Abstract
Osteoclasts (OCs) are the exclusive bone resorptive cell, they derive from monocyte/macrophage precursors, which can circulate within the hematopoietic cell pool or be resident in a number of tissues. The maintenance of an adequate bone mass depends on the controlled and timely removal of old, damaged bone. The increase of OC activity is observed in many pathologies characterised by bone loss, such as osteoporosis, rheumatoid arthritis, bone metastasis, periprosthetic osteolysis in aseptic loosening of arthroplasty and also in pediatric diseases, such as phenilketonuria and 21-hydroxylase deficiency. During the bone resorption process there is an intense cross-talk between immune system cells and OCs. In particular, T cells release factors and cytokines, which rule osteoclastogenesis, and on the other hand, OCs produce factors that act on T cells. A primary mediator of osteoclastogenesis is the receptor activator of nuclear factor-κβ-RANK ligand-osteoprotegerin system, but also other cytokines promote OC activation according to the different pathologies. This review summarizes the main mechanisms promoting osteoclastogenesis in diseases characterised by bone loss, focusing on factors and cytokines involved in this process and on the interaction between OCs and T cells.
Mechanisms of osteoclast formation
Osteoclasts (OCs) are multinucleated cells, originated by the differentiation of monocyte/macrophage precursor cells. The OC precursors circulate in the peripheral blood, expressing CD11b–c, CD14 and receptor activator of nuclear factor-κβ (RANK).1 The OC formation is driven by contact with mesenchymal cells in bone, which express RANK ligand (RANKL).2 RANKL is a member of the tumor necrosis factor (TNF) superfamily, it exists as membrane-bound protein,3 cleaved in a soluble form (sRANKL) by metalloproteinases.4,5 In vitro, in the absence of stromal cells, RANKL can activate mature OCs in a dose-dependent manner6 whereas in vivo it can lead rapidly to the resorption of bone by activating pre-existing OCs.7,8 RANKL inhibition through its natural decoy receptor osteoprotegerin (OPG) prevents bone loss in postmenopausal osteoporosis and cancer metastasis. Several diseases associated with bone loss are linked to the alteration in RANKL to OPG axis.9 Other factors have been described to affect osteoclastogenesis, such as parathyroid hormone, parathyroid hormone-related protein, 1,25(OH)2D3, glucocorticoids, interleukin (IL)-1, IL-6, IL-7, IL-11, TNF-α and prostaglandin-E2.10,11,12 Many of these factors exert most of their osteoclastogenic activity by inducing RANKL expression on osteoblasts (OBs).2,13
OCs and T cell cross-talk
The interactions between T cells and OCs represent an intriguing aspect of the new and complex osteoimmunology research field. Bone cells express surface molecules, which are essential for the expansion of hemopoietic stem cells from which all cells of the mammalian immune system derive and at the same time various immunoregulatory cytokines influence the fate of bone cells. Now it is clear that a host of immune factors, including co-stimulatory receptors, cytokines such as interferon (IFN)-γ and TNF, and T and B lymphocytes regulate bone cell development, bone turnover and are involved in the pathogenesis of bone disease.14 sRANKL is secreted by activated T cells and represents a crucial link between bone metabolism and the immune system, directly regulating osteoclastogenesis and bone remodeling, and explaining why autoimmune diseases,15 cancers,16 periprosthetic osteolysis,17 phenylketonuria (PKU)18 and osteoporosis19 result in a systemic and local bone loss. RANK and RANKL also modulate the immunity through dendritic cells (DCs) because they increase the ability of DCs to stimulate naïve T-cell proliferation and enhance DC survival. Moreover, T cells also release IL-7, a regulator of bone homeostasis, which promotes osteoclastogenesis by upregulating T-cell-derived cytokines, such as RANKL.20 Cytokines such as TNF-α, IL-1 and IL-18 can boost the activating effect of T cells on OCs, because they upregulate RANKL expression on T cells.21 A negative link between T-cell activation and bone resorption is represented by IFN-γ that was shown to block RANKL signaling and thus to inhibit OC differentiation in vitro.22 In contrast with this in vitro effects, IFN-γ in animal model of estrogen deficiency and rheumatoid arthritis (RA) promotes the OC formation and bone resorption.23,24 These data underline the controversial role of IFN-γ in osteoclastogenesis.
Recently, investigators focused on the OC modulatory activity of T cells, showing that OCs can present antigenic peptides to T cells, inducing FoxP3 expression in CD8+ T cells.25 In this way, CD8/FoxP3+ cells function as T-regulatory cells, able to cause an inappropriate activation of the immune response.25 The cellular responses in cell-to-cell interactions between T cells and OCs are regulated through reciprocal CD137/CD137L and RANK/RANKL interactions. CD137 is a co-stimulatory member of the TNF receptor induced by T-cell receptor activation. It is capable to transduce signals in both directions, through the receptor and into the cell that expresses the ligand. CD137L is expressed on DCs and OC precursors: in vitro CD137L ligation suppresses osteoclastogenesis, by inhibiting the multinucleation process. On the other hand, RANKL expressed on T cells binds to RANK on OCs, producing a reverse signal in T cells able to enhance apoptosis.26
OCs and T cells in Ra
In RA, inflammation of the synovial joints at last causes the formation of a hyperplastic structure called the pannus, which invades the joint space destroying cartilage and bone. OCs are found mainly at the site of focal erosion, where they destroy the mineralised cartilage and subchondral bone. The destruction of these tissues leads to deep resorption pits, which are filled by inflammatory cells. An inflammatory milieu induces naïve T cells to differentiate in Th17 cells, a specialized inflammatory subset, capable of inducing RANKL expression on OBs and synovial fibroblasts. IL-17 also enhances local inflammation and increases the production of inflammatory cytokines, which further promote RANKL expression and activity. Therefore, Th17 cells are the link between T cell response and bone destruction in RA.27 A potential role for naturally occurring regulatory T cell in RA has been proposed and the imbalance between pro-inflammatory Th17 cells and T-regulatory cells seems to be determinant.28 In fact, an increase of T-regulatory cell number improves clinical signs of arthritis and suppressed local and systemic bone destruction.29 Synovial tissues of patients with RA also produce many factors regulating bone resorption, such as TNF-α, IL-1 and IL-6, which amplify OC differentiation, activation and consequent bone destruction.30 Inhibitors that target these pro-inflammatory cytokines have been approved for the treatment of RA, contrasting the OC formation and the release of pro-osteoclastogenic factors by mesenchymal cells.31 For example, data from clinical trials confirm that inhibiting TNF-α activity in RA protects against bone erosion.32 These effects are due to the direct reduction of OC-mediated bone loss and increase of osteoblast-mediated bone formation. A humanized anti-IL-6 receptor monoclonal antibody has been approved for RA treatment because it reduces disease activity and bone loss through the inhibition of osteoclastogenesis.33,34
In addition to the typical pro-inflammatory cytokines, Rifas and Weitzmann35 described a novel cytokine, called secreted osteoclastogenic factor of activated T cells, which promotes OC formation in the absence of OBs and RANKL. Secreted osteoclastogenic factor of activated T cell induces IL-6 production by OBs, thus it indirectly activates osteoclastogenesis and may exacerbate inflammation and bone destruction under inflammatory conditions such as RA.
Recently, investigators demonstrated that RANKL with macrophage colony-stimulating factor can induce transdifferentiation of immature DCs to the OC lineage and that this process is significantly enhanced by RA synovial fluid.36 DCs are antigen-presenting cells, but they could function as OC precursors in inflammatory conditions, transforming into DC-derived OC. Moreover, DCs modulate T-cell activity through the RANK/RANKL pathway and other cytokines associated with osteoclastogenesis. Thus, DCs could function as an osteo-immune interface, contributing to bone loss in inflammatory diseases.37
Literature reports other factors, expressed in synovial tissues, which may rule bone resorption. In particular, a peptide derived from T-cell leukemia translocation-associated gene protein, expressed in synovial tissues from RA patients, has been demonstrated to be able to inhibit pit formation by mature human OCs and to suppress the formation of large OCs in the culture. The use of polyclonal antibodies against T-cell leukemia translocation-associated gene protein inhibited the formation of large OCs in the cultures, preventing OC precursor cellular fusion.38 Moreover, T-cell protein tyrosine phosphatase, an important regulator of hematopoiesis and cytokine signaling, has been recognized to have a role in the pathogenesis of RA. T-cell protein tyrosine phosphatase deficiency has been associated with a bone mass reduction, an increased OC activity, an infiltration of mixed inflammatory cell types and the release of pro-inflammatory cytokines in the knee joints.39
In summary, the identification of the cytokines and factors involved in the inflammatory processes in RA allowed treatment advances for this disease, and further improvements are advisable.
OCs and T cells in osteoporosis
Osteoporosis is characterised by a systemic impairment of bone mass, strength and microarchitecture, which increases the propensity of fragility fractures. Osteoporosis includes many skeletal disorders such as postmenopausal, senile osteoporosis and idiopathic osteoporosis. The first two clinically overlap, whereas the third is used for premenopausal women and young men with osteoporotic fragility fractures without identifiable secondary causes. Osteoporosis is a multifactorial disease: genetic, nutrition and lifestyle determinants have been recognized to cooperate in its pathogenesis. From a biological point of view, in osteoporotic patients there is a decreased bone mass and increased fragility, caused by an upregulated OC activity and a reduced bone-forming activity. In patients with fragility fractures, bone marrow cells are mainly responsible for the pro-osteoclastogenic macrophage colony-stimulating factor and RANKL production, whereas bone releases OB inhibitors, such as DKK-1 and sclerostin.40 These two molecules own to the Wnt protein family, which is determinant in maintaining bone mass. DKK-1 and sclerostin are thought to be involved in the pathogenesis of arthritis,41 glucocorticoid-induced osteoporosis and disuse osteoporosis.42 In the early postmenopause period there is an acute phase of estrogen deficiency, characterised by a rapid bone loss, which is crucial for the onset of postmenopausal osteoporosis.43 Estrogen deficiency causes an increase in the immune function, which culminates in an overproduction of TNF-α through a complex mechanism mediated by activated T cells, antigen-presenting cells and the cytokines, such as IFN-γ, IL-7 and TGF-β.44 TNF-α stimulates the OC formation and bone resorption both directly and by augmenting the sensitivity of maturing OCs to RANKL. Studies in mice suggest that activated T cells are the most relevant source of TNF-α in conditions of estrogen deficiency.45 In fact, in animal model, the adoptive transfer of wild-type T cells restores the capacity of ovariectomy to induce bone loss, while transfer of T cells from TNF-null mice does not.46 As ovariectomy increases the proliferation of unstimulated T cells and the number of bone marrow T cells, in vivo estrogens may regulate the number of TNF-α-producing T cells, rather than TNF gene expression. This hypothesis is confirmed by the finding that in vitro estrogen treatment of cultured T cells did not blunt the ability of T cells from ovariectomized mice to produce TNF-α.46 Recently, T-cell co-stimulatory molecule CD40 ligand has been demonstrated to have a pivotal role in stimulating T-cell activation and TNF production by T cells, thus promoting osteoclastogenesis.47
In postmenopausal women, the production of pro-inflammatory cytokines is greater than in premenopausal subjects and it is related to estrogen deficiency.48 In peripheral blood mononuclear cells (PBMCs) derived from women affected by postmenopausal osteoporosis, a significant increase in spontaneous OC formation and bone-resorbing activity in vitro have been described.49 This phenomenon is due to an increase of circulating OC precursors and to an enhanced TNF-α and RANKL production in patients compared with healthy controls. Moreover, in postmenopausal osteoporosis women, T cells showed a more active phenotype, stimulating osteoclastogenesis, compared with premenopausal and postmenopausal healthy women.19 The major part of the data concerning T cell and OC interactions in osteoporosis derived from animal models, thus it is advisable to further improve the study of this relationship in humans.
OCs and T cells in bone metastases
Bone metastases represent a common cause of morbidity in patients suffering different types of cancer and are commonly classified in osteolytic and osteoblastic. Osteotropic tumors, such as the breast, lung, kidney and prostate cancer, may show both aspects. In the osteolytic metastases the bone destruction is mediated by the OCs, even though the OC activation varies depending on the tumors. The affinity of some tumors to grow in bone results from the special microenvironment provided by bone. Activated OCs resorb bone and release growth factors from the bone matrix, which stimulate the growth of metastatic tumor cells. The last, in turn, secrete additional factors that act on bone cells, creating a vicious cycle and consequent bone metastases. The most prominent cause of bone destruction in metastases is parathyroid hormone-related protein, which stimulates OC bone resorption and is secreted by many cancer types.50 Moreover, tumor cells secrete many cytokines, such as IL-6, IL-8 and IL-11, which increase the OC number, survival and activity.
The immune system and particularly T cells have a fundamental role in metastases formation, because the bone microenvironment is a reservoir of immune cell types. In breast cancer patients, memory T cells have been found in the bone marrow, suggesting their role in cancer immune surveillance.51 Interestingly, some antibone metastatic therapies also showed immunomodulatory effects. For example, the blockade of TGF-β at metastatic sites may locally activate an antitumor T-cell response, because normally TGF-β, released in bone marrow by OC activity, inhibits T-cell proliferation.52 Zoledronic acid, used as antiresorptive agent, can activate cytotoxic γ/δ-T cells and inhibit populations of myeloid-derived cells with T-cell-suppressor capabilities.53 Another recent work by Zhang et al.54 provides compelling evidence that a condition of immune deficiency can interfere with the antitumor effects of OC blockade, and CD8+ T cells are able to reduce the growth of tumor cells in the bone, regardless of OC functionality.
Further proof of the direct dialogue between T cells and OCs has been derived by studies conducted on patients affected by osteotropic solid tumors. The PBMCs of patients affected by solid tumors with bone metastases show an increase of circulating OC precursors compared with both healthy controls and cancer patients without bone metastases.16 These OC precursors differentiate into mature, multinucleated and bone-resorbing OCs in vitro, without adding pro-osteoclastogenic factors. This spontaneous osteoclastogenesis in vitro depends on T cells, which release TNF-α and RANKL. In fact, T-cell-depleted PBMCs did not differentiate into OCs without adding macrophage colony-stimulating factor and RANKL.16 IL-7, released by tumor cells,20,55 regulates the production of pro-osteoclastogenic factors by T cells. IL-7 rules T- and B-cell proliferation, but it has also a role in bone homeostasis.56 In bone metastatic patients IL-7 sera levels were found to be significantly higher than in non-bone metastatic patients and in healthy controls.57 This serum IL-7 increase seems to directly depend on tumor production: a strong IL-7 expression was detected in tumor masses originated in a human-in-mice model of bone metastases and in human bone metastatic biopsies.55 These data support an active role of IL-7 in promoting bone metastatization.
Multiple myeloma (MM) is a B-cell neoplasm characterised by lytic bone disease. The cross-talk between MM cells and OCs is critical for the osteoclastogenesis induction and the OB inhibition.58 In the pathogenesis of MM bone disease, myeloma cells induce an OPG/RANKL imbalance: RANKL production is increased and OPG is decreased. Several studies suggest that myeloma cells upregulate RANKL and downregulate IFN-γ, which is known to be a strong suppressor of osteoclastogenesis. In MM, IL-7 also stimulates osteoclastogenesis through the upregulation of RANKL production by T cells and its level in the sera of MM patients is high.59 In culture of PBMCs obtained from myeloma patients, OCs developed without adding exogenous stimulating factors and this osteoclastogenesis is dependent on RANKL and T cells.60 Furthermore, T cells from MM patients also overexpress OPG and TNF-related apoptosis-inducing ligand, which binds OPG and promote the increased osteoclastogenesis.61 These findings suggest that targeting the RANK/RANKL/OPG pathway could be efficacious to control bone metastases.
OCs and T cells in periprosthetic osteolysis
Loosening of total hip arthroplasty caused by periprosthetic osteolysis is a major clinical problem and makes revision surgery essential for the patients. The periprosthetic bone-destruction phenomenon is due to local inflammatory reaction to implant wear debris.62 Activated periprosthetic cells secrete cytokines and chemokines, which induce the recruitment of inflammatory cells, the formation of osteolytic granulomas and affect the bone remodeling. Wear debris induce phagocytosis by macrophages, which activate the OC signaling pathway. In periprosthetic osteolysis, OC activity is increased and mainly dependent on RANKL, even though a TNF-α-dependent osteoclatogenesis has been described.63 Loosened total hip arthroplasty patients had higher levels of inflammatory cytokines IL-6, IL-8 and IL-10 in the synovial fluid than primary total hip arthroplasty patients.62 Moreover, there is a positive correlation among the levels of IL-6, IL-8, IL-10 and RANKL in the synovial fluid or RANKL expression on osteoblastic stromal cells in the periprosthetic bone marrow. IL-6 increases bone resorption by stimulating RANKL production in OBs and enhancing RANKL sensitivity in OCs. IL-8 induces RANKL expression in OBs and directly stimulates osteoclastogenesis and bone resorption. Further downstream signaling by wear debris overlaps with that of TNF and RANKL and activate kinases and molecules involved in OC differentiation and activation.64
In vitro spontaneous osteoclastogenesis has been documented, after cultures of PBMCs derived from periprosthetic osteolysis patients. This osteoclastogenesis is RANKL and T-cell dependent as it was inhibited by RANK-Fc addition in cell culture and by T-cell depletion.17 In these patients, T cells are close to OCs in the periprosthetic tissues, suggesting their interaction. Local CD8+ T cells show a regulatory phenotype, expressing CD25 and FoxP3, whereas CD4+ T cells do not express activation markers. The regulatory phenotype of CD8+ T cells may explain the inhibition of effector CD4+ T cells, which are often found inactive in periprosthetic tissue. Thus, a possible pathogenic mechanism of periprosthetic osteolysis may be represented by T cells that initially proliferate and support osteoclatogenesis through the RANK/RANKL pathway. Later, OCs may provide a negative feedback on T cells, leading to the activation of regulatory CD8+ T cells and subsequent inhibition of CD4+ T cells.17 The role of T cells in supporting osteolysis may suggest the use of preventive treatment with bisphosfonates, because T cells prevent OC apoptosis induced by these molecules.65
OCs and T cells in pediatric disease
The literature widely documented pediatric disesases with a bone impairment, using both radiological and ultrasound methods.66 PKU is an inborn error of amino-acid metabolism resulting from deficiency of phenylalanine metabolism. An early protein-restricted diet integrated with phenylalanine-free medical foods successfully prevents the irreversible developmental delay characteristic of the natural course of the disease, by maintaining plasma phenylalanine concentrations in the non-neurotoxic. Poor compliance to dietary prescriptions is common during adolescence, as the risk of the mental retardation due to hyperphenylalaninemia seems to be insignificant at this age. However, this laxity of dietary restriction has been related to systemic complications of PKU in adulthood, such as bone impairment. An increased spontaneous osteoclastogenesis in vitro was documented in a cohort of PKU patients,67 consistent with the increased bone resorption markers in affected patients. This osteoclastogenesis in PKU depends on an increased number of circulating OC precursors. TNF-α seems to stimulate and be regulated by OC precursors, whereas OC maturation depends on RANKL and T cells. Among PBMCs of PKU patients T cells showed an activated phenotype. The level of spontaneous osteoclastogenesis and the T-cell activation state correlated with PKU patients' bone condition. Thus, the finding of a specific sub-population of activated T cells accounting for spontaneous osteoclastogenesis infers a dysfunctional immune system activation in PKU patients. Moreover, there is a direct correlation between plasma phenylalanine concentration and spontaneous osteoclastogenesis in PKU patients, suggesting that hyperphenylalaninemia could enhance OC differentiation and consequently promote bone resorption.18
Another childhood pathological condition relating to bone impairment is 21-hydroxylase deficiency (21-OHD). 21-OHD is the most common cause of congenital adrenal hyperplasia, resulting from deletions or mutations of the P450 21-hydroxylase gene (CYP21).68 Children with 21-OHD need chronic glucocorticoid therapy as soon as they are diagnosed with the disease, to both replace the congenital deficit in cortisol synthesis and to reduce the androgen secretion by the adrenal cortex. In a cohort of 21-OHD patients, a slight reduction in bone mass has been documented as well as the spontaneous formation of mature, multinucleated and bone-resorbing OCs in unstimulated PBMC culture.69 This spontaneous osteoclastogenesis seems to correlate directly with the higher number of circulating OC precursors present in 21-OHD patients compared with controls, and demonstrates that OC precursors are markedly increased in the circulation of 21-OHD patients.69 Osteoclastogenesis in 21-OHD patients' PBMC cultures was dependent on T cells, which produce macrophage colony-stimulating factor and RANKL. In particular, T cells from patients expressed high levels of RANKL and low levels of OPG compared with controls. In fact, RANKL was significantly elevated in the sera from 21-OHD patients, whereas OPG decreased, thus the RANKL to OPG ratio was higher in the sera of 21-OHD patients than in controls. The high levels of OC precursors, the T-cell-dependent osteoclastogenesis and the high RANK to OPG ratio in 21-OHD patients could explain the slight reduction in the bone mineral density of these subjects. The prevention of bone loss is mandatory for children, thus the osteoimmunology field needs further investigation in pediatric diseases, to identify treatments able to preserve the status and development of bone in children.
Concluding remarks and future directions
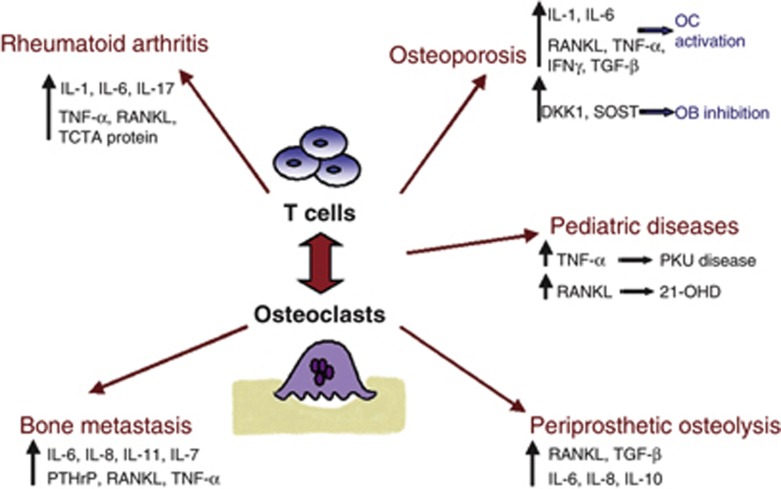
The osteoimmunology field focused on cellular and molecular mechanisms of the immune system that impact bone loss. In particular, the study of the interaction between OCs and T cells allowed to better define the pathophysiology of many diseases characterized by bone destruction, identifying different mediators of bone loss. In fact, numerous cytokines that were first shown to regulate immune cell function have also been demonstrated to regulate bone cells and influence skeletal health (Figure 1). Conversely, products of bone cells are critical for the engraftment of marrow in bone, the normal development of the hematopoietic and immune systems and provide niche for long-term memory B and T cells. Many inhibitors of pro-osteoclastogenic cytokines and factors have been developed and many others are currently under investigation. New antiresorptive agents targeting the cross-talk between OC and T cell could represent a successful strategy to treat diseases characterized by unbalanced bone remodeling.
Figure 1. Schematic representation of OC and T-cell cross-talk, resulting in the upregulation of multiple cytokines and factors, which lead to OC activation and increased bone resorption, associated with several diseases.
Footnotes
The authors declare no conflict of interest.
References
- Fujikawa Y, Quinn JM, Sabokbar A, McGee JO, Athanasou NA. The human osteoclast precursor circulates in the monocyte fraction. Endocrinology 1996;137:4058–4060. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003;423:337–342. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res 2000;15:2–12. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 1998;95:3597–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum L, Wong BR, Josien R, Becherer JD, Erdjument-Bromage H, Schlondorff J et al. Evidence for a role of a tumor necrosis factor-alpha (TNF-alpha)-converting enzyme-like protease in shedding of TRANCE, a TNF family member involved in osteoclastogenesis and dendritic cell survival. J Biol Chem 1999;274:13613–13618. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998;93:165–176. [DOI] [PubMed] [Google Scholar]
- Burgess TL, Qian Y, Kaufman S, Ring BD, Van G, Capparelli C et al. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol 1999;145:527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller K, Murphy C, Kirstein B, Fox SW, Chambers TJ. TNFalpha potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology 2002;143:1108–1118. [DOI] [PubMed] [Google Scholar]
- Bostanci N, Ilgenli T, Emingil G, Afacan B, Han B, Toz H et al. Gingival crevicular fluid levels of RANKL and OPG in periodontal diseases: implications of their relative ratio. J Clin Periodontol 2007;34:370–376. [DOI] [PubMed] [Google Scholar]
- Clines GA, Guise TA. Hypercalcaemia of malignancy and basic research on mechanisms responsible for osteolytic and osteoblastic metastasis to bone. Endocr Relat Cancer 2005;12:549–583. [DOI] [PubMed] [Google Scholar]
- Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 2000;106:1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone 1999;25:255–259. [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 1999;20:345–357. [DOI] [PubMed] [Google Scholar]
- Pacifici R. The immune system and bone. Arch Biochem Biophys 2010;503:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima T, Wada T, Penninger JM. RANKL and RANK as novel therapeutic targets for arthritis. Curr Opin Rheumatol 2003;15:280–287. [DOI] [PubMed] [Google Scholar]
- Roato I, Grano M, Brunetti G, Colucci S, Mussa A, Bertetto O et al. Mechanisms of spontaneous osteoclastogenesis in cancer with bone involvement. FASEB J 2005;19:228–230. [DOI] [PubMed] [Google Scholar]
- Roato I, Caldo D, D'Amico L, D'Amelio P, Godio L, Patane S et al. Osteoclastogenesis in peripheral blood mononuclear cell cultures of periprosthetic osteolysis patients and the phenotype of T cells localized in periprosthetic tissues. Biomaterials 2010;31:7519–7525. [DOI] [PubMed] [Google Scholar]
- Roato I, Porta F, Mussa A, D'Amico L, Fiore L, Garelli D et al. Bone impairment in phenylketonuria is characterized by circulating osteoclast precursors and activated T cell increase. PLoS One 2010;5:e14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amelio P, Grimaldi A, Di Bella S, Brianza SZ, Cristofaro MA, Tamone C et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone 2008;43:92–100. [DOI] [PubMed] [Google Scholar]
- Roato I, Brunetti G, Gorassini E, Grano M, Colucci S, Bonello L et al. IL-7 up-regulates TNF-alpha-dependent osteoclastogenesis in patients affected by solid tumor. PLoS One 2006;1:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai SM, Matsuno H, Nakamura H, Nishioka K, Yudoh K. Interleukin-18 enhances monocyte tumor necrosis factor alpha and interleukin-1beta production induced by direct contact with T lymphocytes: implications in rheumatoid arthritis. Arthritis Rheum 2004;50:432–443. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature 2000;408:600–605. [DOI] [PubMed] [Google Scholar]
- Cenci S, Toraldo G, Weitzmann MN, Roggia C, Gao Y, Qian WP et al. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci USA 2003;100:10405–10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X et al. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest 2007;117:122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesel JR, Buchwald ZS, Aurora R. Cross-presentation by osteoclasts induces FoxP3 in CD8+ T cells. J Immunol 2009;182:5477–5487. [DOI] [PubMed] [Google Scholar]
- Senthilkumar R, Lee HW. CD137L- and RANKL-mediated reverse signals inhibit osteoclastogenesis and T lymphocyte proliferation. Immunobiology 2009;214:153–161. [DOI] [PubMed] [Google Scholar]
- Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 2006;203:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistala K, Wedderburn LR. Th17 and regulatory T cells: rebalancing pro- and anti-inflammatory forces in autoimmune arthritis. Rheumatology (Oxford) 2009;48:602–606. [DOI] [PubMed] [Google Scholar]
- Zaiss MM, Frey B, Hess A, Zwerina J, Luther J, Nimmerjahn F et al. Regulatory T cells protect from local and systemic bone destruction in arthritis. J Immunol 2010;184:7238–7246. [DOI] [PubMed] [Google Scholar]
- Schett G, Teitelbaum SL. Osteoclasts and Arthritis. J Bone Miner Res 2009;24:1142–1146. [DOI] [PubMed] [Google Scholar]
- Karmakar S, Kay J, Gravallese EM. Bone damage in rheumatoid arthritis: mechanistic insights and approaches to prevention. Rheum Dis Clin North Am 2010;36:385–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen JS, Han C, van der Heijde D, Emery P, Bathon JM, Keystone E et al. Infliximab treatment maintains employability in patients with early rheumatoid arthritis. Arthritis Rheum 2006;54:716–722. [DOI] [PubMed] [Google Scholar]
- Maini RN, Taylor PC, Szechinski J, Pavelka K, Broll J, Balint G et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum 2006;54:2817–2829. [DOI] [PubMed] [Google Scholar]
- Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008;371:987–997. [DOI] [PubMed] [Google Scholar]
- Rifas L, Weitzmann M. A novel T cell cytokine, secreted osteoclatogenic factor of activated T cells, induces osteoclast formation in a RANKL-independent manner. Arthritis Rheum 2009;60:3324–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivollier A, Mazzorana M, Tebib J, Piperno M, Aitsiselmi T, Rabourdin-Combe C et al. Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood 2004;104:4029–4037. [DOI] [PubMed] [Google Scholar]
- Alnaeeli M, Park J, Mahamed D, Penninger JM, Teng YT. Dendritic cells at the osteo-immune interface: implications for inflammation-induced bone loss. J Bone Miner Res 2007;22:775–780. [DOI] [PubMed] [Google Scholar]
- Kotake S, Nanke Y, Kawamoto M, Yago T, Udagawa N, Ichikawa N et al. T-cell leukemia translocation-associated gene (TCTA) protein is required for human osteoclastogenesis. Bone 2009;45:627–639. [DOI] [PubMed] [Google Scholar]
- Doody KM, Bussieres-Marmen S, Li A, Paquet M, Henderson JE, Tremblay ML. T cell protein tyrosine phosphatase deficiency results in spontaneous synovitis and subchondral bone resorption in mice. Arthritis Rheum 2012;64:752–761. [DOI] [PubMed] [Google Scholar]
- D'Amelio P, Roato I, D'Amico L, Veneziano L, Suman E, Sassi F et al. Bone and bone marrow pro-osteoclastogenic cytokines are up-regulated in osteoporosis fragility fractures. Osteoporos Int 2011;22:2869–2877. [DOI] [PubMed] [Google Scholar]
- Appel H, Ruiz-Heiland G, Listing J, Zwerina J, Herrmann M, Mueller R et al. Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum 2009;60:3257–3262. [DOI] [PubMed] [Google Scholar]
- Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res 2009;24:1651–1661. [DOI] [PubMed] [Google Scholar]
- Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest 2006;116:1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici R. T cells and post menopausal osteoporosis in murine models. Arthritis Res Ther 2007;9:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest 2000;106:1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G et al. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA 2001;98:13960–13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Tawfeek H, Bedi B, Yang X, Adams J, Gao KY et al. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc Natl Acad Sci USA 2011;108:768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard-Poenaru O, Roux C, Blanque R, Gardner C, de Vemejoul MC, Cohen-Solal ME. Bone-resorbing cytokines from peripheral blood mononuclear cells after hormone replacement therapy: a longitudinal study. Osteoporos Int 2001;12:769–776. [DOI] [PubMed] [Google Scholar]
- D'Amelio P, Grimaldi A, Pescarmona GP, Tamone C, Roato I, Isaia G. Spontaneous osteoclast formation from peripheral blood mononuclear cells in postmenopausal osteoporosis. FASEB J 2005;19:410–412. [DOI] [PubMed] [Google Scholar]
- Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest 1996;98:1544–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerer M, Rocha M, Bai L, Umansky V, Solomayer EF, Bastert G et al. Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int J Cancer 2001;92:96–105. [PubMed] [Google Scholar]
- Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res 2007;13 (18 Part 1): 5262–5270. [DOI] [PubMed] [Google Scholar]
- Schilbach K, Geiselhart A, Handgretinger R. Induction of proliferation and augmented cytotoxicity of gammadelta T lymphocytes by bisphosphonate clodronate. Blood 2001;97:2917–2918. [DOI] [PubMed] [Google Scholar]
- Zhang K, Kim S, Cremasco V, Hirbe AC, Novack DV, Weilbaecher K et al. CD8+ T cells regulate bone tumor burden independent of osteoclast resorption. Cancer Res 2011;71:4799–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roato I, Caldo D, Godio L, D'Amico L, Giannoni P, Morello E et al. Bone invading NSCLC cells produce IL-7: mice model and human histologic data. BMC Cancer 2010;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MR, Shepherd R, Leavey JK, Gao Y, Grassi F, Schnell FJ et al. An IL-7-dependent rebound in thymic T cell output contributes to the bone loss induced by estrogen deficiency. Proc Natl Acad Sci USA 2005;102:16735–16740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roato I, Gorassini E, Buffoni L, Lyberis P, Ruffini E, Bonello L et al. Spontaneous osteoclastogenesis is a predictive factor for bone metastases from non-small cell lung cancer. Lung Cancer 2008;61:109–116. [DOI] [PubMed] [Google Scholar]
- Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 2003;349:2483–2494. [DOI] [PubMed] [Google Scholar]
- Giuliani N, Colla S, Sala R, Moroni M, Lazzaretti M, La Monica S et al. Human myeloma cells stimulate the receptor activator of nuclear factor-kappa B ligand (RANKL) in T lymphocytes: a potential role in multiple myeloma bone disease. Blood 2002;100:4615–4621. [DOI] [PubMed] [Google Scholar]
- Colucci S, Brunetti G, Rizzi R, Zonno A, Mori G, Colaianni G et al. T cells support osteoclastogenesis in an in vitro model derived from human multiple myeloma bone disease: the role of the OPG/TRAIL interaction. Blood 2004;104:3722–3730. [DOI] [PubMed] [Google Scholar]
- Brunetti G, Colucci S, Rizzi R, Mori G, Colaianni G, Oranger A et al. The role of OPG/TRAIL complex in multiple myeloma: the OPG/TRAIL complex in an in vitro osteoclastogenesis model derived from human multiple myeloma-bone disease. Ann NY Acad Sci 2006;1068:334–340. [DOI] [PubMed] [Google Scholar]
- Wang CT, Lin YT, Chiang BL, Lee SS, Hou SM. Over-expression of receptor activator of nuclear factor-kappaB ligand (RANKL), inflammatory cytokines, and chemokines in periprosthetic osteolysis of loosened total hip arthroplasty. Biomaterials 2010;31:77–82. [DOI] [PubMed] [Google Scholar]
- Sabokbar A, Kudo O, Athanasou NA. Two distinct cellular mechanisms of osteoclast formation and bone resorption in periprosthetic osteolysis. J Orthop Res 2003;21:73–80. [DOI] [PubMed] [Google Scholar]
- Abbas S, Clohisy JC, Abu-Amer Y. Mitogen-activated protein (MAP) kinases mediate PMMA-induction of osteoclasts. J Orthop Res 2003;21:1041–1048. [DOI] [PubMed] [Google Scholar]
- Sutherland KA, Rogers HL, Tosh D, Rogers MJ. RANKL increases the level of Mcl-1 in osteoclasts and reduces bisphosphonate-induced osteoclast apoptosis in vitro. Arthritis Res Ther 2009;11:R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta F, Spada M, Lala R, Mussa A. Phalangeal quantitative ultrasound in children with phenylketonuria: a pilot study. Ultrasound Med Biol 2008;34:1049–1052. [DOI] [PubMed] [Google Scholar]
- Porta F, Roato I, Mussa A, Repici M, Gorassini E, Spada M et al. Increased spontaneous osteoclastogenesis from peripheral blood mononuclear cells in phenylketonuria. J Inherit Metab Dis 2008;31 (Suppl 2): S339–S342. [DOI] [PubMed] [Google Scholar]
- White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev 2000;21:245–291. [DOI] [PubMed] [Google Scholar]
- Faienza MF, Brunetti G, Colucci S, Piacente L, Ciccarelli M, Giordani L et al. Osteoclastogenesis in children with 21-hydroxylase deficiency on long-term glucocorticoid therapy: the role of receptor activator of nuclear factor-kappaB ligand/osteoprotegerin imbalance. J Clin Endocrinol Metab 2009;94:2269–2276. [DOI] [PubMed] [Google Scholar]