Abstract
Osteoporosis is a disease of high bone remodeling with an imbalance of bone resorption over bone formation, resulting in decreased bone mineral density and deterioration of bone microarchitecture. From the emerging understandings of the molecular and cellular regulators of bone remodeling, potential new targets for therapeutic intervention for this disease have been identified. Cathepsin K (CatK), a cysteine protease produced by osteoclasts, is the primary enzyme mediating the degradation of the demineralized bone matrix. Current genetic and pharmacological evidence from studies in multiple preclinical species have consistently demonstrated that inhibition of CatK results in the reduction of bone resorption while allowing bone formation to continue. Early results from clinical studies with several investigational CatK inhibitors indicate that the impact of CatK inhibition on bone formation is distinct from that of either the bisphosphonates or the anti-receptor activator of nuclear factor-κB ligand antibody, denosumab. Odanacatib, a highly selective, reversible and potent inhibitor of CatK, is currently in phase III clinical trials for the treatment of postmenopausal osteoporosis.
Introduction
Cathepsin K (CatK) is a member of the papain family of lysosomal cysteine proteases and is abundantly expressed by osteoclasts.1,2 This enzyme is the major protease responsible for the degradation of type I collagen, which constitutes approximately 90% of the bone organic matrix. CatK is capable of degrading collagen type I not only in the telopeptide regions, but also at multiple sites in the triple helical domains.3,4,5 The remaining 10% of non-collageneous bone matrix proteins, including osteocalcin, osteopontin, osteonectin, proteoglycans and a number of bone growth factors, may also be substrates of CatK.5,6
The CatK gene (CTSK) resides in human chromosome 1q21 and is encoded by approximately 12.1 kb of genomic DNA spanning 8 exons and 7 introns.7 In osteoclasts, expression of CatK is regulated by the receptor activator of nuclear factor-κB ligand (RANKL) via activation of NFATc1.8,9 Loss-of-function mutations in CTSK lead to pycnodysostosis, a rare autosomal recessive disorder associated with bone sclerosis in humans.10,11,12 Affected individuals typically have short stature, osteosclerosis with increased risk of non-traumatic fractures, clavicular dysplasia, acro-osteolysis of the distal phalanges, skull deformities associated with frontal bossing, delayed suture closure and dental abnormalities.10,11,12
Targeted disruption of CatK in mice generally leads to high bone mass of the long bones and vertebrae.13,14,15,16 Transgenic mice that overexpress CatK have reduced trabecular bone volume as a result of accelerated bone turnover.17 There were subtle differences on the bone phenotype in CatK−/− mice as reported by various laboratories. CatK knock-out (CatK−/−) mice, either with mixed C57BL/6J and 129Sv background14,16,18 or back-crossed to C57BL/6J background,15 have normal bone length and skull development, suggesting redundancy of collagenase activities in endochondral and intramembranous bone formation during murine skeletal development.13,14,15,16,18 Moreover, although the CatK−/− mice on the mixed genetic background have higher bone mineral density (BMD) at the central femur and a positive correlation between ultimate load and bone mineral content,16 the knock-out mice on C57BL/6J genetic background were reported to maintain maximal load to fracture, but with increased bone brittleness as compared with wild-type mice.15 Interestingly, Chen et al.19 generated CatK−/− mice on 129/Sv background that led to a human pcynodysostosis-like phenotype, including high osteoclast number on bone surface and shorter long bones as compared with the wild-type littermates. Osteoclasts isolated from these CatK−/− mice also lacked normal apoptosis and senescence.19 Unlike the bone phenotype found in CatK−/− mice and in pycnodysostotic patients that have disruption of CatK expression since birth, pharmaceutical intervention of CatK activity in the adult skeleton may not have a negative impact on bone quality evidenced by a number of preclinical studies as described below.
CatK is not exclusively expressed in the osteoclast. Its expression has been found in fibroblasts from bone,20 embryonic lung,21 skin22—albeit at levels much lower than that in osteoclasts. CatK expression is also induced in synovial fibroblasts and macrophages of arthritic joints,23 smooth muscle cells and macrophages of atherosclerotic plague,24 white adipocytes,25 and in breast and prostate tumors.26,27 Recent studies also reported detectable CatK expression in osteoblasts28 and osteocytic cell lines.29 It is not clear whether CatK has any rate-limiting role(s) in the non-osteoclastic cell types, as CatK activity is restricted to the low pH environment. Moreover, unlike osteoclasts predominantly expressing CatK for bone resorption, fibroblasts or other cell types also express multiple cathepsins, which could rescue the lysosomal processing activities in the absence of CatK under physiological or certain pathological conditions.30
The potential role of CatK as a pharmaceutical target for the treatment of osteoporosis and diseases associated with high bone turnover has been extensively reviewed.1,31,32,33,34,35,36,37 The structures, efficacy and selectivity profiles of low-molecular-weight inhibitors of CatK were also recently published.1,38 Due to expression detected in cell types other than osteoclasts, it seems conceivable that inhibition of CatK may be beneficial in other diseases, including rheumatoid arthritis, osteoarthritis, metastatic bone disease or arthrosclerosis. Recent reviews have extensively covered the potential indications for the CatK inhibitors.1,30,34,39 Therefore, the following perspective is intended to focus only on the novel mechanism(s) of CatK inhibition in regulating bone resorption and formation, and to provide an update on recent findings from preclinical studies and on recent developments of CatK inhibitors, which have advanced to clinical trials for the treatment of osteoporosis.
Bone resorption
CatK enzyme is abundantly detected in osteoclasts along the bone resorption surfaces,40,41 and in intracellular lysosomes and transcytotic vesicles.41,42 CatK is synthesized as an inactive precursor (pre-pro CatK) and auto-catalytically activated to the mature CatK under acidic condition in the lysosomes or resorption lacunae.43 CatK inhibitors have been designed to be antiresorptives by directly targeting the inhibition of matrix degradation by the osteoclast without affecting either cell formation or survival. Indeed, the absence of CatK in mice has no effect on the differentiation and fusion of osteoclast progenitors in vivo, and the number of multinucleated tartrate resistant alkaline phosphatase (TRAP) 5b positive osteoclasts tends to increase in CatK−/− mice.16 In pycnodysostotic patients, the osteoclast number in transilial biopsies also appears to be the same as in normal controls.11,20 Moreover, bone biopsies from pycnodysostosis patients show retention of undigested collagen fibrils in the resorption lacunae and cytoplasmic vacuoles, suggesting that the absence of normal functioning CatK also disrupts intracellular matrix degradation.20
On the bone surface, activated osteoclasts organize the sealing zone to allow polarized secretion toward the bone surface; the ruffled border membrane is formed as the result of active vesicular transport to deliver the proteins important for bone resorption.44,45,46 The proton ATPase and CatK are the primary molecules mediating first acidification, and second, collagen digestion, respectively, in the activated osteoclasts.34,42 To efficiently remove the degraded bone matrix proteins, osteoclasts also transport large fragments of partially digested matrix proteins via transcytotic vesicles and release these products to the membrane opposite the bone surface.42,46,47 Recent studies demonstrate that treatment with odanacatib, a selective CatK inhibitor blocks the degradation of bone matrix proteins by osteoclasts and causes them to make small shallow resorption lacunae in contrast to untreated osteoclasts, which generate typical deep trail-like resorption lacunae.41 In addition, odanacatib also reduces intracellular processing of bone matrix proteins, and transcytotic removal and transport of degraded matrix proteins in osteoclasts, thus resulting in an overall reduction in resorption efficiency. It is also important to note that osteoclasts, which were isolated from CatK−/− mice or treated with low-molecular-weight inhibitors, maintain normal cell differentiation, migration, polarization, survival and secretory functionality.41 Hence, inhibition of CatK seems to reduce only osteoclastic matrix degradation without significantly affecting other cellular activities.
Inhibition of CatK also positively regulates its own expression. In an attempt to compensate for CatK deficiency and a reduction of bone resorption, the increase in osteoblast activity results in elevation of mRNA levels for RANKL in the CatK−/− bone.18 This elevated bone marrow RANKL/OPG ratio in CatK−/− mice leads to an increase in osteoclast number and also directly upregulates CatK transcription.18 Although effectively reducing estrogen-deficient induced bone loss, the same RANKL-mediated pathway may be responsible for the significant increases in the number of viable osteoclasts on bone surface, reported from studies with ovariectomized monkeys treated with odanacatib.48 At the cellular level, treatment of osteoclasts with odanacatib also significantly induces accumulation of intracellular vesicles containing high concentrations of CatK and TRAP5b, without affecting the osteoclast life span.41 The vesicular retention in osteoclasts in vitro is also seen histologically in cells from monkeys and patients treated with CatK inhibitors.48,49 In addition to the direct upregulation of CatK expression by RANKL, interaction with the inhibitors has also been shown to stabilize the mature enzyme conformation, at the same time inhibiting the self-destruction of unengaged enzymes, a known mechanism common among several classes of proteases, including the cathepsins.33,43,50 The drug-induced intracellular retention of CatK may explain the rapid rate of resolution in bone-turnover markers upon discontinuation of treatment with a CatK inhibitor in humans.51
CatK inhibitors have been designed to reversibly block the human enzyme, and consequently these compounds have limited potency for the rat and mouse enzymes due to the low degree of amino acid homology between the respective enzymes.34 Non-human primates and rabbits have been the species selected to evaluate antiresorptive efficacy of CatK inhibitors in vivo.34 In two different studies, treatment of ovariectomized (OVX) cynomolgus monkeys for 9 months with relacatib, a non-selective inhibitor of cathepsins or for 18 months with balicatib, a lysosomotropic CatK inhibitor, demonstrated a potent and dose-dependent reduction in the bone-resorption markers, serum C-telopeptide (sCTX) and urinary N-telopeptide (uNTX) of collagen type I, and prevention of bone loss.52,53 Histomorphometry indicated that these agents also reduced indices of bone resorption at both trabecular and cortical sites.52,53 Similarly, odanacatib, a more potent, non-lysosomotropic and selective CatK inhibitor was evaluated in estrogen-deficient models of bone loss in rabbits and rhesus monkeys.48,54 In these studies, odanacatib also demonstrated dose-related BMD gains in the vertebrae and femurs, and reduction of bone-resorption markers sCTX and uNTX, whereas osteoclast numbers were clearly elevated as determined by serum TRAP5b levels and histomorphometric analysis.48,54 More recently, ONO-5443, another potent non-lysosomotropic CatK inhibitor with limited selectivity toward cathepsins S, L and B dose-dependently reduced plasma calcium levels increased by parathyroid hormone-related peptide in thyroparathyroidectomized rats and decreased serum and urine CTX levels in monkeys.55
Bone formation
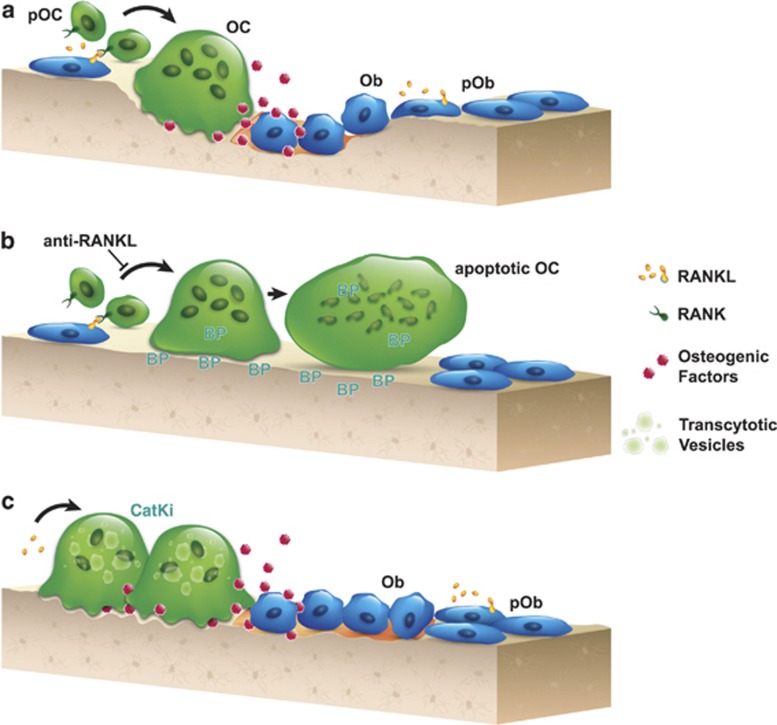
There is ample evidence that bone formation is tightly coupled to bone resorption during the remodeling process. Bone remodeling is necessary for skeletal growth, maintenance of normal bone structure and repair of older damaged bone.56 Activated osteoclasts have been proposed to be the source of what is required for the osteoblastic bone formation response during remodeling (Figure 1a).57,58 Bisphosphonates and the anti-RANKL antibody denosumab are known to produce a marked and sustained reduction of bone formation, following the inhibition of bone resorption.59 Although the anti-RANKL antibody denosumab potently diminishes the formation of mature osteoclasts, uptake of bisphosphonates by osteoclasts inhibits bone resorption by blocking the organization of the sealing zone and eventually inducing osteoclast apoptosis (Figure 1b).59 Hence, these antiresorptives share a common mechanistic consequence in generally producing a functional osteoclast-poor condition, thus decreasing the communication to osteoblasts. Unlike bisphosphonates or denosumab, inhibition of CatK leads to a functional osteoclast-rich environment, suggesting that the inhibition of CatK may selectively reduce the removal of mineralized matrix while maintaining the normal 'coupling' signaling to osteoblastic bone formation (Figure 1c).
Figure 1.
Schematic representations depict (a) the normal coupling process of bone resorption and formation during the remodeling process. Elevated production of RANKL by osteoblastic cells results in differentiation and activation of osteoclasts at remodeling sites. Coupling factors derived from the resorbed bone matrix or directly from the activated osteoclasts, stimulate the recruitment and maturation of osteoblasts to initiate bone formation on the existing resorption surface. (b) Denosumab blocks osteoclastogenesis, and bisphosphonate induces the loss of ruffled border and eventual osteoclast apoptosis. These therapies lead to little to no resorption surface and fewer numbers of osteoclasts on bone. (c) Treatment with a CatK inhibitor reduces osteoclastic resorption efficiency and retards transcytotic trafficking of matrix removal. This does not prevent other osteoclast functions, such as the generation of a shallow resorption surface and the release of osteogenic factors; together, these functions initiate osteoblast bone formation. OC, osteoclast; pOC, osteoclast progenitor; Ob, osteoblast; pOb, osteoblast progenitor; BP, bisphosphonate; CatKi, cathepsin K inhibitor.
CatK-deficient mice have reduced osteoclastic bone resorption, but with high bone-formation rate in trabecular and cortical bones.16 Moreover, mice with targeted deletion of the CatK gene in osteoclasts exhibited the same phenotype as found in the global CatK−/− mice, including osteopetrosis with trends of elevated osteoclast number and significant increase in the bone formation rate, whereas animals with the deletion of this gene in osteoblasts failed to show any skeletal phenotype.60 This provides genetic evidence that inhibition of CatK produced by osteoclasts may enhance the communication from osteoclasts to osteoblasts.
In a recently published study, skeletally mature OVX rabbits were treated with odanacatib or a lesser selective inhibitor L-006235, and were compared with rabbits treated with the bisphosphonate alendronate.54 All agents provided full protection against estrogen-deficiency-induced bone loss. However, unlike alendronate, treatment with the CatK inhibitors resulted in little to no reduction in bone formation rate in both trabecular and cortical surfaces, as compared with vehicle-treated controls.54 In contrast, pharmacological studies with CatK inhibitors in OVX non-human primates have produced mixed results. At the respective doses of relacatib, balicatib or odanacatib that fully prevented estrogen-deficiency-induced BMD loss in the spine and hip of ovariectomized monkeys, these CatK inhibitors inhibited trabecular bone turnover at multiple skeletal sites similar to standard bone resorption inhibitors.48,52,53 However, it was demonstrated that these agents also maintained endocortical bone formation as compared with vehicle-treated controls. Unexpectedly, balicatib as well as odanacatib stimulated periosteal bone formation, particularly in the femur, thus, preferentially increasing cortical bone mass and dimension (thickness) in the hip of the monkeys.53,61 The treatment-related increase in cortical dimension is predicted to produce a positive impact on bone strength in animals treated with CatK inhibitors. In summary, although behaving as effective antiresorptives in all studied preclinical species, the genetic or pharmacological inhibition of CatK maintains or even stimulates bone formation at all bone sites in the mouse and rabbit. This mechanism seems to preferentially build cortical bones in non-human primates, where treatment-related stimulation of periosteal bone formation suggests that CatK inhibitors may have anabolic properties not found with other standard bone-resorption inhibitors.
As depicted in Figure 1, bisphosphonates or the anti-RANKL antibody denosumab reduced the osteoclastic bone-resorption surface. Inhibition of CatK in osteoclasts only reduces the resorption efficiency with little effects on other normal osteoclastic functions. These osteoclasts are still capable of generating shallow resorption surfaces; hence, they are continuing to release osteogenic factors derived from either the viable osteoclasts or the resorbed bone matrix. There is evidence to show that treatment with a CatK inhibitor dramatically increases the local concentrations of matrix-derived bone growth factors, such as insulin-like growth factor-1 (IGF-1) and bone morphogenetic protein (BMP-2).6 Active transforming growth factor-β1 is another factor released from bone during bone resorption, which directs the migration of mesenchymal stem cells to support osteoblast differentiation during bone remodeling.62 Thus, it is reasonable to propose that the locally released matrix-derived osteogenic factors, including IGF-1, transforming growth factor-β1 and BMPs, as well as the matrix proteins, could be the physiological substrates for CatK during the resorption process. Inhibition of CatK may lead to an enhancement of local concentrations of these 'coupling' factors.
Inhibition of CatK also maintains or increases osteoclast numbers in multiple preclinical species. There is emerging evidence that multiple factors are secreted by osteoclasts, which mediates communication between osteoclasts and osteoblasts to balance bone resorption and formation (Figure 1).56 The 'coupling' factors may also include the soluble factors, such as sphingosine-1-phosphate, Wnt10b and BMP-6,63 or operate through direct cell–cell interactions via osteoclast-derived ephrinB2 and its receptor EphB4 on osteoblasts.64,65 These osteogenic factors influence either the recruitment or differentiation of osteoblast progenitors to mature osteoblasts. More recently, semaphorin 4D, an exon-guidance molecule secreted by osteoclasts, has been shown to suppress osteoblast differentiation by binding to its receptor, Plexin-B1.66
Although the above 'coupling' model may fit the action of the CatK inhibitors on maintaining bone formation on the remodeling endocortical surface, the mechanism behind treatment-related formation in the femoral periosteal modeling surface of OVX monkeys treated with balicatib or odanacatib requires further investigations. A recent study demonstrated the expression of CatK in osteocytes in lactating rats, and proposed its role in osteocytic removal of perilacunar/canalicular matrix during the reproductive cycle.67,68 The presence of CatK in osteocytes also implicate its potential role in modulating responses of the long bones to mechanical loading; and thus, stimulation of periosteal bone formation may be part of the reponse.
The overall preclinical data demonstrate that CatK inhibitors are a novel class of antiresorptives that block bone resorption while maintaining bone formation. Compared with other antiresorptive drugs, these new agents build cortical bone at mechanically relevant sites, and might be particularly effective in reducing non-vertebral fractures such as the wrist and hip.
CatK inhibitors in clinical trials
Several CatK inhibitors have advanced into clinical development, and Table 1 summarizes the different preclinical and clinical profiles of these inhibitors. Relacatib (GlaxoSmithKline, Brentford, UK) a pan-cathepsin inhibitor, was discontinued following Phase I studies.69 Balicatib (AAE-581, Novartis, Basel, Switzerland) is a highly selective nitrile-based CatK inhibitor in enzyme-based assays; however, because of its lysosomotropic property, the selectivity profile of this compound was compromised in whole-cell assays (Table 1).70,71 In a 1-year dose-ranging study that enrolled 675 postmenopausal women with osteopenia/osteoporosis, the two top doses of balicatib at 25 and 50 mg reduced sCTX by 61% and uNTx by 55%, without any change in the bone-formation markers osteocalcin, bone-specific alkaline phosphatase and N-terminal propeptide of type I collagen.32,36 Although there were dose-dependent BMD increases in lumbar spine and hip of 4.5 and 2.2%, respectively, its development was discontinued after 1 year, reportedly because of skin-related adverse events including rashes and scleroderma-like skin thickening.72,73 The skin findings observed in this trial could be due to the accumulated high concentration of this drug in the lysosomes, leading to cross-inhibition of multiple cathepsins and marked reduction of dermal-matrix turnover.70
Table 1. Summary of differences between the cathepsin K inhibitors in clinical trials.
| Compounds | Characteristics | Preclinical findings | Clinical findings | References |
|---|---|---|---|---|
| MIV-701/ MIV-710 | MIV-701IC50 (nm)CatK=2.5CatL and S >100 μmCatB and F >10 μmMIV-710 (structure unknown) | In osteoclast, bone-resorption assay in vitro showed elevation of IGF-1 and bone morphogenetic protein, BMP-2 | MIV-701Completed Phase I study (100, 200 and 300 mg, QD) in postmenopausal women for 2 weeks. The 300-mg daily dose showed reduction in CTX by 65% and no reduction of osteocalcin.MIV-71050-mg QD dose reduced CTX by 75% | 1,86 |
| Relacatib (SB-462795) | α-Heteroatom cyclic ketoneNon-lysosomotropicKi (nM)CatK=0.041CatL=0.068CatB=13CatS=1.6CatV=0.063 | OVX-cynomolgus treated orally with relacatib at 1, 3, or 10 mg kg−1 per day for 9 months, compared with alendronate.Rapidly reduced NTX and dose dependently preserved BMD. By histomorphometry, reduced cancellous remodeling as did alendronate. In cortical bone, preserved osteonal bone formation at low and medium doses. | Discontinued after a Phase I study because of drug–drug interactions with paracetamol, ibuprofen and atorvastatin | 1,36,52 |
| Balicatib (AAE-581) | Nitrile-basedLysosomotropicIC50 (nM)CatK=1.4CatL=503CatB=4800CatS=65 μM | OVX-cynomolgus dosed orally gavage with balicatib at 0, 3, 10 and 50 mg kg−1 twice daily for 18 months.In spine and femur, all three doses of balicatib increased BMD relative to vehicle. Histomorphometric indices showed treatment-related reduction in bone turnover in trabecular vertebra and femoral neck. Higher periosteal bone formation rates in treated groups | In a Phase II trial, postmenopausal women with spine T score <−2.0, dosed with 5, 10, 25, 50 mg daily for 12 months.Dose-dependent reduction in CTX and NTX with no change in BSAP and P1NP, and osteocalcinIncreased BMD at the spine and hipDiscontinued after Phase II study because of dose-related increased incidence of skin reactions, including puritus and morphea-like syndrome. | 1,36,53 |
| Odanacatib (MK-0822) | Nitrile-basedNon-lysosomotropicIC50 (nM)CatK=0.2CatL=2995CatB=1034CatS=60CatV=762CatF=795Cat C, H, Z >10 μM | In OVX-rabbits, demonstrated to prevent BMD loss and preserved bone formation rate at all bone sites.In OVX-rhesus, dosed at 6 and 30 mg kg−1 per day for 21 months prevented BMD loss at spine and hip.Histomorphometry study demonstrated treatment-related reduction in trabecular bone remodeling. In cortical femur, this compound preserved endocortical and stimulated periosteal bone formation | Postmenopausal women with low BMD dosed with 3, 10, 25 or 50 mg once weekly.Except the 3-mg dose, demonstrated a dose-dependent reduction in bone-resorption markers (CTX and NTX) and transient modest reduction in bone-formation markers (BSAP and P1NP) in the first year. By the second year, bone-formation markers were no different from that in placebo.Continuous BMD gains at the total hip, lumbar spine and femoral neck in the extension to 5 years. Rapid resolution of effects in BMD post discontinuation of treatment. Well tolerated and no major side effects observed after 5-yearsOdanacatib is currently in Phase III trials | 1,36,48,61 |
| ONO 5334 |
Hydrazine-basedNon-lysosomotropicKi (nM)CatK=0.1CatL=1.7CatB=32CatS=0.83CatC=2500 |
In intact cynomolgus, ONO-5334 (0.3, 3, 30 mg kg−1 per day, oral daily) rapidly decreased CTX, showed no effect on bone-formation markers, osteocalcin and BSAP |
Postmenopausal women with osteoporosis dosed with 50 mg twice daily, 100 and 300 mg daily, versus alendronate 70 mg once weekly.Reduction on bone-resorption markers and BMD gains in spine and hip of the 300-mg dose group were similar to ALNShowed no to little reduction in bone formation markers |
36,55 |
Abbreviations: BMD, bone mineral density; BMP-2, bone morphogenetic protein-2; BSAP; bone-specific alkaline phosphatase; Cat, cathepsin; CTX, C-telopeptide; IGF-1, insulin-like growth factor-1; NTX, N-telopeptide; OVX, ovariectomized; P1NP, N-terminal propeptide of type I collagen; QD, every day.
ONO-5334 (ONO Pharmaceutical, Osaka, Japan) is a hydrazine-based potent non-lysosomotropic inhibitor of CatK with modest selectivity profile against cathepsins S (Table 1).55 This compound has advanced into a Phase II trial, which enrolled 285 women with postmenopausal osteoporosis.74 Note that women with low bone turnover defined as uCTX-1 below 200 μg mmol−1 of creatinine were excluded from this trial. The women were randomized to receive placebo, alendronate 70 mg weekly, or ONO-5334 at 50 mg twice daily, 100 or 300 mg once daily. The 12-month efficacy and safety results for this CatK inhibitor were recently reported.74 ONO-5334 provided dose-related reduction of bone-resorption markers (sCTX and uNTX) with little or no reduction of bone-formation markers compared with alendronate. The 300-mg dose of ONO-5334 resulted in bone-resorption marker reductions comparable to those with alendronate. Moreover, unlike alendronate, the CatK inhibitor at this dose significantly elevated the osteoclast marker TRAP5b. Compared with placebo, the 300-mg dose of ONO-5334 achieved significant increases in BMD at the lumbar spine, total hip and femoral neck by 5.1, 3 and 2.6%, respectively, comparable to those of alendronate after 1 year. Despite its limited selectivity profile, there were no clinically relevant safety concerns reported after 1 year of therapy. No difference in the rate of dermatological adverse events was observed across all study groups.
Odanacatib (MK-0822, Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA) is a non-lysosomotropic, nitrile-based, highly selective CatK inhibitor (Table 1).75,76,77 In Phase I clinical trials, odanacatib was tested as once daily or weekly regimens, and was demonstrated to have an apparent half-life of 66–93 h in humans, supporting the use of this drug with a once weekly dosing regimen.78 Dose ranging of odanacatib demonstrated dose proportionality in plasma exposure up to 10 mg, at which optimal reduction of bone-resorption markers, uNTX and sCTX were also achieved. As these Phase I clinical studies were ⩽3 weeks of dosing, the long-term treatment-related effects on bone-formation markers were not definitively established until Phase II study.78
In a placebo-controlled Phase II study, the effects of weekly oral doses of odanacatib (3, 10, 25 and 50 mg) were assessed in 399 postmenauposal women with low BMD (T-score ⩽−2.0 and ⩾−3.5).79 After 12 months of treatment, odanacatib at the highest dose tested (50 mg once weekly) increased BMD at the lumbar spine by 3.4%, femoral neck by 2.5%, and reduced uNTx by 58%, compared with placebo. After 24 months, the 50-mg once weekly dose resulted in increases in BMD versus placebo of 5.7% at the lumbar spine, 4.1% at the total hip and 4.7% at the femoral neck sites. Although uNTX remained below baseline, the sCTX level tended to return toward baseline during the first 24 months. More importantly, in the first 12 months of treatment, odanacatib 50 mg only modestly reduced the levels of bone-formation markers, bone-specific alkaline phosphatase and N-terminal propeptide of type I collagen by approximately 20 and 30%, respectively, significantly less than that seen with the bisphosphonates (alendronate decreases bone-specific alkaline phosphatase by ∼45%, N-terminal propeptide of type I collagen by ∼60% versus placebo80). After 24 months, the levels of bone-formation markers in the odanacatib 50-mg dose gradually drifted back toward baseline. Of note, iliac crest biopsies were collected after fluorochrome double-labeling in a subset of 32 women from all treatment groups. Histomorphometry from this limited number of biopsies showed no qualitative abnormalities. Interestingly, compared with the historical findings on biopsy studies with bisphosphonates and denosumab,59 activation frequency and bone-formation rate in the odanacatib-treated biopsies showed no remarkable differences from the placebo group. Overall, all doses were well-tolerated and clinical adverse events, particularly skin and respiratory events, were balanced in the treatment groups versus placebo.79
The results from the year 3 Phase II extension included 189 patients from the original study, who were re-randomized to odanacatib 50 mg once weekly or placebo after the initial 2 years.51 The patients treated continuously with the odanacatib 50-mg dose for 3 years increased lumbar spine BMD by 7.9% at the spine, total hip by 5.8% and the femoral neck by 5.0% from baseline.51 Bone-resorption markers did not change significantly during the third year of treatment, whereas the levels of bone-formation markers were no longer different from those in the placebo group.51 The osteoclast marker TRAP5b levels were raised above baseline during the 3 years of treatment, but were not significantly different from placebo levels. In the group of patients who had received odanacatib for 2 years and then switched to placebo, treatment discontinuation resulted in BMD loss at all sites, which returned to levels above or at the baseline. Bone-turnover markers increased above baseline within a month post discontinuation of odanacatib, but this increase largely resolved by the end of year 3.51 Unlike the bisphosphonates, the rapid resolution of effect seen with odanacatib is expected with the reversible kinetics of a non-bone binding agent and has been recently reviewed.81 Indeed, the resolution of effects of odanacatib is similar to that seen with the non-bisphosphonate osteoporosis treatments, including estrogens, selective estrogen receptor modulators and denosumab.82,83,84 The overall adverse event rates were balanced in all treatment groups with a greater number of non-complicated urinary tract infections in the odanacatib groups.
More recently, the 5-year extension data of the odanacatib Phase II study was reported.85 Women who received odanacatib 50 mg weekly for 5 years had continued increases in BMD from baseline at the lumbar spine (11.9%), total hip (8.5%) and femoral neck (9.5%). Additionally, the level of urinary NTX was decreased by 67.4%, whereas the levels of serum bone-specific alkaline phosphatase remained relatively unchanged from baseline. The levels of TRAP5b in the odanacatib-treated patients were not different from placebo controls. The 5-year results from the odanacatib trial showed a progressive (almost at a linear rate) increase in bone mass in osteopenic/osteoporotic patients at multiple bone sites, particularly at the hip. This finding clearly differentiates odanacatib from the historical results of alendronate in the Fracture Intervention Trial Long-term Extension,80 in which patients who received alendronate 10 mg daily showed early increased rates of BMD gain in the spine progressively slowing down after 3 years of treatment. Hip BMD in these patients also gained significantly in response to alendronate, reaching a plateau with continued treatment beyond 3 years.80
To further assess the long-term effects of odanacatib on safety and efficacy in osteoporotic postmenopausal women, the Phase II study has been extended out to 10 years. A global multicenter placebo-controlled Phase III fracture outcome trial with 50 mg once weekly of odanacatib has enrolled more than 16 000 postmenopausal women with osteoporosis and is currently in its third year. Other smaller Phase III BMD trials with odanacatib include a special imaging trial and a study in postmenopausal osteoporotic women previously treated with alendronate. A Phase III trial in osteoporotic men has recently been initiated.
Summary and perspectives
Unlike bisphosphonates or the anti-RANKL antibody, inhibition of CatK in osteoclasts reduces only the resorption efficiency of the cells. Other normal osteoclastic functions, such as differentiation, migration, polarization and survival remain unaltered, leading to a proposed model in which CatK-inhibited osteoclasts continue to release matrix- or cellular-derived 'coupling' factors to recruit, differentiate or enhance osteoblast bone-formation activity. The exact molecular mechanism(s) linking CatK inhibition and bone formation remains largely unknown, and is predicted to involve complex networks of cell-to-cell communications. However, the current model cannot be used to explain the bone site-specific action of the CatK inhibitors on cortical versus trabecular bones, and the apparent anabolic action on the periosteal modeling surface in ovariectomized monkeys.
Despite this lack of understandings at the cellular levels, the current genetic and pharmacological evidence from the preclinical and early clinical studies with several investigational CatK inhibitors consistently demonstrates that the mechanism of CatK inhibition is distinct from that mediated by bisphosphonates or denosumab. A safe and efficacious CatK inhibitor that blocks bone resorption, but allows progressive bone formation, is expected to provide long-term benefits to patients with osteoporosis, potentially restoring BMD toward its original peak bone mass. The results from the ongoing Phase III fracture outcome study with odanacatib will confirm if the theoretical advantages in bone mass gained by this mechanism over bisphosphonates translate into better fracture risk reduction.
Acknowledgments
I thank C Livezey for his excellent figure illustration; A Leung, M Flicker, T Lombardi and A De Papp for their careful edits and helpful comments on this manuscript.
Footnotes
Le T Duong is an employee of Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA.
References
- Bromme D, Lecaille F. Cathepsin K inhibitors for osteoporosis and potential off-target effects. Expert Opin Investig Drugs 2009;18:585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake FH, Dodds RA, James IE, Connor JR, Debouck C, Richardson S et al. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J Biol Chem 1996;271:12511–12516. [DOI] [PubMed] [Google Scholar]
- Garnero P, Borel O, Byrjalsen I, Ferreras M, Drake FH, McQueney MS et al. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem 1998;273:32347–32352. [DOI] [PubMed] [Google Scholar]
- Atley LM, Mort JS, Lalumiere M, Eyre DR. Proteolysis of human bone collagen by cathepsin K: characterization of the cleavage sites generating by cross-linked N-telopeptide neoepitope. Bone 2000;26:241–247. [DOI] [PubMed] [Google Scholar]
- Bossard MJ, Tomaszek TA, Thompson SK, Amegadzie BY, Hanning CR, Jones C et al. Proteolytic activity of human osteoclast cathepsin K. Expression, purification, activation, and substrate identification. J Biol Chem 1996;271:12517–12524. [DOI] [PubMed] [Google Scholar]
- Fuller K, Lawrence KM, Ross JL, Grabowska UB, Shiroo M, Samuelsson B et al. Cathepsin K inhibitors prevent matrix-derived growth factor degradation by human osteoclasts. Bone 2008;42:200–211. [DOI] [PubMed] [Google Scholar]
- Rood JA, Van Horn S, Drake FH, Gowen M, Debouck C. Genomic organization and chromosome localization of the human cathepsin K gene (CTSK). Genomics 1997;41:169–176. [DOI] [PubMed] [Google Scholar]
- Balkan W, Martinez AF, Fernandez I, Rodriguez MA, Pang M, Troen BR. Identification of NFAT binding sites that mediate stimulation of cathepsin K promoter activity by RANK ligand. Gene 2009;446:90–98. [DOI] [PubMed] [Google Scholar]
- Troen BR. The regulation of cathepsin K gene expression. Ann NY Acad Sci 2006;1068:165–172. [DOI] [PubMed] [Google Scholar]
- Gelb BD, Shi GP, Chapman HA, Desnick RJ. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science 1996;273:1236–1238. [DOI] [PubMed] [Google Scholar]
- Motyckova G, Fisher DE. Pycnodysostosis: role and regulation of cathepsin K in osteoclast function and human disease. Curr Mol Med 2002;2:407–421. [DOI] [PubMed] [Google Scholar]
- Schilling AF, Mulhausen C, Lehmann W, Santer R, Schinke T, Rueger JM et al. High bone mineral density in pycnodysostotic patients with a novel mutation in the propeptide of cathepsin K. Osteoporos Int 2007;18:659–669. [DOI] [PubMed] [Google Scholar]
- Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, Rommerskirch W et al. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci USA 1998;95:13453–13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen M, Lazner F, Dodds R, Kapadia R, Feild J, Tavaria M et al. Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res 1999;14:1654–1663. [DOI] [PubMed] [Google Scholar]
- Li CY, Jepsen KJ, Majeska RJ, Zhang J, Ni R, Gelb BD et al. Mice lacking cathepsin K maintain bone remodeling but develop bone fragility despite high bone mass. J Bone Miner Res 2006;21:865–875. [DOI] [PubMed] [Google Scholar]
- Pennypacker B, Shea M, Liu Q, Masarachia P, Saftig P, Rodan S et al. Bone density, strength, and formation in adult cathepsin K (-/-) mice. Bone 2009;44:199–207. [DOI] [PubMed] [Google Scholar]
- Kiviranta R, Morko J, Uusitalo H, Aro HT, Vuorio E, Rantakokko J. Accelerated turnover of metaphyseal trabecular bone in mice overexpressing cathepsin K. J Bone Miner Res 2001;16:1444–1452. [DOI] [PubMed] [Google Scholar]
- Kiviranta R, Morko J, Alatalo SL, NicAmhlaoibh R, Risteli J, Laitala-Leinonen T et al. Impaired bone resorption in cathepsin K-deficient mice is partially compensated for by enhanced osteoclastogenesis and increased expression of other proteases via an increased RANKL/OPG ratio. Bone 2005;36:159–172. [DOI] [PubMed] [Google Scholar]
- Chen W, Yang S, Abe Y, Li M, Wang Y, Shao J et al. Novel pycnodysostosis mouse model uncovers cathepsin K function as a potential regulator of osteoclast apoptosis and senescence. Hum Mol Genet 2007;16:410–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts V, Hou WS, Rialland X, Tigchelaar W, Saftig P, Bromme D et al. Cathepsin K deficiency in pycnodysostosis results in accumulation of non-digested phagocytosed collagen in fibroblasts. Calcif Tissue Int 2003;73:380–386. [DOI] [PubMed] [Google Scholar]
- Buhling F, Waldburg N, Gerber A, Hackel C, Kruger S, Reinhold D et al. Cathepsin K expression in human lung. Adv Exp Med Biol 2000;477:281–286. [DOI] [PubMed] [Google Scholar]
- Runger TM, Quintanilla-Dieck MJ, Bhawan J. Role of cathepsin K in the turnover of the dermal extracellular matrix during scar formation. J Invest Dermatol 2007;127:293–297. [DOI] [PubMed] [Google Scholar]
- Hou WS, Li Z, Gordon RE, Chan K, Klein MJ, Levy R et al. Cathepsin k is a critical protease in synovial fibroblast-mediated collagen degradation. Am J Pathol 2001;159:2167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest 1998;102:576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Junfeng H, Tianhong L, Lu W, Shulin C, Yu Z et al. Cathepsin K in adipocyte differentiation and its potential role in the pathogenesis of obesity. J Clin Endocrinol Metab 2006;91:4520–4527. [DOI] [PubMed] [Google Scholar]
- Littlewood-Evans AJ, Bilbe G, Bowler WB, Farley D, Wlodarski B, Kokubo T et al. The osteoclast-associated protease cathepsin K is expressed in human breast carcinoma. Cancer Res 1997;57:5386–5390. [PubMed] [Google Scholar]
- Brubaker KD, Vessella RL, True LD, Thomas R, Corey E. Cathepsin K mRNA and protein expression in prostate cancer progression. J Bone Miner Res 2003;18:222–230. [DOI] [PubMed] [Google Scholar]
- Mandelin J, Hukkanen M, Li TF, Korhonen M, Liljestrom M, Sillat T et al. Human osteoblasts produce cathepsin K. Bone 2006;38:769–777. [DOI] [PubMed] [Google Scholar]
- Jia J, Yao W, Guan M, Dai W, Shahnazari M, Kar R et al. Glucocorticoid dose determines osteocyte cell fate. Faseb J 2011;25:3366–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen-Mankonen HJ, Morko J, Vuorio E. Role of cathepsin K in normal joints and in the development of arthritis. Curr Drug Targets 2007;8:315–323. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Kaleta J, Bromme D. The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv Drug Deliv Rev 2005;57:973–993. [DOI] [PubMed] [Google Scholar]
- Vasiljeva O, Reinheckel T, Peters C, Turk D, Turk V, Turk B. Emerging roles of cysteine cathepsins in disease and their potential as drug targets. Curr Pharm Des 2007;13:387–403. [DOI] [PubMed] [Google Scholar]
- Lecaille F, Bromme D, Lalmanach G. Biochemical properties and regulation of cathepsin K activity. Biochimie 2008));90:208–226. [DOI] [PubMed] [Google Scholar]
- Rodan SB, Duong LT. Cathepsin K – a new molecular target for osteoporosis. IBMS BoneKEy 2008;5:16–24. [Google Scholar]
- Stoch SA, Wagner JA. Cathepsin K inhibitors: a novel target for osteoporosis therapy. Clin Pharmacol Ther 2008;83:172–176. [DOI] [PubMed] [Google Scholar]
- Costa AG, Cusano NE, Silva BC, Cremers S, Bilezikian JP. Cathepsin K: its skeletal actions and role as a therapeutic target in osteoporosis. Nat Rev Rheumatol 2011;7:447–456. [DOI] [PubMed] [Google Scholar]
- Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet 2011;377:1276–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black WC. Peptidomimetic inhibitors of cathepsin K. Curr Top Med Chem 2011;10:745–751. [DOI] [PubMed] [Google Scholar]
- Lafarge JC, Naour N, Clement K, Guerre-Millo M. Cathepsins and cystatin C in atherosclerosis and obesity. Biochimie 92:1580–1586. [DOI] [PubMed] [Google Scholar]
- Yamaza T, Tsuji Y, Goto T, Kido MA, Nishijima K, Moroi R et al. Comparison in localization between cystatin C and cathepsin K in osteoclasts and other cells in mouse tibia epiphysis by immunolight and immunoelectron microscopy. Bone 2001;29:42–53. [DOI] [PubMed] [Google Scholar]
- Leung P, Pickarski M, Zhuo Y, Masarachia PJ, Duong LT. The effects of the cathepsin K inhibitor odanacatib on osteoclastic bone resorption and vesicular trafficking. Bone 2011;49:623–635. [DOI] [PubMed] [Google Scholar]
- Vaaraniemi J, Halleen JM, Kaarlonen K, Ylipahkala H, Alatalo SL, Andersson G et al. Intracellular machinery for matrix degradation in bone-resorbing osteoclasts. J Bone Miner Res 2004;19:1432–1440. [DOI] [PubMed] [Google Scholar]
- Rieman DJ, McClung HA, Dodds RA, Hwang SM, Holmes MW, James IE et al. Biosynthesis and processing of cathepsin K in cultured human osteoclasts. Bone 2001;28:282–289. [DOI] [PubMed] [Google Scholar]
- Duong LT, Rodan GA. Regulation of osteoclast formation and function. Rev Endocr Metab Disord 2001;2:95–104. [DOI] [PubMed] [Google Scholar]
- Vaananen HK, Laitala-Leinonen T. Osteoclast lineage and function. Arch Biochem Biophys 2008;473:132–138. [DOI] [PubMed] [Google Scholar]
- Coxon FP, Taylor A. Vesicular trafficking in osteoclasts. Semin Cell Dev Biol 2008;19:424–433. [DOI] [PubMed] [Google Scholar]
- Mulari M, Vaaraniemi J, Vaananen HK. Intracellular membrane trafficking in bone resorbing osteoclasts. Microsc Res Tech 2003;61:496–503. [DOI] [PubMed] [Google Scholar]
- Masarachia PJ, Pennypacker BL, Pickarski M, Scott KR, Wesolowski GA, Smith SY et al. Odanacatib reduces bone turnover and increases bone mass in the lumbar spine of skeletally mature ovariectomized rhesus monkeys. J Bone Miner Res 2012;27:509–523. [DOI] [PubMed] [Google Scholar]
- Chappard D, Libouban H, Mindeholm L, Basle MF, Legrand E, Audran M. The cathepsin K inhibitor AAE581 induces morphological changes in osteoclasts of treated patients. Microsc Res Tech 2010;73:726–732. [DOI] [PubMed] [Google Scholar]
- McQueney MS, Amegadzie BY, D'Alessio K, Hanning CR, McLaughlin MM, McNulty D et al. Autocatalytic activation of human cathepsin K. J Biol Chem 1997;272:13955–13960. [DOI] [PubMed] [Google Scholar]
- Eisman JA, Bone HG, Hosking DJ, McClung MR, Reid IR, Rizzoli R et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: three-year continued therapy and resolution of effect. J Bone Miner Res 2011;26:242–251. [DOI] [PubMed] [Google Scholar]
- Stroup GB, Kumar S, Jerome CP. Treatment with a potent cathepsin K inhibitor preserves cortical and trabecular bone mass in ovariectomized monkeys. Calcif Tissue Int 2009;85:344–355. [DOI] [PubMed] [Google Scholar]
- Jerome C, Missbach M, Gamse R. Balicatib, a cathepsin K inhibitor, stimulates periosteal bone formation in monkeys. Osteoporos Int 2011;22:3001–3011. [DOI] [PubMed] [Google Scholar]
- Pennypacker BL, Duong LT, Cusick TE, Masarachia PJ, Gentile MA, Gauthier JY et al. Cathepsin K inhibitors prevent bone loss in estrogen-deficient rabbits. J Bone Miner Res 2011;26:252–262. [DOI] [PubMed] [Google Scholar]
- Ochi Y, Yamada H, Mori H, Nakanishi Y, Nishikawa S, Kayasuga R et al. Effects of ONO-5334, a novel orally-active inhibitor of cathepsin K, on bone metabolism. Bone 2011;49:1351–1356. [DOI] [PubMed] [Google Scholar]
- Martin T, Gooi JH, Sims NA. Molecular mechanisms in coupling of bone formation to resorption. Crit Rev Eukaryot Gene Expr 2009;19:73–88. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med 2005;11:76–81. [DOI] [PubMed] [Google Scholar]
- Henriksen K, Neutzsky-Wulff AV, Bonewald LF, Karsdal MA. Local communication on and within bone controls bone remodeling. Bone 2009;44:1026–1033. [DOI] [PubMed] [Google Scholar]
- Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 2011;48:677–692. [DOI] [PubMed] [Google Scholar]
- Lotinun S, Kiviranta R, Alzate J, Vuorio E, Horne W, Sabatakos G et al. Osteoclast-Targeted Deletion of Cathepsin K in Mice Increases Bone Formation Whereas Deletion in Osteoblasts Has No Effect. J Bone Miner Res 2010;25:S1–S81. [Google Scholar]
- Cusick T, Chen CM, Pennypacker BL, Pickarski M, Kimmel D, Scott BB et al. Odanacatib treatment increases hip bone mass and cortical thickness by preserving endocortical bone formation and stimulating periosteal bone formation in the ovariectomized adult rhesus monkey. J Bone Miner Res 2012;27:524–537. [DOI] [PubMed] [Google Scholar]
- Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med 2009;15:757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci USA 2008;105:20764–20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab 2006;4:111–121. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Allan EH, Ho PW, Gooi JH, Quinn JM, Gillespie MT et al. Communication between ephrinB2 and EphB4 within the osteoblast lineage. Adv Exp Med Biol 2011;658:51–60. [DOI] [PubMed] [Google Scholar]
- Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med 2011;17:1473–1480. [DOI] [PubMed] [Google Scholar]
- Qing H, Ardeshirpour L, Pajevic P, Dusevich V, Jähn K, Kato S et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res 2012;27:1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quing H, Bonewald L. Osteocyte remodeling of the perilacunar and pericanalicular matrix. Int J Oral Sci 2009;1:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Dare L, Vasko-Moser JA, James IE, Blake SM, Rickard DJ et al. A highly potent inhibitor of cathepsin K (relacatib) reduces biomarkers of bone resorption both in vitro and in an acute model of elevated bone turnover in vivo in monkeys. Bone 2007;40:122–131. [DOI] [PubMed] [Google Scholar]
- Falgueyret JP, Desmarais S, Oballa R, Black WC, Cromlish W, Khougaz K et al. Lysosomotropism of basic cathepsin K inhibitors contributes to increased cellular potencies against off-target cathepsins and reduced functional selectivity. J Med Chem 2005;48:7535–7543. [DOI] [PubMed] [Google Scholar]
- Black WC, Percival MD. The consequences of lysosomotropism on the design of selective cathepsin K inhibitors. Chembiochem 2006;7:1525–1535. [DOI] [PubMed] [Google Scholar]
- Peroni A, Zini A, Braga V, Colato C, Adami S, Girolomoni G. Drug-induced morphea: report of a case induced by balicatib and review of the literature. J Am Acad Dermatol 2008;59:125–129. [DOI] [PubMed] [Google Scholar]
- Runger TM, Adami S, Benhamou CL, Czerwinski E, Farrerons J, Kendler DL et al. Morphea-like skin reactions in patients treated with the cathepsin K inhibitor balicatib. J Am Acad Dermatol 2012;66:e89–e96. [DOI] [PubMed] [Google Scholar]
- Eastell R, Nagase S, Ohyama M, Small M, Sawyer J, Boonen S et al. Safety and efficacy of the cathepsin K inhibitor ONO-5334 in postmenopausal osteoporosis: the OCEAN study. J Bone Miner Res 2011;26:1303–1312. [DOI] [PubMed] [Google Scholar]
- Gauthier JY, Chauret N, Cromlish W, Desmarais S, Duong le T, Falgueyret JP et al. The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg Med Chem Lett 2008;18:923–928. [DOI] [PubMed] [Google Scholar]
- Desmarais S, Masse F, Percival MD. Pharmacological inhibitors to identify roles of cathepsin K in cell-based studies: a comparison of available tools. Biol Chem 2009;390:941–948. [DOI] [PubMed] [Google Scholar]
- Perez-Castrillon JL, Pinacho F, De Luis D, Lopez-Menendez M, Duenas Laita A. Odanacatib, a new drug for the treatment of osteoporosis: review of the results in postmenopausal women. J Osteoporos 2010;2010:pii: 401581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoch SA, Zajic S, Stone J, Miller DL, Van Dyck K, Gutierrez MJ et al. Effect of the cathepsin K inhibitor odanacatib on bone resorption biomarkers in healthy postmenopausal women: two double-blind, randomized, placebo-controlled phase I studies. Clin Pharmacol Ther 2009;86:175–182. [DOI] [PubMed] [Google Scholar]
- Bone HG, McClung MR, Roux C, Recker RR, Eisman JA, Verbruggen N et al. Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J Bone Miner Res 2011;25:937–947. [DOI] [PubMed] [Google Scholar]
- Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. Jama 2006;296:2927–2938. [DOI] [PubMed] [Google Scholar]
- Bauer DC. Discontinuation of odanacatib and other osteoporosis treatments: here today and gone tomorrow? J Bone Miner Res 2011;26:239–241. [DOI] [PubMed] [Google Scholar]
- Neele SJ, Evertz R, De Valk-De Roo G, Roos JC, Netelenbos JC. Effect of 1 year of discontinuation of raloxifene or estrogen therapy on bone mineral density after 5 years of treatment in healthy postmenopausal women. Bone 2002;30:599–603. [DOI] [PubMed] [Google Scholar]
- Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 2008;43:222–229. [DOI] [PubMed] [Google Scholar]
- Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 2011;96:972–980. [DOI] [PubMed] [Google Scholar]
- Binkley N, Bone H, Gilchrist N, Langdahl B, Resch H, Rodriguez-Portales J et al. Treatment with the cathepsin K inhibitor odanacatib in postmenopausal women with low BMD: 5 year results of a Phase 2 trial. J Bone Miner Res 2011;26:SA0453. [Google Scholar]
- Podgorski I. Future of anticathepsin K drugs: dual therapy for skeletal disease and atherosclerosis? Future Med Chem 2009;1:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]