Abstract
The osteoarthritic diseases are common disorders characterized by progressive destruction of the articular cartilage in the joints, and associated with remodeling of the subchondral bone, synovitis and the formation of bone outgrowths at the joint margins, osteophytes. From the clinical perspective, osteoarthritis leads to joint pain and loss of function. Osteoarthritis is the leading cause of progressive disability. New data from genetic, translational and basic research have demonstrated that pathways with essential roles in joint and bone development also contribute to the postnatal homeostasis of the articular cartilage and are involved in osteoarthritis, making these potential therapeutic targets. Other systems of interest are the tissue-destructive enzymes that break down the extracellular matrix of the cartilage as well as mediators of inflammation that contribute to synovitis. However, the perspective of a durable treatment over years to decades highlights the need for a personalized medicine approach encompassing a global view on the disease and its management, thereby including nonpharmaceutical approaches such as physiotherapy and advanced surgical methods. Integration of novel strategies based on their efficacy and safety with the identification of individuals at risk and optimal individual rehabilitation management remains a major challenge for the medical community in particular, as the incidence of osteoarthritis is likely to further increase with the overall aging of the population.
Introduction
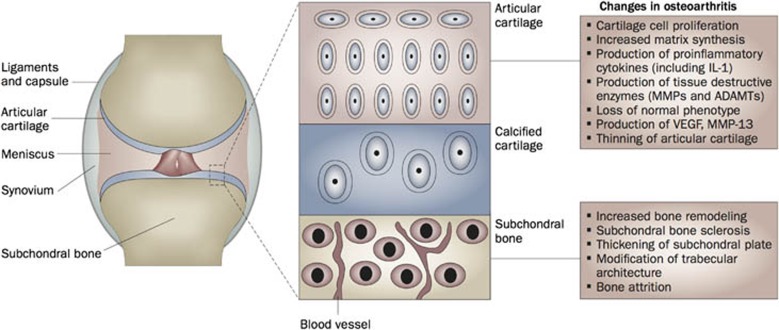
Much alike silence can bring new meaning to words or music, joints, the empty spaces between the bones, fulfill essential roles in the skeleton. The synovial joints are specialized structures organized around a cavity that connect the different bones of the skeleton and that allow movement within well-defined ranges and along specific axes. Different tissues functionally cooperate within the joint organ to meet the required balance between connecting and articulating the skeletal elements. The articular cartilage typically caps the proximal and distal ends of the bone, providing a smooth and pressure-deformable buffer zone that supports movement (Figure 1). The underlying or subchondral bone forms a complex interface with the articular cartilage with critical roles in stress and load distribution. The synovium lines the inner cavity. It is composed of a thin pseudo-epithelial lining layer populated by synovial fibroblasts and tissue-resident macrophages and synthesizes lubricating molecules such as lubricin and hyaluronan. The sublining zone is well-vascularized and the source of nourishment by diffusion for the articular cartilage which is nonvascular. The joint capsule, a strong tension-resistant connective tissue encloses the joint. Ligaments provide further strength and limit the range of motion.
Figure 1. The bone–cartilage unit is at the center of joint function and disease.
The joint enables movement by concerted interaction between its different tissues. Progressive development of osteoarthritis results in activation of different processes and pathways in the distinct tissues and cells of the joint. The current paradigm suggests that these changes evolve simultaneously and that osteoarthritis is not simply a disease of the cartilage or bone. New evidence supports the existence of several types of communication between cartilage and bone. ADAMTs, a disintegrin and metalloproteinase with thrombospondin motifs; IL-1, interleukin-1; MMP, matrix metalloproteinase; VEGF, vascular endothelial growth factor (reproduced from Lories and Luyten5).
Joint diseases—osteoarthritis
Synovial joints can be affected by a wide array of clinical problems.1,2,3 Trauma is common and may require surgical intervention. Patients may fully recover from joint injuries but may also experience long-term consequences, as a damaged joint likely has a suboptimal function, increasing the risk for accelerated tear and wear. In addition, different autoimmune and autoinflammatory diseases primarily affect the synovial joints. The specific reason why autoimmune phenomena have a preference to involve the joint is still unclear. Biomechanical factors leading to local cell stress as well as the immune-privileged microenvironment within the synovium with the presence of tissue-resident macrophages have been suggested to have a role.4
Osteoarthritis is the most common joint disease. This chronic condition has long time been considered as degenerative joint disease but its pathophysiology is undoubtedly much more complex than a simple wear and tear or aging phenomenon.1,4,5 Osteoarthritis can be considered a common denominator for a wide range of diseases, all affecting joints and leading to progressive damage, pain and loss of joint function. Typical features include loss of articular cartilage exemplified on X-rays by loss of joint space width, subchondral bone sclerosis and cyst formation, bony outgrowths at the joint margins called osteophytes and synovitis, all contributing to signs and symptoms. Osteoarthritis may involve a single large or small joint, a restricted number of joints but can also be polyarticular, including both the peripheral and axial skeleton. The diseases have a complex pathogenesis to which both genetic and acquired factors contribute.6 The acquired factors are commonly associated with abnormal loads and strains on the joint, for instance owing to disproportionate weight or owing to excessive labor or recreational physical activity. Genetic factors likely contribute by affecting the intrinsic quality of the articular cartilage and bone or by influencing anatomic factors such as the alignment or shape of the joint.6 Normal loads in a joint with an abnormal anatomy as well as abnormal and repetitive loads in a structurally normal joint may lead to osteoarthritis.5
Despite its high and increasing prevalence in society, therapeutic options for osteoarthritis patients are still limited.1 Beyond lifestyle measures, such as weight reduction and specific exercise programs, most patients are dependent on painkillers and nonsteroidal antiinflammatory drugs, as disease or structure modifying drugs preserving or restoring joint structure and function are not yet available. Food supplements based on cartilage extracellular matrix components such as glucosamine and chondroitine are widely used but their specific place in osteoarthritis treatment remains heavily debated despite some encouraging clinical trial results.7,8,9 In addition to visco-supplementation in selected patients, severe cases require surgical interventions such as load-changing osteotomy or joint replacement.1 In this review, we discuss some of the recent developments in osteoarthritis research, their translational importance and further directions that are emerging from the perspective that the synovial joint is an organ in which the different tissues functionally cooperate under physiological circumstances, and in which they are all affected and eventually lead to progression of disease. In this context, developmental signaling pathways critical for tissue growth and differentiation, likely, also have critical roles in postnatal homeostasis and may be targets for the development of regenerative strategies.
Progress in genetics identifies a key role for developmental signaling pathways in osteoarthritis
The articular cartilage is a specialized and unique tissue. It is composed of the articular chondrocytes embedded in a specific extracellular matrix (Figure 1). This matrix contains type-II collagen and large sulfated proteoglycans such as aggrecan. The specific properties of these extracellular matrix molecules provide the articular cartilage its biomechanical characteristics.10 The collagen fibers are responsible for resistance against tensile stretch, whereas the abundant negative charges on the macro-molecular proteoglycans attract water molecules that can be shifted within the tissue, giving its capacity to deform and adapt upon loading. The articular chondrocytes synthesize their own matrix and have distinct characteristics according to the layer of the cartilage where they reside. The superficial or tangential layer is cell-rich and the cells have a flattened shape. Their matrix consists mainly of type-II collagen with only a small amount of proteoglycans. These superficial chondrocytes also produce lubricin and thereby contribute to boundary lubrication. Underneath this layer, round-shaped cells are found with abundant matrix surrounding them. This intermediate zone is rich in proteoglycans. In the deeper layer, chondrocytes are larger to become hypertrophic-like chondrocytes embedded in the lowest calcified layer. The frontier between uncalcified and calcified cartilage is recognized under the microscope as the tidemark (on hematoxilin–eosin staining). In general, chondrocytes are considered to have low metabolic activity, maintaining synthesis of the proteoglycans and have a limited regeneration potential.
The articular cartilage is distinct from other types of cartilage tissue found in the body during development and growth.11 In contrast to the temporary cartilage templates essential in development and in the growth plate, articular chondrocytes do not undergo terminal differentiation and will not calcify their extracellular matrix, except in the deeper layers that form the interface with the subchondral bone. From the developmental perspective, joint formation is a specific process in skeletal development.11 The prospective joint is first recognized as the joint interzone, a layer of cells that does not undergo the classical chondrogenic differentiation but that will form the joint cavity and the different structures of the joint. These joint interzones are involved in the differentiation of the articular cartilage although the latter's specific development and growth is probably occurring at the interphase between the joint cavity and the bone in the postnatal phase. Signaling pathways associated with skeletal and joint development likely contribute to postnatal joint homeostasis and have a role in joint diseases such as osteoarthritis as outlined below.4,5
Genetic research into susceptibility genes for osteoarthritis has been a challenging exercise, which can be explained by several factors, including difficulties in defining phenotypes, the time-dependent evolution of the disease and its multifactorial nature, characterized by complex interactions between genetic and acquired or environmental factors.12 Although some associations have been debated as they are not consistent among different cohorts, genes associated with developmental signaling cascades such as bone morphogenetic proteins (BMP) and wingless-type (Wnt) signaling have been identified as susceptibility factors (Table 1).6 Among these, frizzled-related protein (FRZB), a secreted Wnt modulator, and growth and differentiation factor-5 (GDF5), a growth factor belonging to the BMP family appear of specific relevance. Other examples are Asporin and SMAD3, molecules linked to the transforming growth factor-beta (TGFβ) signaling cascade.13,14 Coincidently FRZB and GDF5 proteins were originally isolated and identified from a chondrogenic extract of bovine articular cartilage.15,16 Gdf5 is expressed in the developing joint interzone and therefore associated with joint development. Its postnatal expression is not limited to the articular cartilage but it is also found in the synovium and ligaments. Spontaneous loss-of-function mutations in mice result in shortened limbs, indicating problems in the endochondral bone formation cascade, and in multiple joint fusions suggesting effects on joint formation itself.17 These mice also lack a number of ligaments, again highlighting the role of GDF5 in the development of joint as an organ. Loss of function mutations in humans (genetic syndromes) show similar skeletal dysplasia phenotypes.18,19,20 Variation in the GDF5 gene is associated with osteoarthritis and the risk allele is associated with lower expression levels.21
Table 1. Osteoarthritis (OA) susceptibility genes linked to developmental signaling cascades.
| Pathway | Gene | Type of study | Summary |
|---|---|---|---|
| Transforming growth factor (TGF)-β/bone morphogenetic protein (BMP) | GDF5 | Candidate gene study and meta-analysis21,33 | Chondrogenic molecule with key roles in joint development—low-expression-level allele associated with OA |
| Asporin | Genome-wide association study13 | Extracellular matrix molecule inhibiting TGFβ-receptor association | |
| SMAD3 | Candidate gene study14 | Intracellular signaling molecule in the TGFβ cascade | |
| Wingless type (Wnt) | DOT1L | Genome-wide association study for cartilage thickness36 | Histone-methyltransferase associated with Wnt/βcatenin/TCF signaling |
| |
FRZB |
Sib-pair genome scan and candidate gene study—not confirmed in meta-analysis33,34,35 |
Extracellular Wnt modulator—Frzb−/− mice show increased cartilage damage in induced OA models.35 |
Abbreviations: BMP, bone morphogenetic proteins; FRZB, frizzled-related protein; GDF5, growth and differentiation factor-5; OA, Osteoarthritis; TGF, Tranforming growth factor; Wnt, Wingless type.
Somewhat surprisingly, osteoarthritis susceptibility in Gdf5 haploinsufficient mice could not be linked to functional failure of the articular cartilage but appeared associated with joint stability and abnormalities of the underlying bone.22 Nevertheless, modulation of the GDF5 cascade and extension of BMP signaling represents a therapeutic target to stimulate chondrocyte anabolism and repair with proof of principle studies in animal models available and trials in patients underway.23,24,25 As BMPs are powerful morphogens, such approaches are not straightforward and ectopic bone formation should be contained and avoided, in particular in the synovium, if local therapy is considered. In general, safety remains an issue, when modulating these major signaling pathways that are also involved in many postnatal biological processes. This is exemplified by recent safety warnings with regard to the use of the BMP2 device for spine fusion.26
FRZB was originally identified as a Wnt antagonist27,28 although recent data suggest that it is better defined as a Wnt signaling modulator.29 The Wnt pathway is involved in many developmental processes going from axis formation to the specific differentiation of cells and tissues into organs. Some Wnts such as Wnt9a are associated with the joint interzone and its expression shows an overlap with Gdf5.30 Evidence from a series of elegant mouse model studies suggest that both lack and excess of Wnt signaling within the articular cartilage leads to a disruption of the stable phenotype of the articular chondrocytes and accelerated spontaneous osteoarthritis.31,32 Polymorphisms in FRZB have been associated with hip osteoarthritis although the effect is not consistent over different groups.33,34 Frzb−/− mice show increased susceptibility to cartilage damage in induced models of osteoarthritis.35 We could associate this observation with increased Wnt signaling and destructive enzyme expression within the articular cartilage but also with abnormalities in bone. Further evidence for a role of Wnt signaling in joint formation and disease is provided by the association of polymorphisms in the DOT1L gene with hip cartilage thickness and osteoarthritis.36 DOT1L is a methyltransferase involved in the beta-catenin-TCF Wnt transcription complex. Knockdown of Dot1l in developing chondrocytes inhibits chondrogenic differentiation including expression of essential extracellular matrix molecules such as type-II collagen and aggrecan.36
The articular chondrocytes in osteoarthritis: embarking on a suicide mission?
The first sign of osteoarthritis at the cellular and molecular level within the articular cartilage appears to be a shift in the quiescent state of the articular chondrocytes. Different factors, for example, repetitive loading or acute trauma and inflammation, trigger the articular chondrocytes to become active (Figure 1). The cells start to produce additional extracellular matrix molecules. However, at the same time, the chondrocytes also produce proinflammatory cytokines such as interleukin-1 and tissue destructive enzymes such as matrix metalloproteinases (MMPs) and ADAMTS (A Disintegrin And Metalloproteinase with Thrombospondin Motifs). In the short term, anabolic signals are apparently able to compete with the destructive cascades but in the long term these protective mechanisms fail and progressive loss of cartilage with cell death and depletion of the extracellular matrix evolves.
Much research has been done to identify the enzymes responsible for tissue destruction and the factors that upregulate them. Obviously, enzymes and cytokines may represent relatively straightforward therapeutic targets. With regard to cartilage destruction, a critical role for ADAMTS enzymes has been discovered in mice.37,38,39 ADAMTS4 and ADAMTS5 can act as aggrecanases breaking down the complex structure of the proteoglycans. Collagenases (matrix metalloproteinases (MMP)-13 and 1) can break down type-II collagen. The collagen structure appears to remain protected until proteoglycan breakdown has occurred. However, once the fibrous network is also degraded, irreversible cartilage damage is likely.10 Proof of principle studies in rodent models have shown that ADAMTS and MMPs can be successfully targeted. Nevertheless, the translation of these concepts into successful human clinical trials and market approval for drugs has been proven difficult as the effect size was not large or unexpected systemic toxicity excluded further development of the drugs.38
As mentioned above, different factors can contribute to the onset of osteoarthritis. Some aspects of mechanical stress and inflammation are discussed below. In addition, recent evidence implicates disintegration of the pericellular matrix as a critical feature in the onset of cartilage damage.40 This specific matrix surrounding the chondrocytes appears to protect the cells from interacting with molecules in the interterritorial matrix. The serine proteinase high-temperature requirement A1 has been attributed a critical role in this process. Such interactions include activation of the discoidin domain receptor 2 with type-II collagen.40 Type-II collagen but also fibronectin fragments may trigger local MMP13 upregulation.41
Loss of the chondrocyte's stable phenotype—a role for damage and aging
One particular feature of osteoarthritis progression is the loss of the articular chondrocyte's stable phenotype.1,5,10 Some of the cells, in particular in the deeper layers of the cartilage, start expressing markers of chondrocyte hypertrophy including expression of collagen type X. The concurrent change in matrix composition and increased calcification is likely changing the biomechanical properties of the tissue and further contributing to its progressive destruction. From the pathology perspective, this can also be recognized as advancement of the tidemark and increased vascularization, most likely secondary to increased vascular endothelial growth factor production.
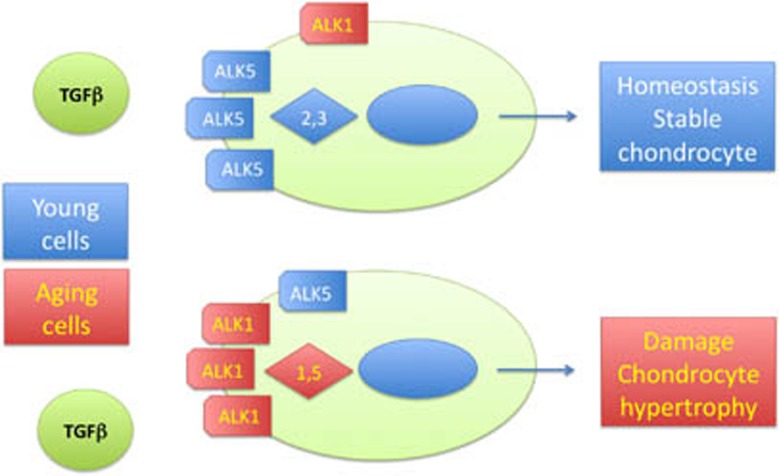
An intriguing aspect of cartilage aging and osteoarthritis progression has recently been described.42 TGFβ has an important role in cartilage homeostasis and normally signals to the activin-like kinase 5 (ALK5) receptor leading to intracellular activation of smad2 and 3 molecules (Figure 2). With aging, the ratio between the ALK5 and alternative ALK1 receptor appears to change, leading to preferential activation of smad1 and 5 molecules within the cell by TGFβ–ALK1 binding at the cell surface.42 This specific cascade is associated with increased MMP13 production and with terminal differentiation of the chondrocytes, two features that may contribute to osteoarthritis.
Figure 2. Transforming growth factor (TGF)β in young and aging cartilage.
The ratio between activin-like kinase 5 (ALK5) and 1 receptors is shifted as the cartilage cells age leading to increased smad1,5 signaling instead of smad 2,3 signaling. Activation of the smad1, 5 cascade triggers production of matrix metalloproteinase 13 and chondrocytes hypertrophy.
Other features associated with cartilage damage and aging are the upregulation of inflammatory and damage sensing receptors such as toll-like receptors,43 and receptor for advanced glycation end products.44 Activation of these receptors by cartilage damage as well as by proinflammatory cytokines contributes to the complex detrimental cascade of cartilage damage. As the disease progresses, these different cascades and events appear to function as positive feedback mechanisms with an avalanche-like effect on the destruction of the joints, the associated loss of function and with an increase in pain.
The articular cartilage and the underlying bone: a functional relationship determining the outcome of osteoarthritis
The role of the subchondral bone, the part of the bone that forms the interface with the articular cartilage, in the onset and progression of disease has been long debated.5 Most researchers will now defend an integrated view in which both tissues have an interactive role in pathology. Subchondral bone sclerosis and activation of local remodeling is among the earliest signs of the disease and can be recognized in some individuals on X-rays before cartilage damage or osteophyte formation becomes apparent. Although the bone metabolism is intensified, the anabolic efforts do not necessarily result in stronger bone as mineralization, an essential feature to gain bone strength, is often not capable of maintaining pace with the formation of new bone matrix.5 Similarly, local changes in bone architecture may weaken rather than strengthen the bone. Under all circumstances, it appears likely that changes at the bone–cartilage interface result in altered loading within both cartilage and bone, thereby affecting the homeostasis of both tissues and contributing to the disease process. From this perspective, both stronger bones, providing increased resistance to transfer of loads, and weaker bones, providing less support, are likely to convey susceptibility to osteoarthritis development.5 Taking into account the functional interaction between the cartilage and the subchondral bone, this could also be considered a therapeutic target. Indeed, emerging evidence from animal models but also clinical trials in humans supports the idea that regulation of the bone remodeling cycle could be beneficial in the treatment of osteoarthritis.45,46
Inflammation, an old player back into the game
Joint inflammation is the primary phenomenon in diseases such as rheumatoid arthritis and spondyloarthritis. From the clinical perspective of the rheumatologist, inflammation in patients with osteoarthritis tends to be less severe and more easily manageable with nonsteroidal antiinflammatory drugs or arthrocentesis followed by local steroid injection. Inflammation was therefore long time considered to be a bystander phenomenon. Recent evidence is challenging this perspective in two ways.47 Inflammation likely contributes to progressive joint destruction by boosting the expression of tissue destructive enzymes. In addition inflammation is proposed as a primary driving force for the progression of disease.
Indeed, recent studies have highlighted that some of the inflammatory and immune responses found in the synovium of patients with osteoarthritis may be more specific than commonly anticipated.48,49 In addition, the synovial fat tissue or fat pad is also suggested to contribute to the disease processes with the production of cytokines and adipokines.50 These data highlight the potential contribution of inflammation not only to signs and symptoms but also to the pathological processes. Whether antiinflammatory drugs should therefore be preferred over conventional pain killers remains an open question that is often debated.51 The consequences of inhibiting cyclooxygenase enzymes with nonsteroidal antiinflammatory have been extensively studied in both cartilage and bone biology. However, potential effects on structural progression of osteoarthritis have been contradictory and current concepts remain largely based on in vitro experiments. This is further complicated by the hypothesis that more efficient pain control could result in increased joint use and strain in vivo, thereby accelerating disease progression. Of interest, specific targeting of cytokines using biologics has been evaluated in a trial in patients with hand osteoarthritis.52 This subtype is typically associated with relatively severe inflammation and therefore an obvious candidate to perform these studies. The emerging data do not suggest that specific targeting of tumor necrosis factor has major effects. Given the high costs of biologics and the potential side effects, it does not seem likely that these drugs will be introduced for large groups of patients with osteoarthritis although they may be beneficial in specific subsets.
The road ahead for therapies in osteoarthritis
Basic and translational research efforts in the field have suggested different therapeutic targets in the last decade. As outlined above, these targets face a difficult road from discovery toward application. The osteoarthritic diseases are very heterogeneous and different processes are likely to have a leading role in individual patients and joints. Moreover, osteoarthritis is often a slow process gradually evolving over years. Treatment strategies will likely vary over time. As patients, in particular elderly individuals, may have distinct and multiple comorbidities, drug treatments may not be sustainable over a longer time period. This clearly demonstrates the clinical need for personalized medicine approaches, individual risk factor assessment and prediction models. Identification of patients at risk for rapid progression is likely to become an important filter in the daily management strategies. The perspective of treatment options should also not be limited to drug interventions. Specific physiotherapy and rehabilitation programs, lifestyle measures and further refinement of surgical interventions will continue to contribute to the management of osteoarthritis patients, in particular in high-risk individuals. Finally, in the emerging era of tissue engineering, early stages of the disease, in particular posttraumatic cartilage injuries, may be good candidates for cell-based therapies with or without specific growth factors and carrier strategies. The further design of regenerative medicine approaches will likely be at the crossroads of interdisciplinary medicine combining biological and engineering paradigms.53,54 The increasing evidence that signaling pathways critical in skeletal and joint development are also at the center of homeostatic, destructive and regenerative processes in the joint with osteoarthritis identifies these pathways as targets that could bridge the gap between development and regeneration. The recent identification of a small molecule named Kartogenin demonstrates that chemical stimulation of in vivo cartilage differentiation and repair may become feasible.55
Conclusion
Significant progress has been made in osteoarthritis research (Table 2). Although most research has focused on the biology and pathology of the articular cartilage, novel insights support the concept of a joint organ of which all tissues are involved in homeostatic and pathological processes. Signaling cascades associated with bone and joint development also appear critical in adult life as supported by genetic and preclinical data. Nevertheless, the development of specific, long-lasting and effective therapies remains a major challenge that will likely benefit from a more multidisciplinary and systems biology driven approach, bridging some gaps between biological and tissue engineering concepts.53,54
Table 2. Key messages on osteoarthritis.
| Key message 1: | Joint are specialized skeletal structures that connect the bones and allow movement within specific ranges. Their optimal function requires cooperation and homeostasis of the different tissues that make up its structure. |
| Key message 2: | Osteoarthritis is not the normal aging of the joint but a complex disease to which both genetic and acquired factors contribute. |
| Key message 3: | Current treatment options are limited to symptom control or surgical intervention. Novel targets include developmental signaling cascades and cell therapies. |
| Key message 4: | The development of targeted therapies faces many challenges including the need for long-term treatment with low risk of side effects. |
Footnotes
The authors declare no conflict of interest.
References
- Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet 2011;377:2115–2126. [DOI] [PubMed] [Google Scholar]
- Dougados M, Baeten D. Spondyloarthritis. Lancet 2011;377:2127–2137. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–2219. [DOI] [PubMed] [Google Scholar]
- Lories R. The balance of tissue repair and remodeling in chronic arthritis. Nat Rev Rheumatol 2011;7:700–707. [DOI] [PubMed] [Google Scholar]
- Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol 2011;7:43–49. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Spector TD. Genetic epidemiology of hip and knee osteoarthritis. Nat Rev Rheumatol 2011;7:23–32. [DOI] [PubMed] [Google Scholar]
- Wandel S, Juni P, Tendal B, Nuesch E, Villiger PM, Welton NJ et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010;341:c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Bruyere O et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 2001;357:251–256. [DOI] [PubMed] [Google Scholar]
- Clegg DO, Reda DJ, Harris CL, Klein MA, O'Dell JR, Hooper MM et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 2006;354:795–808. [DOI] [PubMed] [Google Scholar]
- Heinegard D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol 2011;7:50–56. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Bhattaram P. Vertebrate skeletogenesis. Curr Top Dev Biol 2010;90:291–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhof HJ, Meulenbelt I, Akune T, Arden NK, Aromaa A, Bierma-Zeinstra SM et al. Recommendations for standardization and phenotype definitions in genetic studies of osteoarthritis: the TREAT-OA consortium. Osteoarthritis Cartilage 2011;19:254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizawa H, Kou I, Iida A, Sudo A, Miyamoto Y, Fukuda A et al. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat Genet 2005;37:138–144. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Spector TD, Tamm A, Kisand K, Doherty SA, Dennison EM et al. Genetic variation in the SMAD3 gene is associated with hip and knee osteoarthritis. Arthritis Rheum 2010;62:2347–2352. [DOI] [PubMed] [Google Scholar]
- Chang SC, Hoang B, Thomas JT, Vukicevic S, Luyten FP, Ryba NJ et al. Cartilage-derived morphogenetic proteins. New members of the transforming growth factor-beta superfamily predominantly expressed in long bones during human embryonic development. J Biol Chem 1994;269:28227–28234. [PubMed] [Google Scholar]
- Hoang B, Moos M Jr, Vukicevic S, Luyten FP. Primary structure and tissue distribution of FRZB, a novel protein related to Drosophila frizzled, suggest a role in skeletal morphogenesis. J Biol Chem 1996;271:26131–26137. [DOI] [PubMed] [Google Scholar]
- Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee SJ. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature 1994;368:639–643. [DOI] [PubMed] [Google Scholar]
- Thomas JT, Kilpatrick MW, Lin K, Erlacher L, Lembessis P, Costa T et al. Disruption of human limb morphogenesis by a dominant negative mutation in CDMP1. Nat Genet 1997;17:58–64. [DOI] [PubMed] [Google Scholar]
- Polinkovsky A, Robin NH, Thomas JT, Irons M, Lynn A, Goodman FR et al. Mutations in CDMP1 cause autosomal dominant brachydactyly type C. Nat Genet 1997;17:18–19. [DOI] [PubMed] [Google Scholar]
- Thomas JT, Lin K, Nandedkar M, Camargo M, Cervenka J, Luyten FP. A human chondrodysplasia due to a mutation in a TGF-beta superfamily member. Nat Genet 1996;12:315–317. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Mabuchi A, Shi D, Kubo T, Takatori Y, Saito S et al. A functional polymorphism in the 5′ UTR of GDF5 is associated with susceptibility to osteoarthritis. Nat Genet 2007;39:529–533. [DOI] [PubMed] [Google Scholar]
- Daans M, Luyten FP, Lories RJ. GDF5 deficiency in mice is associated with instability-driven joint damage, gait and subchondral bone changes. Ann Rheum Dis 2011;70:208–213. [DOI] [PubMed] [Google Scholar]
- Boon MR, van der Horst G, van der Pluijm G, Tamsma JT, Smit JW, Rensen PC. Bone morphogenetic protein 7: a broad-spectrum growth factor with multiple target therapeutic potency. Cytokine Growth Factor Rev 2011;22:221–229. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Pike MC, Jonas BL, Kissin E, Krop J, McAlindon T. Phase 1 safety and tolerability study of BMP-7 in symptomatic knee osteoarthritis. BMC Musculoskelet Disord 2010;11:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Muneta T, Takahashi T, Ju YJ, Tsuji K, Sekiya I. Intra-articular injections of bone morphogenetic protein-7 retard progression of existing cartilage degeneration. J Orthop Res 2010;28:1502–1506. [DOI] [PubMed] [Google Scholar]
- Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 2011;11:471–491. [DOI] [PubMed] [Google Scholar]
- Lin K, Wang S, Julius MA, Kitajewski J, Moos M Jr, Luyten FP. The cysteine-rich frizzled domain of Frzb-1 is required and sufficient for modulation of Wnt signaling. Proc Natl Acad Sci USA 1997;94:11196–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Krinks M, Lin K, Luyten FP, Moos M Jr. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell 1997;88:757–766. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci 2008;121:737–746. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell 2001;104:341–351. [DOI] [PubMed] [Google Scholar]
- Zhu M, Chen M, Zuscik M, Wu Q, Wang YJ, Rosier RN et al. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum 2008;58:2053–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res 2009;24:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelou E, Chapman K, Meulenbelt I, Karassa FB, Loughlin J, Carr A et al. Large-scale analysis of association between GDF5 and FRZB variants and osteoarthritis of the hip, knee, and hand. Arthritis Rheum 2009;60:1710–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin J, Dowling B, Chapman K, Marcelline L, Mustafa Z, Southam L et al. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci USA 2004;101:9757–9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lories RJ, Peeters J, Bakker A, Tylzanowski P, Derese I, Schrooten J et al. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum 2007;56:4095–4103. [DOI] [PubMed] [Google Scholar]
- Castano-Betancourt MC, Cailotto F, Kerkhof H, Cornelis FM, Doherty S, Hart D et al. Genome-wide association and functional studies identify the DOT1L gene to be involved in cartilage thickness and osteoarthritis. Proc Natl Acad Sci USA 2012;109:8218–8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 2005;434:644–648. [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Malfait F, Barve RA, Shieh HS, Malfait AM. A review of the ADAMTS family, pharmaceutical targets of the future. Curr Pharm Des 2009;15:2359–2374. [DOI] [PubMed] [Google Scholar]
- Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature 2005;434:648–652. [DOI] [PubMed] [Google Scholar]
- Xu L, Polur I, Servais JM, Hsieh S, Lee PL, Goldring MB et al. Intact pericellular matrix of articular cartilage is required for unactivated discoidin domain receptor 2 in the mouse model. Am J Pathol 2011;179:1338–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury TT, Schulz RM, Rai SS, Thuemmler CB, Wuestneck N, Bader A et al. Biomechanical modulation of collagen fragment-induced anabolic and catabolic activities in chondrocyte/agarose constructs. Arthritis Res Ther 2010;12:R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kraan PM, Blaney Davidson EN, van den Berg WB. A role for age-related changes in TGF beta signaling in aberrant chondrocyte differentiation and osteoarthritis. Arthritis Res Ther 2010;12:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelbergen RF, Blom AB, van den Bosch MH, Sloetjes A, Abdollahi-Roodsaz S, Schreurs BW et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on toll-like receptor 4. Arthritis Rheum 2012;64:1477–1487. [DOI] [PubMed] [Google Scholar]
- Steenvoorden MM, Huizinga TW, Verzijl N, Bank RA, Ronday HK, Luning HA et al. Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum 2006;54:253–263. [DOI] [PubMed] [Google Scholar]
- Shirai T, Kobayashi M, Nishitani K, Satake T, Kuroki H, Nakagawa Y et al. Chondroprotective effect of alendronate in a rabbit model of osteoarthritis. J Orthop Res 2011;29:1572–1577. [DOI] [PubMed] [Google Scholar]
- Cooper C, Reginster JY, Chapurlat R, Christiansen C, Genant H, Bellamy N et al. Efficacy and safety of oral strontium ranelate for the treatment of knee osteoarthritis: rationale and design of randomised, double-blind, placebo-controlled trial. Curr Med Res Opin 2012;28:231–239. [DOI] [PubMed] [Google Scholar]
- Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol 2011;23:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanzello CR, McKeon B, Swaim BH, DiCarlo E, Asomugha EU, Kanda V et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum 2011;63:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanzello CR, Umoh E, Pessler F, Diaz-Torne C, Miles T, Dicarlo E et al. Local cytokine profiles in knee osteoarthritis: elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthritis Cartilage 2009;17:1040–1048. [DOI] [PubMed] [Google Scholar]
- Klein-Wieringa IR, Kloppenburg M, Bastiaansen-Jenniskens YM, Yusuf E, Kwekkeboom JC, El-Bannoudi H et al. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann Rheum Dis 2011;70:851–857. [DOI] [PubMed] [Google Scholar]
- Lories RJ. Changing the outcome of osteoarthritis: still a challenge for cyclooxygenase 2 inhibitors. Arthritis Rheum 2012;64:37–39. [DOI] [PubMed] [Google Scholar]
- Verbruggen G, Wittoek R, Cruyssen BV, Elewaut D. Tumour necrosis factor blockade for the treatment of erosive osteoarthritis of the interphalangeal finger joints: a double blind, randomised trial on structure modification. Ann Rheum Dis 2011;71:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenas P, Moos M, Luyten FP. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part II: from genes to networks: tissue engineering from the viewpoint of systems biology and network science. Tissue Eng Part B Rev 2009;15:395–422. [DOI] [PubMed] [Google Scholar]
- Lenas P, Moos M, Luyten FP. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part I: from three-dimensional cell growth to biomimetics of in vivo development. Tissue Eng Part B Rev 2009;15:381–394. [DOI] [PubMed] [Google Scholar]
- Johnson K, Zhu S, Tremblay MS, Payette JN, Wang J, Bouchez LC et al. A stem cell-based approach to cartilage repair. Science 2012;336:717–721. [DOI] [PubMed] [Google Scholar]