Abstract
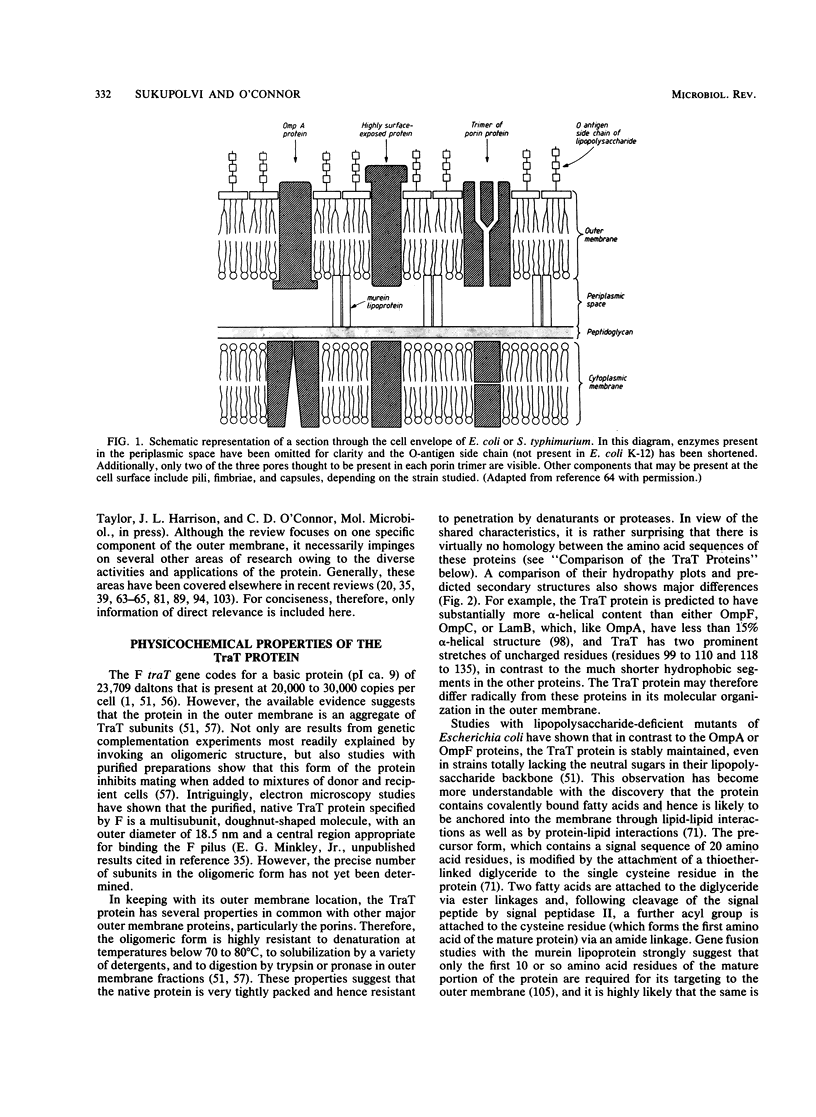
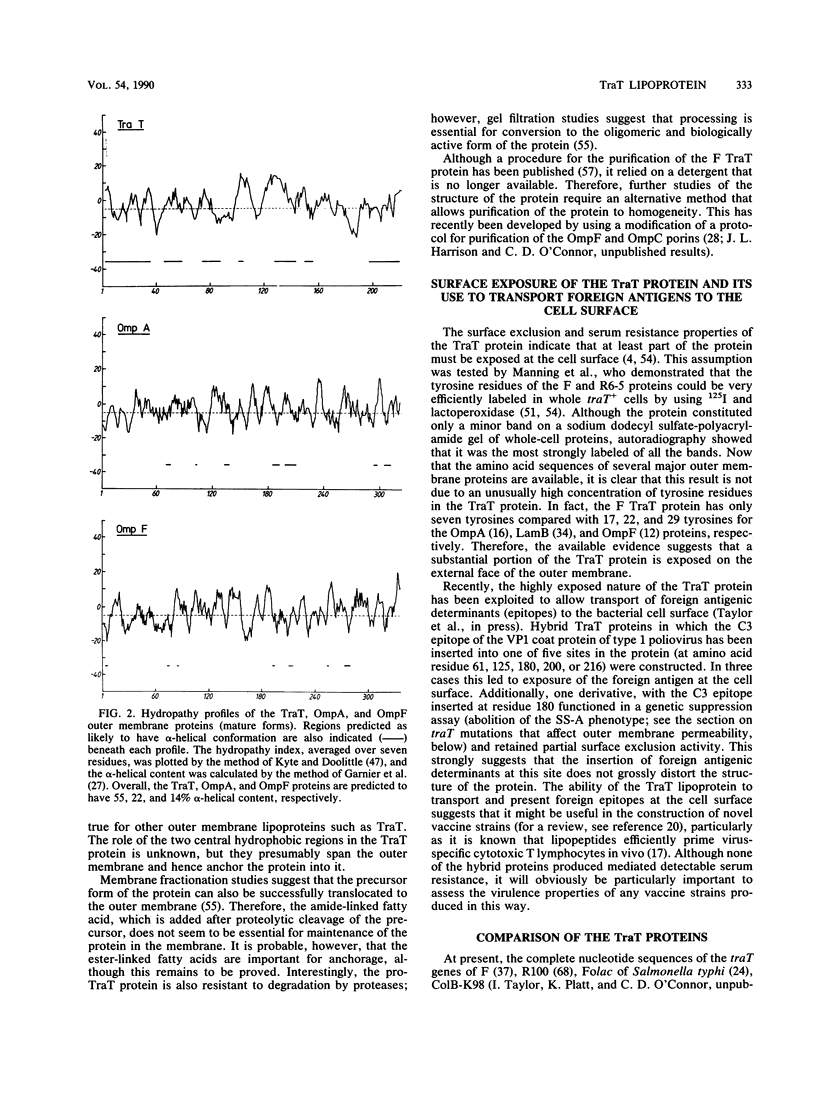
The TraT protein is a cell-surface-exposed, outer membrane lipoprotein specified by large, usually conjugative, F-like plasmids. Two biological activities have been associated with the protein: (i) prevention of self-mating of cells carrying identical or closely related conjugative plasmids, by blocking the formation of stable mating aggregates; and (ii) resistance to the bactericidal activities of serum, possibly by inhibiting the correct assembly or efficient functioning of the terminal membrane attack complex of complement. The protein therefore interacts not only with components of the outer membrane but also with specific external agents. In conjugative plasmids the traT gene lies within the region necessary for the conjugal transfer of DNA (tra), although its expression is not necessarily dependent on the expression of other tra genes. Recently, however, the gene has been discovered in isolation from other tra genes in nonconjugative virulence-associated plasmids, providing further evidence that the TraT protein may have a role in pathogenesis. The nucleotide sequences of several traT genes have been determined, and comparison of the corresponding amino acid sequences suggests that a central region of five amino acid residues flanked by hydrophobic domains determines the specificity of the protein in surface exclusion. Additionally, studies of mutants with different amino acid alterations within the hydrophobic domains have shown that insertion of charged residues disrupts normal outer membrane integrity. This review considers our current knowledge of the distribution, structure, and biological role(s) of the protein. Recent applications of the protein in studies of the unusual permeability properties of the outer membrane and for the transport of foreign antigenic determinants to the bacterial cell surface are also discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Kennedy N., Skurray R. Cell--cell interactions in conjugating Escherichia coli: role of traT protein in surface exclusion. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5104–5108. doi: 10.1073/pnas.74.11.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Manning P. A., Kusecek B., Schwuchow S., Willetts N. A genetic analysis of F sex factor cistrons needed for surface exclusion in Escherichia coli. J Mol Biol. 1980 Apr 25;138(4):779–795. doi: 10.1016/0022-2836(80)90065-0. [DOI] [PubMed] [Google Scholar]
- Agüero M. E., Aron L., DeLuca A. G., Timmis K. N., Cabello F. C. A plasmid-encoded outer membrane protein, TraT, enhances resistance of Escherichia coli to phagocytosis. Infect Immun. 1984 Dec;46(3):740–746. doi: 10.1128/iai.46.3.740-746.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balligand G., Laroche Y., Cornelis G. Genetic analysis of virulence plasmid from a serogroup 9 Yersinia enterocolitica strain: role of outer membrane protein P1 in resistance to human serum and autoagglutination. Infect Immun. 1985 Jun;48(3):782–786. doi: 10.1128/iai.48.3.782-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns M. M., Davies D. L., Hardy K. G. Cloned fragments of the plasmid ColV,I-K94 specifying virulence and serum resistance. Nature. 1979 Jun 28;279(5716):778–781. doi: 10.1038/279778a0. [DOI] [PubMed] [Google Scholar]
- Binns M. M., Mayden J., Levine R. P. Further characterization of complement resistance conferred on Escherichia coli by the plasmid genes traT of R100 and iss of ColV,I-K94. Infect Immun. 1982 Feb;35(2):654–659. doi: 10.1128/iai.35.2.654-659.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitter-Suermann D., Peters H., Jürs M., Nehrbass R., Montenegro M., Timmis K. N. Monoclonal antibody detection of IncF group plasmid-encoded TraT protein in clinical isolates of Escherichia coli. Infect Immun. 1984 Nov;46(2):308–313. doi: 10.1128/iai.46.2.308-313.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The properties of sex pili, the viral nature of "conjugal" genetic transfer systems, and some possible approaches to the control of bacterial drug resistance. CRC Crit Rev Microbiol. 1971 May;1(1):105–160. doi: 10.3109/10408417109104479. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Cheah K. C., Ray A., Skurray R. Expression of F plasmid traT: independence of traY----Z promoter and traJ control. Plasmid. 1986 Sep;16(2):101–107. doi: 10.1016/0147-619x(86)90068-5. [DOI] [PubMed] [Google Scholar]
- Chen R., Schmidmayr W., Krämer C., Chen-Schmeisser U., Henning U. Primary structure of major outer membrane protein II (ompA protein) of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4592–4596. doi: 10.1073/pnas.77.8.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of beta-turns. Biophys J. 1979 Jun;26(3):367–383. doi: 10.1016/S0006-3495(79)85259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuba P. J., Leon M. A., Banerjee A., Palchaudhuri S. Cloning and DNA sequence of plasmid determinant iss, coding for increased serum survival and surface exclusion, which has homology with lambda DNA. Mol Gen Genet. 1989 Apr;216(2-3):287–292. doi: 10.1007/BF00334367. [DOI] [PubMed] [Google Scholar]
- Clément J. M., Hofnung M. Gene sequence of the lambda receptor, an outer membrane protein of E. coli K12. Cell. 1981 Dec;27(3 Pt 2):507–514. doi: 10.1016/0092-8674(81)90392-5. [DOI] [PubMed] [Google Scholar]
- Deres K., Schild H., Wiesmüller K. H., Jung G., Rammensee H. G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989 Nov 30;342(6249):561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- Donaldson D. M., Roberts R. R., Larsen H. S., Tew J. G. Interrelationship between serum beta-lysin, lysozyme, and the antibody-complement system in killing Escherichia coli. Infect Immun. 1974 Sep;10(3):657–666. doi: 10.1128/iai.10.3.657-666.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman C. J., Ni Bhriain N., Higgins C. F. DNA supercoiling and environmental regulation of virulence gene expression in Shigella flexneri. Nature. 1990 Apr 19;344(6268):789–792. doi: 10.1038/344789a0. [DOI] [PubMed] [Google Scholar]
- Dougan G., Hormaeche C. E., Maskell D. J. Live oral Salmonella vaccines: potential use of attenuated strains as carriers of heterologous antigens to the immune system. Parasite Immunol. 1987 Mar;9(2):151–160. doi: 10.1111/j.1365-3024.1987.tb00496.x. [DOI] [PubMed] [Google Scholar]
- Falkow S., Baron L. S. EPISOMIC ELEMENT IN A STRAIN OF SALMONELLA TYPHOSA. J Bacteriol. 1962 Sep;84(3):581–589. doi: 10.1128/jb.84.3.581-589.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietta A., Romero E., Siccardi A. G. Effect of some R factors on the sensitivity of rough Enterobacteriaceae to human serum. Infect Immun. 1977 Nov;18(2):278–282. doi: 10.1128/iai.18.2.278-282.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Paranchych W. Nucleotide sequence of the surface exclusion genes traS and traT from the IncF0 lac plasmid pED208. J Bacteriol. 1986 Jun;166(3):713–721. doi: 10.1128/jb.166.3.713-721.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. A., Allen H., Burkly L. C., Sherman D. H., Waneck G. L., Widera G. Molecular biology of the H-2 histocompatibility complex. Science. 1986 Jul 25;233(4762):437–443. doi: 10.1126/science.3726537. [DOI] [PubMed] [Google Scholar]
- Frost L. S., Finlay B. B., Opgenorth A., Paranchych W., Lee J. S. Characterization and sequence analysis of pilin from F-like plasmids. J Bacteriol. 1985 Dec;164(3):1238–1247. doi: 10.1128/jb.164.3.1238-1247.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gehring K. B., Nikaido H. Existence and purification of porin heterotrimers of Escherichia coli K12 OmpC, OmpF, and PhoE proteins. J Biol Chem. 1989 Feb 15;264(5):2810–2815. [PubMed] [Google Scholar]
- Gulig P. A., Curtiss R., 3rd Plasmid-associated virulence of Salmonella typhimurium. Infect Immun. 1987 Dec;55(12):2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett J., Kotlarski I., Mathan V., Francki K., Rowley D. The colonization of Peyer's patches by a strain of Salmonella typhimurium cured of the cryptic plasmid. J Infect Dis. 1986 Jun;153(6):1119–1125. doi: 10.1093/infdis/153.6.1119. [DOI] [PubMed] [Google Scholar]
- Ham L. M., Cram D., Skurray R. Transcriptional analysis of the F plasmid surface exclusion region: mapping of traS, traT, and traD transcripts. Plasmid. 1989 Jan;21(1):1–8. doi: 10.1016/0147-619x(89)90081-4. [DOI] [PubMed] [Google Scholar]
- Havekes L. M., Lugtenberg B. J., Hoekstra W. P. Conjugation deficient E. coli K12 F- mutants with heptose-less lipopolysaccharide. Mol Gen Genet. 1976 Jul 5;146(1):43–50. doi: 10.1007/BF00267981. [DOI] [PubMed] [Google Scholar]
- Hovi M., Sukupolvi S., Edwards M. F., Rhen M. Plasmid-associated virulence of Salmonella enteritidis. Microb Pathog. 1988 May;4(5):385–391. doi: 10.1016/0882-4010(88)90066-6. [DOI] [PubMed] [Google Scholar]
- Inokuchi K., Mutoh N., Matsuyama S., Mizushima S. Primary structure of the ompF gene that codes for a major outer membrane protein of Escherichia coli K-12. Nucleic Acids Res. 1982 Nov 11;10(21):6957–6968. doi: 10.1093/nar/10.21.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen-Ihler K. A., Minkley E. G., Jr The conjugation system of F, the fertility factor of Escherichia coli. Annu Rev Genet. 1986;20:593–624. doi: 10.1146/annurev.ge.20.120186.003113. [DOI] [PubMed] [Google Scholar]
- Jacobson A. Role of F pili in the penetration of bacteriophage fl. J Virol. 1972 Oct;10(4):835–843. doi: 10.1128/jvi.10.4.835-843.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalajakumari M. B., Guidolin A., Buhk H. J., Manning P. A., Ham L. M., Hodgson A. L., Cheah K. C., Skurray R. A. Surface exclusion genes traS and traT of the F sex factor of Escherichia coli K-12. Determination of the nucleotide sequence and promoter and terminator activities. J Mol Biol. 1987 Nov 5;198(1):1–11. doi: 10.1016/0022-2836(87)90452-9. [DOI] [PubMed] [Google Scholar]
- Joiner K. A. Complement evasion by bacteria and parasites. Annu Rev Microbiol. 1988;42:201–230. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Scales R., Warren K. A., Frank M. M., Rice P. A. Mechanism of action of blocking immunoglobulin G for Neisseria gonorrhoeae. J Clin Invest. 1985 Nov;76(5):1765–1772. doi: 10.1172/JCI112167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A. Studies on the mechanism of bacterial resistance to complement-mediated killing and on the mechanism of action of bactericidal antibody. Curr Top Microbiol Immunol. 1985;121:99–133. doi: 10.1007/978-3-642-45604-6_6. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Warren K. A., Brown E. J., Swanson J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. IV. C5b-9 forms high molecular weight complexes with bacterial outer membrane constituents on serum-resistant but not on serum-sensitive Neisseria gonorrhoeae. J Immunol. 1983 Sep;131(3):1443–1451. [PubMed] [Google Scholar]
- Joiner K. A., Warren K. A., Tam M., Frank M. M. Monoclonal antibodies directed against gonococcal protein I vary in bactericidal activity. J Immunol. 1985 May;134(5):3411–3419. [PubMed] [Google Scholar]
- Jones G. W., Rabert D. K., Svinarich D. M., Whitfield H. J. Association of adhesive, invasive, and virulent phenotypes of Salmonella typhimurium with autonomous 60-megadalton plasmids. Infect Immun. 1982 Nov;38(2):476–486. doi: 10.1128/iai.38.2.476-486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanukollu U., Bieler S., Hull S., Hull R. Contribution of the traT gene to serum resistance among clinical isolates of enterobacteriaceae. J Med Microbiol. 1985 Feb;19(1):61–67. doi: 10.1099/00222615-19-1-61. [DOI] [PubMed] [Google Scholar]
- Kennedy N., Beutin L., Achtman M., Skurray R., Rahmsdorf U., Herrlich P. Conjugation proteins encoded by the F sex factor. Nature. 1977 Dec 15;270(5638):580–585. doi: 10.1038/270580a0. [DOI] [PubMed] [Google Scholar]
- Korpela K., Ranki M., Sukupolvi S., Mäkelä P. H., Rhen M. Occurrence of Salmonella typhimurium virulence plasmid-specific sequences in different serovars of Salmonella. FEMS Microbiol Lett. 1989 Mar;49(1):49–54. doi: 10.1016/0378-1097(89)90340-6. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Beutin L., Achtman M. Outer membrane of Escherichia coli: properties of the F sex factor traT protein which is involved in surface exclusion. J Bacteriol. 1980 Apr;142(1):285–294. doi: 10.1128/jb.142.1.285-294.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Morelli G., Fisseau C. RNA-polymerase binding sites within the tra region of the F factor of Escherichia coli K-12. Gene. 1984 Jan;27(1):121–123. doi: 10.1016/0378-1119(84)90245-2. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Reeves P. Further characterization of the recipient ability of Escherichia coli K-12 bacteriophage-resistant mutants. J Bacteriol. 1977 Apr;130(1):540–541. doi: 10.1128/jb.130.1.540-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Timmis J. K., Moll A., Timmis K. N. Mutants that overproduce TraTp, a plasmid-specified major outer membrane protein of Escherichia coli. Mol Gen Genet. 1982;187(3):426–431. doi: 10.1007/BF00332623. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Jr, Ippen-Ihler K. Identification of a membrane protein associated with expression of the surface exclusion region of the F transfer operon. J Bacteriol. 1977 Mar;129(3):1613–1622. doi: 10.1128/jb.129.3.1613-1622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkley E. G., Jr Purification and characterization of pro-TraTp, the signal sequence-containing precursor of a secreted protein encoded by the F sex factor. J Bacteriol. 1984 May;158(2):464–473. doi: 10.1128/jb.158.2.464-473.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkley E. G., Jr, Willetts N. S. Overproduction, purification and characterization of the F traT protein. Mol Gen Genet. 1984;196(2):225–235. doi: 10.1007/BF00328054. [DOI] [PubMed] [Google Scholar]
- Moll A., Manning P. A., Timmis K. N. Plasmid-determined resistance to serum bactericidal activity: a major outer membrane protein, the traT gene product, is responsible for plasmid-specified serum resistance in Escherichia coli. Infect Immun. 1980 May;28(2):359–367. doi: 10.1128/iai.28.2.359-367.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro M. A., Bitter-Suermann D., Timmis J. K., Agüero M. E., Cabello F. C., Sanyal S. C., Timmis K. N. traT gene sequences, serum resistance and pathogenicity-related factors in clinical isolates of Escherichia coli and other gram-negative bacteria. J Gen Microbiol. 1985 Jun;131(6):1511–1521. doi: 10.1099/00221287-131-6-1511. [DOI] [PubMed] [Google Scholar]
- Morona R., Krämer C., Henning U. Bacteriophage receptor area of outer membrane protein OmpA of Escherichia coli K-12. J Bacteriol. 1985 Nov;164(2):539–543. doi: 10.1128/jb.164.2.539-543.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux P., Willetts N. Promoters in the transfer region of plasmid F. Basic Life Sci. 1985;30:605–614. doi: 10.1007/978-1-4613-2447-8_42. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer-membrane permeability of bacteria. Crit Rev Microbiol. 1986;13(1):1–62. doi: 10.3109/10408418609108734. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor C. D., Timmis K. N. Highly repressible expression system for cloning genes that specify potentially toxic proteins. J Bacteriol. 1987 Oct;169(10):4457–4462. doi: 10.1128/jb.169.10.4457-4462.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata R. T., Levine R. P. Characterization of complement resistance in Escherichia coli conferred by the antibiotic resistance plasmid R100. J Immunol. 1980 Oct;125(4):1494–1498. [PubMed] [Google Scholar]
- Ogata R. T., Winters C., Levine R. P. Nucleotide sequence analysis of the complement resistance gene from plasmid R100. J Bacteriol. 1982 Aug;151(2):819–827. doi: 10.1128/jb.151.2.819-827.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson-Delafield J., Martinez R. J., Lehrer R. I. Microbicidal cationic proteins in rabbit alveolar macrophages: a potential host defense mechanism. Infect Immun. 1980 Oct;30(1):180–192. doi: 10.1128/iai.30.1.180-192.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal N. B., Minkley E. G., Jr The product of the F sex factor traT surface exclusion gene is a lipoprotein. J Biol Chem. 1984 May 10;259(9):5357–5360. [PubMed] [Google Scholar]
- Rashtchian A., Crooks J. H., Levy S. B. traJ independence in expression of traT on F. J Bacteriol. 1983 May;154(2):1009–1012. doi: 10.1128/jb.154.2.1009-1012.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Cheah K. C., Skurray R. An F-derived conjugative cosmid: analysis of tra polypeptides in cosmid-infected cells. Plasmid. 1986 Sep;16(2):90–100. doi: 10.1016/0147-619x(86)90067-3. [DOI] [PubMed] [Google Scholar]
- Reynard A. M., Beck M. E., Cunningham R. K. Effects of antibiotic resistance plasmids on the bactericidal activity of normal rabbit serum. Infect Immun. 1978 Mar;19(3):861–866. doi: 10.1128/iai.19.3.861-866.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynard A. M., Beck M. E. Plasmid-mediated resistance to the bactericidal effects of normal rabbit serum. Infect Immun. 1976 Sep;14(3):848–850. doi: 10.1128/iai.14.3.848-850.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen M., Sukupolvi S. The role of the traT gene of the Salmonella typhimurium virulence plasmid for serum resistance and growth within liver macrophages. Microb Pathog. 1988 Oct;5(4):275–285. doi: 10.1016/0882-4010(88)90100-3. [DOI] [PubMed] [Google Scholar]
- Ried G., Henning U. A unique amino acid substitution in the outer membrane protein OmpA causes conjugation deficiency in Escherichia coli K-12. FEBS Lett. 1987 Nov 2;223(2):387–390. doi: 10.1016/0014-5793(87)80324-1. [DOI] [PubMed] [Google Scholar]
- Riede I., Eschbach M. L. Evidence that TraT interacts with OmpA of Escherichia coli. FEBS Lett. 1986 Sep 15;205(2):241–245. doi: 10.1016/0014-5793(86)80905-x. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Janzer J., Head J. Influence of lipopolysaccharide and protein in the cell envelope on recipient capacity in conjugation of Salmonella typhimurium. J Bacteriol. 1981 Oct;148(1):283–293. doi: 10.1128/jb.148.1.283-293.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurray R. A., Nagaishi H., Clark A. J. Molecular cloning of DNA from F sex factor of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1976 Jan;73(1):64–68. doi: 10.1073/pnas.73.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa B. A., Moore D., Ippen-Ihler K. Physiology of F-pilin synthesis and utilization. J Bacteriol. 1983 Feb;153(2):962–968. doi: 10.1128/jb.153.2.962-968.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukupolvi S., O'Connor D. Amino acid alterations in a hydrophobic region of the TraT protein of R6-5 increase the outer membrane permeability of enteric bacteria. Mol Gen Genet. 1987 Nov;210(1):178–180. doi: 10.1007/BF00337776. [DOI] [PubMed] [Google Scholar]
- Sukupolvi S., O'Connor D., Edwards M. F. The traT protein is able to normalize the phenotype of a plasmid-carried permeability mutation of Salmonella typhimurium. J Gen Microbiol. 1986 Aug;132(8):2079–2085. doi: 10.1099/00221287-132-8-2079. [DOI] [PubMed] [Google Scholar]
- Sukupolvi S., Vaara M., Helander I. M., Viljanen P., Mäkelä P. H. New Salmonella typhimurium mutants with altered outer membrane permeability. J Bacteriol. 1984 Aug;159(2):704–712. doi: 10.1128/jb.159.2.704-712.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukupolvi S., Vaara M. Salmonella typhimurium and Escherichia coli mutants with increased outer membrane permeability to hydrophobic compounds. Biochim Biophys Acta. 1989 Dec 6;988(3):377–387. doi: 10.1016/0304-4157(89)90011-7. [DOI] [PubMed] [Google Scholar]
- Sukupolvi S., Vuorio R., Qi S. Y., O'Connor D., Rhen M. Characterization of the traT gene and mutants that increase outer membrane permeability from the Salmonella typhimurium virulence plasmid. Mol Microbiol. 1990 Jan;4(1):49–57. doi: 10.1111/j.1365-2958.1990.tb02014.x. [DOI] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W., Hughes C. Plasmid carriage and the serum sensitivity of enterobacteria. Infect Immun. 1978 Oct;22(1):10–17. doi: 10.1128/iai.22.1.10-17.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K. N., Boulnois G. J., Bitter-Suermann D., Cabello F. C. Surface components of Escherichia coli that mediate resistance to the bactericidal activities of serum and phagocytes. Curr Top Microbiol Immunol. 1985;118:197–218. doi: 10.1007/978-3-642-70586-1_11. [DOI] [PubMed] [Google Scholar]
- Van der Ley P., Bekkers A., Van Meersbergen J., Tommassen J. A comparative study on the phoE genes of three enterobacterial species. Implications for structure-function relationships in a pore-forming protein of the outer membrane. Eur J Biochem. 1987 Apr 15;164(2):469–475. doi: 10.1111/j.1432-1033.1987.tb11080.x. [DOI] [PubMed] [Google Scholar]
- Vogel H., Jähnig F. Models for the structure of outer-membrane proteins of Escherichia coli derived from raman spectroscopy and prediction methods. J Mol Biol. 1986 Jul 20;190(2):191–199. doi: 10.1016/0022-2836(86)90292-5. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Arai T., Hattori T. Effects of cell wall polysaccharide on the mating ability of Salmonella typhimurium. Nature. 1970 Jan 3;225(5227):70–71. doi: 10.1038/225070a0. [DOI] [PubMed] [Google Scholar]
- Willetts N., Maule J. Interactions between the surface exclusion systems of some F-like plasmids. Genet Res. 1974 Aug;24(1):81–89. doi: 10.1017/s0016672300015093. [DOI] [PubMed] [Google Scholar]
- Willetts N., Maule J. Specificities of IncF plasmid conjugation genes. Genet Res. 1986 Feb;47(1):1–11. doi: 10.1017/s0016672300024447. [DOI] [PubMed] [Google Scholar]
- Willetts N., Skurray R. The conjugation system of F-like plasmids. Annu Rev Genet. 1980;14:41–76. doi: 10.1146/annurev.ge.14.120180.000353. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Yu F., Inouye M. A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell. 1988 May 6;53(3):423–432. doi: 10.1016/0092-8674(88)90162-6. [DOI] [PubMed] [Google Scholar]



