Abstract
Locomotor activity (LMA) is a simple and easily performed measurement of behavior in mice and other rodents. Improvements in video tracking software (VTS) have allowed it to be coupled to LMA testing, dramatically improving specificity and sensitivity when compared to the line crossings method with manual scoring. In addition, VTS enables high-throughput experimentation. While similar to automated video tracking used for the open field test (OFT), LMA testing is unique in that it allows mice to remain in their home cage and does not utilize the anxiogenic stimulus of bright lighting during the active phase of the light-dark cycle. Traditionally, LMA has been used for short periods of time (mins), while longer movement studies (hrs-days) have often used implanted transmitters and biotelemetry. With the option of real-time tracking, long-, like short-term LMA testing, can now be conducted using videography. Long-term LMA testing requires a specialized, but easily constructed, cage so that food and water (which is usually positioned on the cage top) does not obstruct videography. Importantly, videography and VTS allows for the quantification of parameters, such as path of mouse movement, that are difficult or unfeasible to measure with line crossing and/or biotelemetry. In sum, LMA testing coupled to VTS affords a more complete description of mouse movement and the ability to examine locomotion over an extended period of time.
Keywords: Neuroscience, Issue 76, Behavior, Neurobiology, Anatomy, Physiology, Psychology, Animal, Exploratory Behavior, Behavioral Research, Psychoneuroimmunology, Locomotion, Neuroimmune, high throughput, sickness behavior, noninvasive, video recording, imaging, animal model
Protocol
1. Short-term LMA Set Up and Procedure
- Short-term LMA cage set up
- Test short-term LMA within the home cage of a single-housed mouse. To facilitate VTS-dependent image analysis, use bedding in high contrast with mouse color (i.e. dark colored bedding for white mice and light colored bedding for black mice).
- During short-term LMA testing, cover cages with clear ¼ in Plexiglas with 11 x ¼ in diameter holes to support appropriate ventilation.
- Short-term LMA testing room
- Test mice in a clean environment in which they have been acclimated to for at least 24 hr 8.
- To illuminate the testing environment, use brooder clamp lights with red bulbs (< 125 lumens). Prior to videography, adjust the angle and position of the lights to remove glare or reflections from the Plexiglas cage tops.
- Remove or avoid room noise above 70 dB. Use a white noise generator if intermittent background noise is unavoidable during mouse testing.
- Cover the testing surface upon which the cages are placed in the testing room with butcher or lab bench paper of similar color to the cage bedding, in case the cage floor is exposed during videography.
- Short-term LMA videography
- Center and place a ceiling-mounted night vision-capable video camera with at least 120 GB of memory above the recording area in the testing room. Do not exceed the resolution capability of the VTS used by placing too many cages within the video camera view frame (e.g. Ethovision XT 7 by Noldus Information Technology (Leesburg VA) can simultaneously track 8 mice in real-time).
- Short-term LMA testing procedure
- Test short-term LMA during the active/dark phase of the light cycle. Make sure the time relative to the light cycle is the same across all groups, as LMA follows a crepuscular pattern, with more activity during the early and later periods of the dark cycle 1. Include both experimental and control subjects in each group of recorded mice to ensure potential effects of temporal differences in LMA are minimized.
- Individually house and acclimate mice at least 24 hr prior to the start of LMA testing 8.
- Place the cage dividers and cages (containing individual mice) directly under the camera. Remove all nesting/enrichment material from the cages, as this may obstruct the view of the mouse (e.g. if the mouse burrows/covers itself with nesting material) and interfere with VTS accuracy. Place a clear Plexiglas lid on each cage recorded.
- When videoing several cages simultaneously, be sure that the resolution is compatible with the chosen VTS. To ease VTS analysis, position the cages identically within each divider (i.e. upper right hand corner).
- Start recording. Begin each video with a slate that identifies all relevant experimental information to ensure proper identification of the video independent of any additional non-video documentation. Use a timer or stopwatch to track duration of LMA testing (5-10 min). If timing information is lost, recover it from the video record.
- For repeated measures using the short-term LMA method, allow mice access to food and water during the non-measurement intervals.
- During testing, keep any experimenters out of sight of the mice so as not to act as an external visual cue.
- When testing is finished, return/replace nesting/enrichment material, to reduce the potential for development of anxiety-like behavior 8.
- Short-term LMA analysis
- Store the video recordings from the testing session to an archival media compatible with the VTS used. For Ethovision XT 7, a hard drive disk (HDD) is used.
- Replay the videos in the VTS using its associated media player, analyzing the videos in real-time. In Figure 1, the detection variables were: 2 cm/sec begins tracking; 1.75 cm/sec halts tracking.
- Initially, choose the entire cage floor as the region of interest (ROI) for tracking. For advanced analyses, such as assessment of thigmotaxis, create multiple intra-cage ROIs.
- For high-throughput studies, analyze all cages within the video simultaneously with individual all-cage floor ROIs.
- Use the VTS to calculate the total distance moved (cm), velocity of movement (cm/sec) and duration of movement (sec). For Ethovision XT 7, export the data generated into Microsoft Excel for storage and additional analysis.
2. Long-term LMA Testing Set Up and Procedure
- Long-term LMA testing cage construction
- Cut a 5.5 cm diameter hole in the short-end wall of a standard mouse cage. Create a covering for the hole with ¼ in galvanized steel mesh. Cut the mesh, creating several ½ in openings to allow mouse access to food. Burnish any cut mesh to remove sharp edges.
- Use additional mesh to create an external hopper directly connected to the mesh used to cover the 5.5 cm opening. Connect the hopper to the mesh used to cover the hole, and attach the assembly to the cage using aluminum rivets.
- Construct a water bottle from a standard 50 ml conical (Corning) and a 1-hole rubber stopper (Fisher) that is over-drilled to allow for insertion of no-drip sipper tube (Sta-Pure Systems). Secure the water bottle to the cage with Velcro tape (3M) and insert the sipper tube through the mesh covered opening.
- During long-term LMA testing, cover cages with clear ¼ in Plexiglas with 11 x ¼ in diameter holes to support appropriate ventilation.
- Long-term LMA testing room
- Long-term LMA testing room set up is identical to short-term LMA. See section 1.2 for procedure.
- Long-term LMA videography
- Center and place a ceiling-mounted infrared, security-style, camera directly linked to a PC above the recording area in the testing room. Only record the number of cages at once that results in individual cage resolution within the capability of the VTS used (e.g. Ethovision XT 7 can simultaneously track 8 mice in real-time).
- Long-term LMA testing procedure
- Test long-term LMA during the active/dark phase of the light cycle. Make sure the time relative to the light cycle is the same across all groups, as LMA follows a crepuscular pattern with more activity during the early and later periods of the dark cycle 1. Test both experimental and control subjects in each group of recorded mice to ensure minimization of potential effects of temporal differences in LMA.
- Individually house and acclimate the test mice in the modified cages for at least 24 hr.
- Place the cage dividers and cages (containing individual mice) directly under the camera. Remove all nesting/enrichment material from the cages to ensure an unobstructed of view of the mouse. Mouse burrowing into the nesting material will interfere with the ability of the VTS to track the mouse. Place a clear Plexiglas lid on each of the cages to be recorded.
- When recording several cages at once, be sure the resolution is compatible with the chosen VTS. To ease VTS analysis, position cages identically within each divider (i.e. upper right hand corner).
- Long-term LMA testing is generally analyzed in real-time, and a video record is usually not created. A video record can be recorded by using a secondary camera or via a DVD recorder linked to the primary camera. Be sure to keep detailed records of individual mouse and group information.
- Set up the VTS for real-time analysis. The entire cage floor should encompass the ROI for tracking. Set up ROIs for all cages being recorded. Use the VTS to calculate the total distance moved (cm), velocity of movement (cm/sec) and duration of movement (sec). In Figure 2, the detection variables were: 2 cm/sec begins tracking; 1.75 cm/sec halts tracking.
- Begin recording mouse long-term LMA. Monitor the duration of long-term LMA testing via the clock associated with the analyzing computer.
- During testing, keep all experimenters out of sight of the mice so as not to act as an external visual cue.
- When testing is finished, return/replace nesting/enrichment material, to reduce the potential for development of anxiety-like behavior 8.
- Long-term LMA analysis
- Long-term LMA analysis occurs in real-time without the generation of a video record. Export the data into Microsoft Excel for storage and analysis, if using Ethovision XT 7.
- To generate a video record for long-term LMA, use a secondary camera. Be sure the secondary camera has ample storage capacity sufficient to store the potentially lengthy recording(s).
Representative Results
An example of results from a repeated measures, short-term LMA test is shown in Figure 1. This figure shows that after lipopolysaccharide (LPS) administration, mice housed in individually ventilated caging (IVC) recover short-term locomotor activity faster than mice housed in ambient environment caging (AEC) 6. While the initial loss of locomotor activity is similar between LPS injected groups, the recovery to basal LMA is more rapid in IVC-housed mice as was velocity of movement (Figure 1b) and duration of movement (Figure 1c). The use of a repeated measures model with this short-term LMA test allows the observation and comparison of recovery over time between different groups.
A long-term LMA test example is shown in Figure 2. This figure demonstrates mouse locomotion during 12 hr of daily testing over 2 separate but consecutive days. No significant difference in distance moved (Figure 2a), velocity of movement (Figure 2b) or duration of movement (Figure 2c) was found between these 2 days (unpublished pilot data). While there is a significant amount of error due to a small sample size, these results illustrate that consistent and repeatable data can be generated over the course of consecutive days, allowing the potential for longer-term locomotion studies to be performed without the need of implanted transmitters and biotelemetry. Although underpowered, the 1 day versus 2 day difference in distance moved (Figure 2a) appears due to a dissimilarity in the duration of movement (Figure 2c) and not to a difference in velocity (Figure 2b).
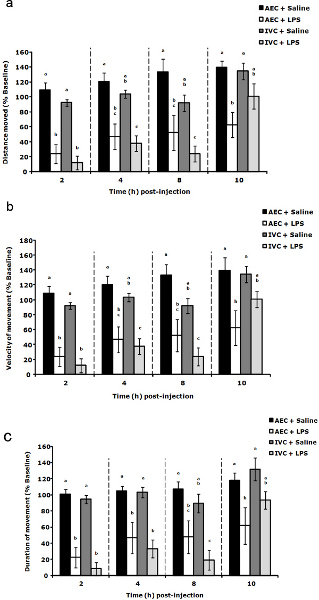 Figure 1. LPS-induced loss of locomotor activity in mice housed in ambient environment caging (AEC) and individually ventilated caging (IVC). Group housed mice (4 per cage) from either AEC or IVC housing were individually housed before the start of short-term LMA testing. Mice were injected with LPS (100 μg/kg) and (a) distance moved (cm), (b) velocity of movement (cm/sec) and (c) duration of movement (s) were evaluated at the times indicated. Results are expressed as percent baseline (pre-LPS) LMA, means ± s.e.m., n = 6. Distance moved main effects: time (F(3,77) = 13.08, P < 0.01), housing type (F(1,77) = 3.69, P = 0.06); time x housing type interaction (F(3,77) = 3.31, P = 0.02). Velocity of movement main effects: time (F(3,77) = 13.08, P < 0.01); housing type (F(1,77) = 3.69, P = 0.06); time x housing type interaction (F(3,77) = 3.31, P = 0.0243). Duration of movement main effects: time (F(3,77) = 12.92, P < 0.01); housing type (F(1,77) = 0.69, P = 0.41); time x housing type interaction (F(3,77) = 3.26, P =0.03). Bars within individual time points without common superscript letters are significantly different (P < 0.05). # Reprinted from: Brain Behav. and Immun., 26 (6), York, J.M., et al., Individually ventilated cages cause chronic low-grade hypoxia impacting mice hematologically and behaviorally, 951-958, Copyright 2012, with permission from Elsevier. Click here to view larger figure.
Figure 1. LPS-induced loss of locomotor activity in mice housed in ambient environment caging (AEC) and individually ventilated caging (IVC). Group housed mice (4 per cage) from either AEC or IVC housing were individually housed before the start of short-term LMA testing. Mice were injected with LPS (100 μg/kg) and (a) distance moved (cm), (b) velocity of movement (cm/sec) and (c) duration of movement (s) were evaluated at the times indicated. Results are expressed as percent baseline (pre-LPS) LMA, means ± s.e.m., n = 6. Distance moved main effects: time (F(3,77) = 13.08, P < 0.01), housing type (F(1,77) = 3.69, P = 0.06); time x housing type interaction (F(3,77) = 3.31, P = 0.02). Velocity of movement main effects: time (F(3,77) = 13.08, P < 0.01); housing type (F(1,77) = 3.69, P = 0.06); time x housing type interaction (F(3,77) = 3.31, P = 0.0243). Duration of movement main effects: time (F(3,77) = 12.92, P < 0.01); housing type (F(1,77) = 0.69, P = 0.41); time x housing type interaction (F(3,77) = 3.26, P =0.03). Bars within individual time points without common superscript letters are significantly different (P < 0.05). # Reprinted from: Brain Behav. and Immun., 26 (6), York, J.M., et al., Individually ventilated cages cause chronic low-grade hypoxia impacting mice hematologically and behaviorally, 951-958, Copyright 2012, with permission from Elsevier. Click here to view larger figure.
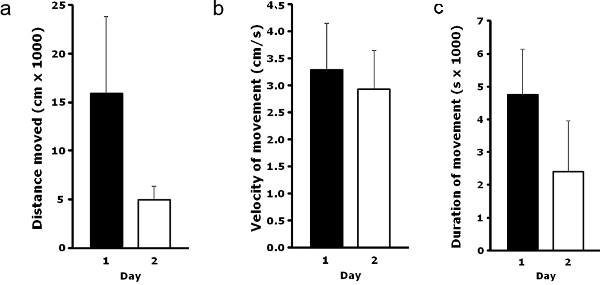 Figure 2. Mouse long-term LMA. Group housed mice (4 per cage) were individually housed before the start of long-term LMA testing. Mice LMA was analyzed in real time for 12 hr (day 1), and in the same mice 1 day later (day 2). Results are expressed as (a) total distance moved (cm x 1,000), (b) velocity of movement (cm/sec) and (c) duration of movement (sec x 1,000) means ± s.e.m., n = 4 (no significant difference between 0 and 24 hr) (unpublished pilot data).
Figure 2. Mouse long-term LMA. Group housed mice (4 per cage) were individually housed before the start of long-term LMA testing. Mice LMA was analyzed in real time for 12 hr (day 1), and in the same mice 1 day later (day 2). Results are expressed as (a) total distance moved (cm x 1,000), (b) velocity of movement (cm/sec) and (c) duration of movement (sec x 1,000) means ± s.e.m., n = 4 (no significant difference between 0 and 24 hr) (unpublished pilot data).
Discussion
Symptoms of sickness in mice include lethargy, loss of interest in social and environmental surroundings, malaise and anorexia 2-3. In mice and other rodents, LMA can be used to evaluate sickness after immune activation 4-5. Additionally, long-term LMA offers the ability to track movement over a 24 hr (or greater) period, allowing analysis of treatment-induced alterations in circadian behavior or disturbances in sleep/wake patterns. LMA offers several advantages over other tests for sickness since it is non-invasive, easily performed as a repeated measure or continuous modality and is minimally confounding. Compared with other methods and systems (e.g. Phenotyper, Intellecage, photobeam systems), LMA analysis with VTS is relatively inexpensive because multiple cages can be recorded/analyzed simultaneously. Although not utilized here, additional analysis of LMA activities like rearing and wall climbing can be achieved through use of a side-mounted camera. With VTS, LMA is high-throughput adaptable. VTS also allows for a more thorough examination of movement including the determination of duration, distance, velocity and path 5. Path tracking is especially powerful since it underlies anxiety behaviors.
Despite its many advantages, a potential pitfall inherent to LMA with VTS is the isolation/single housing of mice for up to (and possibly exceeding) 24 hr. Social isolation can induce a stress response and aggression when prolonged 11. Investigators should be aware of this limitation and take measures to ensure proper experimental control and result interpretation.
During all behavioral testing, including mouse LMA, close attention to pre-experimental considerations is critical to achieving consistent results. Acclimatization to the testing room and test cage environments is essential. Aberrant noise, light and sound are especially interfering as they may provoke a startle response and/or anxiety behavior 7-8. Ideally, LMA testing should occur in a temperature and humidity controlled environment free of noxious odors (especially natural predator odors). A fixed behavioral testing suite that allows for housing of animals for extended periods of time (days) is desirable. The ability to return mice to the animal care facility is advantageous since repeated testing is very useful in the study of aging or chronic diseases.
Finally, LMA testing should be performed in conjunction with almost all other behavioral tests because almost all such tests require mouse movement. Perturbed LMA impacts tests for cognition (Barnes maze, Y maze, T maze, novel object recognition), anxiety-like behavior (Zero maze, open field), pleasure-seeking (wheel running), depressive-like behavior (sucrose/saccharine preference) and sickness (social withdrawal and burrowing) 8-9. By testing short- and long-term LMA, phenotypic differences 10 between mouse sub-strains, genotypes and disease/intervention models can be rapidly evaluated while not significantly influencing subsequent behavioral tests. Furthermore, if significant movement differences are identified appropriate measures can be taken prior to the enactment of more complicated behavioral tests that are limited by the inherent confounds they induce.
Disclosures
Authors declare no conflicts of interest.
Acknowledgments
Support: This research was supported by the National Institutes of Health (DK064862, NS058525 and AA019357 to GGF).
References
- Goulding EH, Schenk AK, Juneja P, MacKay AW, Wade JM, Tecott LH. A robust automated system elucidates mouse home cage behavioral structure. Proc. Natl. Acad. Sci. U.S.A. 2008;105:20575–20582. doi: 10.1073/pnas.0809053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent S, Bluthé RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol. Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. North Am. 2009;29:247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- York JM, et al. The biobehavioral and neuroimmune impact of low-dose ionizing radiation. Brain Behav. Immun. 2012;26:218–227. doi: 10.1016/j.bbi.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York JM, et al. Individually ventilated cages cause chronic low-grade hypoxia impacting mice hematologically and behaviorally. Brain Behav. Immun. 2012;26:951–958. doi: 10.1016/j.bbi.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M, et al. Refining rodent husbandry: the mouse. Report of the Rodent Refinement Working Party. Lab. Anim. 1998;32:233–259. doi: 10.1258/002367798780559301. [DOI] [PubMed] [Google Scholar]
- York JM, Blevins NA, Baynard T, Freund GG. Mouse testing methods in psychoneuroimmunology. Methods in Molecular Biology. 2012;934:243–276. doi: 10.1007/978-1-62703-071-7_13. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of rodents. Comp. Med. 2003;53:140–146. [PubMed] [Google Scholar]
- Karelina K, et al. Social isolation alters neuroinflammatory response to stroke. Proc. Natl. Acad. Sci. U.S.A. 2009;106:5895–5900. doi: 10.1073/pnas.0810737106. [DOI] [PMC free article] [PubMed] [Google Scholar]


