent mesenchymal stem cells with immunosuppressive and anti-inflammatory properties capable to protect against experimental inflammatory bowel disease. Stem Cells. 2011;29:251–262. doi: 10.1002/stem.569. [DOI] [PubMed] [Google Scholar]
- 20.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roddy GW, Oh JY, Lee RH, et al. Action at a distance: Systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TSG-6. Stem Cells. 2011;29:1572–1579. doi: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- 22.Zeng X, Zeng YS, Ma YH, et al. Bone marrow mesenchymal stem cells in a three dimensional gelatin sponge scaffold attenuate inflammation, promote angiogenesis and reduce cavity formation in experimental spinal cord injury. Cell Transplant. 2011 doi: 10.3727/096368911X566181. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodwin M, Sueblinvong V, Eisenhauer P, et al. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells. 2011;29:1137–1148. doi: 10.1002/stem.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun L, Wang D, Liang J, et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467–2475. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 26.Leroux L, Descamps B, Tojais NF, et al. Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther. 2010;18:1545–1552. doi: 10.1038/mt.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uccelli A, Prockop DJ. Why should mesenchymal stem cells (MSCs) cure autoimmune diseases? Curr Opin Immunol. 2010;22:768–774. doi: 10.1016/j.coi.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Shi Y, Hu G, Su J, et al. Mesenchymal stem cells: A new strategy for immunosuppression and tissue repair. Cell Res. 2010;20:510–518. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 29.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 30.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller I, Kordowich S, Holzwarth C, et al. Application of multipotent mesenchymal stromal cells in pediatric patients following allogeneic stem cell transplantation. Blood Cells Mol Dis. 2008;40:25–32. doi: 10.1016/j.bcmd.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Prasad VK, Lucas KG, Kleiner GI, et al. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant. 2011;17:534–541. doi: 10.1016/j.bbmt.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Kebriaei P, Isola L, Bahceci E, et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:804–811. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Wu KH, Chan CK, Tsai C, et al. Effective treatment of severe steroid-resistant acute graft-versus-host disease with umbilical cord-derived mesenchymal stem cells. Transplantation. 2011;91:1412–1416. doi: 10.1097/TP.0b013e31821aba18. [DOI] [PubMed] [Google Scholar]
- 35.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 36.Carrion F, Nova E, Ruiz C, et al. Autologous mesenchymal stem cell treatment increased T regulatory cells with no effect on disease activity in two systemic lupus erythematosus patients. Lupus. 2010;19:317–322. doi: 10.1177/0961203309348983. [DOI] [PubMed] [Google Scholar]
- 37.Ciccocioppo R, Bernardo ME, Sgarella A, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60:788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 38.Duijvestein M, Vos AC, Roelofs H, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: Results of a phase I study. Gut. 2010;59:1662–1669. doi: 10.1136/gut.2010.215152. [DOI] [PubMed] [Google Scholar]
- 39.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (Prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nayan M, Paul A, Chen G, et al. Superior therapeutic potential of young bone marrow mesenchymal stem cells by direct intramyocardial delivery in aged recipients with acute myocardial infarction: In vitro and in vivo investigation. J Tissue Eng. 2011;2011:741213. doi: 10.4061/2011/741213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Blanc K, Samuelsson H, Gustafsson B, et al. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007;21:1733–1738. doi: 10.1038/sj.leu.2404777. [DOI] [PubMed] [Google Scholar]
- 42.Fouillard L, Bensidhoum M, Bories D, et al. Engraftment of allogeneic mesenchymal stem cells in the bone marrow of a patient with severe idiopathic aplastic anemia improves stroma. Leukemia. 2003;17:474–476. doi: 10.1038/sj.leu.2402786. [DOI] [PubMed] [Google Scholar]
- 43.Jiang R, Han Z, Zhuo G, et al. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: A pilot study. Front Med. 2011;5:94–100. doi: 10.1007/s11684-011-0116-z. [DOI] [PubMed] [Google Scholar]
- 44.Lee PH, Kim JW, Bang OY, et al. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther. 2008;83:723–730. doi: 10.1038/sj.clpt.6100386. [DOI] [PubMed] [Google Scholar]
- 45.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connick P, Kolappan M, Patani R, et al. The mesenchymal stem cells in multiple sclerosis (MSCIMS) trial protocol and baseline cohort characteristics: An open-label pre test: Post test study with blinded outcome assessments. Trials. 2011;12:62. doi: 10.1186/1745-6215-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi MR, Kim HY, Park JY, et al. Selection of optimal passage of bone marrow-derived mesenchymal stem cells for stem cell therapy in patients with amyotrophic lateral sclerosis. Neurosci Lett. 2010;472:94–98. doi: 10.1016/j.neulet.2010.01.054. [DOI] [PubMed] [Google Scholar]
- 48.Honmou O, Houkin K, Matsunaga T, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790–1807. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng L, Xie DY, Lin BL, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: Short-term and long-term outcomes. Hepatology. 2011 doi: 10.1002/hep.24434. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Kharaziha P, Hellstrom PM, Noorinayer B, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: A phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21:1199–1205. doi: 10.1097/MEG.0b013e32832a1f6c. [DOI] [PubMed] [Google Scholar]
- 51.Lu D, Chen B, Liang Z, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: A double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92:26–36. doi: 10.1016/j.diabres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 52.Yamada Y, Ueda M, Hibi H, et al. A novel approach to periodontal tissue regeneration with mesenchymal stem cells and platelet-rich plasma using tissue engineering technology: A clinical case report. Int J Periodontics Restorative Dent. 2006;26:363–369. [PubMed] [Google Scholar]
- 53.Rasulov MF, Vasilchenkov AV, Onishchenko NA, et al. First experience of the use bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull Exp Biol Med. 2005;139:141–144. doi: 10.1007/s10517-005-0232-3. [DOI] [PubMed] [Google Scholar]
- 54.Mannon PJ. Remestemcel-L: Human mesenchymal stem cells as an emerging therapy for Crohn's disease. Expert Opin Biol Ther. 2011;11:1249–1256. doi: 10.1517/14712598.2011.602967. [DOI] [PubMed] [Google Scholar]
- 55.Kishk NA, Gabr H, Hamdy S, et al. Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil Neural Repair. 2010;24:702–708. doi: 10.1177/1545968310369801. [DOI] [PubMed] [Google Scholar]
- 56.Liang J, Zhang H, Hua B, et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: A pilot clinical study. Ann Rheum Dis. 2010;69:1423–1429. doi: 10.1136/ard.2009.123463. [DOI] [PubMed] [Google Scholar]
- 57.Fang B, Song Y, Lin Q, et al. Human adipose tissue-derived mesenchymal stromal cells as salvage therapy for treatment of severe refractory acute graft-vs.-host disease in two children. Pediatr Transplant. 2007;11:814–817. doi: 10.1111/j.1399-3046.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- 58.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahmood A, Lu D, Lu M, et al. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–702. doi: 10.1227/01.neu.0000079333.61863.aa. discussion 702–693. [DOI] [PubMed] [Google Scholar]
- 61.Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 62.Wynn RF, Hart CA, Corradi-Perini C, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 63.Belema-Bedada F, Uchida S, Martire A, et al. Efficient homing of multipotent adult mesenchymal stem cells depends on FROUNT-mediated clustering of CCR2. Cell Stem Cell. 2008;2:566–575. doi: 10.1016/j.stem.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Cheng Z, Ou L, Zhou X, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 65.Rüster B, Gottig S, Ludwig RJ, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 66.Ko IK, Kim BG, Awadallah A, et al. Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther. 2010;18:1365–1372. doi: 10.1038/mt.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ries C, Egea V, Karow M, et al. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: Differential regulation by inflammatory cytokines. Blood. 2007;109:4055–4063. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]
- 68.Ding Y, Xu D, Feng G, et al. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes. 2009;58:1797–1806. doi: 10.2337/db09-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi M, Li J, Liao L, et al. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: Role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92:897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- 70.Ren G, Zhao X, Zhang L, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dvorak HF. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 72.Spaeth E, Klopp A, Dembinski J, et al. Inflammation and tumor microenvironments: Defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–738. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- 73.Studeny M, Marini FC, Champlin RE, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 74.Ren C, Kumar S, Chanda D, et al. Cancer gene therapy using mesenchymal stem cells expressing interferon-β in a mouse prostate cancer lung metastasis model. Gene Ther. 2008;15:1446–1453. doi: 10.1038/gt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seo SH, Kim KS, Park SH, et al. The effects of mesenchymal stem cells injected via different routes on modified IL-12-mediated antitumor activity. Gene Ther. 2011;18:488–495. doi: 10.1038/gt.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loebinger MR, Eddaoudi A, Davies D, et al. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69:4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ren C, Kumar S, Chanda D, et al. Therapeutic potential of mesenchymal stem cells producing interferon-α in a mouse melanoma lung metastasis model. Stem Cells. 2008;26:2332–2338. doi: 10.1634/stemcells.2008-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roger M, Clavreul A, Venier-Julienne MC, et al. Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials. 2010;31:8393–8401. doi: 10.1016/j.biomaterials.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 79.Cheng H, Kastrup CJ, Ramanathan R, et al. Nanoparticulate cellular patches for cell-mediated tumoritropic delivery. ACS Nano. 2010;4:625–631. doi: 10.1021/nn901319y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 81.Kawada H, Fujita J, Kinjo K, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–3587. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 82.Dennis JE, Konstantakos EK, Arm D, et al. In vivo osteogenesis assay: A rapid method for quantitative analysis. Biomaterials. 1998;19:1323–1328. doi: 10.1016/s0142-9612(97)00170-1. [DOI] [PubMed] [Google Scholar]
- 83.Ohgushi H, Kotobuki N, Funaoka H, et al. Tissue engineered ceramic artificial joint–ex vivo osteogenic differentiation of patient mesenchymal cells on total ankle joints for treatment of osteoarthritis. Biomaterials. 2005;26:4654–4661. doi: 10.1016/j.biomaterials.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 84.Kon E, Muraglia A, Corsi A, et al. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 2000;49:328–337. doi: 10.1002/(sici)1097-4636(20000305)49:3<328::aid-jbm5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 85.Solchaga LA, Temenoff JS, Gao J, et al. Repair of osteochondral defects with hyaluronan- and polyester-based scaffolds. Osteoarthritis Cartilage. 2005;13:297–309. doi: 10.1016/j.joca.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 86.Crisostomo PR, Wang Y, Markel TA, et al. Human mesenchymal stem cells stimulated by TNF-α, LPS, or hypoxia produce growth factors by an NF κB- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675–C682. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 87.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. [Google Scholar]
- 88.Xu G, Zhang Y, Zhang L, et al. The role of IL-6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochem Biophys Res Commun. 2007;361:745–750. doi: 10.1016/j.bbrc.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cao W, Yang Y, Wang Z, et al. Leukemia inhibitory factor inhibits T helper 17 cell differentiation and confers treatment effects of neural progenitor cell therapy in autoimmune disease. Immunity. 2011;35:273–284. doi: 10.1016/j.immuni.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 90.Hahn JY, Cho HJ, Kang HJ, et al. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51:933–943. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 91.Schnabel LV, Lynch ME, van der Meulen MC, et al. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J Orthop Res. 2009;27:1392–1398. doi: 10.1002/jor.20887. [DOI] [PubMed] [Google Scholar]
- 92.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-γ in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 93.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 94.Augello A, Tasso R, Negrini SM, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 95.Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 97.González MA, Gonzalez-Rey E, Rico L, et al. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 98.Delalat B, Pourfathollah AA, Soleimani M, et al. Isolation and ex vivo expansion of human umbilical cord blood-derived CD34+ stem cells and their cotransplantation with or without mesenchymal stem cells. Hematology. 2009;14:125–132. doi: 10.1179/102453309X402250. [DOI] [PubMed] [Google Scholar]
- 99.Yanez R, Lamana ML, Garcia-Castro J, et al. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 100.Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 101.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Danielyan L, Schafer R, von Ameln-Mayerhofer A, et al. Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson disease. Rejuvenation Res. 2011;14:3–16. doi: 10.1089/rej.2010.1130. [DOI] [PubMed] [Google Scholar]
- 103.Sakaida I, Terai S, Yamamoto N, et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304–1311. doi: 10.1002/hep.20452. [DOI] [PubMed] [Google Scholar]
- 104.Granero-Moltó F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27:1887–1898. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang QZ, Su WR, Shi SH, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen L, Tredget EE, Liu C, et al. Analysis of allogenicity of mesenchymal stem cells in engraftment and wound healing in mice. PLoS One. 2009;4:e7119. doi: 10.1371/journal.pone.0007119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lazarus HM, Koc ON, Devine SM, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11:389–398. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]


