Abstract
Although the initial promise of cardiac cell-based therapy was based on the concept that stem cells engraft into diseased tissue and differentiate into beating cardiomyocytes, it is now clear that successful cell-based tissue repair involves a more complex orchestration of cellular and molecular events. Many lessons about successful tissue repair can be gleaned from the results of early-stage clinical trials. This body of work shows that cell-based therapy (with various cell sources and delivery methods) effectively prevents and reverses the remodeling process, the sine qua non of the myocardial injury reaction and anatomic substrate for subsequent clinical events. The potentially favorable remodeling responses to cell therapy have prompted a search for mechanisms of action beyond cell repopulation and guided future clinical trial design by providing more clear focus on pathophysiological endpoints signifying favorable responses to cell-based therapy. Perhaps the most important mechanistic insight is that endogenous stem/precursor cells have the potential to participate in tissue healing. With regard to the phenotype of cellular response, it is clear that parameters of remodeling, such as infarct size and ventricular dimensions, should be directly measured, thereby necessitating the use of sophisticated imaging modalities, such as cardiac magnetic resonance imaging or multidetector computed tomography. These new insights offer an optimistic outlook on the state of cell-based therapeutics for cardiac disease and suggest that pivotal clinical trials are warranted. Here, we review lessons learned from clinical trials and evaluate the choice and assessment of endpoints to best predict efficacy of cell therapy.
Keywords: Cell transplantation, Mesenchymal stem cells, Ventricular remodeling, Myocardial infarction, Magnetic resonance imaging, Heart failure
Introduction
The past decade has witnessed major advancements in the investigation of mechanisms of action, as well as clinical application of cell-based therapy for heart disease due to ischemic injury [1–3]. A large number of clinical trials have been conducted using a variety of cell sources, delivery methods, and patient characteristics. Although the totality of evidence, as shown in three meta-analyses [4–6], supports a beneficial effect of cardiac cell-based therapy, the field remains highly controversial because of concerns over inconsistency of results and modest effect on global cardiac function, assessed as left ventricular ejection fraction (LVEF). Furthermore, some have argued that more basic stem cell biology research is a prerequisite for further clinical translation [1]. Here we review the lessons learned from completed and ongoing clinical trials of cardiac cell-based therapy and propose that parameters of remodeling, including ventricular dimensions and infarct size, are the best predictors of clinical efficacy.
Historical Perspective
The translation of stem cell biology research into the clinical arena began shortly after preclinical experimental studies were first published. For instance, Strauer et al. [7, 8] conducted and published the results of intracoronary bone marrow mononuclear cell infusion in patients with chronic myocardial infarction (MI) within a few months of the publication of the work of Orlic et al. in 2001 [9], which showed that bone marrow-derived stem cells regenerate infarcted myocardium in mice. By 2006, several important bone marrow-derived stem cell trials had been conducted, notably the Reinfusion of Enriched Progenitor Cell and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial [10], the Bone Marrow Transfer to Enhance ST-Elevation Infarct Regeneration trial (BOOST) [11], and the Autologous Bone Marrow-Derived Stem-Cell Transfer in Patients with ST-Segment Elevation Myocardial Infarction double-blind, randomized control trial [12]. Together, these studies reported an increase in LVEF of 3.3%–5.9% in patients treated with intracoronary bone marrow-derived stem cells. This modest magnitude of LVEF increase was corroborated in two meta-analyses, which evaluated 13 clinical trials with a total of 811 patients [5] and 18 studies with a total of 999 patients [4] and found significant improvements in LVEF of 2.99% and 3.66% in the cell-treated groups, respectively. Furthermore, a more recent meta-analysis of seven randomized controlled clinical trials, which included a total of 237 patients, reported a similarly modest increase in LVEF of 3.7% in patients who received intracoronary circulating progenitor cells or intracoronary/intramyocardial peripheral blood stem cells [6].
Evaluating the efficacy of cardiac cell-based therapy has posed a challenge because of the differences in study design characteristics, including cell sources, culture techniques, timing and method of cell delivery, patient demographics, type of myocardial injury, and type and method of assessment of clinical endpoints. Although the interpretation of the early wave of trials was challenged by variation in these design characteristics, the primary objections raised to the trials were the perceived low magnitude of LVEF increase and the lack of concomitant mechanistic insights [1, 6]. Indeed, although LVEF was chosen as the primary endpoint in the first wave of trials, additional, more comprehensive data from these investigations provide some instrumental insights. In this context, it is clear that cell-based therapy should be evaluated from the perspective of the pathophysiology of remodeling postinjury. As cell-based therapy should either prevent or reverse remodeling given its role as a regenerative strategy, greater attention should be paid to existing data from clinical trials that support the notion that successful cell-based therapy prevents, reduces, and even reverses ventricular injury and remodeling [3, 12–15].
Cell Type
Most clinical trials have used autologous whole bone marrow as a cell therapeutic [5, 7, 8, 11, 12, 14–16]. However, extensive investigation is under way using bone marrow-derived mesenchymal stem cells (MSCs) [3, 13] and cardiac stem cells (CSCs) (NCT00474461, NCT00893360). Although early results are encouraging for these preparations, much work is required to compare cell preparations and optimize their production. Regardless of cell type, considerations of ventricular remodeling are likely to be operative as the best way to compare the efficacy of various cell preparations.
Remodeling
Why Is Remodeling the Better Endpoint?
An appreciation of effective cell-based therapy requires consideration of the pathophysiology of myocardial injury following infarction, a process termed remodeling [17]. The key architectural and constitutional changes that occur in the ventricle after MI are defined as remodeling. In the infarct zone, remodeling results in acute wall thinning because of proteolysis of the extracellular matrix (ECM) [18]. This is followed by an increased production of ECM that results in collagenous scar in the infarct zone and interstitial fibrosis in the noninfarcted zone. The interstitial fibrosis in the noninfarcted zones increases tissue stiffness and decreases cardiomyocyte survival, resulting in an increased risk of arrhythmias. The loss of balance in the ECM greatly affects the performance of the heart, leading to chamber dilatation and contractile dysfunction [18]. In addition, the neoangiogenic response after MI is insufficient to provide the adequate myocardial perfusion necessary to meet the demands of the hypertrophied myocardial wall, leading to further loss of cardiomyocytes and fibrous tissue formation [19]. Accordingly, proangiogenic and antifibrotic effects are highly likely to contribute to the success of a cell therapeutic preparation.
From the point of view of trial design, the pathophysiology of ventricular remodeling calls into question the choice of LVEF as an endpoint for cell-based therapy trials. LVEF, the percentage of end-diastolic volume ejected with each contraction (left ventricular [LV] stroke volume/end-diastolic volume), is highly load dependent [1, 20]. Therefore, LVEF can vary with ventricular preload, afterload, and contractility and thus reflects integrated cardiovascular performance, in addition to chamber dimensions. Other factors following cardiac damage can influence LVEF; for example, myocardial injury activates compensatory neurohormonal systems that increase peripheral vasoconstriction, which can decrease LVEF by increased afterload [20]. Cell therapy is designed to prevent or reverse remodeling by repopulation of injured myocardial segments, thereby protecting the contractility of these segments (regional contractility). Thus, the intrinsic cardiovascular compensatory mechanisms could result in small changes in LVEF, which reflects global contractility. As such, the resulting benefits from cardiac cell-based therapy would be best predicted by a reduction in chamber dimensions and infarct size and improvement in regional contractility, as shown by Janssens et al. [12] and Williams et al. [3]. These beneficial changes may be masked by the sole use of LVEF as an outcome measure.
There are two therapeutic strategies by which to approach ventricular remodeling, namely prevention and reversal. In the preventive strategy, treatment is provided in the acute setting of MI, whereas once there is an established scar, the goal would be to achieve reversal of remodeling. The Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) [15], REPAIR-AMI [10], and Prochymal studies [13], among others, evaluated the efficacy and safety of cell therapy in the acute MI setting, demonstrating a therapeutic effect on attenuation of remodeling (Fig. 1). With regard to reversal of remodeling, early run-in data from the Transendocardial Autologous Cells in Ischemic Heart Failure (TAC-HFT) trial [3] support the ability of MSCs to achieve reverse remodeling in the chronic MI setting.
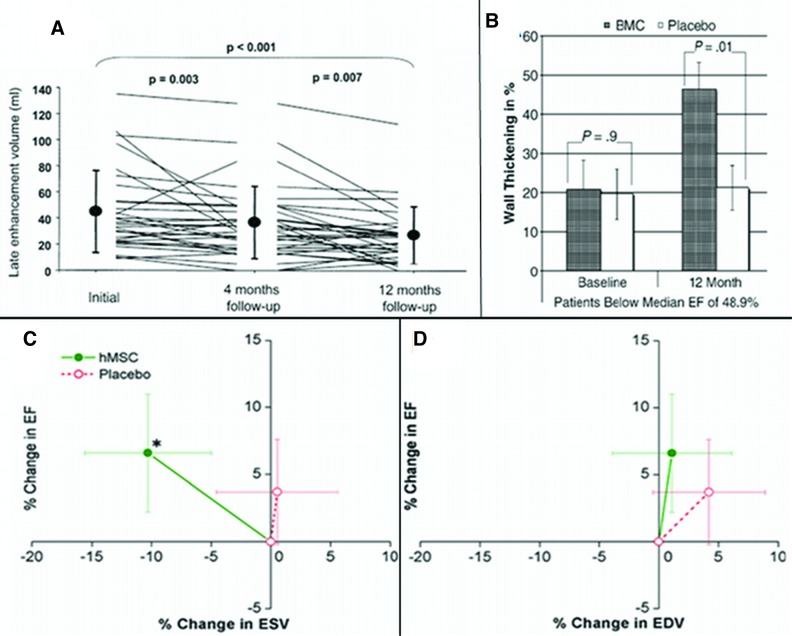
Figure 1.
Effect of cell therapy on remodeling. (A): Reduction in infarct volume assessed by delayed enhancement MRI. Reprinted with permission from Elsevier (Schächinger et al.) [15]. (B): Reduction in wall thickening. Reprinted with permission from Elsevier (Dill et al.) [21]. (C): Decline in ESV (*p < .005 vs. baseline). (D): No increase in EDV. Reprinted with permission from Elsevier (Hare et al.) [13]. Abbreviations: BMC, bone marrow cell; EDV, end diastolic volume; EF, ejection fraction; ESV, end systolic volume; hMSC, human mesenchymal stem cells.
In the TOPCARE-AMI trial [15], a subanalysis of 37 patients from a total of 59 patients who received intracoronary bone marrow-derived progenitors demonstrated a significant reduction in scar tissue volume by delayed enhancement with magnetic resonance imaging (MRI). TOPCARE-AMI is one of the first clinical trials demonstrating evidence of significant infarct size reduction that was sustained after a year of follow-up (Fig. 1A). Similarly, a cardiac MRI substudy of the REPAIR-AMI trial [21] highlights the importance of remodeling changes. This subanalysis of 54 patients, from a total of 204, demonstrated a significant effect of cell therapy on wall thickening, which indicated attenuation of remodeling, as well as the importance of targeting a “sicker population,” because the beneficial effect was observed only in patients with ejection fraction <48% (Fig. 1B). The beneficial effects on remodeling are consistent with those observed in our Prochymal trial cardiac MRI substudy [13]. In this substudy, patients who received allogeneic MSCs intravenously after reperfusion exhibited a decline in end systolic volume (Fig. 1C) and no increase in end-diastolic volume (Fig. 1D), indicating prevention of remodeling. On the other hand, patients receiving placebo had evidence of progressive left ventricular chamber enlargement. Moreover, the first report of our TAC-HFT pilot study [3] has shown evidence of the impact of stem cell therapy on reverse remodeling with reduction of infarct size and improvement of contractility.
Although the early wave of trials was criticized for inconsistency and an overall low magnitude of benefit, their results did in fact also support a favorable remodeling effect. The study performed by Janssens et al. [12] is highly instructive to consider in this regard. Ironically, this study, which used cardiac MRI to measure MI size and LVEF, was reported as a negative study because there were no differences in the LVEF between the cell-treated and placebo groups. However, improved regional function was reported in the bone marrow stem cell-treated group. Moreover, and highly importantly, cell therapy in this study significantly reduced MI size by approximately 28%. Similarly, the first clinical trial with intracoronary blood-derived progenitor cells [22] also reported that the cell-treated group had a significant infarct size reduction at 3 months. In a randomized controlled clinical trial by Hendrikx et al. [14], 10 patients with a postinfarction nonviable scar received bone marrow-derived mononuclear cells intramyocardially after coronary artery bypass graft (CABG). A significant improvement in systolic wall thickening in the infarct area, assessed by cardiac MRI, was observed at 4 months. The results of the study indicated a significant recovery of regional contractile function, although global LVEF was not significantly improved. Collectively, these studies support the notion that reduction in MI size and improvement in regional contractile function should be the endpoints in future clinical trials.
Role of Preclinical Models in Assessing Phenotype and Mechanism
A recent meta-analysis [23] demonstrated that preclinical studies in large animal models are translatable to humans and reliably predict the outcome of clinical trials. In this regard, our group has shown in a swine model of chronic MI that surgical injection of autologous MSCs results in a reduction in infarct size and an increase in regional contractility [24]. These results have been reproduced in patients in the phase I pilot of TAC-HFT [3]. The outcomes after a year of follow-up of the first eight patients who received injections of autologous bone marrow progenitor cells (mononuclear cells or MSCs, n = 4) revealed significant reduction of the infarct size and improvement in the regional contractility as assessed by cardiac MRI. The improvement in regional contractility of the left ventricle, by peak Eulerian circumferential strain in the treated infarct zone, strongly correlated with reduction of end systolic and end diastolic volumes. Once this study is completed, its outcomes will give us more insights that will aid in the design of future clinical trials.
Assessment of Remodeling
Given that remodeling independently predicts cardiovascular mortality [25], it could be an attractive surrogate for assessing the clinical efficacy of cardiac cell-based therapy. The ideal assessment method should be one able to analyze cardiac structural and functional changes, including infarct size, perfusion of the myocardial wall, ventricular motion, ventricular volumes, and chamber dimensions. Serial noninvasive imaging studies using echocardiography (ECHO); stress perfusion imaging, such as single-photon emission computed tomography (SPECT) or positron emission tomography (PET); multidetector computed tomography (MDCT); and cardiac MRI have the potential to demonstrate time-dependent changes in cardiac structure and function. ECHO is considered first choice in the workup of heart failure patients to evaluate ventricular volumes, such as LVEF, and it also permits the analysis of strain, which is a measure of tissue deformation, a load-independent measure of regional cardiac function for ventricular motion [26]. However, this method lacks accurate visualization of tissue viability, which is essential in the recognition of the infarct zone for cell therapy. On the other hand, SPECT and PET are routinely used in clinical practice to detect ischemia, viability, and myocardial perfusion. Nevertheless, these nuclear methods have limitations in sensitivity as a consequence of impaired spatial resolution or mitigation of artifacts, which are less concerning in perfusion cardiac MRI [27]. Perfusion MRI and delayed enhancement by MDCT or MRI allow the visualization of different tissue characteristics and provide viability information from the myocardial layers, such as epicardium, midwall, and endocardium. Tagging analysis provides strain information from the myocardial layers as well. This is an important analysis of cardiac regional function that is very useful for the understanding of changes in cardiac contractility [28]. Moreover, cardiac MRI permits the measurement of ventricular volumes and chamber dimensions, as well as the analysis of cardiac global function. Indeed, measurement of LVEF by cardiac MRI compares well with ECHO [29]. The versatility of cardiac MRI for different analyses has made this method the gold standard for assessment of myocardial viability [30]. MDCT is also an evolving method to address ventricular function and viability. Nieman et al. have demonstrated that delayed enhancement with computed tomography compares well with cardiac MRI [31]. Accordingly, cardiac MRI and MDCT are emerging as the lead techniques to assess remodeling after cell therapy.
Why Does Cell Therapy Work?
Lessons Learned from Studies Using MSCs
MSCs are a rare population of self-renewing, multipotent cells present in the bone marrow. Although MSCs represent 0.001%–0.01% of all nucleated bone marrow cells, they can be expanded in vitro under specific conditions. These multipotent cells can differentiate into osteoblasts, chondrocytes, and adipocytes [32], as well as endothelial cells, vascular smooth muscle cells, and cardiomyocytes [33]. However, although there is in vitro and preclinical evidence that MSCs differentiate into cardiomyocyte-like cells with sarcomeric organization when injected into the adult myocardium, it appears that a low percentage of cells engraft, independently of cell source, dose, and route of delivery [33–35]. Thus, the mechanisms of action of MSC-induced cardiac regeneration in preclinical and clinical studies have been attributed by some to paracrine effects [35]. MSCs and MSC-derived paracrine factors have the potential to prevent or reverse pathological remodeling by modulating endogenous processes, such as fibrosis, neovascularization, and recruitment of CSCs.
Antifibrotic Effects of MSCs
Accumulating evidence supports the notion that MSCs reduce myocardial fibrosis and thereby attenuate LV dilatation [36]. MSCs have been shown to modulate the expression of ECM proteins and matrix metalloproteinases and to secrete antiapoptotic and antifibrotic factors, such as hepatocyte growth factor and adrenomedullin [35, 37]. Indeed, MSC-conditioned medium has been shown to decrease type I and III collagen expression, attenuate proliferation of fibroblasts, and upregulate expression of elastin [38]. Together, these effects lead to a degradation of ECM proteins that results in reduction of myocardial fibrosis and favorable changes in remodeling. The reduction of infarct size seen in various preclinical and clinical studies is at least in part mediated by this mechanism of action.
Effects of MSCs on Neovascularization
MSCs have also been shown to secrete angiogenic cytokines, such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF)-2, FGF-7, monocyte chemoattractant protein-1, platelet-derived growth factor, placental growth factor, and transforming growth factor-β [35]. These paracrine mediators are regulated by multiple factors following ischemia. In swine models, the intramyocardial injection of MSCs led to increased VEGF expression and increased vessel density associated with improved myocardial function [34], supporting neovascularization [39–41] as an important mechanism underlying the beneficial effects of MSC therapy on myocardial remodeling.
Stimulation of Endogenous CSCs by MSCs
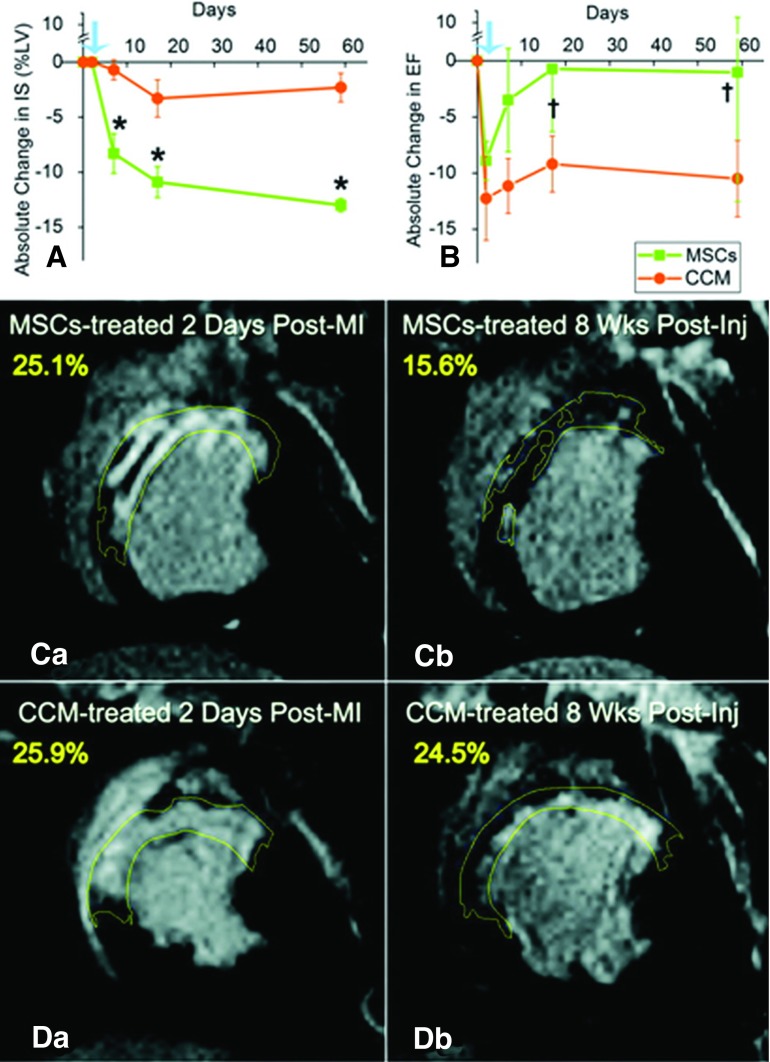
There is growing evidence that MSCs stimulate endogenous CSC recruitment, proliferation, and differentiation as a key mechanism promoting the regenerative potential of the injured myocardium [34]. To test this hypothesis, we administered transendocardial injections of allogeneic MSCs from male swine into female pigs following MI [34]. In addition to demonstrating the cardiogenic differentiation capacity of MSCs, this study revealed for the first time an increase in the proliferation of endogenous CSCs in the MSC-treated group, enhanced lineage commitment of the CSCs, and reconstitution of niche-like structures. Importantly, cardiac MRI documented a reduction of infarct size as early as 4 days after cell injection, which was progressive over 8 weeks (Fig. 2).
Figure 2.
Impact of MSCs and concentrated conditioned medium (CCM) on IS. (A): ≈50% reduction in IS (*p < 0.002). (B): Ejection fraction restoration toward normal (†p = .042 and †p = .026 within MSC group). Blue arrows indicate day before injection. (C) and (D): Delayed-enhancement of MSC-treated (C) and CCM-treated (D) animals before and 8 weeks after injections. Reprinted with permission from Wolters Kluwer Health (Hatzistergos et al.) [34]. Abbreviations: CCM, concentrated conditioned medium; Inj, injection; IS, infarct size; LV, left ventricle; MI, myocardial infarction; MSC, mesenchymal stem cell.
The stimulation of endogenous CSCs by MSCs requires a complex molecular interaction and is a crucial component of the beneficial cell therapeutic effects. Shabbir et al. [42] demonstrated in a hamster heart failure model that 1 month after intramuscular administration of MSCs, capillary and myocyte nuclear densities were enhanced by approximately 30% and 80%, respectively, apoptosis was attenuated by approximately 60%, and fibrosis was reduced by approximately 50%. In this study, the increase in circulating levels of trophic factors, such as hepatocyte growth factor, leukemia inhibitory factor, and macrophage colony-stimulating factor, were involved in the mobilization and proliferation of c-kit+ CSCs. There is also evidence that VEGF not only is involved in promoting neovascularization but also interacts with stem cell-derived factor (SDF) and is involved in the mobilization and migration of stem cells, contributing to the overall repair of the injured myocardium [34, 42–44]. This process appears to be mediated by SDF-1α/CXCR4 activation. Tang et al. [45] demonstrated that genetically modified MSCs overexpressing VEGF induced the release of SDF in both transplanted MSCs and endogenous cardiac cells. This process markedly reduced infarct size and improved cardiac function. Together, these studies support the unique role of MSCs in the activation of endogenous CSCs for their reparative effect in the injured myocardium. Whether additional therapeutic interventions to optimize exogenous and endogenous stem cell function, such as growth factor administration, gene therapy, or modulation with small molecules or pharmacologic approaches, would safely enhance cardiac repair and regenerative capacity is the focus of intense investigation [46].
Future Directions
CSCs can be isolated and expanded from human myocardial samples using minimally invasive procedures [2, 47, 48]. Different populations have been identified and characterized as c-kit+ cells, Sca-1+ cells, side population cells, and cells expressing Islet-1. These cells offer the promise of a highly cardiogenic source of cells for cell therapy. Urbanek et al. found evidence of this in samples obtained from hypertrophied left ventricular walls of patients undergoing aortic valve surgery [49]. Messina et al. found self-adherent clusters called cardiospheres after culturing atrial or ventricular biopsy specimens. These cardiospheres expressed endothelial and stem cell markers (including c-kit+), and cells derived from them displayed morphologic and phenotypical patterns characteristic of cardiomyocytes [50]. In addition, Smith et al. found cardiospheres made up of a mixed population of cardiac progenitors, fibroblasts, and mesenchymal cells after examining right ventricular biopsies [51]. Currently, the phase I clinical trial Cardiac Stem Cell Infusion in Patients with Ischemic Cardiomyopathy (SCIPIO) is enrolling patients and consists of administering c-kit+ CSCs via intracoronary delivery in patients with ischemic cardiomyopathy after taking a biopsy from the right atrial appendage during CABG (NCT00474461). Moreover, the phase I Randomized, Dose Escalation Study of the Safety and Efficacy of Intracoronary Delivery of Cardiosphere-Derived Stem Cells in Patients with Ischemic Left Ventricular Dysfunction and a Recent Myocardial Infarction (CADUCEUS), an ongoing study in which patient follow-up will be for 12 months, will evaluate cardiac parameters every 6 months by cardiac MRI (NCT00893360). These clinical trials evaluating cardiac-derived progenitor/stem cells will potentially offer new insights regarding the efficacy of cell therapy on myocardial remodeling and regeneration.
Conclusion
Cardiac cell-based therapy holds enormous promise as a strategy aimed at preventing or reversing myocardial remodeling after injury and promoting tissue regeneration. An early wave of trials using bone marrow revealed a modest but statistically significant increase in LVEF. More detailed evaluation of existing human phenotypic data supports the notion that cell-based therapy has the capacity for infarct size reduction and improved regional contraction in infracted segments. Preclinical models (primarily using large animals, such as pigs and sheep) have been instrumental in advancing phenotypic and mechanistic insights underlying cell therapy. The field has advanced rapidly, and now numerous cell types, including MSCs and their precursors, as well as CSCs, are under evaluation. It is clear that cell therapy holds major promise as a durable and sustainable therapeutic strategy and that successful cell therapy involves a complex interplay between cell types and an orchestration of events, including reduction of fibrosis, neovascularization, and cell repopulation. Existing mechanistic studies support the importance of the release of trophic factors from cell therapeutic agents, in addition to cell engraftment, differentiation, and stimulation of endogenous cell recruitment. This enhanced understanding of phenotypic response and mechanistic appreciation of the underpinnings of cell-based therapy can be harnessed for improved trial design, as well as for development of newer generations of cell products that have greater efficacy.
Acknowledgments
Dr. Hare is supported by NIH Grants R01-HL094849, P20-HL101443, R01-HL084275, R01-HL107110, and U54-HL081028.
Author Contributions
V.Y.S.: conception and design, collection/assembly of data, manuscript writing; I.H.S.: collection/assembly of data, manuscript writing; J.M.H.: conception and design, financial support, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selem S, Hatzistergos KE, Hare JM. Cardiac stem cells: Biology and therapeutic applications. In: Atala A, Lanza R, Thomson JA, editors. Principles of Regenerative Medicine. San Diego, CA: Elsevier Inc; 2011. pp. 327–346. [Google Scholar]
- 3.Williams AR, Trachtenberg B, Velazquez DL, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circ Res. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: A systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Rendon E, Brunskill SJ, Hyde CJ, et al. Autologous bone marrow stem cells to treat acute myocardial infarction: A systematic review. Eur Heart J. 2008;29:1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 6.Wen Y, Meng L, Ding Y, et al. Autologous transplantation of blood-derived stem/progenitor cells for ischaemic heart disease. Int J Clin Pract. 2011;65:858–865. doi: 10.1111/j.1742-1241.2011.02715.x. [DOI] [PubMed] [Google Scholar]
- 7.Strauer BE, Brehm M, Zeus T, et al. [Intracoronary, human autologous stem cell transplantation for myocardial regeneration following myocardial infarction] Dtsch Med Wochenschr. 2001;126:932–938. doi: 10.1055/s-2001-16579-2. [DOI] [PubMed] [Google Scholar]
- 8.Strauer BE, Brehm M, Zeus T, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 9.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 10.Schächinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 11.Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: Eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 12.Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 13.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendrikx M, Hensen K, Clijsters C, et al. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: Results from a randomized controlled clinical trial. Circulation. 2006;114(1 suppl):I101–I107. doi: 10.1161/CIRCULATIONAHA.105.000505. [DOI] [PubMed] [Google Scholar]
- 15.Schächinger V, Assmus B, Britten MB, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: Final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44:1690–1699. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Schächinger V, Erbs S, Elsasser A, et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: Final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27:2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 17.Pfeffer MA, Braunwald E. Ventricular enlargement following infarction is a modifiable process. Am J Cardiol. 1991;68:127D–131D. doi: 10.1016/0002-9149(91)90270-u. [DOI] [PubMed] [Google Scholar]
- 18.Lindsey ML, Mann DL, Entman ML, et al. Extracellular matrix remodeling following myocardial injury. Ann Med. 2003;35:316–326. doi: 10.1080/07853890310001285. [DOI] [PubMed] [Google Scholar]
- 19.Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 20.Harrison RW, Thakkar RN, Senzaki H, et al. Relative contribution of preload and afterload to the reduction in cardiac output caused by nitric oxide synthase inhibition with L-N(G)-methylarginine hydrochloride 546C88. Crit Care Med. 2000;28:1263–1268. doi: 10.1097/00003246-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Dill T, Schächinger V, Rolf A, et al. Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: Results of the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. Am Heart J. 2009;157:541–547. doi: 10.1016/j.ahj.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Erbs S, Linke A, Adams V, et al. Transplantation of blood-derived progenitor cells after recanalization of chronic coronary artery occlusion: First randomized and placebo-controlled study. Circ Res. 2005;97:756–762. doi: 10.1161/01.RES.0000185811.71306.8b. [DOI] [PubMed] [Google Scholar]
- 23.van der Spoel TI, Jansen of Lorkeers SJ, Agostoni P, et al. Human relevance of pre-clinical studies in stem cell therapy: Systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc Res. 2011;91:649–658. doi: 10.1093/cvr/cvr113. [DOI] [PubMed] [Google Scholar]
- 24.Schuleri KH, Feigenbaum GS, Centola M, et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foley PW, Chalil S, Khadjooi K, et al. Left ventricular reverse remodelling, long-term clinical outcome, and mode of death after cardiac resynchronization therapy. Eur J Heart Fail. 2011;13:43–51. doi: 10.1093/eurjhf/hfq182. [DOI] [PubMed] [Google Scholar]
- 26.Paterson DI, OMeara E, Chow BJ, et al. Recent advances in cardiac imaging for patients with heart failure. Curr Opin Cardiol. 2011;26:132–143. doi: 10.1097/HCO.0b013e32834380e7. [DOI] [PubMed] [Google Scholar]
- 27.Schwitter J. Perfusion cardiovascular magnetic resonance: Will it replace SPECT? Dialogues Cardiovasc Med. 2007;12:114–122. [Google Scholar]
- 28.Shehata ML, Cheng S, Osman NF, et al. Myocardial tissue tagging with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009;11:55. doi: 10.1186/1532-429X-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nesser HJ, Mor-Avi V, Gorissen W, et al. Quantification of left ventricular volumes using three-dimensional echocardiographic speckle tracking: Comparison with MRI. Eur Heart J. 2009;30:1565–1573. doi: 10.1093/eurheartj/ehp187. [DOI] [PubMed] [Google Scholar]
- 30.Danias PG. Cardiovascular magnetic resonance imaging: An opportunity for fruitful collaborations. Hellenic J Cardiol. 2007;48:53–54. [PubMed] [Google Scholar]
- 31.Nieman K, Shapiro MD, Ferencik M, et al. Reperfused myocardial infarction: Contrast-enhanced 64-Section CT in comparison to MR imaging. Radiology. 2008;247:49–56. doi: 10.1148/radiol.2471070332. [DOI] [PubMed] [Google Scholar]
- 32.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 33.Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirotsou M, Jayawardena TM, Schmeckpeper J, et al. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50:280–289. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berry MF, Engler AJ, Woo YJ, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290:H2196–H2203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 37.Xu X, Xu Z, Xu Y, et al. Effects of mesenchymal stem cell transplantation on extracellular matrix after myocardial infarction in rats. Coron Artery Dis. 2005;16:245–255. doi: 10.1097/00019501-200506000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Ohnishi S, Sumiyoshi H, Kitamura S, et al. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Lett. 2007;581:3961–3966. doi: 10.1016/j.febslet.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 39.Schuleri KH, Amado LC, Boyle AJ, et al. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. Am J Physiol Heart Circ Physiol. 2008;294:H2002–H2011. doi: 10.1152/ajpheart.00762.2007. [DOI] [PubMed] [Google Scholar]
- 40.Tang YL, Zhao Q, Zhang YC, et al. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117:3–10. doi: 10.1016/j.regpep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y, Wang S, Yu Z, et al. Direct injection of autologous mesenchymal stromal cells improves myocardial function. Biochem Biophys Res Commun. 2009;390:902–907. doi: 10.1016/j.bbrc.2009.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shabbir A, Zisa D, Suzuki G, et al. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: A noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296:H1888–H1897. doi: 10.1152/ajpheart.00186.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakanishi C, Yamagishi M, Yamahara K, et al. Activation of cardiac progenitor cells through paracrine effects of mesenchymal stem cells. Biochem Biophys Res Commun. 2008;374:11–16. doi: 10.1016/j.bbrc.2008.06.074. [DOI] [PubMed] [Google Scholar]
- 44.Urbich C, Aicher A, Heeschen C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Tang JM, Wang JN, Zhang L, et al. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res. 2011;91:402–411. doi: 10.1093/cvr/cvr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanashiro-Takeuchi RM, Schulman IH, Hare JM. Pharmacologic and genetic strategies to enhance cell therapy for cardiac regeneration. J Mol Cell Cardiol. 2011;51:619–625. doi: 10.1016/j.yjmcc.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D'Amario D, Fiorini C, Campbell PM, et al. Functionally competent cardiac stem cells can be isolated from endomyocardial biopsies of patients with advanced cardiomyopathies. Circ Res. 2011;108:857–861. doi: 10.1161/CIRCRESAHA.111.241380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urbanek K, Quaini F, Tasca G, et al. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:10440–10445. doi: 10.1073/pnas.1832855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Messina E, De AL, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 51.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]