Abstract
Human eosinophils display directed chemotactic activity toward an array of soluble chemokines. Eosinophils have been observed to migrate to draining lymph nodes in experimental models of allergic inflammation, yet it is unknown whether eosinophils express CCR7, a key chemokine receptor in coordinating leukocyte trafficking to lymph nodes. The purpose of this study is to demonstrate expression of CCR7 by human eosinophils and functional responses to CCL19 and CCL21, the known ligands of CCR7. Human eosinophils were purified by negative selection from healthy donors. CCR7 expression of freshly purified, unstimulated eosinophils and of IL-5–primed eosinophils was determined by flow cytometry and Western blot. Chemotaxis to CCL19 and CCL21 was measured in transwell assays. Shape changes to CCL19 and CCL21 were analyzed by flow cytometry and microscopy. Calcium fluxes of fluo-4 AM–loaded eosinophils were recorded by flow cytometry after chemokine stimulation. ERK phosphorylation of CCL19- and CCL21-stimulated eosinophils was measured by Western blot and Luminex assay. Human eosinophils expressed CCR7 as demonstrated by flow cytometry and Western blots. Eosinophils exhibited detectable cell surface expression of CCR7. IL-5–primed eosinophils exhibited chemotaxis toward CCL19 and CCL21 in a dose-dependent fashion. Upon stimulation with CCL19 or CCL21, IL-5–primed eosinophils demonstrated dose-dependent shape changes with polarization of F-actin and exhibited calcium influxes. Finally, primed eosinophils stimulated with CCL19 or CCL21 exhibited increased phosphorylation of ERK in response to both CCR7 ligands. We demonstrate that human eosinophils express CCR7 and have multipotent responses to the known ligands of CCR7.
Keywords: basic immunology, chemokines, eosinophils
Clinical Relevance
Several lines of evidence have demonstrated that leukocytes traffic to lymph nodes under the direction of the chemokine receptor CCR7. We demonstrate here the novel findings that human eosinophils express CCR7 and have multipotent responses to the known ligands of CCR7, CCL19, and CCL21. These findings are important in that they suggest that eosinophil migration to lymph nodes may be facilitated by the presence of functional CCR7, potentially relevant to allergic airways inflammation and eosinophilic pulmonary diseases.
Eosinophils are granulocytes that have been implicated as integral components of Th2 inflammation associated with allergic airways inflammation. Although eosinophils have important functions that are related to the release of cytotoxic cationic granule proteins and of lipid mediators, including leukotriene C4, they also have diverse immunoregulatory functions in innate and adaptive immunity (1).
Eosinophils display directed chemotactic activity toward an array of soluble chemokines, including several chemokines that bind to the eotaxin receptor CCR3 (2–6). Although CCR3 is a highly expressed chemokine receptor, eosinophils express several additional chemokine receptors (7, 8). Chemokines have important effects on eosinophils beyond chemotaxis, causing them to be activated and modulating their immunoregulatory functions, as most prominently demonstrated by the multipotent effects of CCL11 on eosinophils (6, 9–11).
Several types of leukocytes, including lymphocytes and dendritic cells, migrate to lymph nodes under the direction of the chemokines CCL19 and CCL21 and their receptor CCR7, facilitating leukocyte interactions, such as antigen presentation, within lymph nodes (12). We have previously demonstrated in a murine model of airways inflammation that eosinophils traffic to regional lymph nodes after exposure to antigen and function as professional antigen-presenting cells (13). Other researchers have observed eosinophil migration to draining lymph nodes in murine allergic inflammation (14–16). Although a prior report described inducible CCR7 expression in an eosinophilic leukemia cell line, it is unknown whether primary human eosinophils express CCR7 (17). That report also described chemotaxis of human eosinophils to CCL21 but only after stimulation with high concentrations of GM-CSF, IL-3, and IFN-γ (17).
Here we document the expression of CCR7 by primary human eosinophils. We show that IL-5–primed eosinophils exhibit chemotaxis, shape changes, calcium fluxes, and ERK phosphorylation in response to CCL19 and CCL21, the known ligands of CCR7. These findings identify CCR7 as functionally present in human eosinophils and potentially important in modulating their activation and immunoregulatory roles.
Materials and Methods
Eosinophil Purification
Venous blood specimens were collected after informed consent under protocols approved by the Institutional Review Board at Beth Israel Deaconess Medical Center. Eosinophils were isolated from peripheral blood of normal and mildly atopic blood donors by negative selection (StemCell Technologies, Vancouver, BC, Canada) as previously described (18). Eosinophil purity, assessed by Hema 3 staining (Fisher Scientific, Waltham, MA) of cytocentrifuge smears, was routinely greater than 99% of nucleated cells. Viability, assessed by trypan blue (Life Technologies, Grand Island, NY) exclusion, was routinely greater than 99%.
Eosinophil Priming
For experiments using IL-5–primed cells, purified eosinophils were suspended in RPMI 1640 (supplemented with 10% FBS and 1% penicillin/streptomycin) with 10 ng/ml recombinant human IL-5 (R&D Systems, Minneapolis, MN) and incubated at 37°C for 48 hours.
Antibodies and Reagents
Anti-CCR7 rabbit polyclonal antibody (Abcam, Cambridge, MA) and corresponding blocking peptide (Abcam), anti–phospho-ERK1/2 mAb (clone D13.14.4E; Cell Signaling, Danvers, MA), anti-ERK1/2 mAb (clone 137F5; Cell Signaling), and rabbit polyclonal anti–glyceraldehyde 3-phosphate dehydrogenase antibody (GAPDH) (Sigma-Aldrich, St. Louis, MO) were used for Western blotting. Anti-CCR7 unconjugated mAb (clone E75; Abcam), Alexa 647-conjugated and Alexa 488-conjugated anti-CCR7 mAbs (clone G043H7; Biolegend, San Diego, CA), and PE-conjugated anti–Siglec-8 mAb (clone 7C9; Biolegend) were used for flow cytometry. Recombinant human CCL19 and CCL21 were from R&D Systems.
Chemotaxis Assays
Transwell permeable supports containing polycarbonate membranes with 5.0-μm pores (Corning, Lowell, MA) were used. IL-5–primed eosinophils were resuspended in RPMI 1640 with 0.1% ovalbumin, 24 mM Hepes, and 2 mM glutamine at a concentration of 2 × 106 eosinophils per ml. The lower chamber below the transwell contained 600 μl of chemokine-containing medium or control medium. Eosinophil suspensions (100 μl) were added to the upper chamber. Eosinophil suspensions added directly to control medium without a transwell were used as the reference standard for calculating the fraction of migrating cells. Wells for each experiment were set up in duplicate. Plates were incubated for 1 hour at 37°C. Transwell inserts were removed, 6 μl of 0.5 M EDTA was added to each well, and plates were incubated for 10 minutes at 37°C. Cells were counted in triplicate for 15 seconds by flow cytometry.
Eosinophil Shape Changes
Eosinophil shape changes were imaged using phase microscopy with an inverted microscope (Nikon TE-300). Analyses of eosinophil cytoskeletal rearrangements by F-actin staining and measurement of shape changes by flow cytometry were performed as previously described (19).
Measurement of Intracellular Calcium
Eosinophils (1.5 × 106 cells/ml) were loaded with 1 μM fluo-4 AM (Life Technologies) in 1% FBS in Hanks’ balanced salt solution for 30 minutes at room temperature before chemokine stimulation. Fluorescence changes were recorded over time by flow cytometry.
Luminex Assay of Protein Phosphorylation
IL-5–primed eosinophils were subjected to lysis in buffer containing 1% Triton X-100, 10% glycerol, and 0.5% hexadecyl trimethylammonium bromide, supplemented by protease inhibitor cocktail (Roche, Basel, Switzerland) and phosphatase inhibitor cocktail (Sigma-Aldrich). The quantities of phosphorylated ERK1/2 and of GAPDH in lysates were assayed using commercially available multiplex kits (Millipore, Billerica, MA).
Results
CCR7 Expression by Human Eosinophils
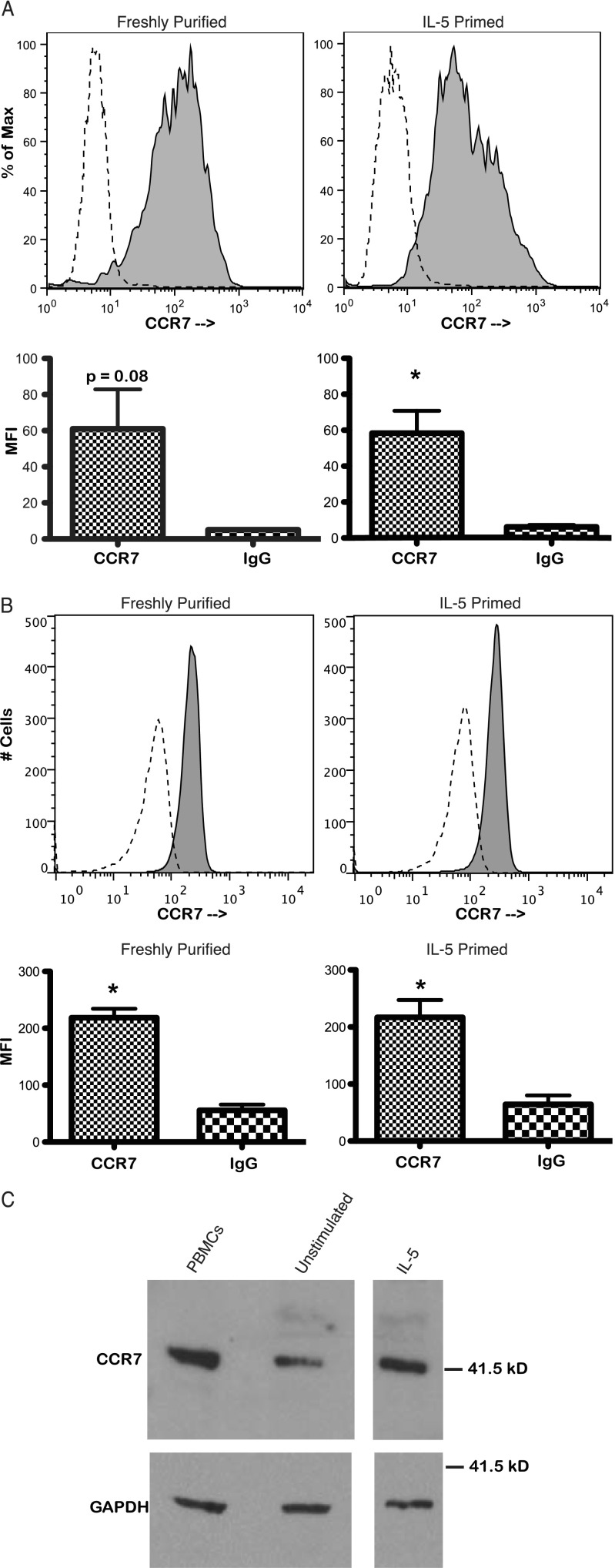
We first sought to demonstrate expression of CCR7 on the surface of human eosinophils. The eosinophil-specific marker Siglec-8 was expressed by all cells after purification of eosinophils from whole blood, indicative of a pure eosinophil population (see Figure E1 in the online supplement). Viability of eosinophils by propidium iodide staining was routinely 97 to 99% after 48 hours of incubation in IL-5–containing medium before antibody staining (data not shown). Eosinophils were stained with propidium iodide after primary and secondary antibody staining, and only cells that demonstrated viability by propidium iodide exclusion were included in the analysis. Cell surface staining for CCR7 on nonpermeabilized human eosinophils, both freshly purified and IL-5–primed, was performed and demonstrated surface expression of CCR7 (Figure 1A and Figure E2; four independent experiments from four separate donors each for freshly purified and IL-5–primed conditions; three donors were common to freshly purified and IL-5–primed conditions).
Figure 1.
CCR7 expression by human eosinophils. Flow cytometry events representing intact cells were analyzed, and debris was excluded by forward- and side-scatter gating. Shaded histograms represent CCR7 staining and are overlaid on unshaded dashed histograms representing irrelevant isotype control staining. (A) Flow cytometry of unstimulated, freshly purified eosinophils and IL-5–primed eosinophils for surface expression of CCR7. Eosinophils demonstrating viability by propidium iodide exclusion were included in the analysis. The histogram plots show single experiments representative of four independent experiments for unstimulated eosinophils and IL-5–stimulated eosinophils. The set of histograms displayed is from the same donor. Data are expressed as percentage of maximum cell count (% of Max). Forward scatter versus CCR7 fluorescence plots of these data are presented in Figure E2. Bar graphs depict geometric mean fluorescence intensity (MFI) averaged over the four donors tested for the freshly purified, unstimulated condition (P = 0.08, paired t test; bars represent SEM) and the IL-5–primed condition (*P < 0.03, paired t test; bars represent SEM). (B) Flow cytometry of unstimulated, freshly purified eosinophils and IL-5–primed eosinophils for total CCR7 expression. Eosinophils were fixed in 2% paraformaldehyde and permeabilized with 90% methanol before staining. The histogram plots are from a single donor and are representative of four separate experiments from four separate donors. Forward scatter versus CCR7 fluorescence plots of these data are presented in Figure E3. Bar graphs depict geometric MFI averaged over the four donors tested (*P < 0.005, paired t test; bars represent SEM). (C) Western blot for CCR7 of freshly purified, unstimulated eosinophils and IL-5–primed eosinophils. Peripheral blood mononuclear cells (PBMCs) from the donor providing eosinophils served as a positive control. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression served as a loading control. Data are representative of four independent experiments.
To confirm the expression of CCR7 by human eosinophils, flow cytometry was performed for the expression of the total pool of CCR7 in permeabilized, freshly purified, unstimulated human eosinophils and in permeabilized, IL-5–primed (48 h) human eosinophils. Eosinophils were fixed in 2% paraformaldehyde and permeabilized with 90% methanol before antibody staining. Freshly purified, unstimulated eosinophils and eosinophils primed for 48 hours with IL-5 demonstrated uniform expression of CCR7 (Figure 1B and Figure E3). For both unstimulated and IL-5–primed eosinophils, geometric means (mean fluorescence intensity) of aggregate data from four donors showed statistically significant expression of CCR7 (Figure 1B; paired t test, P < 0.005 for both conditions; all four donors were common to both conditions). When an alternative permeabilization method was used using 0.1% saponin, similar results were observed, indicating that the CCR7 signal was not an artifact of the permeabilization process (Figure E4). In addition, Western blots for CCR7 were performed in unstimulated eosinophils and IL-5–primed eosinophils and showed expression of CCR7 in both conditions (Figure 1C; data representative of four independent experiments). When a CCR7 blocking peptide against the primary antibody was present during primary antibody incubation, specific CCR7 signal was diminished on Western blots (Figure E5).
Human Eosinophil Chemotaxis to CCL19 and CCL21
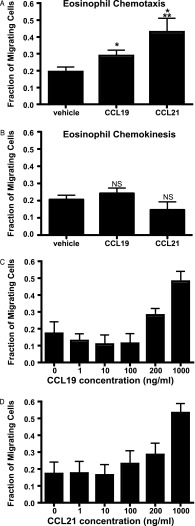
IL-5–primed eosinophils showed statistically significant chemotaxis (Wilcoxon matched pairs test, P < 0.05) to CCL19 and CCL21 (200 ng/ml) compared with vehicle control (Figure 2A; data aggregated from 11 independent experiments). Eosinophil chemotaxis to 200 ng/ml CCL21 was greater than chemotaxis to 200 ng/ml CCL19 (Wilcoxon matched pairs test, P < 0.05). Migration of IL-5–primed eosinophils was not due to nondirectional chemokinetic activity, as assayed by transwell migration with 200 ng/ml chemokine present in the upper chamber only (Figure 2B). Dose-dependent chemotaxis of IL-5–primed human eosinophils was observed for CCL19 (Figure 2C) and CCL21 (Figure 2D) (P < 0.05, one-way ANOVA for both CCL19 and CCL21; data aggregated from three experiments for each chemokine). Chemotaxis for both chemokines increased in a dose-dependent fashion.
Figure 2.
Chemotaxis of IL-5–primed human eosinophils to CCL19 and CCL21. (A) Aggregate chemotaxis data averaged from 11 separate donors. CCL19 and CCL21 concentration was 200 ng/ml in the lower chamber. Migrating cells are expressed as a fraction of cells counted when added directly without the presence of a transwell. For all chemotaxis experiments, counts are representative of intact eosinophils gated by forward- and side-scatter characteristics. *P < 0.05 compared with vehicle control; **P < 0.05 compared with CCL19 (Wilcoxon matched pairs test; bars represent SEM). (B) Chemokinesis assay with 200 ng/ml of CCL19 or CCL21 in upper chamber did not show statistically significant nondirectional chemokinetic activity (Wilcoxon matched pairs test). NS = not significant compared with vehicle control. Bars represent SEM. Data are aggregated from three separate experiments from three separate donors. (C and D) Dose-response of eosinophil chemotaxis to CCL19 (C) and CCL21 (D). For CCL19 and CCL21, chemotaxis increased as the concentration of chemokine in the lower chamber increased (P < 0.05, one-way ANOVA for CCL19 and CCL21). Data are aggregated from three independent experiments from three separate donors.
Shape Change of Human Eosinophils to CCL19 and CCL21
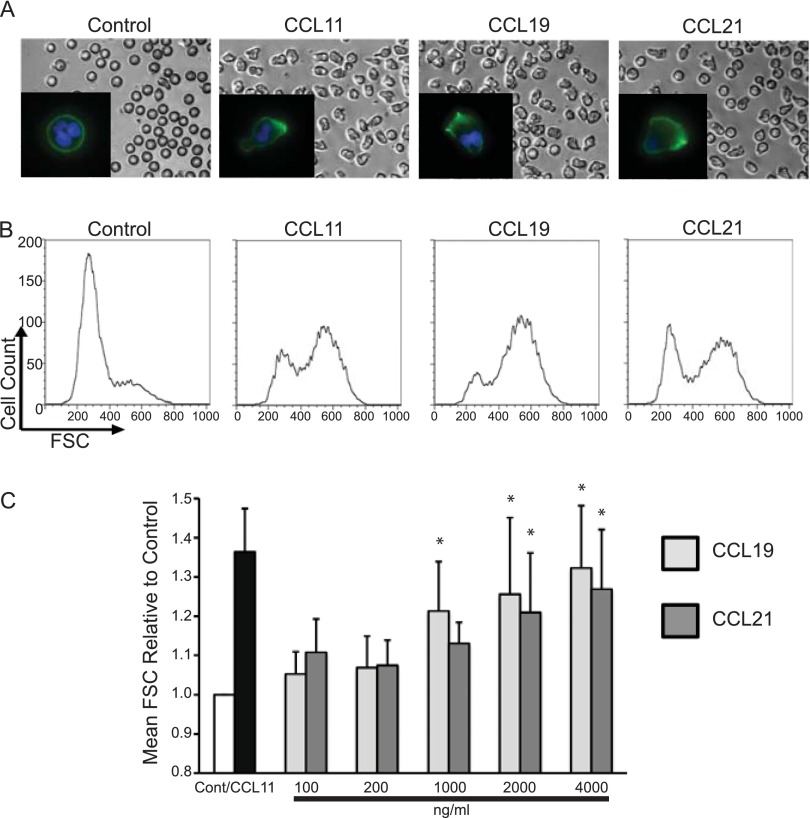
IL-5–primed eosinophils demonstrated elongated shape change in response to stimulation for 10 minutes with CCL11 (100 ng/ml), CCL19 (2 μg/ml), and CCL21 (2 μg/ml) compared with rounded untreated cells (Figure 3A). The inset images (Figure 3A) demonstrate F-actin rearrangement and polarization by Alexa 488–phalloidin staining (green), with nuclei stained by Hoescht dye (blue). Shape change was confirmed by increased flow cytometric forward scatter, which showed a marked shift to a larger forward-scatter state with CCL19 and CCL21 stimulation (Figure 3B and Figure E6). Mean forward scatter increased with escalating doses of CCL19 and CCL21 (Figure 3C).
Figure 3.
CCR7-mediated shape changes and F-actin rearrangement. (A) Visualization of shape change by microscopy. Eosinophils were cultured with IL-5 for 48 hours and then stimulated with vehicle, CCL11 (100 ng/ml), CCL19 (2 μg/ml), or CCL21 (2 μg/ml) for 10 minutes. F-actin staining with Alexa 488–conjugated phalloidin appears in green, and Hoescht nuclear staining appears in blue (insets). (B) Quantification of shape changes at above chemokine doses and duration by flow cytometric analysis of forward scatter (FSC). Histograms of shape change data are from a single experiment representative of four separate experiments from four separate donors. Forward-scatter versus side-scatter plots of these data are presented in Figure E6. (C) Aggregate data from shape change experiments (four experiments). Bar graphs demonstrate a concentration-dependent (10-min stimulation) increase in mean forward scatter expressed as a ratio to vehicle-treated control cells (Cont). (Dunnett’s multiple comparisons test, *P < 0.05).
Calcium Influx of Human Eosinophils with CCL19 and CCL21 Stimulation
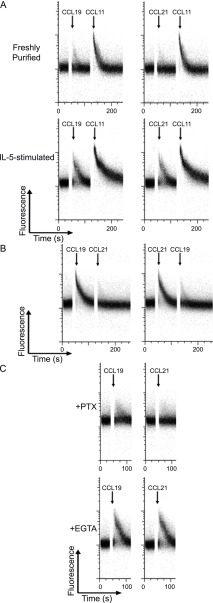
Freshly purified eosinophils demonstrated a small but observable calcium influx when stimulated with CCL19 or CCL21 (1 μg/ml) (Figure 4A). Calcium influx with CCL11 stimulation (100 ng/ml) served as a positive control and was not affected by preceding stimulation with CCR7 ligands. IL-5–primed eosinophils had robust calcium influx detectable by flow cytometry when stimulated by CCL19 and CCL21. There was no desensitization to CCL11-mediated calcium influx from the preceding calcium influx induced by CCR7 ligands (Figure 4A). CCL19- and CCL21-mediated calcium influx in IL-5–primed eosinophils had mutual cross-desensitization at high dose (5 μg/ml), consistent with action of both chemokines through the same receptor (Figure 4B). In addition, inhibition of calcium influx by pertussis toxin was observed, consistent with the relevant receptor being a G protein–coupled CC chemokine receptor (Figure 4C). Extracellular calcium chelation with EGTA in calcium-free medium did not inhibit CCL19- and CCL21-mediated calcium influx, indicative of intracellular release of calcium after stimulation with CCR7 ligands (Figure 4C).
Figure 4.
CCL19- and CCL21-induced calcium flux. (A) Purified unstimulated eosinophils and IL-5–primed eosinophils were loaded with fluo-4 AM, and calcium flux was recorded using flow cytometry. Baseline was established for 35 seconds before chemokines were added. Arrows indicate time of addition of CCL19 (1 μg/ml), CCL21 (1 μg/ml), or CCL11 (100 ng/ml). Data are representative of four separate experiments from separate donors. (B) Cross-desensitization of CCL19 and CCL21 for calcium flux. Calcium flux of IL-5–primed eosinophils was measured as above. Arrows indicate time of addition of CCL19 (5 μg/ml) or CCL21 (5 μg/ml). Data are representative of three separate experiments from separate donors. (C) In the upper panel, IL-5–primed eosinophils were pretreated with pertussis toxin (PTX) (1 μg/ml). In the lower panel, eosinophils were resuspended in calcium-free medium with 2 mM EGTA for 10 minutes before the addition of CCL19 and CCL21. Arrows indicate time of addition of CCL19 (1 μg/ml) or CCL21 (1 μg/ml). Data are representative of three separate experiments from separate donors.
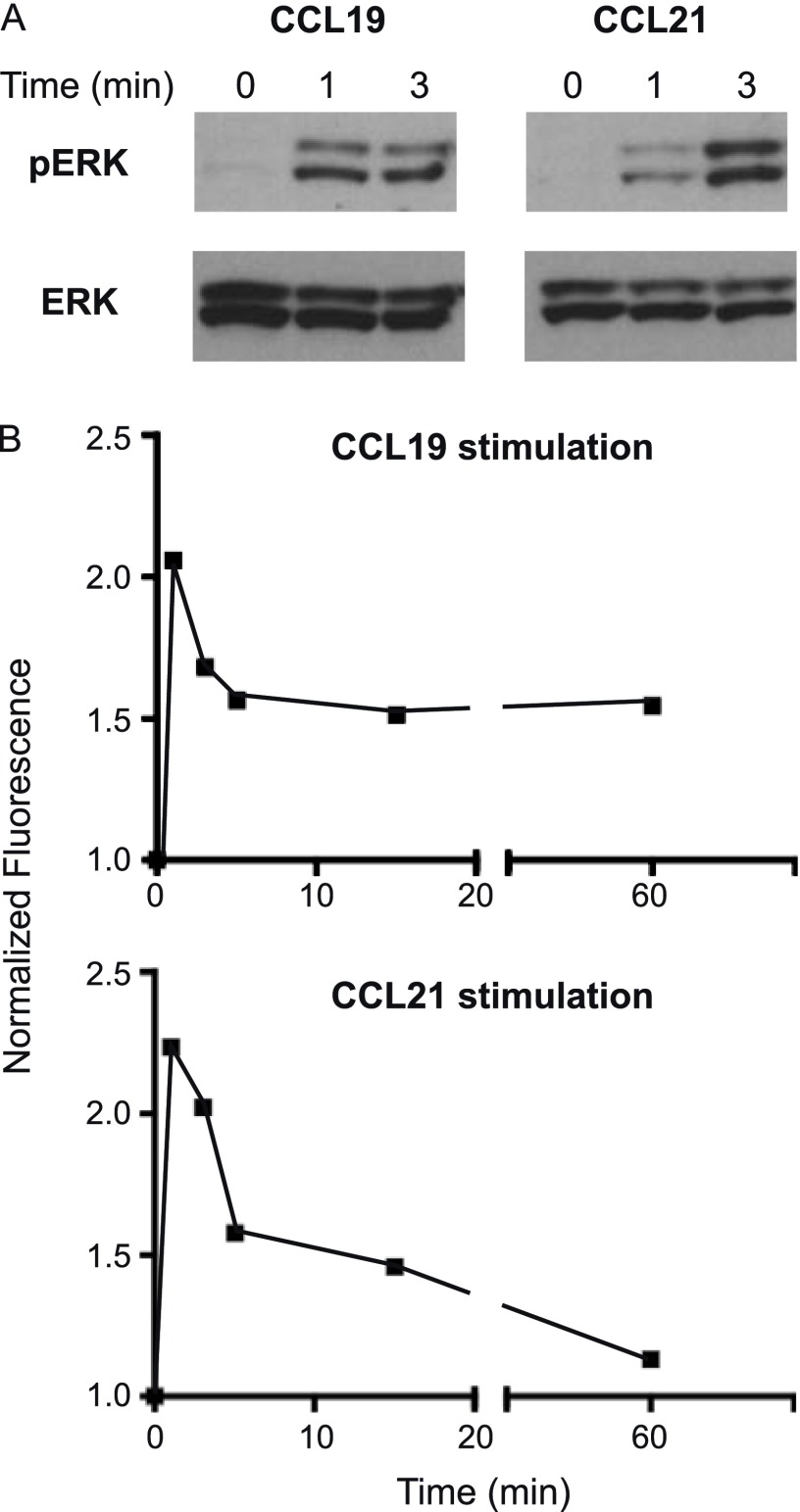
ERK Phosphorylation with CCL19 and CCL21 Stimulation
We assayed phosphorylation of ERK in IL-5–primed human eosinophils after incubation with 500 ng/ml of CCL19 and CCL21. Western blots showed an increase in phosphorylated ERK1/2 at 1- and 3-minute time points with CCL19 and CCL21 stimulation (Figure 5A). Phospho-ERK was only minimally detected in unstimulated cells (time 0). To measure the time course over a longer scale, we used a Luminex bead–based approach to test eosinophils from four separate donors, measuring the ratio of the signal for phospho-ERK to the signal for GAPDH. We found that the early induction of phospho-ERK by CCL19 and CCL21 abated at later time points, returning almost to baseline in the case of CCL21 (Figure 5B).
Figure 5.
CCR7-mediated ERK phosphorylation. IL-5–primed eosinophils were incubated in CCL19 (500 ng/ml) or CCL21 (500 ng/ml). (A) Western blots demonstrate an increase in phospho-ERK1/2 (pERK) after stimulation with CCL19 and CCL21. Western blots for total ERK1/2 (ERK) are included as controls. (B) ERK1/2 phosphorylation over an extended time course was assayed by Luminex multiplex assay and normalized to glyceraldehyde 3-phosphate dehydrogenase values detected in eosinophil lysates at specified time points. Data are mean values from four separate donors and expressed as the ratio to unstimulated cells (time 0).
Discussion
We demonstrate that human eosinophils express the chemokine receptor CCR7 and are functionally responsive to CCL19 and CCL21, the known ligands of CCR7. Our data definitively show that primary human eosinophils express CCR7 at the protein level. The detection of CCR7 on the cell surface is consistent with its functional relevance in human eosinophils. Furthermore, eosinophils have not only demonstrable surface expression of CCR7 in nonpermeabilized cells but significant stores of intracellular CCR7 as well. The expression of CCR7 by human eosinophils is supported by its detection in permeabilized cells using two distinct permeabilization methods (methanol and saponin permeabilization) and detection by Western blot analysis.
Prior reports of intracellular pools of chemokine receptors in leukocytes have been a source of controversy. Specifically, a report of intracellular staining of lymphocytes of CCR5 detected by flow cytometry (20) was refuted by a subsequent study suggesting that the detection of intracellular CCR5 was an artifact of the permeabilization method (21). We therefore used methanol permeabilization and saponin permeabilization to confirm our results. Both methods have been successfully used in demonstrating intracellular cytokine or chemokine receptor expression by human eosinophils (22, 23). In addition, we confirmed by Western blots the expression of CCR7 protein by human eosinophils.
It has previously been shown that antigen-loaded mouse eosinophils instilled intratracheally traffic to regional draining lymph nodes, where they interact with CD4+ T cells expressing antigen-specific T-cell receptor (13). This interaction leads to the activation and proliferation of T cells and to the production of Th2 cytokines, specifically IL-4 (13). However, whether the migration of eosinophils to draining lymph nodes in mouse models of allergic inflammation is mediated by CCR7 has not been established. Recent data by Jacobsen and colleagues indicate that CCR7 may not be necessary for accumulation of eosinophils in lymph nodes given that eosinophils from CCR7−/− mice also migrate to draining lymph nodes (24). However, the importance of CCR7-mediated chemotaxis to the movement of eosinophils in humans remains unknown. Our data establish that human eosinophils are capable of expressing CCR7 and of responding in a multifaceted manner, including chemotaxis, to stimulation by the ligands of CCR7. There has been additional recent precedent for the migration of granulocyte populations in response to CCR7, with human neutrophils showing chemotactic activity to CCL19 and CCL21 (25).
Unlike in the one prior report of eosinophil chemotaxis to CCL21 (17), eosinophils were primed here in a more physiologic fashion with IL-5 before functional assays, with multiple lines of evidence supporting the importance of IL-5 priming in facilitating multipotent responses of eosinophils to chemokine stimuli (26–29). Therefore, we used IL-5 priming in all of the experiments demonstrating functional effects of stimulation with CCR7 ligands. Chemotaxis was observed with submicromolar concentrations of CCL19 and CCL21, fully supportive of migration of eosinophils in the presence of physiologically relevant concentrations of these chemokines. We found that CCL21 is a more potent stimulus for eosinophil chemotaxis than CCL19. These data are in accordance with recent findings of greater potency of CCL21 than CCL19 in inducing murine dendritic cell chemotaxis in a three-dimensional experimental model (30).
Eosinophil shape change is consistent with a relationship between chemoattractant-induced responses and the earliest phases of leukocyte recruitment from the microcirculation (31). Because shape change must occur before or during its movement, we examined eosinophil shape change using microscopy and flow cytometry, observing this phenomenon by both methods with CCL19 and CCL21 stimulation of IL-5–primed human eosinophils. We found that eosinophil shape change to stimulation by CCR7 ligands is a concentration-dependent process. Significantly, eosinophils undergo cytoskeletal rearrangement with F-actin polarization in response to CCL19 and CCL21, further supporting a role for these chemokines in eosinophil motility and recruitment.
Chemokines induce rapid fluxes in intracellular calcium after receptor binding (32). We found CCL19- and CCL21-induced calcium influx in IL-5–primed eosinophils as additional evidence of functional binding of these chemokines to human eosinophils. One advantage of using a flow cytometric approach to analysis of calcium flux is the ability to assess the proportion of cells responding to a given ligand (33). Only a small population of fresh eosinophils responded to CCL19 or CCL21, although effective calcium influx was elicited by CCL11 (eotaxin-1). In contrast, a large proportion of total IL-5–primed eosinophils responded to CCL19 or CCL21.
It is possible in mice and humans that the actions of CCL19 and CCL21 occur through an alternate and unidentified receptor to CCR7. Conflicting data exist on the ability of human CCL21 to signal through CXCR3 as an alternative to CCR7 (34–36). The data that we present demonstrating cross-desensitization of calcium influx by CCL19 and CCL21 suggest that the two chemokines act on the same receptor in IL-5–primed human eosinophils. CCR7 remains the only receptor that definitively has functional binding to both CCL19 and CCL21. Although human eosinophils express CXCR3 (37), given our detection of CCR7 in IL-5–primed eosinophils and the multiple effects seen of CCL19 and CCL21 stimulation on IL-5–primed eosinophils, our findings likely represent signaling through CCR7.
CCL19 and CCL21 also induced the early phosphorylation of ERK in IL-5–primed human eosinophils. Although data from Western blot and Luminex analysis are concordant in their findings of early ERK phosphorylation, the precise point of peak phosphorylation cannot be definitively identified between the 1- and 3-minute time points, perhaps partially due to variability between donors. In dendritic cells, CCL19 and CCL21 stimulation activates MAP kinase members and Rho, which independently regulate chemotaxis and migratory speed, respectively (38). ERK phosphorylation in eosinophils may parallel that observed in dendritic cells, regulating chemotaxis and associated events such as shape change and calcium flux.
The data presented here demonstrate that the ligands of CCR7, CCL19, and CCL21 have wide-ranging effects on human eosinophils, including chemotaxis, fully consistent with the expression of functional CCR7 by eosinophils and potentially relevant to allergic airways inflammation and eosinophilic pulmonary disease.
Acknowledgments
Acknowledgments
The authors thank Kristen Young for technical assistance in isolation of human eosinophils and Jason Xenakis for technical assistance with flow cytometry.
Footnotes
This work was supported by National Institutes of Health grants R01AI051645 and R01/R37AI020241 (P.F.W.) and F32AI081513 (P.A.) and by the Uehara Memorial Foundation (S.U.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2012-0499OC on February 28, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Akuthota P, Wang HB, Spencer LA, Weller PF. Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin Exp Allergy. 2008;38:1254–1263. doi: 10.1111/j.1365-2222.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmermann N, Hershey GK, Foster PS, Rothenberg ME. Chemokines in asthma: cooperative interaction between chemokines and IL-13. J Allergy Clin Immunol. 2003;111:227–242. doi: 10.1067/mai.2003.139. [DOI] [PubMed] [Google Scholar]
- 3.Ponath PD, Qin S, Post TW, Wang J, Wu L, Gerard NP, Newman W, Gerard C, Mackay CR. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med. 1996;183:2437–2448. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daugherty BL, Siciliano SJ, DeMartino JA, Malkowitz L, Sirotina A, Springer MS. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med. 1996;183:2349–2354. [Google Scholar]
- 5.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 6.Bandeira-Melo C, Herbst A, Weller PF. Eotaxins: contributing to the diversity of eosinophil recruitment and activation. Am J Respir Cell Mol Biol. 2001;24:653–657. doi: 10.1165/ajrcmb.24.6.f209. [DOI] [PubMed] [Google Scholar]
- 7.Liu LY, Jarjour NN, Busse WW, Kelly EA. Chemokine receptor expression on human eosinophils from peripheral blood and bronchoalveolar lavage fluid after segmental antigen challenge. J Allergy Clin Immunol. 2003;112:556–562. doi: 10.1016/s0091-6749(03)01798-6. [DOI] [PubMed] [Google Scholar]
- 8.Trivedi SG, Lloyd CM. Eosinophils in the pathogenesis of allergic airways disease. Cell Mol Life Sci. 2007;64:1269–1289. doi: 10.1007/s00018-007-6527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandeira-Melo C, Sugiyama K, Woods LJ, Weller PF. Cutting edge: eotaxin elicits rapid vesicular transport-mediated release of preformed IL-4 from human eosinophils. J Immunol. 2001;166:4813–4817. doi: 10.4049/jimmunol.166.8.4813. [DOI] [PubMed] [Google Scholar]
- 10.Fujisawa T, Kato Y, Nagase H, Atsuta J, Terada A, Iguchi K, Kamiya H, Morita Y, Kitaura M, Kawasaki H, et al. Chemokines induce eosinophil degranulation through CCR-3. J Allergy Clin Immunol. 2000;106:507–513. doi: 10.1067/mai.2000.108311. [DOI] [PubMed] [Google Scholar]
- 11.Bandeira-Melo C, Phoofolo M, Weller PF. Extranuclear lipid bodies, elicited by CCR3-mediated signaling pathways, are the sites of chemokine-enhanced leukotriene C4 production in eosinophils and basophils. J Biol Chem. 2001;276:22779–22787. doi: 10.1074/jbc.M101436200. [DOI] [PubMed] [Google Scholar]
- 12.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 13.Wang HB, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J Immunol. 2007;179:7585–7592. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duez C, Dakhama A, Tomkinson A, Marquillies P, Balhorn A, Tonnel AB, Bratton DL, Gelfand EW. Migration and accumulation of eosinophils toward regional lymph nodes after airway allergen challenge. J Allergy Clin Immunol. 2004;114:820–825. doi: 10.1016/j.jaci.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000;105:945–953. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi HZ, Xiao CQ, Li CQ, Mo XY, Yang QL, Leng J, Chen YQ. Endobronchial eosinophils preferentially stimulate T helper cell type 2 responses. Allergy. 2004;59:428–435. doi: 10.1046/j.1398-9995.2003.00405.x. [DOI] [PubMed] [Google Scholar]
- 17.Jung YJ, Woo SY, Jang MH, Miyasaka M, Ryu KH, Park HK, Seoh JY. Human eosinophils show chemotaxis to lymphoid chemokines and exhibit antigen-presenting-cell-like properties upon stimulation with IFN-gamma, IL-3 and GM-CSF. Int Arch Allergy Immunol. 2008;146:227–234. doi: 10.1159/000115891. [DOI] [PubMed] [Google Scholar]
- 18.Akuthota P, Shamri R, Weller PF.Isolation of human eosinophils. Curr Protoc Immunol 2012;98:7.31.1–7.31.8 [DOI] [PMC free article] [PubMed]
- 19.Radke AL, Reynolds LE, Melo RC, Dvorak AM, Weller PF, Spencer LA. Mature human eosinophils express functional Notch ligands mediating eosinophil autocrine regulation. Blood. 2009;113:3092–3101. doi: 10.1182/blood-2008-05-155937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Achour L, Scott MG, Shirvani H, Thuret A, Bismuth G, Labbé-Jullié C, Marullo S. CD4–CCR5 interaction in intracellular compartments contributes to receptor expression at the cell surface. Blood. 2009;113:1938–1947. doi: 10.1182/blood-2008-02-141275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilch-Cooper HA, Sieg SF, Hope TJ, Koons A, Escola JM, Offord R, Veazey RS, Mosier DE, Clagett B, Medvik K, et al. Circulating human CD4 and CD8 T cells do not have large intracellular pools of CCR5. Blood. 2011;118:1015–1019. doi: 10.1182/blood-2010-05-282509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu LY, Bates ME, Jarjour NN, Busse WW, Bertics PJ, Kelly EA. Generation of Th1 and Th2 chemokines by human eosinophils: evidence for a critical role of TNF-alpha. J Immunol. 2007;179:4840–4848. doi: 10.4049/jimmunol.179.7.4840. [DOI] [PubMed] [Google Scholar]
- 23.Spencer LA, Melo RC, Perez SA, Bafford SP, Dvorak AM, Weller PF. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc Natl Acad Sci USA. 2006;103:3333–3338. doi: 10.1073/pnas.0508946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobsen EA, Zellner KR, Colbert D, Lee NA, Lee JJ. Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. J Immunol. 2011;187:6059–6068. doi: 10.4049/jimmunol.1102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beauvillain C, Cunin P, Doni A, Scotet M, Jaillon S, Loiry ML, Magistrelli G, Masternak K, Chevailler A, Delneste Y, et al. CCR7 is involved in the migration of neutrophils to lymph nodes. Blood. 2011;117:1196–1204. doi: 10.1182/blood-2009-11-254490. [DOI] [PubMed] [Google Scholar]
- 26.Warringa RA, Schweizer RC, Maikoe T, Kuijper PH, Bruijnzeel PL, Koenderman L. Modulation of eosinophil chemotaxis by interleukin-5. Am J Respir Cell Mol Biol. 1992;7:631–636. doi: 10.1165/ajrcmb/7.6.631. [DOI] [PubMed] [Google Scholar]
- 27.Schweizer RC, Welmers BA, Raaijmakers JA, Zanen P, Lammers JW, Koenderman L. RANTES- and interleukin-8-induced responses in normal human eosinophils: effects of priming with interleukin-5. Blood. 1994;83:3697–3704. [PubMed] [Google Scholar]
- 28.Sedgwick JB, Quan SF, Calhoun WJ, Busse WW. Effect of interleukin-5 and granulocyte-macrophage colony stimulating factor on in vitro eosinophil function: comparison with airway eosinophils. J Allergy Clin Immunol. 1995;96:375–385. doi: 10.1016/s0091-6749(95)70057-9. [DOI] [PubMed] [Google Scholar]
- 29.Bates ME, Green VL, Bertics PJ. ERK1 and ERK2 activation by chemotactic factors in human eosinophils is interleukin 5-dependent and contributes to leukotriene C(4) biosynthesis. J Biol Chem. 2000;275:10968–10975. doi: 10.1074/jbc.275.15.10968. [DOI] [PubMed] [Google Scholar]
- 30.Haessler U, Pisano M, Wu M, Swartz MA. Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19. Proc Natl Acad Sci USA. 2011;108:5614–5619. doi: 10.1073/pnas.1014920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabroe I, Hartnell A, Jopling LA, Bel S, Ponath PD, Pease JE, Collins PD, Williams TJ. Differential regulation of eosinophil chemokine signaling via CCR3 and non-CCR3 pathways. J Immunol. 1999;162:2946–2955. [PubMed] [Google Scholar]
- 32.Rothenberg ME, Ownbey R, Mehlhop PD, Loiselle PM, van de Rijn M, Bonventre JV, Oettgen HC, Leder P, Luster AD. Eotaxin triggers eosinophil-selective chemotaxis and calcium flux via a distinct receptor and induces pulmonary eosinophilia in the presence of interleukin 5 in mice. Mol Med. 1996;2:334–348. [PMC free article] [PubMed] [Google Scholar]
- 33.Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, Kassam N, Smith H, Shi X, Gonzalo JA, Newman W, et al. Cloning of the human eosinophil chemoattractant, eotaxin: expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–612. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soto H, Wang W, Strieter RM, Copeland NG, Gilbert DJ, Jenkins NA, Hedrick J, Zlotnik A. The CC chemokine 6Ckine binds the CXC chemokine receptor CXCR3. Proc Natl Acad Sci USA. 1998;95:8205–8210. doi: 10.1073/pnas.95.14.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenh CH, Cox MA, Kaminski H, Zhang M, Byrnes H, Fine J, Lundell D, Chou CC, Narula SK, Zavodny PJ. Cutting edge: species specificity of the CC chemokine 6Ckine signaling through the CXC chemokine receptor CXCR3: human 6Ckine is not a ligand for the human or mouse CXCR3 receptors. J Immunol. 1999;162:3765–3769. [PubMed] [Google Scholar]
- 36.Dijkstra IM, Hulshof S, van der Valk P, Boddeke HW, Biber K. Cutting edge: activity of human adult microglia in response to CC chemokine ligand 21. J Immunol. 2004;172:2744–2747. doi: 10.4049/jimmunol.172.5.2744. [DOI] [PubMed] [Google Scholar]
- 37.Jinquan T, Jing C, Jacobi HH, Reimert CM, Millner A, Quan S, Hansen JB, Dissing S, Malling HJ, Skov PS, et al. CXCR3 expression and activation of eosinophils: role of IFN-gamma-inducible protein-10 and monokine induced by IFN-gamma. J Immunol. 2000;165:1548–1556. doi: 10.4049/jimmunol.165.3.1548. [DOI] [PubMed] [Google Scholar]
- 38.Riol-Blanco L, Sanchez-Sanchez N, Torres A, Tejedor A, Narumiya S, Corbi AL, Sánchez-Mateos P, Rodríguez-Fernández JL. The chemokine receptor CCR7 activates in dendritic cells two signaling modules that independently regulate chemotaxis and migratory speed. J Immunol. 2005;174:4070–4080. doi: 10.4049/jimmunol.174.7.4070. [DOI] [PubMed] [Google Scholar]