Abstract
Respiratory virus infections cause airway hyperreactivity (AHR). Preventative strategies for virus-induced AHR remain limited. Toll-like receptors (TLRs) have been suggested as a therapeutic target because of their central role in triggering antiviral immune responses. Previous studies showed that concurrent treatment with TLR2/6 and TLR9 agonists reduced lethality and the microbial burden in murine models of bacterial and viral pneumonia. This study investigated the effects of TLR2/6 and TLR9 agonist pretreatment on parainfluenza virus pneumonia and virus-induced AHR in guinea pigs in vivo. Synthetic TLR2/6 lipopeptide agonist Pam2CSK4 and Class C oligodeoxynucleotide TLR9 agonist ODN2395, administered in combination 24 hours before virus infection, significantly reduced viral replication in the lung. Despite a fivefold reduction in viral titers, concurrent TLR2/6 and TLR9 agonist pretreatment did not prevent virus-induced AHR or virus-induced inhibitory M2 muscarinic receptor dysfunction. Interestingly, the TLR agonists independently caused non–M2-dependent AHR. These data confirm the therapeutic antiviral potential of TLR agonists, while suggesting that virus inhibition may be insufficient to prevent virus-induced airway pathophysiology. Furthermore, TLR agonists independently cause AHR, albeit through a distinctly different mechanism from that of parainfluenza virus.
Keywords: Toll-like receptor, airway hyperreactivity, muscarinic receptor, parainfluenza virus
Clinical Relevance
Toll-like receptor (TLR) 2/6 and 9 agonists attenuated parainfluenza virus in the lung. TLR agonists failed to suppress virus-induced airway hyperreactivity (AHR), and may independently induce AHR. Thus, the antiviral therapeutic potential of TLR 2/6 and 9 agonists may be limited by their adverse effects on airway physiology.
Respiratory virus infections commonly cause exacerbations of asthma (1–3) and chronic obstructive pulmonary disease (COPD) (4). In healthy lungs, viruses also cause airway hyperreactivity (AHR; an abnormal tendency of the airways to constrict) (5, 6). Therapies for the prevention of respiratory virus infections are limited to influenza virus (7–10). Currently, no therapies target the respiratory viruses most commonly implicated in asthma and COPD exacerbations, including rhinoviruses and coronaviruses (2), or the prevention of virus-induced AHR.
Respiratory viruses cause AHR by altering the parasympathetic control of bronchoconstriction. Acetylcholine (ACh) released from the parasympathetic vagus nerves activates M3 muscarinic receptors on airway smooth muscle, causing contraction. ACh also activates presynaptic inhibitory M2 muscarinic receptors on the nerves, limiting further ACh release (11). In virus infection, inhibitory M2 receptors are dysfunctional, and this autofeedback mechanism is lost (12). As a result, vagus nerves release more ACh onto airway smooth muscle, potentiating bronchoconstriction (13). Histamine, administered intravenously, causes bronchoconstriction indirectly via activation of the vagus neuronal reflex, and directly through the activation of histamine receptors at the level of airway smooth muscle. Both neuronal and airway smooth muscle physiology can be measured in vivo, using intravenous histamine before and after vagotomy (14). Neuronal inhibitory M2 receptor function and smooth muscle M3 receptor function can be assessed with the M2-selective inhibitor gallamine and intravenous acetylcholine, respectively (13).
Respiratory viruses are detected by Toll-like receptors (TLRs), which are highly conserved pattern-recognition receptors on respiratory epithelia (15, 16), smooth muscle (17), and inflammatory cells (18–21). Ten functional human TLRs have been identified (22). The detection by TLRs of molecular motifs on invading microorganisms triggers immune responses, including the production of interferons, cytokines, and antimicrobial factors via the activation of NF-κB (23). Defective TLR signaling and TLR polymorphisms have been associated with increased susceptibility to infection (24–27).
Because of the central role for TLRs in microbial detection and immune responses, they have garnered interest as therapeutic targets. Recently, concurrent treatment with the synthetic TLR2/6 lipopeptide agonist Pam2CSK4 (Pam2) and the Class C oligodeoxynucleotide TLR9 agonist ODN2395 (ODN) was found to reduce lethality and the microbial burden in murine models of bacterial and viral pneumonia (28, 29). Treatment with the individual TLR agonists separately conferred little protection. Furthermore, the Class C oligodeoxynucleotide TLR9 agonists were superior to Class A or B oligodeoxynucleotides, possibly as a result of the Class C induction of both interferons and NF-κB–related cytokines, compared with interferons (Class A) or NF-κB–related cytokines (Class B) alone (29). In other settings, a TLR9 agonist has been used as a vaccine adjuvant to boost immunity against respiratory syncytial virus (30). Treatment with TLR2/6 agonists or TLR9 agonists has also been shown to reduce allergen-mediated AHR via the suppression of allergic Type 2 T-helper (Th2) cell inflammation (31–33). Despite this body of work, the potential of TLRs as therapeutic targets for the prevention of virus-induced AHR remains unknown.
This work investigated the effects of simultaneous pretreatment with TLR2/6 agonist Pam2 and TLR9 agonist ODN, administered 24 hours before infection, on parainfluenza virus replication and virus-induced AHR in a guinea model of viral pneumonia in vivo. The neural control of airway function in guinea pigs is similar to that in humans, making it a relevant model to investigate mechanisms of virus-induced AHR. We hypothesized that Pam2/ODN pretreatment would attenuate parainfluenza viral titers and alleviate virus-induced AHR. We found that Pam2/ODN significantly reduced viral replication in the guinea pig lung. Despite a fivefold reduction in viral titers, pretreatment did not protect against virus-induced AHR or M2 muscarinic dysfunction. Interestingly, Pam2/ODN caused AHR independent of virus infection, which was not attributable to M2 receptor dysfunction, suggesting that TLR2/6 and TLR9 agonists cause AHR in a different manner from parainfluenza virus in guinea pigs.
Materials and Methods
Animals
Pathogen-free female Hartley guinea pigs (300–400 g; Charles River Breeding Laboratories, Wilmington, MA) were handled in accordance with National Institutes of Health guidelines. Our protocols were approved by the Institutional Animal Care and Use Committee at Oregon Health and Science University.
Study Protocol
Guinea pigs were pretreated with TLR2/6 agonist Pam2 (4 nmol) and TLR9 agonist ODN (1 nmol), or PBS vehicle, delivered as an aerosol into the trachea (referred to as tracheal) with a Penn-Century Microsprayer (Penn-Century, Wyndmoor, PA), or in solution into the right nostril (referred to as nasal), 24 hours before infection. Guinea pigs were infected with 106 tissue culture infectious dose/ml parainfluenza (Sendai) virus or PBS instilled intranasally, as previously described (12). Airway function was assessed in vivo 4 days after virus infection. After the functional experiments, guinea pigs received a lethal dose of anesthesia, and were exsanguinated from the abdominal aorta. Bronchoalveolar lavage (BAL) and peripheral blood leukocyte counts, along with lung viral content, were assessed after the guinea pigs had been killed.
Measurements of Airway Physiology
Guinea pigs were anesthetized with urethane (1.9 g/kg, administered intraperitoneally) and paralyzed with succinylcholine (10 μg/kg · min, administered intravenously), and their jugular veins and right carotid arteries were cannulated. Animals were tracheotomized and ventilated through a tracheal cannula with a rodent respirator (2.5 ml volume, 100 breaths/min; Harvard Apparatus, Inc., South Natick, MA). Peak pulmonary pressures (Ppeak; mm H2O) during each inspiration were measured at the trachea, using a BD DTXplus pressure transducer (Viggo-Spectramed, Oxnard, CA). Increases in Ppeak reflect changes in airflow resistance attributable to changes in airway caliber (34). Bronchoconstriction (measured as an increase in Ppeak over baseline) was induced by histamine (1–5 mg/kg, intravenous) before and after vagotomy, and by acetylcholine (1–10 μg/kg, intravenous) after vagotomy.
Studies of M2 Muscarinic Receptor Function
Bronchoconstrictions were induced by electrical stimulation of the vagus nerves. The vagus nerves were ligated, attached to platinum electrodes, and stimulated at 40-second intervals (8 V, 15 Hz, 2-ms duration, 3 s on, 40 s off). The M2 muscarinic receptor antagonist gallamine (0.1–10 mg/kg, intravenous) was injected after every fourth period of vagal stimulation. The effect of gallamine on vagally induced bronchoconstriction was measured as the ratio of bronchoconstriction in the presence of gallamine to bronchoconstriction in the absence of gallamine.
Virus Isolation and Titration
Viral titers were assessed by real-time RT-PCR from homogenized lung samples, as described in the online supplement.
Drugs and Reagents
Histamine, gallamine, acetylcholine, succinylcholine, and urethane were purchased from Sigma-Aldrich (St. Louis, MO). Pam2 and ODN were obtained from Invivogen (San Diego, CA).
Statistical Analysis
Data are expressed as means ± SEMs. Histamine-induced, gallamine-induced, and acetylcholine-induced responses were analyzed using two-way ANOVA for repeated measures. Baseline data and leukocyte counts were analyzed using one-way ANOVA. Viral titers were compared using the Student t test. All statistical analyses were performed using Prism (GraphPad Software, La Jolla, CA). P < 0.05 was considered significant.
RESULTS
Baseline Physiologic Characteristics
Baseline Ppeak (a measure of baseline airway resistance) before the pharmacologic experiments was significantly increased by virus infection, compared with control guinea pigs (Table 1). Pretreatment with Pam2/ODN partly attenuated virus-induced elevations in baseline bronchoconstriction. Pam2/ODN pretreatment did not affect baseline bronchoconstriction in the absence of virus infection. No significant differences were evident in baseline heart rate, systolic blood pressure, diastolic blood pressure, or weight between groups.
TABLE 1.
BASELINE PHYSIOLOGIC CHARACTERISTICS
| |
Tracheal |
Nasal |
||||||
|---|---|---|---|---|---|---|---|---|
| Control | Pam2/ODN | Virus | Pam2/ODN + Virus | Control | Pam2/ODN | Virus | Pam2/ODN + Virus | |
| Ppeak |
90.0 (2.9) |
99.0 (1.0) |
159.6 (13.8)* |
133.5 (11.7) |
82.5 (4.4) |
80.0 (7.3) |
115.0 (19.1) |
101.7 (6.0) |
| HR |
278.8 (1.3) |
298.8 (5.2) |
297.7 (8.3) |
306.7 (4.3) |
273.3 (14.4) |
291.7 (11.8) |
288.3 (11.7) |
300.8 (12.9) |
| SBP |
47.8 (1.4) |
47.5 (2.9) |
51.4 (3.0) |
52.3 (3.1) |
42.0 (2.9) |
49.9 (2.8) |
46.3 (2.0) |
51.3 (3.6) |
| DBP |
24.0 (2.0) |
22.5 (1.0) |
25.2 (2.4) |
27.0 (2.1) |
19.8 (1.5) |
26.3 (2.8) |
29.0 (2.3) |
27.3 (3.0) |
| Weight | 0.371 (0.0004) | 0.381 (0.013) | 0.345 (0.007) | 0.3734 (0.018) | 0.371 (0.022) | 0.377 (0.026) | 0.356 (0.019) | 0.354 (0.007) |
Definition of abbreviations: DBP, diastolic blood pressure; HR, heart rate; ODN, ODN2395; Pam2, Pam2CSK4; Ppeak, Peak pulmonary pressure; SBP, systolic blood pressure.
Baseline data represent the means ± SEMs before pharmacologic manipulation.
P < 0.05, compared with control samples.
Effect of TLR2/6 and TLR9 Agonist Pretreatment on Parainfluenza Virus Replication
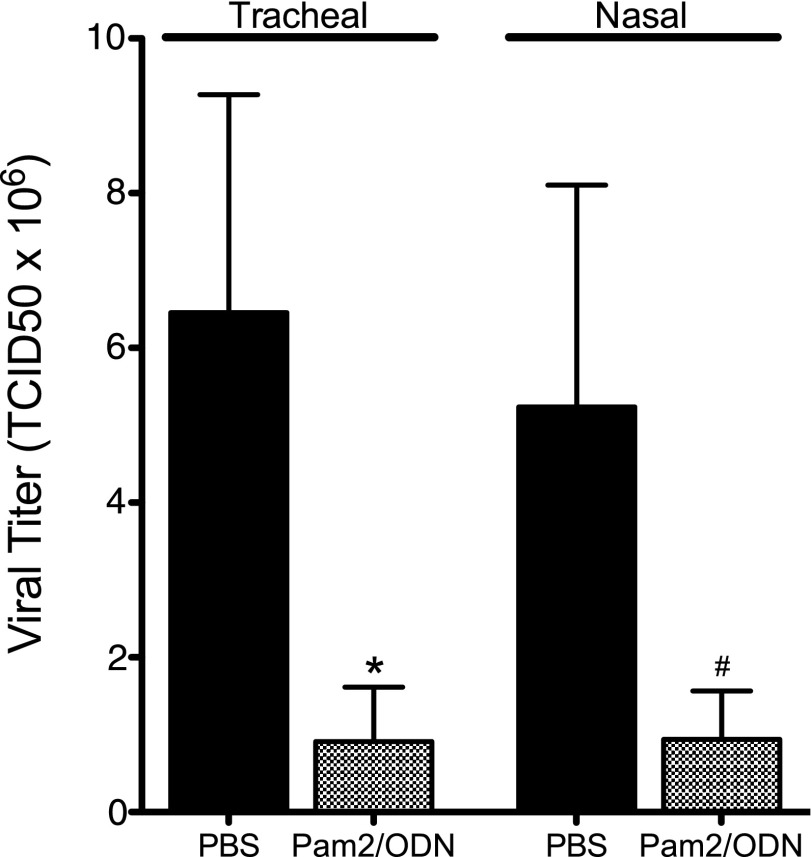
TLR2/6 agonist Pam2 and TLR9 agonist ODN, administered simultaneously 24 hours before infection, reduced parainfluenza virus replication in the lungs (Figure 1). This antiviral effect was profound, resulting in an 80% reduction in parainfluenza virus mRNA 4 days after infection. This treatment effect was present with both tracheal and nasal deliveries of TLR agonists.
Figure 1.
Pam2CSK4 (TLR2/6) and ODN2395 (TLR9) synergistically inhibit parainfluenza virus replication in vivo. Guinea pigs were pretreated simultaneously with Pam2CSK4 (4 nmol) and ODN2395 (1 nmol), or with PBS vehicle, administered as an aerosol directly into the trachea (Tracheal), or as a nasal solution (Nasal), 24 hours before parainfluenza virus infection. Parainfluenza RNA from lung homogenates was quantified by RT-PCR, 4 days after infection. Both tracheal and nasal Pam2/ODN pretreatment inhibited virus replication, although the effect of nasal Pam2/ODN fell short of statistical significance (tracheal, Pam2/ODN, n = 12; PBS, n = 11; *P < 0.05; nasal, Pam2/ODN, n = 6; PBS, n = 10; #P = 0.08). Viral titers were normalized to 18S RNA and transformed to tissue culture infectious dose (TCID) 50 titers using a parainfluenza standard curve quantified by rhesus monkey kidney cell titration. Data shown represent the means ± standard errors of the mean.
Effect of TLR2/6 and TLR9 Agonists on Virus-Induced Vagal-Reflex Airway Hyperreactivity
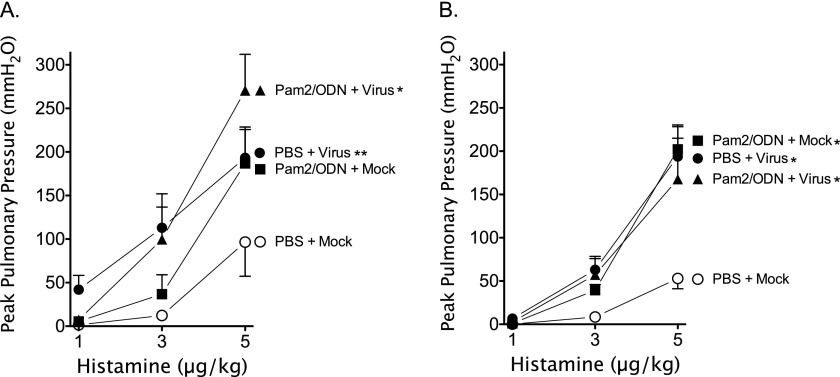
Histamine-induced bronchoconstriction (1–5 μg/kg, intravenous) before vagotomy assesses the neuronal control of airway tone by activating efferent parasympathetic vagus nerves. Both virus infection and Pam2/ODN pretreatment independent of virus infection potentiated histamine-induced vagal-reflex bronchoconstriction in guinea pigs (Figures 2A and 2B). Despite the significant reduction in parainfluenza virus replication in the lung attributable to Pam2/ODN pretreatment, no attenuation of AHR was evident in virus-infected guinea pigs.
Figure 2.
Parainfluenza virus and Pam2CSK4 (TLR2/6)/ODN2395 (TLR9) cause vagal-reflex airway hyperreactivity. Guinea pigs were pretreated simultaneously with Pam2CSK4 (4 nmol) and ODN2395 (1 nmol), or with PBS vehicle, administered as an aerosol directly into the trachea (Tracheal), or as a nasal solution (Nasal), 24 hours before parainfluenza virus or mock infection. Histamine (1–5 μg/kg, intravenous) induced dose-dependent bronchoconstrictions, measured as increases in peak pulmonary pressures (Ppeak), by activating the vagal-reflex innervation of the airways. Parainfluenza infection (PBS + Virus) and Pam2CSK4/ODN2395 pretreatment (Pam2/ODN + Mock) independently, and concomitantly (Pam2/ODN + Virus), potentiated vagal-reflex bronchoconstriction. (A) Aerosolized pretreatment administered into the trachea (n = 4 per group). (B) Nasal solution pretreatment (n = 5 per group). Data shown represent the means ± standard errors of the mean. *P < 0.05, compared with PBS + Mock control group. **P = 0.053, compared with PBS + Mock control group.
Effect of TLR2/6 and TLR9 Agonists on Virus-Induced Non-Neuronal Airway Hyperreactivity
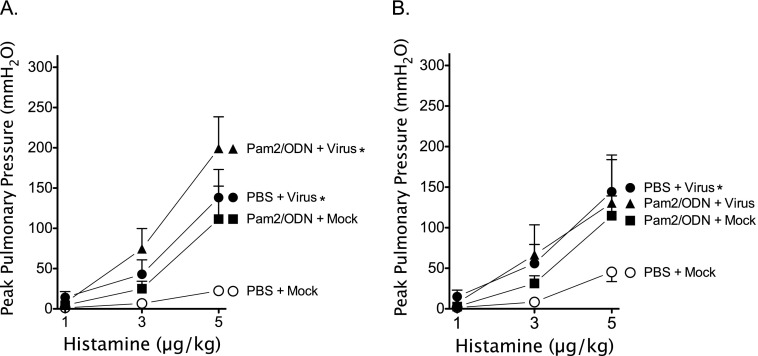
Histamine-induced bronchoconstriction (1–5 μg/kg, intravenous) after vagotomy measures airway responsiveness without vagal-reflex input. Histamine-induced bronchoconstriction after vagotomy was potentiated by virus infection, and by Pam2/ODN pretreatment independent of virus infection (Figures 3A and 3B). Pam2/ODN pretreatment did not attenuate non-neuronal AHR in virus-infected guinea pigs, despite significant reductions in parainfluenza virus replication in the lungs.
Figure 3.
Parainfluenza virus and Pam2CSK4 (TLR2/6)/ODN2395 (TLR9) cause non-neuronal airway hyperreactivity. Guinea pigs were pretreated simultaneously with Pam2CSK4 (4 nmol) and ODN2395 (1 nmol), or with PBS vehicle, administered as an aerosol directly into the trachea (Tracheal), or as a nasal solution (Nasal), 24 hours before parainfluenza virus or mock infection. Histamine (1–5 μg/kg, intravenous) induced dose-dependent bronchoconstrictions, measured as increases in Ppeak, after vagotomy by activating histamine receptors at the level of the airways. Parainfluenza virus infection (PBS + Virus) and Pam2CSK4/ODN2395 pretreatment (Pam2/ODN + Mock) independently, and concomitantly (Pam2/ODN + Virus), potentiated bronchoconstriction. (A) Aerosol pretreatment administered directly into the trachea (n = 4 per group). (B) Nasal solution pretreatment (n = 5 per group). Data shown represent the means ± standard errors of the mean. *P < 0.05, compared with PBS + Mock control group.
Effect of TLR2/6 and TLR9 Agonists on Virus-Induced M2 Muscarinic Receptor Dysfunction
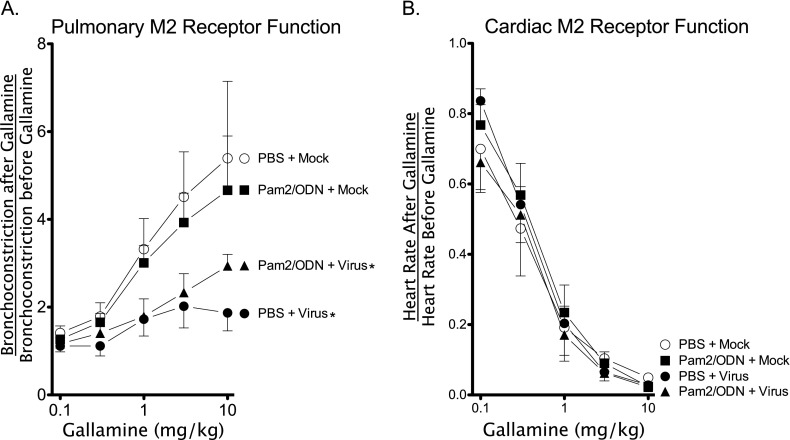
Gallamine (0.1–10 mg/kg, intravenous) increases vagally mediated bronchoconstriction by blocking inhibitory presynaptic vagal M2 muscarinic receptors, thereby increasing acetylcholine release at the neuromuscular junction. Lack of an increase in vagally mediated bronchoconstriction after gallamine administration indicates M2 receptor dysfunction. Virus infection caused M2 muscarinic receptor dysfunction in both vehicle control and Pam2/ODN-pretreated guinea pigs compared with mock-infected control guinea pigs (Figure 4A). Interestingly, although TLR agonists potentiated vagal-reflex and non-neuronal bronchoconstriction in mock-infected animals, they did not cause M2 receptor dysfunction.
Figure 4.
Parainfluenza infection, but not Pam2CSK4 (TLR2/6)/ODN2395 (TLR9), causes pulmonary M2 muscarinic receptor dysfunction. Guinea pigs were pretreated with Pam2CSK4 (4 nmol) and ODN2395 (1 nmol) in combination, or with PBS vehicle, administered as a nasal solution 24 hours before parainfluenza virus or mock infection. Transient bronchoconstrictions were produced by electrical stimulation of the vagus nerves (8 V, 15 Hz, 2-ms duration, 3 seconds on, 40 seconds off). Gallamine (1–10 mg/kg, intravenous), an M2 muscarinic receptor antagonist, was administered after every fourth bronchoconstriction. (A) Gallamine potentiated vagally induced bronchoconstriction by inhibiting M2 muscarinic receptor function in control (PBS + Mock) and in mock-infected Pam2CSK4/ODN2395 pretreated guinea pigs (Pam2/ODN + Mock), but not in virus-infected guinea pigs (PBS + Virus). Pam2CSK4/ODN2395 pretreatment did not prevent virus-induced M2 receptor dysfunction (Pam2/ODN + Virus; n = 5 per group). (B) Gallamine blocked vagally induced decreases in heart rate by inhibiting cardiac myocyte M2 receptors. Gallamine blocked decreases in heart rate similarly in all groups (n = 5 per group). Data shown represent the means ± standard errors of the mean. *P < 0.05, compared with PBS + Mock control group.
Inhibitory M2 muscarinic receptors are also present on cardiac muscle, and reduce heart rate in response to vagal stimulation. Gallamine (0.1–10 mg/kg, intravenous) inhibited vagally mediated bradycardia in all treatment groups (Figure 4B), indicating that neither the virus nor the TLR agonists cause dysfunction of cardiac inhibitory M2 receptors.
Effect of TLR2/6 and TLR9 Agonists on M3 Muscarinic Receptor Function
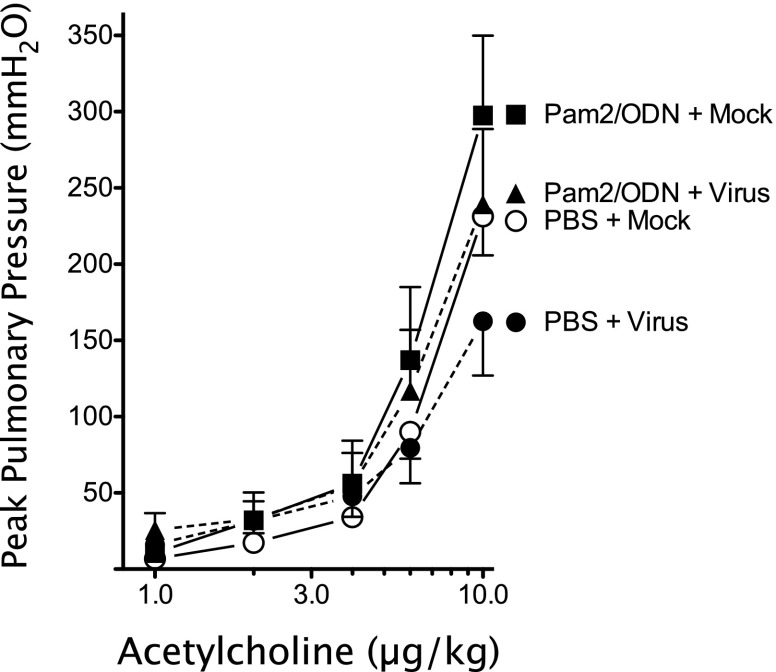
Intravenous acetylcholine causes bronchoconstriction by activating airway smooth muscle M3 muscarinic receptors. No differences in acetylcholine-induced bronchoconstriction (1–10 μg/kg, intravenous) were evident between groups (Figure 5). This demonstrates that airway smooth muscle M3 muscarinic receptors are not affected by virus infection or by TLR 2/6 and TLR9 agonists.
Figure 5.
Pulmonary M3 muscarinic receptor function is unaffected by parainfluenza infection or Pam2CSK4 (TLR2/6)/ODN2395 (TLR9) pretreatment. Acetylcholine (1–10 μg/kg, intravenous) caused bronchoconstriction, measured as increases in Ppeak, by activating airway smooth muscle M3 muscarinic receptors. Acetylcholine-induced bronchoconstriction was similar between groups (n = 5 per group). Data shown represent the means ± standard errors of the mean.
Effects of TLR2/6 and TLR9 Agonists and Virus on Bronchoalveolar Lavage and Peripheral Leukocyte Counts
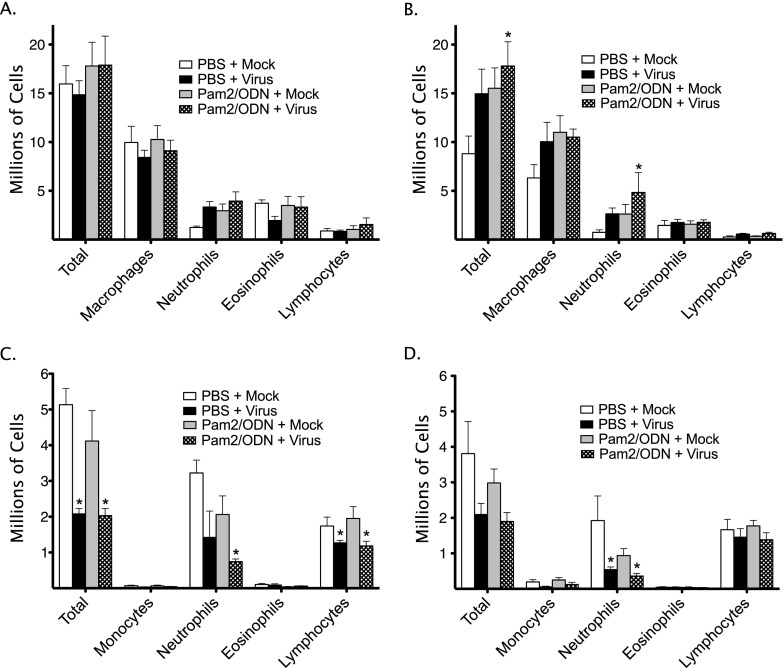
Virus infection and pretreatment with Pam2/ODN increased total BAL leukocytes (Figures 6A and 6B). In both cases, the increase in total leukocytes was largely attributable to increases in macrophages and neutrophils. Treatment with aerosol PBS vehicle, but not nasal PBS vehicle, increased total BAL leukocytes, independent of virus infection or TLR agonists. No differences in eosinophils or lymphocytes were detected between the groups in either the aerosol or nasal treatment cohort.
Figure 6.
Parainfluenza infection and Pam2CSK4 (TLR2/6)/ODN2395 (TLR9) cause pulmonary and systemic inflammatory cell changes. Guinea pigs were pretreated with Pam2CSK4 (4 nmol) and ODN2395 (1 nmol) in combination, or with PBS vehicle, administered as an aerosol directly into the trachea (Tracheal), or as a nasal solution (Nasal), 24 hours before parainfluenza virus or mock infection. Bronchoalveolar (BAL) and peripheral blood total and differential leukocyte counts were evaluated 4 days after virus infection. (A) Tracheal pretreatment BAL counts (n = 6 per group). (B) Nasal pretreatment BAL counts (n = 5 per group). (C) Tracheal pretreatment peripheral leukocyte counts (n = 6 per group). (D) Nasal pretreatment peripheral leukocyte counts (n = 5 per group). Data shown represent the means ± standard errors of the mean. *P < 0.05, compared with PBS + Mock control group.
Virus infection reduced peripheral blood total leukocyte counts (Figures 6C and 6D). Specifically, virus infection decreased lymphocytes and neutrophils. In mock-infected animals, Pam2/ODN pretreatment partly decreased neutrophils, although this effect did not reach statistical significance. No changes in peripheral eosinophils or monocytes were evident.
Discussion
Virus infections of the respiratory tract are a major cause of asthma and COPD exacerbations. Therapeutic strategies for the prevention of virus infection and virus-induced AHR remain limited (7–10). The experiments described in this report were designed to assess the effects of TLR2/6 and TLR9 agonists on parainfluenza virus replication and virus-induced AHR in the guinea pig lung. Pam2 (TLR2/6) and ODN (TLR9) were chosen because of previous work demonstrating their synergistic antimicrobial effects in mice (28, 29). Our data indicate that simultaneous pretreatment with TLR2/6 and TLR9 agonists, administered as an aerosol directly into the trachea or as a nasal solution, decreases viral replication in guinea pig lungs. This work was the first to establish the potent TLR2/6 and TLR9 agonist antiviral effect in this animal model. Moreover, we show that TLR2/6 and TLR9 agonists exert an antiviral effect that is conserved across species, and we demonstrate two therapeutic delivery methods (nasal and inhalational) applicable to future drug development in humans. Furthermore, guinea pig airway nerve function and anatomy resemble those in humans, making it more applicable than a murine model for investigating in vivo dynamic airway responsiveness to a variety of stimuli (35).
TLRs are central to immune responses against invading microbes. The TLR agonists used in these experiments targeted both a virus-sensing TLR (TLR9) and a bacterial-sensing TLR (TLR2/6) (23). Interestingly, the synergistic antimicrobial effect of TLR2/6 and TLR9 agonists was lost when these agonists were administered individually in mice (28, 29). This effect was dependent on the classic TLR–MyD88 signaling pathway, but was not dependent on the presence of leukocytes, suggesting that airway epithelial cells are capable of inducing the TLR agonist response (36, 37). Furthermore, the synergistic effect of TLR9 agonists with TLR2/6 agonists was greatest with Class C oligodeoxynucleotides compared with Class A or B oligodeoxynucleotides, possibly because of the induction by Class C of interferons and the transcription of cytokines via NF-κB, compared with either interferons (Class A) or NF-κB–related cytokines (Class B) alone (38). Determining the relative contributions of these specific pathways to the effects of Pam2/ODN was beyond the scope of this study. However, the available evidence suggests that Pam2/ODN pretreatment synergistically triggers TLR2/6 and TLR9 to promote an antiviral milieu capable of inhibiting viral replication at the onset of infection.
Despite significant reductions in parainfluenza virus replication in vivo attributable to TLR2/6 and TLR9 agonist pretreatment, no improvement in viral-induced vagal-reflex AHR was evident. This lack of improvement in AHR may be attributable to an incomplete inhibition of virus replication in vivo. This is surprising, however, because previous work has demonstrated improvements in virus-induced airway dysfunction with the suppression of viral titers (39). Interestingly, the TLR agonists caused AHR even in the absence of virus infection, which may counteract the positive effects of the suppression of viral replication. This work is the first to establish that TLR2/6 and TLR9 agonists cause AHR in the nonallergic lung. This finding stands in contrast with studies of allergen-sensitized mice, in which individual TLR agonists did not cause AHR (31–33), although the combination of TLR agonists used in the present study was not specifically tested. Despite TLR2/6- and TLR9-mediated reduction of respiratory virus replication, it remains unclear whether this technique may provide net benefit in virus-induced exacerbations of lung disease.
Virus infection and pretreatment with TLR2/6 and TLR9 agonists also induced AHR in vagotomized guinea pigs. Vagotomy removes the efferent vagal-nerve input to the airways, allowing airway smooth muscle responsiveness to histamine to predominate. The TLR agonists did not prevent nonreflex virus-induced AHR, despite significant reductions in viral titers. The link between TLR activation and non-neuronal histamine-induced AHR has not previously been reported, and suggests that TLR agonists and virus infection cause AHR through both neuronal and non-neuronal mechanisms.
TLR agonists and virus infection produced significant changes in BAL and peripheral blood leukocyte counts. The nasal delivery of TLR agonists with or without virus infection produced a doubling of total BAL leukocytes, attributable to significant increases in macrophages and neutrophils. Conversely, nasal TLR agonists caused a decline in systemic neutrophils, but no difference in total leukocytes. Tracheal aerosol TLR2/6 and TLR9 pretreatment also caused an increase in total BAL leukocytes and neutrophils, and a decline in peripheral blood neutrophils. Surprisingly, aerosolized PBS vehicle also increased total BAL leukocytes in control guinea pigs. Despite this leukocyte influx, aerosolized PBS vehicle did not increase bronchoconstriction. This suggests that TLR agonist–mediated AHR is not caused by leukocyte influx into the airway lumen, or that leukocytes require activation by virus or TLR agonists to produce AHR.
Similar to previous work (12), virus infection caused the dysfunction of vagal presynaptic inhibitory M2 muscarinic receptors, but not cardiac M2 receptors. Despite significant reductions in viral titers, pretreatment with TLR2/6 and TLR9 agonists did not prevent virus-induced vagal M2 dysfunction. Interestingly, TLR2/6 and TLR9 pretreatment caused AHR in the absence of virus infection, but did not cause M2 receptor dysfunction. Thus, TLRs produce AHR in a different manner from virus infection. Our data indicate this is not attributable to functional changes in airway smooth muscle M3 muscarinic receptors, because no differences were seen in the airway smooth muscle response to intravenous acetylcholine. Similarly, the potentiation of histamine-induced vagal-reflex and non-neuronal bronchoconstriction by virus and TLR agonists is not likely attributable to changes in the intrinsic contractility of the smooth muscle, because such changes would likely affect the responses to all agonists.
Our results are the first to demonstrate the antiviral effects of TLR2/6 and TLR9 agonist pretreatment on parainfluenza virus infection in guinea pigs, using two therapeutically relevant delivery techniques. Despite significant reductions in virus, the TLR agonists did not protect against virus-induced AHR or virus-induced M2 receptor dysfunction. Interestingly, TLR2/6 and TLR9 agonists produced AHR in both ligand delivery models independent of virus infection, but without dysfunction of the prejunctional inhibitory M2 muscarinic receptors. This suggests that TLR2/6 and TLR9 stimulation can be therapeutically useful for their antiviral effect, but may be limited by the physiological effects of the TLR agonists themselves in the airways.
Footnotes
This work was supported by National Institutes of Health grants HL55543 (A.D.F.), HL54659, HL071795, and ES014601 (D.B.J.), and HL115903 (B.F.D.). M.G.D. is supported by National Institutes of Health grant T-32 HL-83808.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0498OC on February 28, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Atmar RL, Guy E, Guntupalli KK, Zimmerman JL, Bandi VD, Baxter BD, Greenberg SB. Respiratory tract viral infections in inner-city asthmatic adults. Arch Intern Med. 1998;158:2453–2459. doi: 10.1001/archinte.158.22.2453. [DOI] [PubMed] [Google Scholar]
- 2.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O’Toole S, Myint SH, Tyrrell DA, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kherad O, Bridevaux PO, Kaiser L, Janssens JP, Rutschmann O. Viral infection in acute exacerbation of COPD. Rev Med Suisse. 2011;7:2222–2226. [PubMed] [Google Scholar]
- 5.Aquilina AT, Hall WJ, Douglas RG, Jr, Utell MJ. Airway reactivity in subjects with viral upper respiratory tract infections: the effects of exercise and cold air. Am Rev Respir Dis. 1980;122:3–10. doi: 10.1164/arrd.1980.122.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Empey DW, Laitinen LA, Jacobs L, Gold WM, Nadel JA. Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. Am Rev Respir Dis. 1976;113:131–139. doi: 10.1164/arrd.1976.113.2.131. [DOI] [PubMed] [Google Scholar]
- 7.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2005;54:1–40. [PubMed] [Google Scholar]
- 8.Cates CJ, Jefferson TO, Rowe BH. Vaccines for preventing influenza in people with asthma. Cochrane Database Syst Rev. 2008;(2):CD000364. doi: 10.1002/14651858.CD000364.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Jefferson T, Demicheli V, Di Pietrantonj C, Rivetti D. Amantadine and rimantadine for influenza A in adults. Cochrane Database Syst Rev. 2006;2:CD001169. doi: 10.1002/14651858.CD001169.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med. 2005;353:1363–1373. doi: 10.1056/NEJMra050740. [DOI] [PubMed] [Google Scholar]
- 11.Fryer AD, Maclagan J. Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol. 1984;83:973–978. doi: 10.1111/j.1476-5381.1984.tb16539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fryer AD, Jacoby DB. Parainfluenza virus infection damages inhibitory M2 muscarinic receptors on pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol. 1991;102:267–271. doi: 10.1111/j.1476-5381.1991.tb12164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fryer AD, Wills-Karp M. Dysfunction of M2-muscarinic receptors in pulmonary parasympathetic nerves after antigen challenge. J Appl Physiol. 1991;71:2255–2261. doi: 10.1152/jappl.1991.71.6.2255. [DOI] [PubMed] [Google Scholar]
- 14.Undem BJ, Carr MJ. Pharmacology of airway afferent nerve activity. Respir Res. 2001;2:234–244. doi: 10.1186/rr62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene CM, Carroll TP, Smith SG, Taggart CC, Devaney J, Griffin S, O’Neill SJ, McElvaney NG. TLR-induced inflammation in cystic fibrosis and non–cystic fibrosis airway epithelial cells. J Immunol. 2005;174:1638–1646. doi: 10.4049/jimmunol.174.3.1638. [DOI] [PubMed] [Google Scholar]
- 16.Cherfils-Vicini J, Platonova S, Gillard M, Laurans L, Validire P, Caliandro R, Magdeleinat P, Mami-Chouaib F, Dieu-Nosjean MC, Fridman WH, et al. Triggering of TLR7 and TLR8 expressed by human lung cancer cells induces cell survival and chemoresistance. J Clin Invest. 2010;120:1285–1297. doi: 10.1172/JCI36551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sukkar MB, Xie S, Khorasani NM, Kon OM, Stanbridge R, Issa R, Chung KF. Toll-like receptor 2, 3, and 4 expression and function in human airway smooth muscle. J Allergy Clin Immunol. 2006;118:641–648. doi: 10.1016/j.jaci.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Muzio M, Bosisio D, Polentarutti N, D’Amico G, Stoppacciaro A, Mancinelli R, van’t Veer C, Penton-Rol G, Ruco LP, Allavena P, et al. Differential expression and regulation of Toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 19.Juarez E, Nunez C, Sada E, Ellner JJ, Schwander SK, Torres M. Differential expression of Toll-like receptors on human alveolar macrophages and autologous peripheral monocytes. Respir Res. 2010;11:2. doi: 10.1186/1465-9921-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koller B, Bals R, Roos D, Korting HC, Griese M, Hartl D. Innate immune receptors on neutrophils and their role in chronic lung disease. Eur J Clin Invest. 2009;39:535–547. doi: 10.1111/j.1365-2362.2009.02145.x. [DOI] [PubMed] [Google Scholar]
- 21.Wong JP, Christopher ME, Viswanathan S, Karpoff N, Dai X, Das D, Sun LQ, Wang M, Salazar AM. Activation of Toll-like receptor signaling pathway for protection against influenza virus infection. Vaccine. 2009;27:3481–3483. doi: 10.1016/j.vaccine.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 23.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhan U, Lukacs NW, Osterholzer JJ, Newstead MW, Zeng X, Moore TA, McMillan TR, Krieg AM, Akira S, Standiford TJ. TLR9 is required for protective innate immunity in Gram-negative bacterial pneumonia: role of dendritic cells. J Immunol. 2007;179:3937–3946. doi: 10.4049/jimmunol.179.6.3937. [DOI] [PubMed] [Google Scholar]
- 25.Wu Q, Jiang D, Minor MN, Martin RJ, Chu HW. In vivo function of airway epithelial TLR2 in host defense against bacterial infection. Am J Physiol Lung Cell Mol Physiol. 2011;300:L579–L586. doi: 10.1152/ajplung.00336.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carvalho A, Cunha C, Carotti A, Aloisi T, Guarrera O, Di Ianni M, Falzetti F, Bistoni F, Aversa F, Pitzurra L, et al. Polymorphisms in Toll-like receptor genes and susceptibility to infections in allogeneic stem cell transplantation. Exp Hematol. 2009;37:1022–1029. doi: 10.1016/j.exphem.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland AM, Walley KR, Nakada TA, Sham AH, Wurfel MM, Russell JA. A nonsynonymous polymorphism of IRAK4 associated with increased prevalence of Gram-positive infection and decreased response to Toll-like receptor ligands. J Innate Immun. 2011;3:447–458. doi: 10.1159/000323880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuvim MJ, Gilbert BE, Dickey BF, Evans SE. Synergistic TLR2/6 and TLR9 activation protects mice against lethal influenza pneumonia. PLoS ONE. 2012;7:e30596. doi: 10.1371/journal.pone.0030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duggan JM, You D, Cleaver JO, Larson DT, Garza RJ, Guzman Pruneda FA, Tuvim MJ, Zhang J, Dickey BF, Evans SE. Synergistic interactions of TLR2/6 and TLR9 induce a high level of resistance to lung infection in mice. J Immunol. 2011;186:5916–5926. doi: 10.4049/jimmunol.1002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shafique M, Wilschut J, de Haan A. Induction of mucosal and systemic immunity against respiratory syncytial virus by inactivated virus supplemented with TLR9 and NOD2 ligands. Vaccine. 2012;30:597–606. doi: 10.1016/j.vaccine.2011.11.054. [DOI] [PubMed] [Google Scholar]
- 31.Ramaprakash H, Hogaboam CM. Intranasal CPG therapy attenuated experimental fungal asthma in a TLR9-dependent and -independent manner. Int Arch Allergy Immunol. 2010;152:98–112. doi: 10.1159/000265531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kline JN, Waldschmidt TJ, Businga TR, Lemish JE, Weinstock JV, Thorne PS, Krieg AM. Modulation of airway inflammation by CPG oligodeoxynucleotides in a murine model of asthma. J Immunol. 1998;160:2555–2559. [PubMed] [Google Scholar]
- 33.Fuchs B, Knothe S, Rochlitzer S, Nassimi M, Greweling M, Lauenstein HD, Nassenstein C, Muller M, Ebensen T, Dittrich AM, et al. A Toll-like receptor 2/6 agonist reduces allergic airway inflammation in chronic respiratory sensitisation to timothy grass pollen antigens. Int Arch Allergy Immunol. 2010;152:131–139. doi: 10.1159/000265534. [DOI] [PubMed] [Google Scholar]
- 34.Blaber LC, Fryer AD, Maclagan J. Neuronal muscarinic receptors attenuate vagally-induced contraction of feline bronchial smooth muscle. Br J Pharmacol. 1985;86:723–728. doi: 10.1111/j.1476-5381.1985.tb08951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canning BJ, Chou Y. Using guinea pigs in studies relevant to asthma and COPD. Pulm Pharmacol Ther. 2008;21:702–720. doi: 10.1016/j.pupt.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clement CG, Evans SE, Evans CM, Hawke D, Kobayashi R, Reynolds PR, Moghaddam SJ, Scott BL, Melicoff E, Adachi R, et al. Stimulation of lung innate immunity protects against lethal pneumococcal pneumonia in mice. Am J Respir Crit Care Med. 2008;177:1322–1330. doi: 10.1164/rccm.200607-1038OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans SE, Xu Y, Tuvim MJ, Dickey BF. Inducible innate resistance of lung epithelium to infection. Annu Rev Physiol. 2010;72:413–435. doi: 10.1146/annurev-physiol-021909-135909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vollmer J, Krieg AM. Immunotherapeutic applications of CPG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Fryer AD, Yarkony KA, Jacoby DB. The effect of leukocyte depletion on pulmonary M2 muscarinic receptor function in parainfluenza virus–infected guinea-pigs. Br J Pharmacol. 1994;112:588–594. doi: 10.1111/j.1476-5381.1994.tb13115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]