Abstract
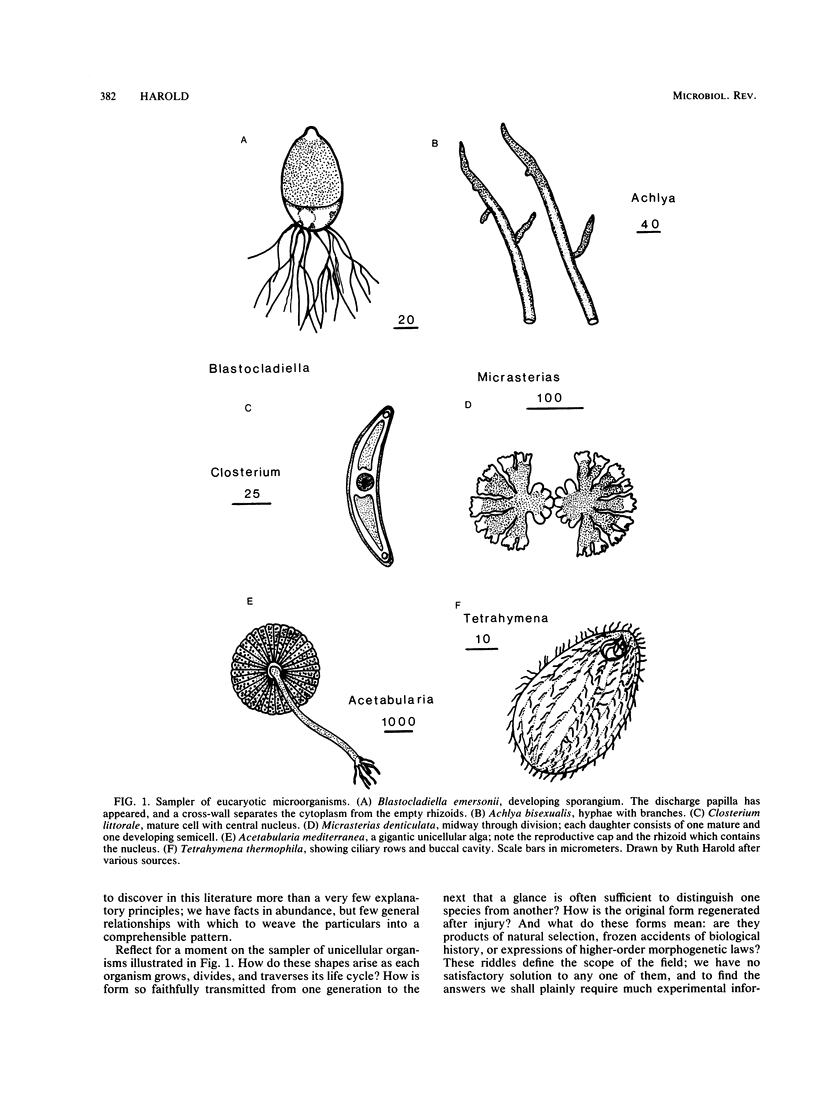
We recognize organisms first and foremost by their forms, but how they grow and shape themselves still largely passes understanding. The objective of this article is to survey what has been learned of morphogenesis of walled eucaryotic microorganisms as a set of problems in cellular heredity, biochemistry, physiology, and organization. Despite the diversity of microbial forms and habits, some common principles can be discerned. (i) That the form of each organism represents the expression of a genetic program is almost universally taken for granted. However, reflection on the findings with morphologically aberrant mutants suggests that the metaphor of a genetic program is misleading. Cellular form is generated by a web of interacting chemical and physical processes, whose every strand is woven of multiple gene products. The relationship between genes and form is indirect and cumulative; therefore, morphogenesis must be addressed as a problem not of molecular genetics but of cellular physiology. (ii) The shape of walled cells is determined by the manner in which the wall is laid down during growth and development. Turgor pressure commonly, perhaps always, supplies the driving force for surface enlargement. Cells yield to this scalar force by localized, controlled wall synthesis; their forms represent variations on the theme of local compliance with global force. (iii) Growth and division in bacteria display most immediately the interplay of hydrostatic pressure, localized wall synthesis, and structural constraints. Koch's surface stress theory provides a comprehensive and quantitative framework for understanding bacterial shapes. (iv) In the larger and more versatile eucaryotic cells, expansion is mediated by the secretion of vesicles. Secretion and ancillary processes, such as cytoplasmic transport, are spatially organized on the micrometer scale. The diversity of vectorial physiology and of the forms it generates is illustrated by examples: apical growth of fungal hyphae, bud formation in yeasts, germination of fucoid zygotes, and development of cells of Nitella, Closterium, and other unicellular algae. (v) Unicellular organisms, no less than embryos, have a remarkable capacity to impose spatial order upon themselves with or without the help of directional cues. Self-organization is reviewed here from two perspectives: the theoretical exploration of morphogens, gradients, and fields, and experimental study of polarization in Fucus cells, extension of hyphal tips, and pattern formation in ciliates. Here is the heart of the matter, yet self-organization remains nearly as mysterious as it was a century ago, a subject in search of a paradigm.
Full text
PDF


























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair W. S., Steinmetz S. A., Mattson D. M., Goodenough U. W., Heuser J. E. Nucleated assembly of Chlamydomonas and Volvox cell walls. J Cell Biol. 1987 Nov;105(5):2373–2382. doi: 10.1083/jcb.105.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A. E., Pringle J. R. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984 Mar;98(3):934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. M., Soll D. R. Differences in actin localization during bud and hypha formation in the yeast Candida albicans. J Gen Microbiol. 1986 Jul;132(7):2035–2047. doi: 10.1099/00221287-132-7-2035. [DOI] [PubMed] [Google Scholar]
- Anderson R. G. The biogenesis of cell structures and the expression of assembly information. J Theor Biol. 1977 Aug 7;67(3):535–548. doi: 10.1016/0022-5193(77)90055-8. [DOI] [PubMed] [Google Scholar]
- Aufderheide K. J., Frankel J., Williams N. E. Formation and positioning of surface-related structures in protozoa. Microbiol Rev. 1980 Jun;44(2):252–302. doi: 10.1128/mr.44.2.252-302.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEISSON J., SONNEBORN T. M. CYTOPLASMIC INHERITANCE OF THE ORGANIZATION OF THE CELL CORTEX IN PARAMECIUM AURELIA. Proc Natl Acad Sci U S A. 1965 Feb;53:275–282. doi: 10.1073/pnas.53.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M., Baba N., Ohsumi Y., Kanaya K., Osumi M. Three-dimensional analysis of morphogenesis induced by mating pheromone alpha factor in Saccharomyces cerevisiae. J Cell Sci. 1989 Oct;94(Pt 2):207–216. doi: 10.1242/jcs.94.2.207. [DOI] [PubMed] [Google Scholar]
- Barker D. G., Johnson A. L., Johnston L. H. An improved assay for DNA ligase reveals temperature-sensitive activity in cdc9 mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1985;200(3):458–462. doi: 10.1007/BF00425731. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S., Bracker C. E., Lippman E., Ruiz-Herrera J. Chitosomes from the wall-less "slime" mutant of Neurospora crassa. Arch Microbiol. 1984 Oct;139(2-3):105–112. doi: 10.1007/BF00401983. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S., Lippman E. Fungal morphogenesis: cell wall construction in Mucor rouxii. Science. 1969 Jul 18;165(3890):302–304. doi: 10.1126/science.165.3890.302. [DOI] [PubMed] [Google Scholar]
- Baum P., Furlong C., Byers B. Yeast gene required for spindle pole body duplication: homology of its product with Ca2+-binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5512–5516. doi: 10.1073/pnas.83.15.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg K. J., Donachie W. D. Cell shape and division in Escherichia coli: experiments with shape and division mutants. J Bacteriol. 1985 Aug;163(2):615–622. doi: 10.1128/jb.163.2.615-622.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck G. B., Brown D. L. Microtubule biogenesis and cell shape in Ochromonas. I. The distribution of cytoplasmic and mitotic microtubules. J Cell Biol. 1973 Feb;56(2):340–359. doi: 10.1083/jcb.56.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracker C. E., Ruiz-Herrera J., Bartnicki-Garcia S. Structure and transformation of chitin synthetase particles (chitosomes) during microfibril synthesis in vitro. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4570–4574. doi: 10.1073/pnas.73.12.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brada D., Schekman R. Coincident localization of secretory and plasma membrane proteins in organelles of the yeast secretory pathway. J Bacteriol. 1988 Jun;170(6):2775–2783. doi: 10.1128/jb.170.6.2775-2783.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley S. H., Quatrano R. S. Sulfation of fucoidin in Fucus embryos. IV. Autoradiographic investigations of fucoidin sulfation and secretion during differentiation and the effect of cytochalasin treatment. Dev Biol. 1979 Dec;73(2):193–205. doi: 10.1016/0012-1606(79)90063-0. [DOI] [PubMed] [Google Scholar]
- Brawley S. H., Quatrano R. S., Wetherbee R. Fine-structural studies of the gametes and embryo of Fucus vesiculosus L. (Phaeophyta). I. Fertilization and pronuclear fusion. J Cell Sci. 1976 Mar;20(2):233–254. doi: 10.1242/jcs.20.2.233. [DOI] [PubMed] [Google Scholar]
- Brawley S. H., Robinson K. R. Cytochalasin treatment disrupts the endogenous currents associated with cell polarization in fucoid zygotes: studies of the role of F-actin in embryogenesis. J Cell Biol. 1985 Apr;100(4):1173–1184. doi: 10.1083/jcb.100.4.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D., White J. G. Cortical flow in animal cells. Science. 1988 Feb 19;239(4842):883–888. doi: 10.1126/science.3277283. [DOI] [PubMed] [Google Scholar]
- Brickell P. M., Tickle C. Morphogens in chick limb development. Bioessays. 1989 Nov;11(5):145–149. doi: 10.1002/bies.950110508. [DOI] [PubMed] [Google Scholar]
- Brinkley B. R. Microtubule organizing centers. Annu Rev Cell Biol. 1985;1:145–172. doi: 10.1146/annurev.cb.01.110185.001045. [DOI] [PubMed] [Google Scholar]
- Brody S., Tatum E. L. The primary biochemical effect of a morphological mutation in Neurospora crassa. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1290–1297. doi: 10.1073/pnas.56.4.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. L., Bouck G. B. Microtubule biogenesis and cell shape in Ochromonas. 3. Effects of herbicidal mitotic inhibitor isopropyl N-phenylcarbamate on shape and flagellum regeneration. J Cell Biol. 1974 May;61(2):514–536. doi: 10.1083/jcb.61.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. M., Jr Cellulose microfibril assembly and orientation: recent developments. J Cell Sci Suppl. 1985;2:13–32. doi: 10.1242/jcs.1985.supplement_2.2. [DOI] [PubMed] [Google Scholar]
- Brown R. M., Jr, Franke W. W., Kleinig H., Falk H., Sitte P. Scale formation in chrysophycean algae. I. Cellulosic and noncellulosic wall components made by the Golgi apparatus. J Cell Biol. 1970 May;45(2):246–271. doi: 10.1083/jcb.45.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. M., Jr Observations on the relationship of the golgi apparatus to wall formation in the marine chrysophycean alga Pleurochrysis scherffelii Pringsheim. J Cell Biol. 1969 Apr;41(1):109–123. doi: 10.1083/jcb.41.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D. Structure, growth, and division of the Bacillus subtilis cell surface. Can J Microbiol. 1988 Apr;34(4):373–380. doi: 10.1139/m88-068. [DOI] [PubMed] [Google Scholar]
- Burman L. G., Park J. T. Molecular model for elongation of the murein sacculus of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1844–1848. doi: 10.1073/pnas.81.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G., Raichler J., Park J. T. Evidence for diffuse growth of the cylindrical portion of the Escherichia coli murein sacculus. J Bacteriol. 1983 Sep;155(3):983–988. doi: 10.1128/jb.155.3.983-988.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B., Goetsch L. A highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol. 1976 Jun;69(3):717–721. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B., Goetsch L. Behavior of spindles and spindle plaques in the cell cycle and conjugation of Saccharomyces cerevisiae. J Bacteriol. 1975 Oct;124(1):511–523. doi: 10.1128/jb.124.1.511-523.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Bowers B. Timing and function of chitin synthesis in yeast. J Bacteriol. 1975 Dec;124(3):1586–1593. doi: 10.1128/jb.124.3.1586-1593.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Roberts R., Bowers B. Synthesis of the yeast cell wall and its regulation. Annu Rev Biochem. 1982;51:763–793. doi: 10.1146/annurev.bi.51.070182.003555. [DOI] [PubMed] [Google Scholar]
- Casperson G. F., Walker N., Bourne H. R. Isolation of the gene encoding adenylate cyclase in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5060–5063. doi: 10.1073/pnas.82.15.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. T. Models of cell differentiation in conidial fungi. Microbiol Rev. 1986 Jun;50(2):95–132. doi: 10.1128/mr.50.2.95-132.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. R., MacAlister T. J., Rothfield L. I. Compartmentalization of the periplasmic space at division sites in gram-negative bacteria. J Bacteriol. 1986 Dec;168(3):1430–1438. doi: 10.1128/jb.168.3.1430-1438.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. Biophysical control of plant cell growth. Annu Rev Plant Physiol. 1986;37:377–405. doi: 10.1146/annurev.pp.37.060186.002113. [DOI] [PubMed] [Google Scholar]
- Dickinson J. R., Williams A. S. The cdc30 mutation in Saccharomyces cerevisiae results in a temperature-sensitive isoenzyme of phosphoglucose isomerase. J Gen Microbiol. 1987 Jan;133(1):135–140. doi: 10.1099/00221287-133-1-135. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Koch A. L. The functions of autolysins in the growth and division of Bacillus subtilis. Crit Rev Microbiol. 1987;15(2):169–222. doi: 10.3109/10408418709104457. [DOI] [PubMed] [Google Scholar]
- Driehuis F., Wouters J. T. Effect of growth rate and cell shape on the peptidoglycan composition in Escherichia coli. J Bacteriol. 1987 Jan;169(1):97–101. doi: 10.1128/jb.169.1.97-101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever W., Nüsslein-Volhard C. A gradient of bicoid protein in Drosophila embryos. Cell. 1988 Jul 1;54(1):83–93. doi: 10.1016/0092-8674(88)90182-1. [DOI] [PubMed] [Google Scholar]
- Drubin D. G., Miller K. G., Botstein D. Yeast actin-binding proteins: evidence for a role in morphogenesis. J Cell Biol. 1988 Dec;107(6 Pt 2):2551–2561. doi: 10.1083/jcb.107.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elorza M. V., Murgui A., Rico H., Miragall F., Sentandreu R. Formation of a new cell wall by protoplasts of Candida albicans: effect of papulacandin B, tunicamycin and Nikkomycin. J Gen Microbiol. 1987 Aug;133(8):2315–2325. doi: 10.1099/00221287-133-8-2315. [DOI] [PubMed] [Google Scholar]
- Farkas V., Kovarík J., Kosinová A., Bauer S. Autoradiographic study of mannan incorporation into the growing cell walls of Saccharomyces cerevisiae. J Bacteriol. 1974 Jan;117(1):265–269. doi: 10.1128/jb.117.1.265-269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field C., Schekman R. Localized secretion of acid phosphatase reflects the pattern of cell surface growth in Saccharomyces cerevisiae. J Cell Biol. 1980 Jul;86(1):123–128. doi: 10.1083/jcb.86.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel J., Nelsen E. M. Discontinuities and overlaps in patterning within single cells. Philos Trans R Soc Lond B Biol Sci. 1981 Oct 7;295(1078):525–538. doi: 10.1098/rstb.1981.0158. [DOI] [PubMed] [Google Scholar]
- Frankel J., Nelsen E. M. Positional reorganization in compound janus cells of Tetrahymena thermophila. Development. 1987 Jan;99(1):51–68. doi: 10.1242/dev.99.1.51. [DOI] [PubMed] [Google Scholar]
- Frankel J. Positional information in unicellular organisms. J Theor Biol. 1974 Oct;47(2):439–481. doi: 10.1016/0022-5193(74)90209-4. [DOI] [PubMed] [Google Scholar]
- Fulton A. B. How do eucaryotic cells construct their cytoarchitecture? Cell. 1981 Apr;24(1):4–5. doi: 10.1016/0092-8674(81)90493-1. [DOI] [PubMed] [Google Scholar]
- Fulton C., Walsh C. Cell differentiation and flagellar elongation in Naegleria gruberi. Dependence on transcription and translation. J Cell Biol. 1980 May;85(2):346–360. doi: 10.1083/jcb.85.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambino J., Bergen L. G., Morris N. R. Effects of mitotic and tubulin mutations on microtubule architecture in actively growing protoplasts of Aspergillus nidulans. J Cell Biol. 1984 Sep;99(3):830–838. doi: 10.1083/jcb.99.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebl M. G., Yochem J., Jentsch S., McGrath J. P., Varshavsky A., Byers B. The yeast cell cycle gene CDC34 encodes a ubiquitin-conjugating enzyme. Science. 1988 Sep 9;241(4871):1331–1335. doi: 10.1126/science.2842867. [DOI] [PubMed] [Google Scholar]
- Goodwin B. C. Problems and prospects in morphogenesis. Experientia. 1988 Aug 15;44(8):633–637. doi: 10.1007/BF01941023. [DOI] [PubMed] [Google Scholar]
- Goodwin B. C. What are the causes of morphogenesis? Bioessays. 1985 Jul;3(1):32–36. doi: 10.1002/bies.950030109. [DOI] [PubMed] [Google Scholar]
- Grain J. The cytoskeleton in protists: nature, structure, and functions. Int Rev Cytol. 1986;104:153–249. doi: 10.1016/s0074-7696(08)61926-9. [DOI] [PubMed] [Google Scholar]
- Grove S. N., Bracker C. E. Protoplasmic organization of hyphal tips among fungi: vesicles and Spitzenkörper. J Bacteriol. 1970 Nov;104(2):989–1009. doi: 10.1128/jb.104.2.989-1009.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson J. H., Elwood H., Ingold A., Kindle K., Sogin M. L. Phylogenetic relationships between chlorophytes, chrysophytes, and oomycetes. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5823–5827. doi: 10.1073/pnas.84.16.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. L., Ramanis Z., Luck D. J. Basal body/centriolar DNA: molecular genetic studies in Chlamydomonas. Cell. 1989 Oct 6;59(1):121–132. doi: 10.1016/0092-8674(89)90875-1. [DOI] [PubMed] [Google Scholar]
- Hanes S. D., Koren R., Bostian K. A. Control of cell growth and division in Saccharomyces cerevisiae. CRC Crit Rev Biochem. 1986;21(2):153–223. doi: 10.3109/10409238609113611. [DOI] [PubMed] [Google Scholar]
- Harold R. L., Harold F. M. Ionophores and cytochalasins modulate branching in Achlya bisexualis. J Gen Microbiol. 1986 Jan;132(1):213–219. doi: 10.1099/00221287-132-1-213. [DOI] [PubMed] [Google Scholar]
- Harold R. L., Harold F. M. Oriented growth of Blastocladiella emersonii in gradients of ionophores and inhibitors. J Bacteriol. 1980 Dec;144(3):1159–1167. doi: 10.1128/jb.144.3.1159-1167.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison L. G. What is the status of reaction-diffusion theory thirty-four years after turing? J Theor Biol. 1987 Apr 21;125(4):369–384. doi: 10.1016/s0022-5193(87)80208-4. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasek J., Rupes I., Svobodová J., Streiblová E. Tubulin and actin topology during zygote formation of Saccharomyces cerevisiae. J Gen Microbiol. 1987 Dec;133(12):3355–3363. doi: 10.1099/00221287-133-12-3355. [DOI] [PubMed] [Google Scholar]
- Hayles J., Nurse P. Cell cycle regulation in yeast. J Cell Sci Suppl. 1986;4:155–170. doi: 10.1242/jcs.1986.supplement_4.10. [DOI] [PubMed] [Google Scholar]
- Heath I. B. A unified hypothesis for the role of membrane bound enzyme complexes and microtubules in plant cell wall synthesis. J Theor Biol. 1974 Dec;48(2):445–449. doi: 10.1016/s0022-5193(74)80011-1. [DOI] [PubMed] [Google Scholar]
- Henning U. L. Determination of cell shape in bacteria. Annu Rev Microbiol. 1975;29:45–60. doi: 10.1146/annurev.mi.29.100175.000401. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Study of cycle of cell wall assembly in Streptococcus faecalis by three-dimensional reconstructions of thin sections of cells. J Bacteriol. 1976 Sep;127(3):1346–1358. doi: 10.1128/jb.127.3.1346-1358.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower M. J., Bruno J., Lucas J. J. Identification of key regulated events early in the life of hybrid animal cells constructed by nuclear transplantation. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5310–5314. doi: 10.1073/pnas.80.17.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb C. L., Hansen W. J., Etcheverry T., Schekman R. Secretory vesicles externalize the major plasma membrane ATPase in yeast. J Cell Biol. 1988 Mar;106(3):641–648. doi: 10.1083/jcb.106.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland I. B. Genetic analysis of the E. coli division clock. Cell. 1987 Feb 13;48(3):361–362. doi: 10.1016/0092-8674(87)90183-8. [DOI] [PubMed] [Google Scholar]
- Holmes J. A., Dutcher S. K. Cellular asymmetry in Chlamydomonas reinhardtii. J Cell Sci. 1989 Oct;94(Pt 2):273–285. doi: 10.1242/jcs.94.2.273. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Aist J. R. Cytoplasmic microtubules and fungal morphogenesis: ultrastructural effects of methyl benzimidazole-2-ylcarbamate determined by freeze-substitution of hyphal tip cells. J Cell Biol. 1980 Oct;87(1):55–64. doi: 10.1083/jcb.87.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J. Ultrastructural analysis of hyphal tip cell growth in fungi: Spitzenkörper, cytoskeleton and endomembranes after freeze-substitution. J Cell Sci. 1981 Apr;48:89–103. doi: 10.1242/jcs.48.1.89. [DOI] [PubMed] [Google Scholar]
- Huffaker T. C., Hoyt M. A., Botstein D. Genetic analysis of the yeast cytoskeleton. Annu Rev Genet. 1987;21:259–284. doi: 10.1146/annurev.ge.21.120187.001355. [DOI] [PubMed] [Google Scholar]
- Huffaker T. C., Thomas J. H., Botstein D. Diverse effects of beta-tubulin mutations on microtubule formation and function. J Cell Biol. 1988 Jun;106(6):1997–2010. doi: 10.1083/jcb.106.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham P. W. The molecular genetics of embryonic pattern formation in Drosophila. Nature. 1988 Sep 1;335(6185):25–34. doi: 10.1038/335025a0. [DOI] [PubMed] [Google Scholar]
- Jacobs C. W., Adams A. E., Szaniszlo P. J., Pringle J. R. Functions of microtubules in the Saccharomyces cerevisiae cell cycle. J Cell Biol. 1988 Oct;107(4):1409–1426. doi: 10.1083/jcb.107.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. F. Localization in the developing Fucus egg and the general role of localizing currents. Adv Morphog. 1968;7:295–328. doi: 10.1016/b978-1-4831-9954-2.50012-4. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F., Nuccitelli R. Electrical controls of development. Annu Rev Biophys Bioeng. 1977;6:445–476. doi: 10.1146/annurev.bb.06.060177.002305. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F., Robinson K. R., Nuccitelli R. Local cation entry and self-electrophoresis as an intracellular localization mechanism. Ann N Y Acad Sci. 1974;238:372–389. doi: 10.1111/j.1749-6632.1974.tb26805.x. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F. The role of ionic currents in establishing developmental pattern. Philos Trans R Soc Lond B Biol Sci. 1981 Oct 7;295(1078):553–566. doi: 10.1098/rstb.1981.0160. [DOI] [PubMed] [Google Scholar]
- Jerka-Dziadosz M., Frankel J. A mutant of Tetrahymena thermophila with a partial mirror-image duplication of cell surface pattern. I. Analysis of the phenotype. J Embryol Exp Morphol. 1979 Jan;49:167–202. [PubMed] [Google Scholar]
- Johnson B. F., Gibson E. J. Autoradiographic analysis of regional cell wall growth of yeasts. III. Saccharomyces cerevisiae. Exp Cell Res. 1966 Mar;41(3):580–591. doi: 10.1016/s0014-4827(66)80108-8. [DOI] [PubMed] [Google Scholar]
- Jong A. Y., Kuo C. L., Campbell J. L. The CDC8 gene of yeast encodes thymidylate kinase. J Biol Chem. 1984 Sep 10;259(17):11052–11059. [PubMed] [Google Scholar]
- Katz D., Rosenberger R. F. A mutation in Aspergillus nidulans producing hyphal walls which lack chitin. Biochim Biophys Acta. 1970 Jun;208(3):452–460. doi: 10.1016/0304-4165(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Adams A. E. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984 Mar;98(3):922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M., Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986 May 9;45(3):329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Kobori H., Yamada N., Taki A., Osumi M. Actin is associated with the formation of the cell wall in reverting protoplasts of the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1989 Dec;94(Pt 4):635–646. doi: 10.1242/jcs.94.4.635. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Biophysics of bacterial walls viewed as stress-bearing fabric. Microbiol Rev. 1988 Sep;52(3):337–353. doi: 10.1128/mr.52.3.337-353.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L., Burdett I. D. Normal pole formation during total inhibition of wall synthesis of Bacillus subtilis. J Gen Microbiol. 1986 Dec;132(12):3441–3449. doi: 10.1099/00221287-132-12-3441. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Burdett I. D. The variable T model for gram-negative morphology. J Gen Microbiol. 1984 Sep;130(9):2325–2338. doi: 10.1099/00221287-130-9-2325. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Higgins M. L., Doyle R. J. Surface tension-like forces determine bacterial shapes: Streptococcus faecium. J Gen Microbiol. 1981 Mar;123(1):151–161. doi: 10.1099/00221287-123-1-151. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Higgins M. L., Doyle R. J. The role of surface stress in the morphology of microbes. J Gen Microbiol. 1982 May;128(5):927–945. doi: 10.1099/00221287-128-5-927. [DOI] [PubMed] [Google Scholar]
- Koch A. L. How bacteria grow and divide in spite of internal hydrostatic pressure. Can J Microbiol. 1985 Dec;31(12):1071–1084. doi: 10.1139/m85-204. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Speculations on the growth strategy of prosthecate bacteria. Can J Microbiol. 1988 Apr;34(4):390–394. doi: 10.1139/m88-070. [DOI] [PubMed] [Google Scholar]
- Koch A. L. The surface stress theory of microbial morphogenesis. Adv Microb Physiol. 1983;24:301–366. doi: 10.1016/s0065-2911(08)60388-4. [DOI] [PubMed] [Google Scholar]
- Kropf D. L., Berge S. K., Quatrano R. S. Actin Localization during Fucus Embryogenesis. Plant Cell. 1989 Feb;1(2):191–200. doi: 10.1105/tpc.1.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf D. L., Caldwell J. H., Gow N. A., Harold F. M. Transcellular ion currents in the water mold Achlya. Amino acid proton symport as a mechanism of current entry. J Cell Biol. 1984 Aug;99(2):486–496. doi: 10.1083/jcb.99.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf D. L. Electrophysiological properties of Achlya hyphae: ionic currents studied by intracellular potential recording. J Cell Biol. 1986 Apr;102(4):1209–1216. doi: 10.1083/jcb.102.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf D. L., Hopkins R., Quatrano R. S. Protein synthesis and morphogenesis are not tightly linked during embryogenesis in Fucus. Dev Biol. 1989 Aug;134(2):451–461. doi: 10.1016/0012-1606(89)90118-8. [DOI] [PubMed] [Google Scholar]
- Kropf D. L., Kloareg B., Quatrano R. S. Cell wall is required for fixation of the embryonic axis in Fucus zygotes. Science. 1988 Jan 8;239(4836):187–190. doi: 10.1126/science.3336780. [DOI] [PubMed] [Google Scholar]
- Lawrence P. A. Background to bicoid. Cell. 1988 Jul 1;54(1):1–2. doi: 10.1016/0092-8674(88)90172-9. [DOI] [PubMed] [Google Scholar]
- Linnemans W. A., Boer P., Elbers P. F. Localization of acid phosphatase in Saccharomyces cerevisiae: a clue to cell wall formation. J Bacteriol. 1977 Aug;131(2):638–644. doi: 10.1128/jb.131.2.638-644.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisziewicz J., Godany A., Förster H. H., Küntzel H. Isolation and nucleotide sequence of a Saccharomyces cerevisiae protein kinase gene suppressing the cell cycle start mutation cdc25. J Biol Chem. 1987 Feb 25;262(6):2549–2553. [PubMed] [Google Scholar]
- MacAlister T. J., Cook W. R., Weigand R., Rothfield L. I. Membrane-murein attachment at the leading edge of the division septum: a second membrane-murein structure associated with morphogenesis of the gram-negative bacterial division septum. J Bacteriol. 1987 Sep;169(9):3945–3951. doi: 10.1128/jb.169.9.3945-3951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matile P., Cortat M., Wiemken A., Frey-Wyssling A. Isolation of glucanase-containing particles from budding Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1971 Mar;68(3):636–640. doi: 10.1073/pnas.68.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh E. M., Ord R. W., Storms R. K. Transcriptional regulation of the cell cycle-dependent thymidylate synthase gene of Saccharomyces cerevisiae. Mol Cell Biol. 1988 Nov;8(11):4616–4624. doi: 10.1128/mcb.8.11.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad T., Archibald A. R., Hancock I. C., Harwood C. R., Hobot J. A. Cell wall assembly in Bacillus subtilis: visualization of old and new wall material by electron microscopic examination of samples stained selectively for teichoic acid and teichuronic acid. J Gen Microbiol. 1989 Mar;135(3):645–655. doi: 10.1099/00221287-135-3-645. [DOI] [PubMed] [Google Scholar]
- Mishra N. C., Tatum E. L. Phosphoglucomutase mutants of Neurospora sitophila and their relation to morphology. Proc Natl Acad Sci U S A. 1970 Jul;66(3):638–645. doi: 10.1073/pnas.66.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. T., Ellis E. A. Sexual morphogenesis in Achlya: ultrastructural basis for the hormonal induction of antheridial hyphae. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1347–1350. doi: 10.1073/pnas.71.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux J. P., Richmond P. A., Taiz L. Control of Cell Elongation in Nitella by Endogenous Cell Wall pH Gradients: MULTIAXIAL EXTENSIBILITY AND GROWTH STUDIES. Plant Physiol. 1980 Feb;65(2):204–210. doi: 10.1104/pp.65.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanney D. L. Molecules and morphologies: the perpetuation of pattern in the ciliated protozoa. J Protozool. 1977 Feb;24(1):27–35. doi: 10.1111/j.1550-7408.1977.tb05277.x. [DOI] [PubMed] [Google Scholar]
- Nelsen E. M., Frankel J., Jenkins L. M. Non-genic inheritance of cellular handedness. Development. 1989 Mar;105(3):447–456. doi: 10.1242/dev.105.3.447. [DOI] [PubMed] [Google Scholar]
- Newport J. Nuclear reconstitution in vitro: stages of assembly around protein-free DNA. Cell. 1987 Jan 30;48(2):205–217. doi: 10.1016/0092-8674(87)90424-7. [DOI] [PubMed] [Google Scholar]
- Ng S. F., Frankel J. 180 degrees rotation of ciliary rows and its morphogenetic implications in Tetrahymena pyriformis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1115–1119. doi: 10.1073/pnas.74.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985 Feb;40(2):405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R. Ionic currents in morphogenesis. Experientia. 1988 Aug 15;44(8):657–666. doi: 10.1007/BF01941026. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R. Oöplasmic segregation and secretion in the Pelvetia egg is accompanied by a membrane-generated electrical current. Dev Biol. 1978 Jan;62(1):13–33. doi: 10.1016/0012-1606(78)90089-1. [DOI] [PubMed] [Google Scholar]
- O'Shea P. S. Physical fields and cellular organisation: field-dependent mechanisms of morphogenesis. Experientia. 1988 Aug 15;44(8):684–694. doi: 10.1007/BF01941030. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Morris N. R. Nuclear movement is beta--tubulin-dependent in Aspergillus nidulans. Cell. 1980 Jan;19(1):255–262. doi: 10.1016/0092-8674(80)90407-9. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Rinehart J. E. Mitochondria and nuclei move by different mechanisms in Aspergillus nidulans. J Cell Biol. 1985 Dec;101(6):2392–2397. doi: 10.1083/jcb.101.6.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y., Miyamoto S., Ohsumi Y., Anraku Y. Calcium-sensitive cls4 mutant of Saccharomyces cerevisiae with a defect in bud formation. J Bacteriol. 1986 Jan;165(1):28–33. doi: 10.1128/jb.165.1.28-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster G. F. On the crawling of cells. J Embryol Exp Morphol. 1984 Nov;83 (Suppl):329–364. [PubMed] [Google Scholar]
- Oster G. F., Perelson A. S. The physics of cell motility. J Cell Sci Suppl. 1987;8:35–54. doi: 10.1242/jcs.1987.supplement_8.3. [DOI] [PubMed] [Google Scholar]
- Pall M. L., Trevillyan J. M., Hinman N. Deficient cyclic adenosine 3',5'-monophosphate control in mutants of two genes of Neurospora crassa. Mol Cell Biol. 1981 Jan;1(1):1–8. doi: 10.1128/mcb.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M. In situ electrophoresis of membrane components. Annu Rev Biophys Bioeng. 1981;10:245–276. doi: 10.1146/annurev.bb.10.060181.001333. [DOI] [PubMed] [Google Scholar]
- Quader H. Cellulose microfibril orientation in Oocystis solitaria: proof that microtubules control the alignment of the terminal complexes. J Cell Sci. 1986 Jul;83:223–234. doi: 10.1242/jcs.83.1.223. [DOI] [PubMed] [Google Scholar]
- Quader H. Morphology and movement of cellulose synthesizing (terminal) complexes in Oocystis solitaria: evidence that microfibril assembly is the motive force. Eur J Cell Biol. 1983 Nov;32(1):174–177. [PubMed] [Google Scholar]
- Quatrano R. S. An ultrastructural study of the determined site of rhizoid formation in Fucus zygotes. Exp Cell Res. 1972 Jan;70(1):1–12. doi: 10.1016/0014-4827(72)90174-7. [DOI] [PubMed] [Google Scholar]
- Quatrano R. S., Griffing L. R., Huber-Walchli V., Doubet R. S. Cytological and biochemical requirements for the establishment of a polar cell. J Cell Sci Suppl. 1985;2:129–141. doi: 10.1242/jcs.1985.supplement_2.7. [DOI] [PubMed] [Google Scholar]
- Quinlan R. A., Pogson C. I., Gull K. The influence of the microtubule inhibitor, methyl benzimidazol-2-yl-carbamate (MBC) on nuclear division and the cell cycle in Saccharomyces cerevisiae. J Cell Sci. 1980 Dec;46:341–352. doi: 10.1242/jcs.46.1.341. [DOI] [PubMed] [Google Scholar]
- Reed S. I., Hadwiger J. A., Lörincz A. T. Protein kinase activity associated with the product of the yeast cell division cycle gene CDC28. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4055–4059. doi: 10.1073/pnas.82.12.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss H. D., Herth W. Nifedipine-sensitive calcium channels are involved in polar growth of lily pollen tubes. J Cell Sci. 1985 Jun;76:247–254. doi: 10.1242/jcs.76.1.247. [DOI] [PubMed] [Google Scholar]
- Reissig J. L., Kinney S. G. Calcium as a branching signal in Neurospora crassa. J Bacteriol. 1983 Jun;154(3):1397–1402. doi: 10.1128/jb.154.3.1397-1402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K. R., Cone R. Polarization of fucoid eggs by a calcium ionophore gradient. Science. 1980 Jan 4;207(4426):77–78. doi: 10.1126/science.207.4426.77. [DOI] [PubMed] [Google Scholar]
- Robinson K. R., Jaffe L. F. Polarizing fucoid eggs drive a calcium current through themselves. Science. 1975 Jan 10;187(4171):70–72. doi: 10.1126/science.1167318. [DOI] [PubMed] [Google Scholar]
- Santos T., del Rey F., Conde J., Villanueva J. R., Nombela C. Saccharomyces cerevisiae mutant defective in exo-1,3-beta-glucanase production. J Bacteriol. 1979 Aug;139(2):333–338. doi: 10.1128/jb.139.2.333-338.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sburlati A., Cabib E. Chitin synthetase 2, a presumptive participant in septum formation in Saccharomyces cerevisiae. J Biol Chem. 1986 Nov 15;261(32):15147–15152. [PubMed] [Google Scholar]
- Schekman R. Protein localization and membrane traffic in yeast. Annu Rev Cell Biol. 1985;1:115–143. doi: 10.1146/annurev.cb.01.110185.000555. [DOI] [PubMed] [Google Scholar]
- Schmid J., Harold F. M. Dual roles for calcium ions in apical growth of Neurospora crassa. J Gen Microbiol. 1988 Sep;134(9):2623–2631. doi: 10.1099/00221287-134-9-2623. [DOI] [PubMed] [Google Scholar]
- Schreurs W. J., Harold F. M. Transcellular proton current in Achlya bisexualis hyphae: relationship to polarized growth. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1534–1538. doi: 10.1073/pnas.85.5.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs W. J., Harold R. L., Harold F. M. Chemotropism and branching as alternative responses of Achlya bisexualis to amino acids. J Gen Microbiol. 1989 Sep;135(9):2519–2528. doi: 10.1099/00221287-135-9-2519. [DOI] [PubMed] [Google Scholar]
- Sclafani R. A., Fangman W. L. Yeast gene CDC8 encodes thymidylate kinase and is complemented by herpes thymidine kinase gene TK. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5821–5825. doi: 10.1073/pnas.81.18.5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Higgins M. L. Problems of cell wall and membrane growth, enlargement, and division. Ann N Y Acad Sci. 1974 May 10;235(0):161–197. doi: 10.1111/j.1749-6632.1974.tb43265.x. [DOI] [PubMed] [Google Scholar]
- Silverman S. J., Sburlati A., Slater M. L., Cabib E. Chitin synthase 2 is essential for septum formation and cell division in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4735–4739. doi: 10.1073/pnas.85.13.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Kupfer A. The directed migration of eukaryotic cells. Annu Rev Cell Biol. 1986;2:337–365. doi: 10.1146/annurev.cb.02.110186.002005. [DOI] [PubMed] [Google Scholar]
- Sloat B. F., Adams A., Pringle J. R. Roles of the CDC24 gene product in cellular morphogenesis during the Saccharomyces cerevisiae cell cycle. J Cell Biol. 1981 Jun;89(3):395–405. doi: 10.1083/jcb.89.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H., Elwood H. J., Alonso R. A., Peattie D. A. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989 Jan 6;243(4887):75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Herman M. A., Staebell M. A. The involvement of cell wall expansion in the two modes of mycelium formation of Candida albicans. J Gen Microbiol. 1985 Sep;131(9):2367–2375. doi: 10.1099/00221287-131-9-2367. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Mitchell L. H. Filament ring formation in the dimorphic yeast Candida albicans. J Cell Biol. 1983 Feb;96(2):486–493. doi: 10.1083/jcb.96.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Sonneborn D. R. Zoospore germination in Blastocladiella emersonii: cell differentiation without protein synthesis? Proc Natl Acad Sci U S A. 1971 Feb;68(2):459–463. doi: 10.1073/pnas.68.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneborn T. M. Gene action in development. Proc R Soc Lond B Biol Sci. 1970 Dec 1;176(1044):347–366. doi: 10.1098/rspb.1970.0054. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld E. M., Koch A. L., Doyle R. J. Cellular location of origin and terminus of replication in Bacillus subtilis. J Bacteriol. 1985 Sep;163(3):895–899. doi: 10.1128/jb.163.3.895-899.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speksnijder J. E., Miller A. L., Weisenseel M. H., Chen T. H., Jaffe L. F. Calcium buffer injections block fucoid egg development by facilitating calcium diffusion. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6607–6611. doi: 10.1073/pnas.86.17.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staebell M., Soll D. R. Temporal and spatial differences in cell wall expansion during bud and mycelium formation in Candida albicans. J Gen Microbiol. 1985 Jun;131(6):1467–1480. doi: 10.1099/00221287-131-6-1467. [DOI] [PubMed] [Google Scholar]
- Stent G. S. Strength and weakness of the genetic approach to the development of the nervous system. Annu Rev Neurosci. 1981;4:163–194. doi: 10.1146/annurev.ne.04.030181.001115. [DOI] [PubMed] [Google Scholar]
- Stubblefield E. A theory for developmental control by a program encoded in the genome. J Theor Biol. 1986 Jan 21;118(2):129–143. doi: 10.1016/s0022-5193(86)80129-1. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Schmid J., Caldwell J. H., Harold F. M. Transcellular ion currents and extension of Neurospora crassa hyphae. J Membr Biol. 1988;101(1):33–41. doi: 10.1007/BF01872817. [DOI] [PubMed] [Google Scholar]
- Tanford C. The hydrophobic effect and the organization of living matter. Science. 1978 Jun 2;200(4345):1012–1018. doi: 10.1126/science.653353. [DOI] [PubMed] [Google Scholar]
- Taschner P. E., Ypenburg N., Spratt B. G., Woldringh C. L. An amino acid substitution in penicillin-binding protein 3 creates pointed polar caps in Escherichia coli. J Bacteriol. 1988 Oct;170(10):4828–4837. doi: 10.1128/jb.170.10.4828-4837.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M., Chen L. B., Fujiwara K. Microtubules and the endoplasmic reticulum are highly interdependent structures. J Cell Biol. 1986 Oct;103(4):1557–1568. doi: 10.1083/jcb.103.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenzi H. F., Flawia M. M., Tellez-Inon M. T., Torres H. N. Control of Neurospora crassa morphology by cyclic adenosine 3', 5'-monophosphate and dibutyryl cyclic adenosine 3', 5'-monophosphate. J Bacteriol. 1976 Apr;126(1):91–99. doi: 10.1128/jb.126.1.91-99.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D des S., Mullins J. T. Role of enzymatic wall-softening in plant morphogenesis: hormonal induction in Achlya. Science. 1967 Apr 7;156(3771):84–85. doi: 10.1126/science.156.3771.84. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Moskalewski S. Microtubules and the organization of the Golgi complex. Exp Cell Res. 1985 Jul;159(1):1–16. doi: 10.1016/s0014-4827(85)80032-x. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen J. O. Wall replication in saccharomyces species: use of fluorescein-conjugated concanavalin A to reveal the site of mannan insertion. J Gen Microbiol. 1972 Sep;72(2):243–247. doi: 10.1099/00221287-72-2-243. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Esmon P. C., Schekman R. Defective plasma membrane assembly in yeast secretory mutants. J Bacteriol. 1984 Dec;160(3):966–970. doi: 10.1128/jb.160.3.966-970.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker J. B. Spatial organization of microtubule-organizing centers and microtubules. J Cell Biol. 1984 Jul;99(1 Pt 2):55s–62s. doi: 10.1083/jcb.99.1.55s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D. Intracellular transport using microtubule-based motors. Annu Rev Cell Biol. 1987;3:347–378. doi: 10.1146/annurev.cb.03.110187.002023. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Walworth N. C., Novick P. J. Purification and characterization of constitutive secretory vesicles from yeast. J Cell Biol. 1987 Jul;105(1):163–174. doi: 10.1083/jcb.105.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster G., Goodwin B. History and structure in biology. Perspect Biol Med. 1981 Autumn;25(1):39–62. doi: 10.1353/pbm.1981.0063. [DOI] [PubMed] [Google Scholar]
- Willison J. H., Brown R. M., Jr Cell wall structure and deposition in Glaucocystis. J Cell Biol. 1978 Apr;77(1):103–119. doi: 10.1083/jcb.77.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B. Bacteriophage T4 assembly and the morphogenesis of subcellular structure. Harvey Lect. 1979;73:203–223. [PubMed] [Google Scholar]
- de Boer P. A., Crossley R. E., Rothfield L. I. A division inhibitor and a topological specificity factor coded for by the minicell locus determine proper placement of the division septum in E. coli. Cell. 1989 Feb 24;56(4):641–649. doi: 10.1016/0092-8674(89)90586-2. [DOI] [PubMed] [Google Scholar]
- de Jonge B. L., Wientjes F. B., Jurida I., Driehuis F., Wouters J. T., Nanninga N. Peptidoglycan synthesis during the cell cycle of Escherichia coli: composition and mode of insertion. J Bacteriol. 1989 Nov;171(11):5783–5794. doi: 10.1128/jb.171.11.5783-5794.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]