Abstract
The skeletal tissue is closely associated with the hematopoietic tissue lodged in its inner cavities. Besides the well-known role of the endosteal osteoblasts in the maintenance of the hematopoietic stem cell (HSC) niche, it is an emerging concept that osteoclasts are involved in the regulation of hematopoiesis as well, although published data are still incomplete and somehow controversial. We reviewed the literature, and report here our perspective on the close relationship between bone resorption and HSC permanence in bone or egress to the circulation. We discussed the pressure that bone diseases exert on the development of hematological alterations, as well as the role of calcium and osteoclast enzymes in the regulation of HSC homeostasis. Genetic studies and preclinical experiments are described, which unveiled how bone disorders and treatments aimed at restoring the bone mass affect hematopoiesis, with consequent clinical implications. We conclude that this new field of investigation must be extended to unequivocally establish the role of osteoclasts in myelopoiesis and lymphopoiesis, and to envision treatments that can help hematological failures to be cured along with the associated bone alterations.
Introduction
The skeleton is characterized not only by a shell of mineralized tissue but also by inner cavities lodging the bone marrow that supplies all peripheral blood elements.1 Osteoblasts are well-known niche components that are indispensable for keeping hematopoiesis lifelong,2 and do so through their interactions with hematopoietic stem cells (HSCs).3 The true nature of the osteoblastic HSC niche is, howover, still controversial. According to recent reports,4 mature osteoblasts are unlikely to support HSCs because the ablation of osteocalcin-positive cells, assumed to be at a late stage of maturation into the osteoblast lineage, does not influence hematopoiesis.5 Likewise, strontium ranelate, which increases the number of mature osteoblasts, does not increase the number of HSCs in the bone marrow.6 In contrast, nestin-positive mesenchymal stromal cells interact with HSCs, while PDGFRα/Sca1-positive cells and early osteoprogenitors are able to support hematopoiesis.7 Furthermore, morphological evidence suggests that pre-osteoblastic cells, which have an aspect similar to fibrotic stromal cells, are better placed to interact with HSCs.4 In fact, HSCs are not juxtaposed in direct contact with endosteal cells, but are generally enriched within three cell diameters from the endosteal surface, a site where pre-osteoblasts rather than mature osteoblasts are located.
Notwithstanding these hot aspects, many signals and pathways involved in the regulation of HSCs by the osteogenic cells are well known,2 as are various regulatory molecules derived from the vascular endothelial niche,7 the innervation,8,9 the systemic circulation and from various local non-cellular components.2 Instead, far less known are the interactions between osteoclasts and hematopoiesis, this being a relatively recent field of investigation that is still incomplete and controversial (Table 1). We will review what is known so far on this component of the hematopoietic niche, discussing potential regulatory pathways and clinical implications.
Table 1. Major findings on the interplay between osteoclasts and hematopoiesis.
| Model | Major findings | Reference |
|---|---|---|
| Ectopic transplantation of bone marrow plug in blood-letting mice | Increased hematopoiesis Reduced bone volume | 11 |
| Ovariectomized rats | Reduced hematopoiesis with time Increased osteoclasts | 16 |
| Osteopetrotic humans and mice | Fibrotic medullary tissue in osteoclast-rich forms | 20,21 |
| Hematopoietic tissue in osteoclast-poor forms | 22,23 | |
| RANKL-treated mice | Increased HSC mobilization Inhibition of HSC anchorage to the niche Cathepsin K-dependent cleavage of CXCL12 Increased MMP-9 Decreased endosteal osteopontin content | 24 |
| MMP-9-deficient mice | MMP-9 required for HSC mobilization | 24,25,26 |
| MMP-9 not required for HSC mobilization | 27,28 | |
| Mice treated with strontium ranelate | Inhibition of osteoclasts Delay in recovery after bone marrow transplantation | 6 |
| Mice treatment with PGE2 | Increased hematopoiesis Disruption of bone trabecular microarchitecture. Stimulation of bone resorption | 29 |
| Calcium sensing receptor-deficient mice | Reduced cellularity in bone marrow Reduced HSC content in bone marrow | 31 |
| Id1−/− mice | Osteoporosis Upregulation of osteoclast-specific genes Increased myeloid differentiation and HSC proliferation | 33 |
| Crebbp−/− mice | Exacerbated myeloid differentiation Reduced bone volume Increased osteoclastogenesis Increased levels of MMP-9 | 37 |
| Bone marrow transplantation in thyroparathyroidectomized rats | Delay in hematocrit recovery Restoration upon PTH treatment | 42 |
| Treatment of mice with erythropoietin and bisphosphonates | Increased erythropoiesis Increased osteoclastogenesis blocked by bisphosphonates Oncostatin M released by erythroid cells in response to erythropoietin, which stimulates osteoclast formation | 43,44,45,46 |
| Treatment of mice with bisphosphonates | Impaired bone marrow engraftment Decrease of HSC number in bone marrow | 48,52 |
| Promotion of proliferation and differentiation of hematopoietic progenitor cells Increase of HSC mobilization | 49 | |
| Genetic knock-down in mice to induce osteopetrosis or osteoporosis, treatment with anti-resorptive agents | Increased HSC mobilization into the peripheral blood in osteopetrotic mice Increased HSC mobilization into the peripheral blood in mice treated with bisphosphonates or RANKL antibody Decreased HSC mobilization in osteoporotic mice Decrease of HSC number in bone marrow | 50 |
| Treatment of mice with bisphosphonates, oc/oc osteopetrotic mice |
Impaired B cells in bone marrow Decrease in expression of CXCL12 and IL-7 Retention of B cells outside the bone marrow niche |
51 |
Abbreviations: HSC, hematopoietic stem cell; MMP-9, matrix metalloproteinase-9; PGE2, prostaglandin E2; PTH, parathyroid hormone.
Lessons from osteoporosis and osteopetrosis
Why should the osteoclast serve as a hematopoietic niche component? The most obvious possibility is with its essential role in creating space into which the hematopoietic tissue expands.10 There are pathological situations that support this hypothesis, and data mostly arise from clinical and preclinical observations in two diametrically opposing bone diseases: the osteoporosis and the osteopetrosis.
Osteoporosis.
An intriguing theory that emerged a few years ago suggested that the constant excessive need for blood cell generation may have a role in the etiology of female osteoporosis. Post-menopausal osteoporosis occurs rapidly after the decline of estrogen production, indicating that preconditions may be already present in the female organism at its onset. Fertile women lose about 70 ml of blood every month, roughly a bit less than 1 l every year and more than 30 l by the end of their fertile life. On the basis of this simple calculation, Gurevitch and Slavin11 hypothesized that there is a hematological etiology of osteoporosis. They suggested that, because blood loss creates developmental pressure on the hematopoietic system, the number of hematopoietic cells increases. These include the monocyte-derived osteoclasts, which intensify resorption and sustain the extension of the space available for hematopoiesis. In support of their proposal, patients affected by hematological diseases accompanied by chronic anemia or hemophilia, or treated with anticoagulants, tend to develop osteoporosis.12,13,14
To demonstrate this theory, they used a preclinical model of ectopic transplantation of a bone marrow plug into the subcapsular space of the mouse kidney, which triggers mesenchymal progenitor cells from the transplant into the development of bone and hematopoietic microenvironment.15 Mice were then subjected to blood-letting for 10 months (about 1/3 of their lifespan). The overall features of the ectopic ossicles developed in the chronically bled mice confirmed the assumption of the authors as they showed more hematopoiesis and less bone development compared with the ossicles formed in mice not subjected to blood withdrawal.11 However, the results using other experimental models were not in line with this hypothesis. In fact, in ovariectomized rats, Lei et al.16 observed that there is a time-dependent bone loss associated with an increase in osteoclast numbers and a parallel reduction in the volume of hematopoietic tissue. Therefore, at least in rats, these observations contradict the notion that osteoporosis is associated with an increase of hematopoiesis.11
Osteopetrosis.
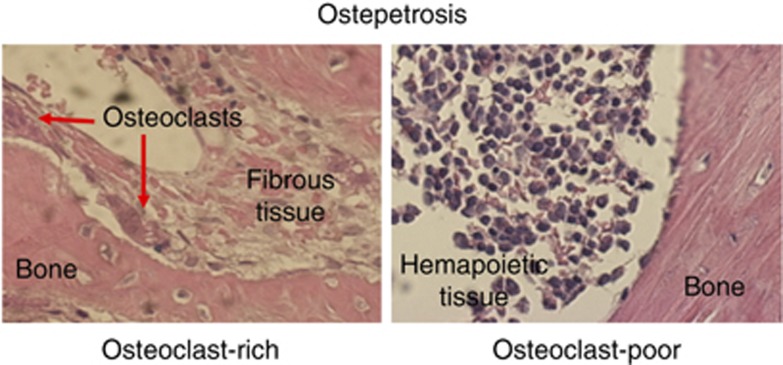
Intriguingly enough, in osteopetrosis, a condition of high bone mass and hematological failure in which osteoclasts are dysfunctional, bone marrow hematopoiesis is impaired regardless of the number of osteoclasts present in the microenvironment.17 In both osteoclast-rich and osteoclast-poor osteopetroses, which differ for the presence of a high number of non-functional osteoclasts in the former and the total absence of osteoclasts in the latter, anemia, pancytopenia and altered blood clothing are similarly observed.17 These events are associated with an insufficient space for hematopoietic tissue to evolve. However, recent observations showed different histological aspects in osteoclast-rich and osteoclast-poor osteopetrosis.18,19 In fact, pathological examination of the bone biopsies of osteoclast-rich osteopetrotic patients refer to 'irregular and massive primary trabeculae surrounded by abnormal fibrous tissue'.20,21 In contrast, Sobacchi et al.22 and Guerrini et al.23 showed a few bone biopsies of osteoclast-poor patients, describing the presence of scant hematopoietic tissue in small medullary spaces, with, however, no fibrosis (Figure 1). As most hallmarks of the two forms of osteopetrosis are very similar except for the presence of high number of osteoclasts versus their absence, it is worth to hypothesize that, at least in this pathological condition, nonfunctional osteoclasts may contribute to the development of fibrosis, possibly inhibiting hematopoiesis.
Figure 1. Hematopoiesis in osteopetrosis.
Histological sections of bone biopsies of patients affected by osteoclast-rich and osteoclast-poor osteopetrosis showing abnormal fibrous tissue in the former and hematopoietic tissue in the latter. Hematoxylin/eosin staining. Original magnification ×40.
Role of osteoclast enzymes
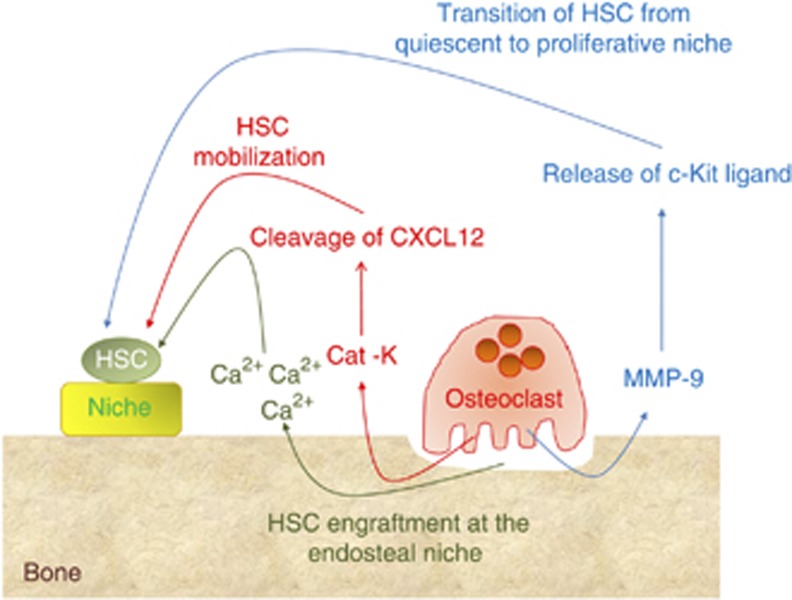
Osteoclasts are ectoenzyme-producing cells.10 Cathepsin K, for example, is the major collagenolytic enzyme released in the osteoclast-resorbing lacuna from the lysosomal compartment, and Kollet et al.24 suggested that osteoclast cathepsin K meditates the cleavage of an important chemokine, CXCL12, responsible for the anchorage of HSCs to the niche, causing the mobilization of immature hematological progenitor cells into the circulation (Figure 2). However, definitive conclusions on the role of cathepsin K in cleaving CXCL12 cannot be drawn yet because of the lack of data in suitable animal models, such as the cathepsin knockout mice.
Figure 2. Role of enzymes and calcium released by osteoclasts on HSC.
Cartoon showing the hypothetical role of cathepsin K (Cat-K), matrix metalloproteinase 9 (MMP-9) and calcium (Ca2+) on HSC mobilization, proliferation and engraftment at the endosteal niche.
Kollet et al.24 also observed that MMP-9 contributes to the recruitment of immature progenitors to the circulation by RANKL-stimulated osteoclasts in a CXCR4-dependent manner, a condition not observed in protein tyrosine phosphatase ɛ-knockout mice, which have defective osteoclasts. This progenitor egress was prevented by calcitonin, a potent inhibitor of osteoclast bone resorption (Table 2). Finally, they observed that RANKL-stimulated osteoclasts reduced osteopontin content along the endosteum, which is considered an important component of the endosteal niche that retains the HSCs in place. Consistent with these results, Heissig et al.25 have demonstrated that recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9-mediated release of c-kit ligand, which permits the transfer of HSCs from the quiescent to the proliferative niche, indirectly suggesting a role of osteoclasts in HSC niche maintenance (Figure 2). Finally, Pelus et al.26 showed that MMP-9 is indeed involved in HSC mobilization induced by the chemokines GRO/β/CXCL2 and GROβτ/CXCL2Δ4, an event neutralized in neutrophil-depleted mice, which also lack MMP-9, and partially blocked by the MMP-9 inhibitor MeOSuc-Ala-Ala-Pro-Cal-CMK.
Table 2. Effects of bone seeking drugs on HSC and hematopoiesis.
| Drug | Effect on HSC and hematopoiesis | Effect on bone | Reference |
|---|---|---|---|
| Calcitonin | Inhibits egress of HSC from bone marrow | Inhibits bone resorption | 24 |
| Strontium ranelate | Delay hematopoietic recovery after HSC transplantation | Stimulates osteoblasts Inhibits osteoclasts | 6 |
| PGE2 | Expands hematopoiesis Increases HSC in bone marrow | Stimulates bone resorption | 29 |
| Erythropoietin | Expands erythropoiesis Induces oncostatin M release | Induces bone loss | 43,44,45,46 |
| PTH | Improves hematocrit Increments HSC in bone marrow Improve HSC engraftment after transplantation | Given intermittently stimulates osteoblasts Given continuously stimulates osteoclasts | 42,53 |
| Bisphosphonates | Reduce HSC in bone marrow Impair HSC engraftment after transplantation Abolish PTH-induced HSC increment in bone marrow Promote proliferation and subsequent differentiation of progenitors Increase HSC mobilization to circulation Decrease numbers of B cells in bone marrow | Induce osteoclast apoptosis Reduce bone resorption Block erythropoietin-induced bone loss | 48,49,50,51,52 |
| Anti-RANKL antibody |
Increases HSC mobilization to circulation |
Inhibits osteoclast formation |
50 |
Abbreviations: HSC, hematopoietic stem cell; PGE2, prostaglandin E2; PTH, parathyroid hormone.
However, other studies argued against MMP-9 having any effect on HSC mobilization. Robinson et al.27 and Levesque et al.28 reported that MMP-9 knockout mice did not show impaired HSC mobilization, and that the engraftment of MMP-9-deficient bone marrow HSCs was not harmed in sublethally irradiated wild-type recipients. Therefore, more insights are necessary to fully understand the relevance of MMP-9 in the regulation of hematopoiesis.
Treatments
Treatment with strontium ranelate, a bone anabolic agent that increases osteoblast number but inhibits osteoclasts, has been observed to cause a delay in hematopoietic recovery after bone marrow transplantation,6 supporting a role for osteoclastic regulation of HSCs (Table 2). It is also believed that prostaglandin E2 expands hematopoiesis not only by increasing HSC number but also through its complex role on the metabolism of osteoblasts and osteoclasts.29 In fact, in mice treated with prostaglandin E2, disruption of bone microarchitecture, possibly due, at least in part, to activation of bone resorption,30 was observed along with an increase of Lineage-Sca-1+c-Kit+ (LSK) bone marrow HSCs29 (Table 2).
Role of calcium
It is known that the activity of osteoclasts elevates the local and systemic calcium ion concentration, and recent studies have demonstrated that the HSC engraftment at the endosteal niche is specified by the calcium-sensing receptor (Figure 2). In fact, Adams et al.31 have shown reduced cellularity and HSC content in the bone marrow of calcium sensing-receptor-deficient mice. In these mice they also observed an increased mobilization of progenitors, suggesting that calcium may have a relevant role in keeping HSC localization in the bone marrow.
Genetic studies
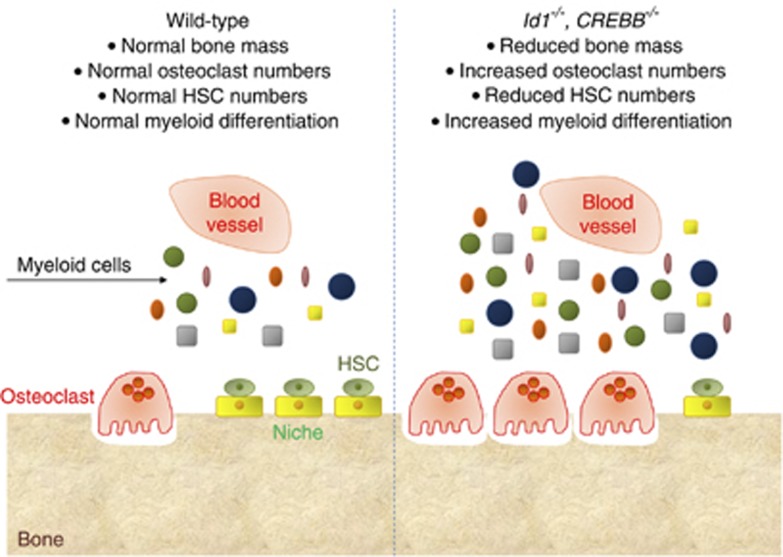
Genetic studies have attempted to demonstrate the molecular mechanisms whereby osteoclasts modulate hematopoiesis. For instance, the inhibitor of differentiation gene (Id1), a transcriptional regulator that prevents basic helix–loop–helix transcription factors from binding the DNA,32 has been found to link bone homeostasis and hematopoiesis (Figure 3). In fact, Id1−/− mice showed an osteoporotic phenotype, with the osteoclast-specific genes, cathepsin K, tartrate-resistant acid phosphatase, and osteoclast-associated receptor upregulated, while the same genes were repressed in osteoclasts overexpressing Id1.33 The hematopoietic compartment of Id1−/− mice showed an increase in myeloid differentiation and HSC proliferation, suggesting that in the absence of Id1 the HSCs are driven towards myeloid differentiation. Id1 has therefore been indicated as a factor in the dynamic cross-talk between osteoclasts and HSCs,33 although further work is necessary to confirm this hypothesis.
Figure 3. Genetic studies.
Cartoon illustrating the effect of deletion in mice of the Id1 and the CREBB genes on bone mass and myelopoiesis.
A recent report has shown that the CREB (cAMP response element-binding)-binding protein (CREBBP), which regulates the hematopoiesis by a HSC-intrinsic effect, has a pivotal role also in the microenvironment-mediated regulation of hematopoiesis.34 CREBBP is a coactivator of transcription that interacts with transcription factors and acetylates histones and other proteins. In humans, chromosomal translocations involving the CREBBP gene have been reported in leukemia35 and myelodysplastic syndromes.36 Defects in Crebbp−/− HSCs include impaired self-renewal and exacerbated myeloid differentiation,37 with a tendency of Crebbp−/− mice to develop hematological malignancies with age.38 It has been observed that Crebbp−/− mice not only poorly support HSCs, promoting excessive myelopoiesis and reducing lymphopoiesis, but also show reduced bone volume due to increased osteoclastogenesis (Figure 3). Interestingly, Crebbp deficiency in the bone marrow microenvironment results in reduced levels of MMP9, a metalloproteinase abundantly expressed in osteoclasts.
Role of bone marrow monocytic cells
Monocytes share with osteoclasts the same origin from the granulocyte/macrophage colony-forming unit. Therefore, in the context of the regulation of hematopoiesis, they may contribute to the HSC niche with mechanisms that, at least in part, may overlap or complement those employed by the osteoclasts. Winkler et al.39 showed that administration of granulocyte colony stimulating factor (G-CSF) depleted the endosteal surface of both endosteal osteoblasts and endosteal macrophages, denominated osteomacs, which was concomitant with the HSC mobilization. Osteomacs could be pivotal in HSC egress because their depletion in Fas-induced apoptosis transgenic mice or by administration of chlodronate-loaded liposomes reduced trophic cytokines at the endosteum and HSC mobilization upon G-CSF administration. The effect of G-CSF appeared to be mediated by the restriction of G-CSF receptor to the monocytic lineage, which was depleted in the bone marrow when the cytokine was administered.40 It is likely that one of the mechanisms underlying the role of monocytic cells in the HSC niche is the production of yet-to-be identified factors that support osteoblasts in vivo, although a direct evidence that this is related to the HSC niche is still lacking. In conditional depletion models of bone marrow mononuclear phagocytes, the expression of CXCL12 was found to be diminished, contributing to HSC egress. Consistently, in this model, the response to CXCR4 antagonists and to G-CFS was enhanced.41
Role of erythropoietin
In 1971, Perris et al.42 had reported an association of the bone marrow response with acute bleeding due to hypocalcemia and release of PTH, observing a delay of hematocrit recovery in thyroparathyroidectomized rats compared with controls, and a restoration upon PTH infusion (Table 2). These observations suggest a close relationship between erythropoiesis and bone resorption.
Erythropoietin is the major physiological cytokine that supports erythropoiesis. Factors released by erythroid cells in response to this hormone regulate the osteoblast/osteoclast coupling. Erythropoietin stimulates osteoclastogenesis in the first instance, followed by an increase in osteoblastogenesis that is induced either by erythropoietin directly or through the expression of bone morphogenetic proteins 2 and 6 by HSCs through the Jak/Stat signaling.43 Oncostatin M, a member of the gp130 family of cytokines, is also induced strongly in erythroblasts in response to erythropoietin.44 Oncostatin M binds to and signals the osteoblasts to produce RANKL, subsequently inducing osteoclast differentiation45 (Table 2). Therefore, oncostatin M contributes to the orchestrated response of erythroid cells to erythropoietin and to the changes in the local bone structures and osteoclast response. Consistent with this knowledge, mice treated with a clinically relevant dose of erythropoietin showed a rapid loss of the trabecular bone volume. Interestingly, bisphosphonates blocked the erythropoietin-induced bone loss and the magnitude of the erythroid response to erythropoietin,46 once again suggesting a close association between bone resorption and myelopoiesis. In fact, the article points to the interplay between erythroid development and skeletal homeostasis, suggesting that bone remodeling is required for attaining sufficient bone marrow space for erythroid expansion. This has been proposed by Suda47 to reflect the phylogenic process of bone marrow formation within the bone cavity.
Anti-resorptive drugs
Recent papers have directly addressed the importance of osteoclast activity in the context of the regulation of hematopoiesis, with, however, contradictory results (Table 2). Lymperi et al.48 showed that inhibition of osteoclast function reduces HSC numbers in vivo. To address this question, they tested the effects of bisphosphonates on hematopoiesis, and observed that mice treated with bisphosphonates displayed a drop in HSCs in the bone marrow. They also observed impairment of the engraftment of bone marrow cells harvested from treated animals. Bisphosphonates also abolished the HSC increment produced by PTH, confirming that this hormone acts on hematopoiesis, at least in part, through the enhancement of osteoclast activity. They also observed that a larger fraction of LSK cells in the bone marrow of treated mice entered the cell cycle, suggesting that osteoclast impairment promotes proliferation and subsequent differentiation of progenitor cells, a finding in line with the observations in the Id1−/− and the Crebbp−/− mice.32,33 HSC impairment was considered a consequence of niche manipulation, because the bone marrow from untreated donor mice transplanted into mice previously treated with bisphosphonates showed a delay in hematopoietic recovery compared with untreated controls. Therefore, the authors concluded that osteoclast function is fundamental for the maintenance of a correct HSC niche.48
In a previous work, however, Takamatsu et al.49 had found that short-term G-CSF administration increased bone resorption concomitant to the egress of HSC. In this circumstance, the bisphosphonate pamidronate reduced the G-CSF-induced bone resorption but, instead of decreasing, it increased the number of HSCs mobilized from bone marrow, suggesting that bone resorption is not the direct cause of HSC mobilization but rather a parallel event.
Miyamoto et al.50 performed an elegant study using various models of osteoclast-deficient mice and also concluded that osteoclasts are dispensable for HSC maintenance and mobilization. They caused HSC mobilization in mice by sequential treatments with G-CSF and counted the number of LSK cells in the peripheral blood. They observed that mobilization of HSCs from bone marrow was increased in bisphosphonate- or RANKL antibody-treated mice compared with controls. Likewise, the number of HSCs mobilized into the blood was higher in mice with osteopetrotic genotypes, including the macrophage colony stimulating factor-, RANKL- and c-Fos-deficient mice. Consistently, mice deficient in the anti-osteoclastogenic cytokine osteoprotegerin, whose phenotype is characterized by increased numbers of osteoclasts and exacerbated bone resorption, exhibited reduced HSC mobilization, leading the authors to conclude that osteoclasts prevent HSC mobilization and may be negative regulators of the hematopoietic system.50 It is worth noting, however, that, according to the involvement of monocytic phagocytes in the regulation of the HSC niche,39,40,41 drugs disrupting osteoclasts could also trigger a positive feedback loop that could affect the homeostasis of bone marrow macrophages through their myeloid progenitors.
A recent report suggested that bone marrow B lymphopoiesis is also regulated by osteoclast activity. Osteopetrosis was induced in normal mice by injections of zoledronic acid, which caused B-cell number to decrease specifically in the bone marrow. This effect was due to a decrease in the expression of CXCL12 and IL-7 by stromal cells, which led to retention of B-cell progenitors outside of the bone marrow niches. Similar results were observed in the osteopetrotic oc/oc mice, suggesting that they were not due to the zoledric acid itself but to the microenvironmental condition created by reduced osteoclast activity.51
Lastly, a very recent paper suggested that osteoclasts promote the formation of the HSC niche by controlling the maturation of osteoblasts.52 In oc/oc mice and in wild-type mice treated with zoledric acid, the frequency of LSK cells in the bone marrow decreased by >90%, whereas that of mesenchymal progenitors increased to 80% of the total number of bone marrow cells compared with 20% in wild-type untreated mice. These mesenchymal progenitors were not capable of recruiting LSK cells and their differentiation into the osteoblast lineage was impaired, suggesting that osteoclasts could affect HSC niche development through their influence on osteoblast differentiation.
Clinical considerations
From this set of data, it is clear though that the field is rapidly expanding, but that a consensus on the role of osteoclasts and bone resorption in the regulation of hematopoiesis is not yet possible because of the heterogeneity of observations and experimental data. However, some considerations can be drawn, especially in the clinical context because treatments with current bone-seeking drugs may have implications on the well-being of patients with hematological failures. For instance, bisphopshonates have been shown to suppress HSC in the bone marrow48,52 and to increase its mobilization into the peripheral blood.48,50 This may implicate that caution must be used for the engraftment of bone marrow when anti-resorptive drugs are used, but could also implicate that treatment with anti-resorptive drugs could improve the yield of HSCs from the peripheral blood of donors in which HSC mobilization is enhanced by treatment with G-CSF for transplantation purpose. Likewise, it is established since the pioneer paper by Calvi et al.53 that PTH increments HSC number in the bone marrow and enhances the engraftment of transplanted HSCs, a finding that has been confirmed more recently by various studies.2 Therefore, administration of PTH can be envisioned as a potential treatment of both donors and hosts to improve the HSC yield in the former and their engraftment in the latter. These implications could be highly relevant also in case of autologous transplant in patients affected by multiple myeloma, a disease that presents with serious bone involvement often treated with anti-resorptive therapy.
In osteopetrotic patients subjected to bone marrow transplantation, a frequent adverse effect due to the appearance of functional osteoclasts is the development of hypercalcemia, for which patients are treated with anti-resorptive drugs.54 It is already known that caution must be used for the treatment of infants with these drugs as they can greatly affect bone turnover with consequences on bone quality. The studies available so far indeed suggest that even more caution is needed as inhibition of bone resorption may also worsen the engraftment and maintenance of HSC, thus compromising the beneficial effects of bone marrow transplantation. Similarly, children with osteogenesis imperfecta or other diseases characterized by severe bone loss are often treated with bisphosphonates.55 Concerns have been raised on the long-term use of bisphosphonates in childhood because, by blocking the bone turnover, they are known to cause a paradoxical bone fragility later in life.56 There is now an additional concern as these kids should probably be monitored also for hematological failures in the light of the putative negative role of osteoclast inactivity on HSC maintenance in bone marrow.
Finally, the regulatory nexus between erythropoietin administration and bone remodeling may also have important clinical implications, for example, in explaining the skeletal deformities in β-thalassemia, which is characterized by increased erythropoietin production. Likewise, patients affected by renal failure are treated with erythropoietin, which may have an impact on their severe osteodystrophy. Moreover, excess of erythropoietin administration in preparation of autologous blood transfusion may have implications, for instance, in patients affected by leukemia or multiple myeloma, considering the serious bone complications observed in these onco-hematological diseases.47
Conclusions
In conclusion, this rapidly expanding field of research is likely to shortly address new questions on the role of osteoclasts in the regulation of hematopoiesis and on the underlying molecular mechanisms. It is necessary though to monitor the new findings, trying to unequivocally clarify whether active osteoclasts are an advantage or a disadvantage for HSC maintenance, mobilization and progression towards myelopoiesis, especially in the clinical context.
Footnotes
The author declares no conflict of interest.
References
- Teti A. Bone development: overview of bone cells and signaling. Curr Osteoporos Rep 2011;9:264–273. [DOI] [PubMed] [Google Scholar]
- Porter RL, Calvi LM. Communications between bone cells and hematopoietic stem cells. Arch Biochem Biophys 2008;473:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ye L, Huang H, He X, Tong WG, Ross J et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003;425:836–841. [DOI] [PubMed] [Google Scholar]
- Askmyr M, Sims NA, Martin TJ, Purton LE. What is the true nature of the osteoblastic hematopoietic stem cell niche? Trends Endocrinol Metab 2009;20:303–309. [DOI] [PubMed] [Google Scholar]
- Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 2010;464:852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymperi S, Horwood N, Marley S, Gordon MY, Cope AP, Dazzi F. Strontium can increase some osteoblasts without increasing hematopoietic stem cells. Blood 2008;111:1173–1181. [DOI] [PubMed] [Google Scholar]
- Lo Celso C, Scadden DT. The haematopoietic stem cell niche at a glance. J Cell Sci 2011;124 (Part 21): 3529–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun S, Hirschi KK. Establishment and regulation of the HSC niche: roles of osteoblastic and vascular compartments. Birth Defects Res C Embryo Today 2010;90:229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006;124:407–421. [DOI] [PubMed] [Google Scholar]
- Peruzzi B, Teti A. The physiology and pathophysiology of the osteoclast. Clin Rev Bone Miner Metab (e-pub ahead of print 2 April 2011; DOI:10.1007/s12018-011-9086-6). [Google Scholar]
- Gurevitch O, Slavin S. The hematological etiology of osteoporosis. Med Hypotheses 2006;67:729–735. [DOI] [PubMed] [Google Scholar]
- Sanner JR, Ramin JE. Osteoporotic, hematopoietic mandibular marrow defect: an osseous manifestation of sickle cell anemia. J Oral Surg 1977;35:986–988. [PubMed] [Google Scholar]
- Gallacher SJ, Deighan C, Wallace AM, Cowan RA, Fraser WD, Fenner JA et al. Association of severe haemophilia A with osteoporosis: a densitometric and biochemical study. Quart J Med 1994;87:181–186. [PubMed] [Google Scholar]
- Bernardi E, Prandoni P. Safety of low molecular weight heparins in the treatment of venous thromboembolism. Expert Opin Drug Saf 2003;2:87–94. [DOI] [PubMed] [Google Scholar]
- Maniatis A, Tavassoli M, Crosby WH. Origin of osteogenic precursor cells in extramedullary marrow implants. Blood 1971;38:569–575. [PubMed] [Google Scholar]
- Lei Z, Xiaoying Z, Xingguo L. Ovariectomy-associated changes in bone mineral density and bone marrow haematopoiesis in rats. Int J Exp Pathol 2009;90:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Fattore A, Cappariello A, Teti A. Genetics, pathogenesis and complications of osteopetrosis. Bone 2008;42:19–29. [DOI] [PubMed] [Google Scholar]
- Del Fattore A, Capannolo M, Rucci N. Bone and bone marrow: the same organ. Arch Biochem Biophys 2010;503:28–34. [DOI] [PubMed] [Google Scholar]
- Del Fattore A, Capannolo M, Teti A. New mechanisms of osteopetrosis. IBMS BoneKEy 2009;6:16–28. [Google Scholar]
- Taranta A, Migliaccio S, Recchia I, Caniglia M, Luciani M, De Rossi G et al. Genotype-phenotype relationship in human ATP6i-dependent autosomal recessive osteopetrosis. Am J Pathol 2003;162:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Fattore A, Peruzzi B, Rucci N, Recchia I, Cappariello A, Longo M et al. Clinical, genetic, and cellular analysis of 49 osteopetrotic patients: implications for diagnosis and treatment. J Med Genet 2006;43:315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobacchi C, Frattini A, Guerrini MM, Abinun M, Pangrazio A, Susani L et al. Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat Genet 2007;39:960–962. [DOI] [PubMed] [Google Scholar]
- Guerrini MM, Sobacchi C, Cassani B, Abinun M, Kilic SS, Pangrazio A et al. Human osteoclast-poor osteopetrosis with hypogammaglobulinemia due to TNFRSF11A (RANK) mutations. Am J Hum Genet 2008;83:64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med 2006;12:657–664. [DOI] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 2002;109:625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelus LM, Bian H, King AG, Fukuda S. Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT/CXCL2delta4. Blood 2004;103:110–119. [DOI] [PubMed] [Google Scholar]
- Robinson SM, Pisarev VM, Chavez JM, Singh RK, Talmadge JE. Use of matrix metalloproteinase (MMP)-9 knockout mice demonstrates that MMP-9 activity is not absolutely required for G-CSF or Flt-3 ligand-induced hematopoietic progenitor cell mobilization or engraftment. Stem Cells 2003;21:417–427. [DOI] [PubMed] [Google Scholar]
- Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C et al. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood 2004;104:65–72. [DOI] [PubMed] [Google Scholar]
- Frisch BJ, Porter RL, Gigliotti BJ, Olm-Shipman AJ, Weber JM, O'Keefe RJ et al. In vivo prostaglandin E2 treatment alters the bone marrow microenvironment and preferentially expands short-term hematopoietic stem cells. Blood 2009;114:4054–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab 2010;21:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 2006;439:599–603. [DOI] [PubMed] [Google Scholar]
- Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell 1990;61:49–59. [DOI] [PubMed] [Google Scholar]
- Chan AS, Jensen KK, Skokos D, Doty S, Lederman HK, Kaplan RN et al. Id1 represses osteoclast-dependent transcription and affects bone formation and hematopoiesis. PLoS One 2009;4:e7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer SN, Zhou Q, Zhou T, Cheng Z, Abboud-Werner SL, Horn D et al. Crebbp haploinsufficiency in mice alters the bone marrow microenvironment, leading to loss of stem cells and excessive myelopoiesis. Blood 2011;118:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow J, Stanton VP Jr, Andresen JM, Becher R, Behm FG, Chaganti RS et al. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet 1996;14:33–41. [DOI] [PubMed] [Google Scholar]
- Taki T, Sako M, Tsuchida M, Hayashi Y. The t(11; 16)(q23;p13) translocation in myelodysplastic syndrome fuses the MLL gene to the CBP gene. Blood 1997;89:3945–3950. [PubMed] [Google Scholar]
- Rebel VI, Kung AL, Tanner EA, Yang H, Bronson RT, Livingston DM. Distinct roles for CREB-binding protein and p300 in hematopoietic stem cell self-renewal. Proc Natl Acad Sci USA 2002;99:14789–14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung AL, Rebel VI, Bronson RT, Ch'ng LE, Sieff CA, Livingston DM et al. Gene dose-dependent control of hematopoiesis and hematologic tumor suppression by CBP. Genes Dev 2000;14:272–277. [PMC free article] [PubMed] [Google Scholar]
- Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 2010;116:4815–4828. [DOI] [PubMed] [Google Scholar]
- Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med 2011;208:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Lucas D, Hidalgo A, Méndez-Ferrer S, Hashimoto D, Scheiermann C et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 2011;208:261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perris AD, MacManus JP, Whitfield JF, Weiss LA. Parathyroid glands and mitotic stimulation in rat bone marrow after hemorrhage. Am J Physiol 1971;220:773–778. [DOI] [PubMed] [Google Scholar]
- Shiozawa Y, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang Z et al. Erythropoietin couples hematopoiesis with bone formation. PLoS One 2010;5:e10853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana P, Menon MP, Bogacheva O, Bogachev O, Niss K, Kapelle WS et al. Erythropoietin modulation of podocalyxin and a proposed erythroblast niche. Blood 2007;110:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EC, McGregor NE, Poulton IJ, Solano M, Pompolo S, Fernandes TJ et al. Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. J Clin Invest 2010;120:582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singbrant S, Russell MR, Jovic T, Liddicoat B, Izon DJ, Purton LE et al. Erythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironment. Blood 2011;117:5631–5642. [DOI] [PubMed] [Google Scholar]
- Suda T. Hematopoiesis and bone remodeling. Blood 2011;117:5556–5557. [DOI] [PubMed] [Google Scholar]
- Lymperi S, Ersek A, Ferraro F, Dazzi F, Horwood NJ. Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood 2011;117:1540–1549. [DOI] [PubMed] [Google Scholar]
- Takamatsu Y, Simmons PJ, Moore RJ, Morris HA, To LB, Lévesque JP. Osteoclast-mediated bone resorption is stimulated during short-term administration of granulocyte colony-stimulating factor but is not responsible for hematopoietic progenitor cell mobilization. Blood 1998;92:3465–3473. [PubMed] [Google Scholar]
- Miyamoto K, Yoshida S, Kawasumi M, Hashimoto K, Kimura T, Sato Y et al. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J Exp Med 2011;208:2175–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Anginot A, Mancini SJ, Schiff C, Carle GF, Wakkach A et al. Osteoclast activity modulates B-cell development in the bone marrow. Cell Res 2011;21:1102–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Abou-Ezzi G, Sitnicka E, Jacobsen SEW, Wakkach A, Blin-Wakkach C. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J Exp Med 2012;209:537–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003;425:841–846. [DOI] [PubMed] [Google Scholar]
- Dini G, Floris R, Garaventa A, Oddone M, De Stefano F, De Marco R et al. Long-term follow-up of two children with a variant of mild autosomal recessive osteopetrosis undergoing bone marrow transplantation. Bone Marrow Transplant 2000;26:219–224. [DOI] [PubMed] [Google Scholar]
- Silverman SL. Bisphosphonate use in conditions other than osteoporosis. Ann NY Acad Sci 2011;1218:33–37. [DOI] [PubMed] [Google Scholar]
- Harcke HT, Stevenson KL, Kecskemethy HH, Bachrach SJ, Grissom LE. Fracture after bisphosphonate treatment in children with cerebral palsy: the role of stress risers. Pediatr Radiol 2012;42:76–81. [DOI] [PubMed] [Google Scholar]