Abstract
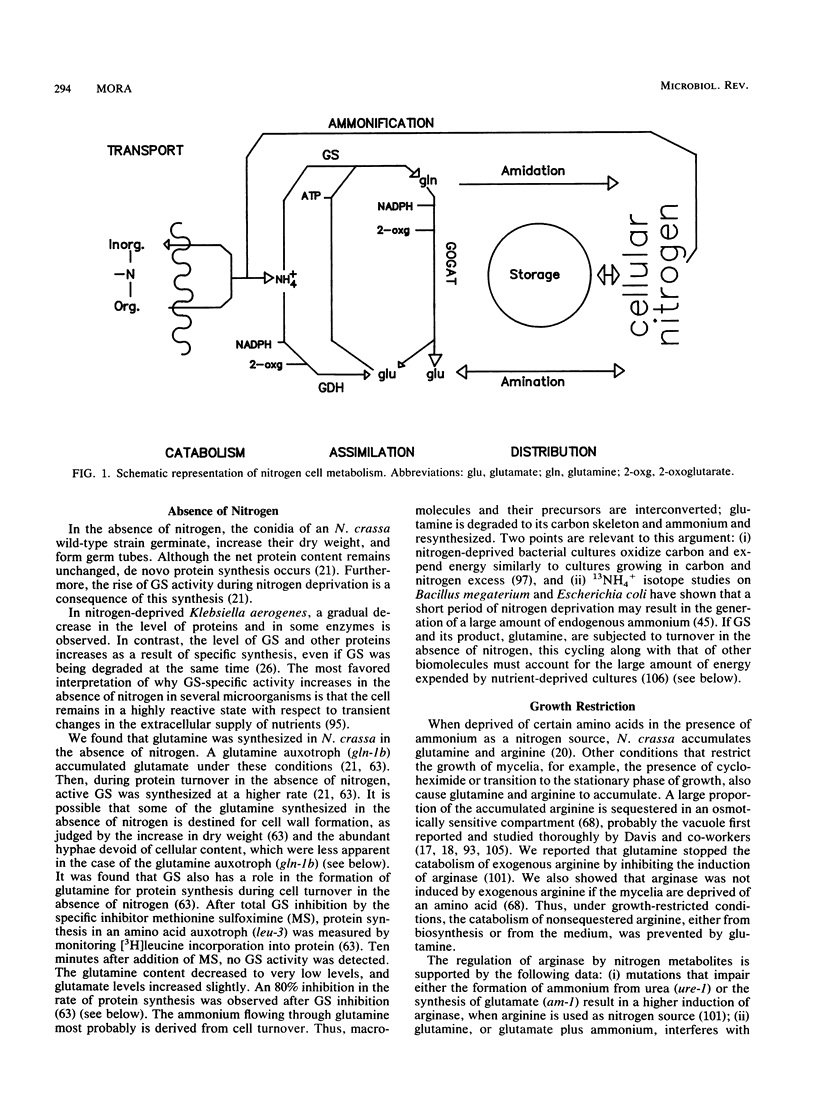
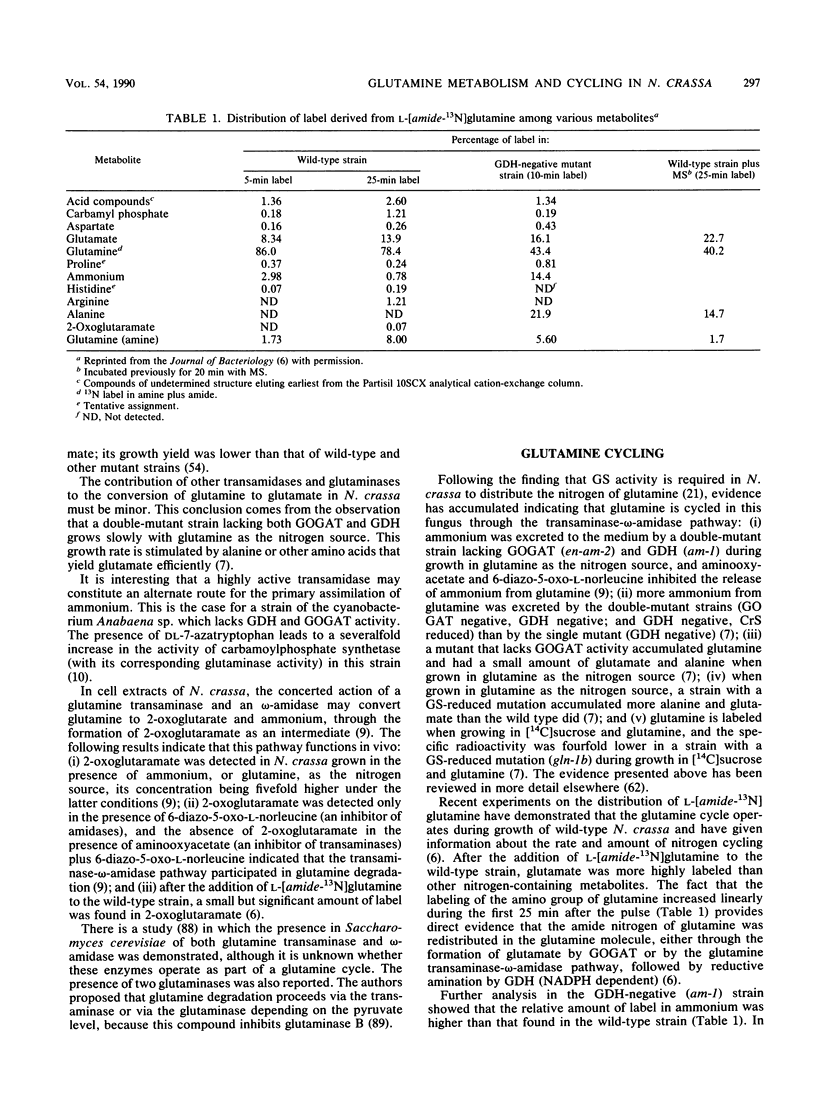
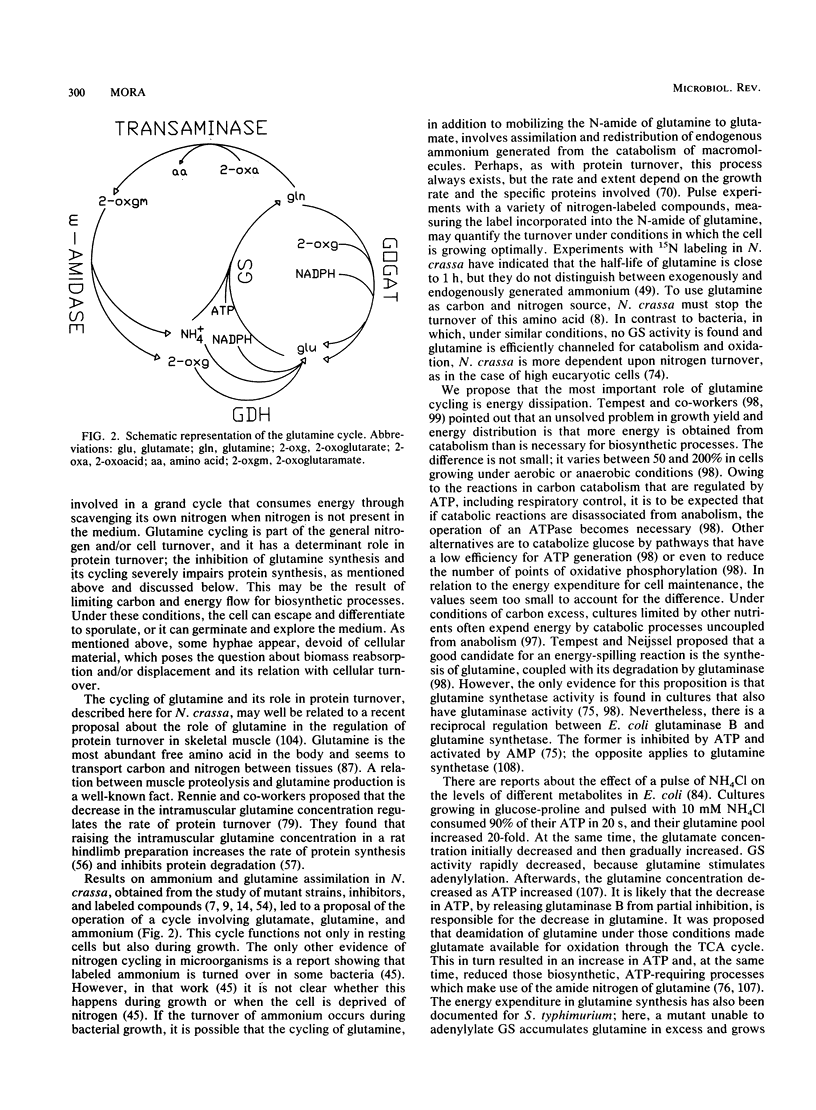
Evidence for the existence of a glutamine cycle in Neurospora crassa is reviewed. Through this cycle glutamine is converted into glutamate by glutamate synthase and catabolized by the glutamine transaminase-omega-amidase pathway, the products of which (2-oxoglutarate and ammonium) are the substrates for glutamate dehydrogenase-NADPH, which synthesizes glutamate. In the final step ammonium is assimilated into glutamine by the action of a glutamine synthetase (GS), which is formed by two distinct polypeptides, one catalytically very active (GS beta), and the other (GS alpha) less active but endowed with the capacity to modulate the activity of GS alpha. Glutamate synthase uses the amide nitrogen of glutamine to synthesize glutamate; glutamate dehydrogenase uses ammonium, and both are required to maintain the level of glutamate. The energy expended in the synthesis of glutamine drives the cycle. The glutamine cycle is not futile, because it is necessary to drive an effective carbon flow to support growth; in addition, it facilitates the allocation of nitrogen or carbon according to cellular demands. The glutamine cycle which dissipates energy links catabolism and anabolism and, in doing so, buffers variations in the nutrient supply and drives energy generation and carbon flow for optimal cell function.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguirre J., Hansberg W. Oxidation of Neurospora crassa glutamine synthetase. J Bacteriol. 1986 Jun;166(3):1040–1045. doi: 10.1128/jb.166.3.1040-1045.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps F., Van den Berghe G., Hers H. G. Evidence for a substrate cycle between AMP and adenosine in isolated hepatocytes. Proc Natl Acad Sci U S A. 1983 May;80(10):2829–2833. doi: 10.1073/pnas.80.10.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J. E., Prival M. J., Magasanik B. Regulation of the synthesis of enzymes responsible for glutamate formation in Klebsiella aerogenes. J Biol Chem. 1973 Sep 10;248(17):6122–6128. [PubMed] [Google Scholar]
- Brooks B., Arch J. R., Newsholme E. A. Effects of hormones on the rate of the triacylglycerol/fatty acid substrate cycle in adipocytes and epididymal fat pads. FEBS Lett. 1982 Sep 20;146(2):327–330. doi: 10.1016/0014-5793(82)80945-9. [DOI] [PubMed] [Google Scholar]
- Calderón J., Cooper A. J., Gelbard A. S., Mora J. 13N isotope studies of glutamine assimilation pathways in Neurospora crassa. J Bacteriol. 1989 Mar;171(3):1772–1774. doi: 10.1128/jb.171.3.1772-1774.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón J., Martínez L. M., Mora J. Isolation and characterization of a Neurospora crassa mutant altered in the alpha polypeptide of glutamine synthetase. J Bacteriol. 1990 Sep;172(9):4996–5000. doi: 10.1128/jb.172.9.4996-5000.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón J., Mora J. Glutamine assimilation pathways in Neurospora crassa growing on glutamine as sole nitrogen and carbon source. J Gen Microbiol. 1989 Oct;135(10):2699–2707. doi: 10.1099/00221287-135-10-2699. [DOI] [PubMed] [Google Scholar]
- Calderón J., Morett E., Mora J. Omega-amidase pathway in the degradation of glutamine in Neurospora crassa. J Bacteriol. 1985 Feb;161(2):807–809. doi: 10.1128/jb.161.2.807-809.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. H., Van Baalen C., Tabita F. R. DL-7-azatryptophan and citrulline metabolism in the cyanobacterium Anabaena sp. strain 1F. J Bacteriol. 1987 Mar;169(3):1114–1119. doi: 10.1128/jb.169.3.1114-1119.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. J., Meister A. The glutamine transaminase-omega-amidase pathway. CRC Crit Rev Biochem. 1977;4(3):281–303. doi: 10.3109/10409237709102560. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. Regulation of cytoplasmic proline levels in Salmonella typhimurium: effect of osmotic stress on synthesis, degradation, and cellular retention of proline. J Bacteriol. 1988 May;170(5):2374–2378. doi: 10.1128/jb.170.5.2374-2378.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daldal F., Fraenkel D. G. Assessment of a futile cycle involving reconversion of fructose 6-phosphate to fructose 1,6-bisphosphate during gluconeogenic growth of Escherichia coli. J Bacteriol. 1983 Jan;153(1):390–394. doi: 10.1128/jb.153.1.390-394.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H. Compartmental and regulatory mechanisms in the arginine pathways of Neurospora crassa and Saccharomyces cerevisiae. Microbiol Rev. 1986 Sep;50(3):280–313. doi: 10.1128/mr.50.3.280-313.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Weiss R. L. Novel mechanisms controlling arginine metabolism in Neurospora. Trends Biochem Sci. 1988 Mar;13(3):101–104. doi: 10.1016/0968-0004(88)90050-3. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Chaloupka J., Vinter V. Turnover of cell walls in microorganisms. Microbiol Rev. 1988 Dec;52(4):554–567. doi: 10.1128/mr.52.4.554-567.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dávila G., Brom S., Mora Y., Palacios R., Mora J. Genetic and biochemical characterization of glutamine synthetase from Neurospora crassa glutamine auxotrophs and their revertants. J Bacteriol. 1983 Dec;156(3):993–1000. doi: 10.1128/jb.156.3.993-1000.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dávila G., Lara M., Guzmán J., Mora J. Relation between structure and function of Neurospora crassa glutamine synthetase. Biochem Biophys Res Commun. 1980 Jan 15;92(1):134–140. doi: 10.1016/0006-291x(80)91530-2. [DOI] [PubMed] [Google Scholar]
- Dávila G., Sánchez F., Palacios R., Mora J. Genetics and physiology of Neurospora crassa glutamine auxotrophs. J Bacteriol. 1978 Jun;134(3):693–698. doi: 10.1128/jb.134.3.693-698.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espín G., Palacios R., Mora J. Glutamine metabolism in nitrogen-starved conidia of Neurospora crassa. J Gen Microbiol. 1979 Nov;115(1):59–68. doi: 10.1099/00221287-115-1-59. [DOI] [PubMed] [Google Scholar]
- Facklam T. J., Marzluf G. A. Nitrogen regulation of amino acid catabolism in Neurospora crassa. Biochem Genet. 1978 Apr;16(3-4):343–354. doi: 10.1007/BF00484090. [DOI] [PubMed] [Google Scholar]
- Fisher S. H., Sonenshein A. L. Bacillus subtilis glutamine synthetase mutants pleiotropically altered in glucose catabolite repression. J Bacteriol. 1984 Feb;157(2):612–621. doi: 10.1128/jb.157.2.612-621.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R. L., Keister D. L. Comparative properties of glutamine synthetases I and II in Rhizobium and Agrobacterium spp. J Bacteriol. 1980 Nov;144(2):641–648. doi: 10.1128/jb.144.2.641-648.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulks R. M., Stadtman E. R. Regulation of glutamine synthetase, aspartokinase, and total protein turnover in Klebsiella aerogenes. Biochim Biophys Acta. 1985 Dec 13;843(3):214–229. doi: 10.1016/0304-4165(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Goldbeter A., Koshland D. E., Jr Energy expenditure in the control of biochemical systems by covalent modification. J Biol Chem. 1987 Apr 5;262(10):4460–4471. [PubMed] [Google Scholar]
- González A., Tenorio M., Vaca G., Mora J. Neurospora crassa mutant impaired in glutamine regulation. J Bacteriol. 1983 Jul;155(1):1–7. doi: 10.1128/jb.155.1.1-7.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward B. E., Hussain A., Wilson R. H., Lyons A., Woodcock V., McIntosh B., Harris T. J. The cloning and nucleotide sequence of cDNA for an amplified glutamine synthetase gene from the Chinese hamster. Nucleic Acids Res. 1986 Jan 24;14(2):999–1008. doi: 10.1093/nar/14.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández G., Mora Y., Mora J. Regulation of glutamine synthesis by glycine and serine in Neurospora crassa. J Bacteriol. 1986 Jan;165(1):133–138. doi: 10.1128/jb.165.1.133-138.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández G., Sánchez-Pescador R., Palacios R., Mora J. Nitrogen source regulates glutamate dehydrogenase NADP synthesis in Neurospora crassa. J Bacteriol. 1983 Apr;154(1):524–528. doi: 10.1128/jb.154.1.524-528.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hottiger T., Schmutz P., Wiemken A. Heat-induced accumulation and futile cycling of trehalose in Saccharomyces cerevisiae. J Bacteriol. 1987 Dec;169(12):5518–5522. doi: 10.1128/jb.169.12.5518-5522.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummelt G., Mora J. NADH-dependent glutamate synthase and nitrogen metabolism in Neurospora crassa. Biochem Biophys Res Commun. 1980 Jan 15;92(1):127–133. doi: 10.1016/0006-291x(80)91529-6. [DOI] [PubMed] [Google Scholar]
- Hummelt G., Mora J. Regulation and function of glutamate synthase in Neurospora crassa. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1688–1694. doi: 10.1016/0006-291x(80)91368-6. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Gerok W., Sies H. Regulation of flux through glutaminase and glutamine synthetase in isolated perfused rat liver. Biochim Biophys Acta. 1983 Jan 25;755(2):272–278. doi: 10.1016/0304-4165(83)90214-3. [DOI] [PubMed] [Google Scholar]
- Kanamori K., Legerton T. L., Weiss R. L., Roberts J. D. Effect of the nitrogen source on glutamine and alanine biosynthesis in Neurospora crassa. An in vivo 15N nuclear magnetic resonance study. J Biol Chem. 1982 Dec 10;257(23):14168–14172. [PubMed] [Google Scholar]
- Kanamori K., Weiss R. L., Roberts J. D. Role of glutamate dehydrogenase in ammonia assimilation in nitrogen-fixing Bacillus macerans. J Bacteriol. 1987 Oct;169(10):4692–4695. doi: 10.1128/jb.169.10.4692-4695.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- Kim C. H., Hollocher T. C. 13N isotope studies on the pathway of ammonia assimilation in Bacillus megaterium and Escherichia coli. J Bacteriol. 1982 Jul;151(1):358–366. doi: 10.1128/jb.151.1.358-366.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S., Hirschman J., Burton D., Jelesko J., Meeks J. C. Covalent modification of bacterial glutamine synthetase: physiological significance. Mol Gen Genet. 1984;197(2):309–317. doi: 10.1007/BF00330979. [DOI] [PubMed] [Google Scholar]
- Lara M., Blanco L., Campomanes M., Calva E., Palacios R., Mora J. Physiology of ammonium assimilation in Neurospora crassa. J Bacteriol. 1982 Apr;150(1):105–112. doi: 10.1128/jb.150.1.105-112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerton T. L., Kanamori K., Weiss R. L., Roberts J. D. 15N NMR studies of nitrogen metabolism in intact mycelia of Neurospora crassa. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1495–1498. doi: 10.1073/pnas.78.3.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerton T. L., Weiss R. L. Mobilization of sequestered metabolities into degradative reactions by nutritional stress in Neurospora. J Bacteriol. 1979 Jun;138(3):909–914. doi: 10.1128/jb.138.3.909-914.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerton T. L., Weiss R. L. Mobilization of vacuolar arginine in Neurospora crassa. Mechanism and role of glutamine. J Biol Chem. 1984 Jul 25;259(14):8875–8879. [PubMed] [Google Scholar]
- Levine R. L., Oliver C. N., Fulks R. M., Stadtman E. R. Turnover of bacterial glutamine synthetase: oxidative inactivation precedes proteolysis. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2120–2124. doi: 10.1073/pnas.78.4.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limón-Lason J., Lara M., Resendiz B., Mora J. Regulation of glutamine synthetase in fed-batch cultures of Neurospora crassa. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1234–1240. doi: 10.1016/0006-291x(77)91425-5. [DOI] [PubMed] [Google Scholar]
- MONDER C., MEISTER A. alpha-Ketoglutaramic acid as a product of enzymic transamination of glutamine in Neurospora. Biochim Biophys Acta. 1958 Apr;28(1):202–203. doi: 10.1016/0006-3002(58)90450-5. [DOI] [PubMed] [Google Scholar]
- MacLennan P. A., Brown R. A., Rennie M. J. A positive relationship between protein synthetic rate and intracellular glutamine concentration in perfused rat skeletal muscle. FEBS Lett. 1987 May 4;215(1):187–191. doi: 10.1016/0014-5793(87)80139-4. [DOI] [PubMed] [Google Scholar]
- MacLennan P. A., Smith K., Weryk B., Watt P. W., Rennie M. J. Inhibition of protein breakdown by glutamine in perfused rat skeletal muscle. FEBS Lett. 1988 Sep 12;237(1-2):133–136. doi: 10.1016/0014-5793(88)80186-8. [DOI] [PubMed] [Google Scholar]
- Miller R. E., Stadtman E. R. Glutamate synthase from Escherichia coli. An iron-sulfide flavoprotein. J Biol Chem. 1972 Nov 25;247(22):7407–7419. [PubMed] [Google Scholar]
- Mora Y., Hernández G., Mora J. Regulation of carbon and nitrogen flow by glutamate synthase in Neurospora crassa. J Gen Microbiol. 1987 Jul;133(7):1667–1674. doi: 10.1099/00221287-133-7-1667. [DOI] [PubMed] [Google Scholar]
- Mosteller R. D., Goldstein R. V., Nishimoto K. R. Metabolism of individual proteins in exponentially growing Escherichia coli. J Biol Chem. 1980 Mar 25;255(6):2524–2532. [PubMed] [Google Scholar]
- Nakamura K., Stadtman E. R. Oxidative inactivation of glutamine synthetase subunits. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2011–2015. doi: 10.1073/pnas.81.7.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R. Neurospora crassa glutamine synthetase. Purification by affinity chromatography and characterization of subunit structure. J Biol Chem. 1976 Aug 10;251(15):4787–4791. [PubMed] [Google Scholar]
- Pine M. J. Turnover of intracellular proteins. Annu Rev Microbiol. 1972;26:103–126. doi: 10.1146/annurev.mi.26.100172.000535. [DOI] [PubMed] [Google Scholar]
- Quinto C., Mora J., Palacios R. Neurospora crassa glutamine synthetase. Role of enzyme synthesis and degradation on the regulation of enzyme concentration during exponential growth. J Biol Chem. 1977 Dec 10;252(23):8724–8727. [PubMed] [Google Scholar]
- Rennie M. J., Hundal H. S., Babij P., MacLennan P., Taylor P. M., Watt P. W., Jepson M. M., Millward D. J. Characteristics of a glutamine carrier in skeletal muscle have important consequences for nitrogen loss in injury, infection, and chronic disease. Lancet. 1986 Nov 1;2(8514):1008–1012. doi: 10.1016/s0140-6736(86)92617-6. [DOI] [PubMed] [Google Scholar]
- Robert F. M., Wong P. P. Isozymes of Glutamine Synthetase in Phaseolus vulgaris L. and Phaseolus lunatus L. Root Nodules. Plant Physiol. 1986 May;81(1):142–148. doi: 10.1104/pp.81.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero D., Dávila G. Genetic and biochemical identification of the glutamate synthase structural gene in Neurospora crassa. J Bacteriol. 1986 Sep;167(3):1043–1047. doi: 10.1128/jb.167.3.1043-1047.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutt H., Holzer H. Biological function of the ammonia-induced inactivation of glutamine synthetase in Escherichia coli. Eur J Biochem. 1972 Mar 15;26(1):68–72. doi: 10.1111/j.1432-1033.1972.tb01740.x. [DOI] [PubMed] [Google Scholar]
- Senior P. J. Regulation of nitrogen metabolism in Escherichia coli and Klebsiella aerogenes: studies with the continuous-culture technique. J Bacteriol. 1975 Aug;123(2):407–418. doi: 10.1128/jb.123.2.407-418.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. T., Pocard J. A., Bernard T., Le Rudulier D. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J Bacteriol. 1988 Jul;170(7):3142–3149. doi: 10.1128/jb.170.7.3142-3149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucki J. W. The optimal efficiency and the economic degrees of coupling of oxidative phosphorylation. Eur J Biochem. 1980 Aug;109(1):269–283. doi: 10.1111/j.1432-1033.1980.tb04792.x. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Weiss R. L., Davis R. H. Use of external, biosynthetic, and organellar arginine by Neurospora. J Bacteriol. 1973 Jul;115(1):284–290. doi: 10.1128/jb.115.1.284-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumar N., Casselton P. J., McNally S. F., Stewart G. R. Occurrence of Isóenzymes of Glutamine Synthetase in the Alga Chlorella kessleri. Plant Physiol. 1984 Feb;74(2):204–207. doi: 10.1104/pp.74.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez F., Calva E., Campomanes M., Blanco L., Guzmán J., Saborío J. L., Palacios R. Heterogeneity of glutamine synthetase polypeptides in Neurospora crassa. J Biol Chem. 1980 Mar 25;255(6):2231–2234. [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Synthesis of glutamate in Aerobacter aerogenes by a hitherto unknown route. Biochem J. 1970 Apr;117(2):405–407. doi: 10.1042/bj1170405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Neijssel O. M. The status of YATP and maintenance energy as biologically interpretable phenomena. Annu Rev Microbiol. 1984;38:459–486. doi: 10.1146/annurev.mi.38.100184.002331. [DOI] [PubMed] [Google Scholar]
- Vaca G., Mora J. Nitrogen regulation of arginase in Neurospora crassa. J Bacteriol. 1977 Sep;131(3):719–725. doi: 10.1128/jb.131.3.719-725.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallari D. S., Jackowski S. Biosynthesis and degradation both contribute to the regulation of coenzyme A content in Escherichia coli. J Bacteriol. 1988 Sep;170(9):3961–3966. doi: 10.1128/jb.170.9.3961-3966.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichido I., Mora Y., Quinto C., Palacios R., Mora J. Nitrogen regulation of glutamine synthetase in Neurospora crassa. J Gen Microbiol. 1978 Jun;106(2):251–259. doi: 10.1099/00221287-106-2-251. [DOI] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Intracellular localization of enzymes of arginine metabolism in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5403–5408. [PubMed] [Google Scholar]
- Westerhoff H. V., Lolkema J. S., Otto R., Hellingwerf K. J. Thermodynamics of growth. Non-equilibrium thermodynamics of bacterial growth. The phenomenological and the mosaic approach. Biochim Biophys Acta. 1982 Dec 31;683(3-4):181–220. doi: 10.1016/0304-4173(82)90001-5. [DOI] [PubMed] [Google Scholar]
- Wootton J. C. Re-assessment of ammonium-ion affinities of NADP-specific glutamate dehydrogenases. Activation of the Neurospora crassa enzyme by ammonium and rubidium ions. Biochem J. 1983 Feb 1;209(2):527–531. doi: 10.1042/bj2090527. [DOI] [PMC free article] [PubMed] [Google Scholar]


