Abstract
Primary ciliary dyskinesia (PCD), resulting from defects in cilia assembly or motility, is caused by mutations in a number of genes encoding axonemal proteins. PCD phenotypes are variable, and include recurrent respiratory tract infections, bronchiectasis, hydrocephaly, situs inversus, and male infertility. We generated knockout mice for the sperm-associated antigen–17 (Spag17) gene, which encodes a central pair (CP) protein present in the axonemes of cells with “9 + 2” motile cilia or flagella. The targeting of Spag17 resulted in a severe phenotype characterized by immotile nasal and tracheal cilia, reduced clearance of nasal mucus, profound respiratory distress associated with lung fluid accumulation and disruption of the alveolar epithelium, cerebral ventricular expansion consistent with emerging hydrocephalus, failure to suckle, and neonatal demise within 12 hours of birth. Ultrastructural analysis revealed the loss of one CP microtubule in approximately one quarter of tracheal cilia axonemes, an absence of a C1 microtubule projection, and other less frequent CP structural abnormalities. SPAG6 and SPAG16 (CP proteins that interact with SPAG17) were increased in tracheal tissue from SPAG17-deficient mice. We conclude that Spag17 plays a critical role in the function and structure of motile cilia, and that neonatal lethality is likely explained by impaired airway mucociliary clearance.
Keywords: primary ciliary dyskinesia, axoneme, central pair, Spag17, cilia
Clinical Relevance
We believe that the data presented in this study are worthy of broad readership because they point out the very significant differences between model organisms (e.g., Chlamydomonas) that have provided the foundation for our understanding of ciliary/flagellar axoneme biology, and mammalian motile cilia function. Our data also provide a hierarchy for the functional roles of central apparatus proteins, based on the phenotypes of mutant mice. Our observations are of relevance to clinical disorders of ciliary dysfunction (e.g., primary ciliary dyskinesia).
Cilia are organelles that play key roles, including the determination of left–right asymmetry in the body; clearing mucus, particles, and fluid out of the airways; and facilitating the flow of cerebrospinal fluid (CSF) (1). Primary ciliary dyskinesia (PCD), which results from defects in the formation and function of cilia and flagella, is a relatively rare disorder affecting 1 in 16,000 individuals worldwide (2). The axoneme, the highly conserved cytoskeletal structure of motile cilia, has a “9 + 2” arrangement consisting of nine outer doublet microtubules surrounding a central microtubule pair. Motility is dependent upon proteins associated with the outer doublet microtubules, including dynein arms, radial spokes, and nexin links.
The sliding of the outer doublet microtubules is modulated by the central pair (CP) apparatus (2–4). The two microtubules in the CP are structurally and biochemically dimorphic, and by convention receive separate designations (e.g., C1 and C2). At least 10 different polypeptides are uniquely associated with the C1 microtubule, and seven are unique to the C2 microtubule (3–6). This biochemical and structural asymmetry is believed to have functional significance with respect to the cilia or flagellar beat and waveform (7).
The mammalian sperm-associated antigen–17 protein (SPAG17) is the orthologue of Chlamydomonas reinhardtii PF6 (8), a protein located on a projection from the C1 CP microtubule in green algae (9). The PF6 protein interacts with a number of other proteins, including calmodulin, and ultimately influences the radial spokes attached to the outer microtubule doublets (10). Consequently, PF6 is thought to be a proximal effector of CP action on the sliding activity of the outer doublets that control cilia or flagellar beat. Chlamydomonas pf6 mutants demonstrate paralyzed flagella, and lack the CP 1a projection (9). The full-length murine SPAG17 protein is 250 kD, like its Chlamydomonas orthologue, and it is found in testes and tissues with motile cilia (8, 11). The present study sought to determine whether mammalian SPAG17 plays an essential role in mammalian motile cilia.
Materials and Methods
Mice
All animal procedures were performed in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. Animals were killed in accordance with protocol AM10297 approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee. Further details are provided in the online supplement.
Targeted Disruption of Spag17
To knock out the Spag17 gene, embryonic stem (ES) cells with conditional potential were obtained from the Knock Out Mouse Project Repository (Davis, CA). The ES cells were used to generate chimeric mice. The chimeric males were crossed to C57BL/6J wild-type females, and the resultant heterozygous offspring were crossed to 129S4/SvJaeSor-Gt(ROSA)26Sortm1(FLP1)Dym/J mice to remove the Neo cassette. The Spag17+/flox mice were used for mating with CMV-Cre (B6.C-Tg(CMV-cre)1Cgn/J) mice to generate Spag17+/−;CMV-Cre mice. Male and female Spag17+/−;CMV-Cre mice were mated, resulting in the expecting Mendelian inheritance for Spag17−/−;CMV-Cre mice. After these matings, the mice demonstrated a mixed background of C57BL/6J–129S4/SvJ. Further details are provided in the online supplement.
Southern Blotting
Genomic DNA was digested with the BglII enzyme and subjected to Southern blot analysis, using a probe for Spag17 detection. Details are provided in the online supplement.
RT-PCR
RT-PCR was performed to amplify the Spag17 message from several tissues, using a primer set specific for murine Spag17. Details are provided in the online supplement.
Western Blotting
Proteins were extracted from murine tracheal tissues and subjected to Western blot analysis, using antibodies against SPAG17, SPAG6, SPAG16, and primary ciliary dyskinesia protein 1 (Pcdp1). Details are provided in the online supplement.
Immunohistochemistry
Brain tissue samples from wild-type and mutant mice were fixed and prepared for SPAG17 immunohistochemistry detection. Details are provided in the online supplement.
Immunofluorescence
Tracheal tissues from wild-type and mutant mice were fixed and prepared for SPAG17 immunofluorescence detection. Details are provided in the online supplement.
Magnetic Resonance Imaging
Two-hour-old wild-type and knockout mice pups were evaluated under magnetic resonance imaging for a morphological comparison of their bodies. Magnetic resonance imaging was performed with Bruker-Biospin Biospec console and a 7-Tesla, 30-cm free bore magnet (Bruker Biospin Corporation, Billerica, MA). Further details are provided in the online supplement.
Electron Microscopy
Ependymal and tracheal tissues from wild-type and mutant mice were fixed and prepared for transmission electron microscopy, according to standard methods. Further details are provided in the online supplement.
Two-Dimensional Image Averaging
Trachea were dissected from the mice pups and placed directly into fixative, and prepared for transmission electron microscopy. Images were first processed in Adobe Photoshop (Adobe Systems Inc., San Jose, CA), and the average intensity projections were generated in ImageJ (http://http://rsb.info.nih.gov/ij/). Further details are provided in the online supplement.
Video Microscopy
Tracheal and nasal tissues were observed with differential interference contrast microscopy. Movies were recorded at 30 frames per second with a Sanyo Hi-Resolution camera (Sanyo Electric Co., Moriguchi City, Japan). Movies were edited using Pinnacle Studio HD software, version 14.0 (Pinnacle Systems, Inc., Mountain View, CA). Further details are provided in the online supplement.
Micro-Computed Tomography
Micro–computed tomography (micro-CT) was performed using an Inveon Micro-CT system (Siemens, Knoxville, TN). Image segmentation, analysis, and volume rendering were performed using Inveon Research Workplace, version 4.0 (Siemens). Further details are provided in the online supplement.
Echocardiography
Echocardiography was performed using the Vevo770 imaging system (Visual Sonics, Inc., Toronto, ON, Canada). Further details are provided in the online supplement.
Results
The Phenotype of SPAG17-Deficient Mice
Mice deficient in SPAG17 were generated through breeding to CMV-Cre, which is expressed in all tissues (Figure 1). Nullizygous pups died within 12 hours of birth, whereas heterozygous mutants were viable and no different from their wild-type littermates. The genotyping of pups immediately after birth demonstrated that the mutant allele was transmitted in a pattern that was not significantly different from that anticipated for Mendelian inheritance (see Table E1 in the online supplement). Longitudinal observations demonstrated that during the first hours after birth, the homozygous pups were indistinguishable from their heterozygous and wild-type littermates (Figure 2A). However, within 6–8 hours after birth, the homozygous mutant mice developed severe respiratory distress, with labored breathing and cyanotic skin (Video E1, and Figure 2B). In addition, the homozygous mutant mice could not suckle, so they lacked “milk spots” in their stomachs (Figure 2B), consistent with abnormal feeding. Furthermore, as shown in Video E2, the homozygous mutant newborn pups displayed a limited capacity for movement and locomotion. All of the longitudinally observed nullizygous mice died within 12 hours of birth, whereas all heterozygous mutant and wild-type mice survived (Table E1).
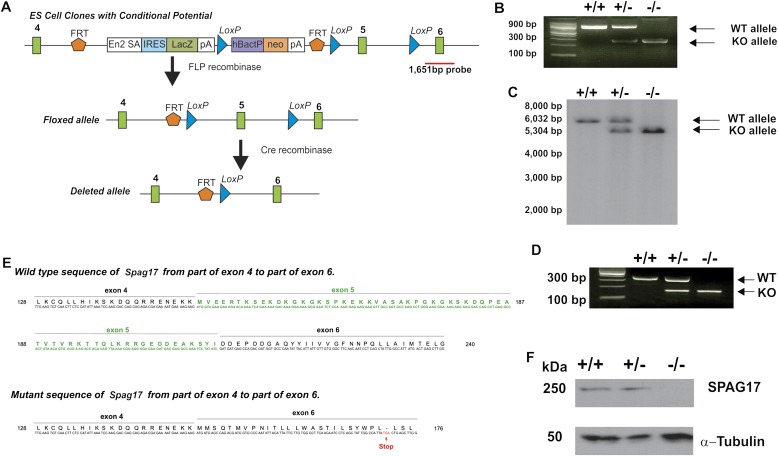
Figure 1.
Targeted disruption of the murine sperm-associated antigen–17 (Spag17) gene. The murine Spag17 gene is located in chromosome 3 and contains 49 exons. An embryonic stem (ES) cell line (C57BL/6N background) in which exon 5 was floxed for conditional targeting (Clone EPD0234_6_G08) was obtained from the Knock Out Mouse Project Repository (Davis, CA). The ES cell clone was used to generate chimeric mice. Chimeric males were crossed to C57BL/6J wild-type females, and the resulting heterozygous offspring were crossed to 129S4/SvJaeSor-Gt(ROSA)26Sortm1(FLP1)Dym/J mice to remove the Neo cassette. Spag17+/flox mice were mated to CMV-Cre mice to generate Spag17+/−;CMV-Cre mice. Male and female Spag17+/−;CMV-Cre mice were mated to create the Spag17−/−;CMV-Cre mice. (A) Schematic representation of the strategy used to disrupt Spag17 gene. Red line shows the probe location used for Southern blotting. ES, embryonic stem; FRT, flippase recognition target; LoxP, Lox-flanked cassette; En2 SA, splicing acceptor region of the En2 gene; IRES, internal ribosome-entry site; LacZ, beta-galactosidase gene; pA, polyadenylation site; neo, neomycin resistance gene; hBactP, human beta-actin promoter. (B) Genotypic analysis applied primers flanking upstream from the first FRT site, and downstream from the third LoxP site. (C) Southern blot analysis used BglII-digested genomic DNA from wild-type, heterozygous, and homozygous mutant mice. (D) Representative RT-PCR used primers in exon 4 (forward) and 6 (reverse). (E) Sequence result for wild-type (WT) and knockout (KO) alleles from RT-PCR products, after thymine-adenine (TA) cloning. (F) Immunoblotting of murine tracheal tissue extracts with rabbit anti-SGAP17 antibody, which reacts with the murine SPAG17 C-terminus. The blot shown is representative of three independent experiments, and tissues from three different mice in each genotype are shown.
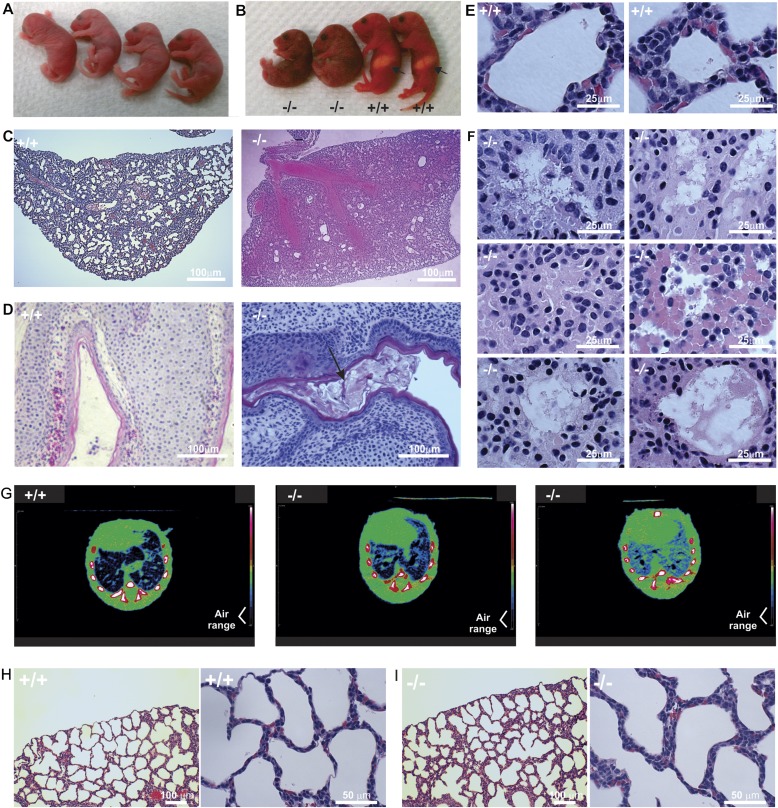
Figure 2.
Deletion of Spag17 gene expression results in hydrocephalus and pulmonary and nasal mucus and fluid congestion. (A) Four littermates with indistinguishable phenotypes within one hour of birth. (B) Pups from the same litter show abnormal phenotypes a few hours after birth. Pups were genotyped by PCR after photograph was taken. Arrows show the presence of milk spot in wild-type mice. (C) Histologic sections from Spag17+/+ and Spag17−/− mice. Lung tissue from Spag17−/− mouse appears to be full of fluid. (D) Paranasal cavities with the presence of mucus (arrow) in a nullizygous mouse. (E) Higher magnification images from Spag17+/+ mouse show normal lung-tissue structure. (F) Higher magnification images from Spag17−/− mouse show fluid infiltration, edema, and the disruption of alveolar epithelia. (G) Representative micro–computed tomography axial images from one Spag17+/+ mouse and two Spag17−/− mice with detectable labored breathing revealed a reduced presence of air in the lungs. (H) Lung histology from Spag17+/+ mouse. (I) Lung histology immediately after birth, before respiratory distress was evident.
To investigate the neonatal lethal phenotype further, magnetic resonance (MR) scanning studies on newborn pups were performed. The axial views from MR scans showed ventricular expansion in the brains of Spag17−/− mice, consistent with hydrocephalus (Figure 3A, red). In addition, lungs from null mice were full of fluid (Figure 3A, green). Examination of brains by histology revealed enlarged lateral ventricles, consistent with the MR scans (Figure 3B). Similarly, the histological examination of lung tissue suggested fluid accumulation, and revealed the disruption of alveolar epithelia (Figures 2C and 2F). Analyses of paranasal sinuses revealed that excessive mucus accumulated in the mutant animals (Figure 2D).
Figure 3.
Mutation of the Spag17 gene results in hydrocephalus and lung fluid accumulation. (A) Two-hour-old wild-type and knockout mice were evaluated using magnetic resonance imaging for a morphological comparison of their bodies. Left: The direction of the scanning images for each section. Right: Axial views from the head, thoracic, and abdominal sections. Arrows in the head sections point to ventricular expansion in the brain of a Spag17−/− mouse, consistent with hydrocephalus. Arrows in the thoracic section show the fluid-filled lung from a null mouse. Arrowhead in the abdominal section from a wild-type mouse identifies the presence of a milk spot, which is absent in the Spag17−/− mouse. (B) Representative axial views from brain tissues stained with hematoxylin and eosin. Arrows indicate the expansion of ventricles.
Micro-CT studies were performed in wild-type and knockout mice. Axial images from Spag17−/− mice with detectable labored breathing reveled a reduced presence of air in the lungs (Figure 2G). In addition, a three-dimensional reconstruction from micro-CT studies indicated less lung expansion in the mutant mice (Video E3).
Although the Spag17 mutants developed a phenotype consistent with PCD, we did not find situs inversus in the Spag17−/− mice, which is in accordance with findings in other CP protein knockout murine models (11–14). Moreover, no cardiac structural defects were observed in nullizygous mice (Figure E1A in the online supplement). However, ultrasound studies showed a reduced heart rate in Spag17−/− cyanotic mice, consistent with severe respiratory distress (Figure E1B).
Because Spag17 knockout mice develop profound respiratory distress within a few hours of birth, we analyzed the lung histology of wild-type and nullizygous pups before they exhibited detectable labored breathing and cyanosis. As shown in Figure 2I, the lung histology from Spag17−/− mice appeared normal. Neither fluid accumulation nor alveolar disruption was observed.
Cilia Motility Defects in SPAG17-Deficient Mice
To determine whether the motility of cilia was affected in the knockout mice, we examined the ciliary movement in tracheal and nasal tissue, using video-microscopy. Most of the cilia from Spag17−/− mice were immotile (Video E4), and only a few cells displayed cilia with an uncoordinated beat (Video E5). The density of cilia in the nullizygous mice appeared to be similar to that in heterozygous mutant and wild-type mice in these videos. In contrast, the cilia of Spag17+/− and Spag17+/+ mice showed normal and coordinated motility (Video E6). Immotile cilia were also observed in nasal respiratory epithelia from mutant mice, whereas normal cilia motility was evident in wild-type mice (Video E7). The tracheas were removed from wild-type and knockout mice and were kept in PBS, with a drop of murine blood cells injected into the tracheal tube. The transit of these blood cells was evaluated using video microscopy. Video E8 shows the movement of blood cells driven by the coordinated ciliary beat in wild-type mice. However, no directed flow was detected, and blood cells were not moved, in the mutant murine tracheal preparations (Video E9).
Axoneme Structural Defects in SPAG17-Deficient Mice
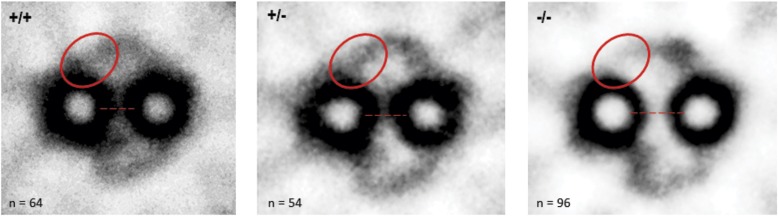
The transmission electron microscopy of cilia from tracheal epithelia demonstrated that 23.7% of the axonemes from homozygous mutant mice were missing one CP microtubule, whereas none of the cilia in tracheas from either heterozygous or wild-type mice demonstrated these defects (Table E2A and Figures 4B and 4D). To analyze the CP projections, two-dimensional image-averaging techniques were performed in the axonemes where the two CPs were present. As shown in Figure 5, the axoneme of Spag17−/− mice appears to lack a C1 projection (i.e., the putative orthologue of the C1a projection of Chlamydomonas). Moreover, the distance between the two CP microtubules was significantly greater in the homozygous mutant than in the wild-type mice, and the density of the bridges between the two CP microtubules was reduced (Table E2B). These abnormalities were not observed in airway cilia from heterozygous mutant or wild-type mice. We also observed that some Spag17 nullizygous mice possessed axonemes with a third central microtubule (“9 + 3”), a phenotype that has not been previously reported in Chlamydomonas or the mouse (Figure E2). These findings suggest that SPAG17 plays both a structural and functional role in the ciliary axoneme.
Figure 4.
Targeted mutation of the Spag17 gene causes a central pair (CP) defect. (A) Transmission electron micrograph from a Spag17+/+ mouse depicts a “9 + 2” microtubule arrangement. (B) Transmission electron micrograph from a Spag17−/− mouse depicts axonemes lacking a microtubule in the CP (arrows). (C) High-magnification electron micrograph from a Spag17+/+ mouse depicts a “9 + 2” microtubule arrangement. (D) High-magnification electron micrographs from a Spag17−/− mouse depict axonemes lacking a microtubule in the central apparatus.
Figure 5.
Spag17 mutant mice demonstrate CP defects. Two-dimensional image-averaging techniques show a reduced bridge density and greater separation between the two CP microtubules (dashed line), and the absence of a C1 projection (ovals), probably the orthologue of the C1a projection of Chlamydomonas, in the knockout mice.
CP Protein Expression in SPAG17-Deficient Mice
Western blotting revealed that the 250-kD SPAG17 protein was absent from the trachea (Figure 1F), and immunofluorescence (Figure E3A) and immunocytochemistry studies (Figure E3B) confirmed the absence of protein in tracheal epithelial cells and brain ependymal cells from Spag17−/− mice.
We also evaluated the expression of SPAG6 (the murine orthologue of a Chlamydomonas protein, PF16, associated with the C1 microtubule), SPAG16 (the murine orthologue of a Chlamydomonas protein, PF20, that is located in the bridge between the C1 and C2 microtubules), and Pcdp1 (the murine orthologue of a Chlamydomonas protein associated with the C1 microtubule) by Western blot analysis in tracheal tissue from Spag17+/+, Spag17+/−, and Spag17−/− mice. The SPAG17 null mice demonstrated significantly increased levels of SPAG6 and SPAG16L proteins compared with wild-type mice (Figures E4A and E4B). The levels of these proteins were also increased in heterozygous mutants, although the increase was not statistically significant. However, Pcdp1 was significantly reduced in Spag17−/− mice compared with wild-type and heterozygous mutants (Figure E4C). These observations could reflect defects in the assembly of CP components that result in accumulation (SPAG6 and SPAG16L) or instability (Pcdp1) proteins in the absence of SPAG17.
Discussion
PCD, characterized by immotile cilia in various tissues and immotile sperm, is a dramatic example of axoneme dysfunction. It is a heterogeneous genetic disorder that affects 1 in 16,000 individuals. Mutations in 12 genes are associated with PCD, mainly those encoding the dynein arms (15), but an estimated two thirds of cases have yet to be assigned a molecular defect (16). The phenotypes of individuals with PCD are variable, but a clear understanding of how specific mutations cause different phenotypes is lacking (15). The discovery that murine mutations in the central apparatus protein genes, Spag6 and Spag16, cause male infertility (and hydrocephalus in the case of Spag6), but not situs inversus (12, 17), supports the notion that lateralization defects in PCD are attributable to the dysfunction of “9 + 0” cilia, which fulfill different roles compared with “9 + 2” cilia (18). However, other phenotypic differences among PCD subjects remain to be explained. Human mutations or putative deleterious genetic variants have been identified in genes encoding central apparatus proteins, including the recent discovery of human Hydin mutations (19). Some of these variants or mutations might also modify the disease phenotype in PCD or other diseases involving pulmonary dysfunction, such as cystic fibrosis (16, 17).
The phenotype in SPAG17-deficient mice of rapid neonatal demise, evidently from respiratory failure, is consistent with airway ciliary dysfunction. Mice deficient in FOXJ1 (C57BL/6J background), a transcription factor that controls ciliogenesis, lack cilia, and most nullizygous offspring die immediately after birth, although a small number (8%) survive the neonatal period (20). Moreover, mice with other targeted mutations that create a PCD phenotype, including Mdnah5−/− (C57BL/6 × CBA/J background) and Dnah11−/− (C3H/HeH background) mutants, have immotile cilia, but survive the neonatal period (21, 22). The fact that SPAG17-deficient mice (C57BL/6J–129S4/SvJ mixed background), which possess cilia with no or impaired motility, all died during the neonatal period may be related to differences in genetic background, which is known to affect the phenotypes of mice with mutations altering cilia function, or may be related to mutation-unique environmental interactions(e.g., with housing conditions). The abnormality in mucociliary clearance of SPAG17 knockout mice, exemplified by the accumulation of mucus in the paranasal sinuses and fluid in the lungs, may have contributed to the respiratory distress of this obligate nasal-breathing species. The facts that the lungs of SPAG17-deficient mice were histologically normal at birth, and that the pups were indistinguishable from their heterozygous and wild-type littermates immediately after delivery, suggest that the respiratory distress was a result of postnatal complications.
We found that cilia in Spag17−/− tracheas failed to clear particles effectively, and the lungs showed fluid accumulation and a disruption of alveolar structure several hours later, which may be a consequence of hypoxic damage. Acute hypoxia increases pulmonary artery pressure, resulting in epithelial malfunction, edema, and lung inflammation (23), leading to respiratory distress. Fluid build-up in the lungs results in death from asphyxia, as suggested by micro-CT observations. The cardiac function abnormality we observed (bradycardia) is consistent with a hypoxic state. Because respiratory failure is usually associated with the low survival of newborn mice (death within 12 hours of birth) (24), our observations suggest that Spag17 knockout neonatal death is attributable to respiratory failure.
Feeding problems are probably also related to respiratory distress and ciliary dysfunction, and may have contributed further to the early neonatal deaths (24). The function of nasal cilia may have impaired olfaction, required for pups to seek their mothers. The inability to feed will result in neonatal death, and nonfeeding pups usually die within a window of 12–24 hours after birth (24).
Although the neonatal SPAG17-deficient mice displayed evidence for evolving hydrocephalus, hydrocephalus per se was not likely the proximate cause of neonatal death. Cilia on ependymal cells facilitate the flow of CSF in the brain, and the congenital hydrocephalus observed in the MR images and histological preparations likely reflected defects in CSF circulation. The development of hydrocephalus is influenced by murine strain, and C57BL/6 mice more commonly develop hydrocephalus than do 129/Sv or mixed background mice (such as the Spag17 knockout we studied). Although most of the knockout mice that developed hydrocephalus died, the ventricular dilatation was modest, and mice with more severe degrees of hydrocephalus (such as the mixed background SPAG6-deficient mice we previously created) survive for a week or two after birth (12).
The axoneme CP is involved in the control of ciliary and flagellar waveforms (2). Although the basic axonemal structure is highly conserved across species, cilia and flagella perform specialized functions unique to the tissue or cell in which they are present. For example, Chlamydomonas flagella can beat with two different waveforms and with high frequency, whereas respiratory mammalian cilia beat with a single waveform and much lower frequency. Recently, O’Toole and colleagues (25) demonstrated that most of the projections associated with each CP microtubule in Chlamydomonas appear to be conserved in human axonemes, although some differences are evident. For example, the C1a projection of Chlamydomonas appears to be more prominent compared with the human C1a projection, and an additional density adjacent to the C1c projection is present in humans. These findings suggest that functional differences may be reflected in small variations of structural organization (25).
The structural phenotype of axonemes in Spag17 mutant mice is distinctive, showing similarities as well as differences in terms of the phenotype of Chlamydomonas axonemes lacking PF6, the protein orthologue of SPAG17. PF6-deficient Chlamydomonas lack the 1a projection off the C1 microtubule (5, 9, 26). We found that SPAG17-deficient mice were also missing a C1 projection, probably the orthologue of the Chlamydomonas C1a projection. In addition, approximately 25% of the tracheal ciliary axonemes were missing one CP microtubule, which is a structural phenotype similar to that in the Chlamydomonas pf16 mutation, which is the orthologue of Spag6. These defects were not evident in the tracheal cilia of wild-type or heterozygous pups.
The loss of one CP microtubule has also been reported in humans with mutations in genes encoding radial spoke head proteins (27, 28). The similarity of structural abnormalities in the CP of the radial spoke head and Spag17 mutants suggests that the nexus between the CP and the radial spokes is critical for stabilizing the axoneme.
SPAG6, the orthologue of Chlamydomonas PF16, is a protein associated with the C1 microtubule. Mice deficient in Spag6 develop hydrocephalus, and 50% of these mice die within 8 weeks of birth. Surviving males are infertile because of impaired sperm motility (12). The axonemes of epididymal sperm tails exhibit ultrastructural abnormalities, including loss of the CP, but the axonemes of cilia in lung or ependymal cells appear to be normal.
SPAG16 is another CP protein, located in the bridge between the C1 and C2 microtubules. It is the orthologue of Chlamydomonas PF20. The Spag16 gene encodes two major transcripts (Spag16L and Spag16S). Mice lacking SPAG16L are generally healthy, and the major phenotype involves male infertility because of a severe sperm motility defect (17, 29). The cilia of tracheal epithelia are motile in the absence of SPAG16L (Video E10). No gross abnormalities were observed in transmission electron micrographs of tracheal cilia in Spag16-deficient mice (17). The Chlamydomonas pf20 mutant exhibits the most significant ultrastructural defects, lacking the entire CP. Thus, a totally different genotype–phenotype relationship exists among mutations in the CP genes of Chlamydomonas and mice, with a hierarchy in axoneme structural defects and functional deficits of pf20 > pf16 > pf6 (most severe to least severe) and Spag17 > Spag6 > Spag16 in mice.
Our previous studies suggested that SPAG17, SPAG6, and SPAG16L form an interactome (8, 11). In a previous Western blot analysis of testes and male germ cells, we found that when SPAG6 was absent (Spag6 nulizygous mice), SPAG16L was also markedly reduced or missing, as was a germ-cell isoform of SPAG17 (8). In contrast, an increase in both SPAG6 and SPAG16 protein was evident in the tracheas of SPAG17-deficient mice. Although the molecular explanation for these observations remains unknown, it could reflect the instability of CP proteins in the absence of SPAG6, or the inability to assemble certain CP components into CP structures in the absence of SPAG17. It may also account for the reduced density of inter-CP microtubule bridges (SPAG16), and may contribute to the absence of the C1 microtubule projection in SPAG17-deficient mice. Interestingly, another putative C1-associated CP protein, Pcdp1, which is not known to be part of the aforementioned interactome, was reduced. Further studies are needed to elucidate the mechanisms underlying these changes in protein content.
The Spag17 knockout mouse described here demonstrates the most severe phenotype of any reported knockout of a CP gene. Mutations of Hydin impair ciliary motility and brain fluid transport, resulting in hydrocephalus (13). Mice lacking Pcdp1 demonstrate an accumulation of mucus in the sinus cavity, hydrocephalus, and sperm with a loss of mature flagella (14). These mutant mice survive the neonatal period, as do the Spag6 and Spag16 mutants already noted. Intriguingly, the phenotypes of deletion in each of these proteins are quite different. One possible explanation for the diversity in phenotypes states that the proximity of the encoded proteins to the radial spokes, which connect to the outer doublets and their dynein motors, determines phenotypic severity. This hierarchy of phenotypic severity underscores the differences between mammalian and nonmammalian axoneme function.
Acknowledgments
Acknowledgments
The authors thank Dr. Elizabeth F. Smith (Department of Biological Sciences, Dartmouth University) for her comments and suggestions, Dr. Jorge Almenara (Anatomic Pathology Research Services, Virginia Commonwealth University) for assistance with the histologic studies, Dr. Lee Lance (Sanford School of Medicine, University of South Dakota) for kindly providing the Pcdp1 antibody, and Dr. Eileen O’Toole (University of Colorado) for assistance with two-dimensional image-averaging analysis.
Footnotes
This work was supported by National Institutes of Health grant HD-37416 (J.F.S.) and a Virginia Commonwealth University Presidential Research Incentive Program Award (Z.Z.). Microscopy was performed at the Department of Anatomy and the Neurobiology Microscopy Facility at Virginia Commonwealth University, supported in part with funding from National Institutes of Health–National Institute of Neurological Disorders and Stroke Center Core Grant P30 NS047463.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0362OC on February 13, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ishikawa H, Marshall W. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 2.Lee L. Mechanisms of mammalian ciliary motility: insights from primary ciliary dyskinesia genetics. Gene. 2011;473:57–66. doi: 10.1016/j.gene.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Goodenough U, Heuser J. Substructure of inner dynein arms, radial spokes, and the central pair/projection complex of cilia and flagella. J Cell Biol. 1985;100:2008–2018. doi: 10.1083/jcb.100.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith E, Lefebvre P. The role of central apparatus components in flagellar motility and microtubule assembly. Cell Motil Cytoskeleton. 1997;38:1–8. doi: 10.1002/(SICI)1097-0169(1997)38:1<1::AID-CM1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.Dutcher S, Huang B, Luck D. Genetic dissection of the central pair microtubules of the flagella of Chlamydomonas reinhardtii. J Cell Biol. 1984;98:229–236. doi: 10.1083/jcb.98.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutcher S. Flagellar assembly in two hundred and fifty easy-to-follow steps. Trends Genet. 1995;11:398–404. doi: 10.1016/s0168-9525(00)89123-4. [DOI] [PubMed] [Google Scholar]
- 7.Inaba K. Sperm flagella: comparative and phylogenetic perspectives of protein components. Mol Hum Reprod. 2011;17:524–538. doi: 10.1093/molehr/gar034. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Jones BH, Tang W, Moss SB, Wei Z, Ho C, Pollack M, Horowitz E, Bennett J, Baker ME, et al. Dissecting the axoneme interactome: the mammalian orthologue of Chlamydomonas PF6 interacts with sperm-associated antigen 6, the mammalian orthologue of Chlamydomonas PF16. Mol Cell Proteomics. 2005;4:914–923. doi: 10.1074/mcp.M400177-MCP200. [DOI] [PubMed] [Google Scholar]
- 9.Rupp G, O’Toole E, Porter ME. The Chlamydomonas PF6 locus encodes a large alanine/proline-rich polypeptide that is required for assembly of a central pair projection and regulates flagellar motility. Mol Biol Cell. 2001;12:739–751. doi: 10.1091/mbc.12.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wargo MJ, Dymek EE, Smith EF. Calmodulin and PF6 are components of a complex that localizes to the C1 microtubule of the flagellar central apparatus. J Cell Sci. 2005;118:4655–4665. doi: 10.1242/jcs.02585. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Zariwala MA, Mahadevan MM, Caballero-Campo P, Shen X, Escudier E, Duriez B, Bridoux A-M, Leigh M, Gerton GL, et al. A heterozygous mutation disrupting the Spag16 gene results in biochemical instability of central apparatus components of the human sperm axoneme. Biol Reprod. 2007;77:864–871. doi: 10.1095/biolreprod.107.063206. [DOI] [PubMed] [Google Scholar]
- 12.Sapiro R, Kostetskii I, Olds-Clarke P, Gerton GL, Radice GL, Strauss JF., III Male infertility, impaired sperm motility, and hydrocephalus in mice deficient in sperm-associated antigen 6. Mol Cell Biol. 2002;22:6298–6305. doi: 10.1128/MCB.22.17.6298-6305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lechtreck K-F, Delmotte P, Robinson ML, Sanderson MJ, Witman GB. Mutations in Hydin impair ciliary motility in mice. J Cell Biol. 2008;180:633–643. doi: 10.1083/jcb.200710162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee L, Campagna DR, Pinkus JL, Mulhern H, Wyatt TA, Sisson JH, Pavlik JA, Pinkus GS, Fleming MD. Primary ciliary dyskinesia in mice lacking the novel ciliary protein Pcdp1. Mol Cell Biol. 2008;28:949–957. doi: 10.1128/MCB.00354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leigh M, Pittman J, Carson J, Ferkol T, Dell S, Davis S, Knowles M, Zariwala M. Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet Med. 2009;11:473–487. doi: 10.1097/GIM.0b013e3181a53562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berg J, Evans J, Leigh M, Omran H, Bizon C, Mane K, Knowles M, Weck K, Zariwala M. Next generation massively parallel sequencing of targeted exomes to identify genetic mutations in primary ciliary dyskinesia: implications for application to clinical testing. Genet Med. 2011;13:218–229. doi: 10.1097/GIM.0b013e318203cff2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Kostetskii I, Tang W, Haig-Ladewig L, Sapiro R, Wei Z, Patel AM, Bennett J, Gerton GL, Moss SB, et al. Deficiency of SPAG16L causes male infertility associated with impaired sperm motility. Biol Reprod. 2006;74:751–759. doi: 10.1095/biolreprod.105.049254. [DOI] [PubMed] [Google Scholar]
- 18.Pazour G, Witman G. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003;15:105–110. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 19.Olbrich H, Schmidts M, Werner C, Onoufriadis A, Loges N, Raidt J, Banki N, Shoemark A, Burgoyne T, Al Turki S, et al. Recessive Hydin mutations cause primary ciliary dyskinesia without randomization of left–right body asymmetry. Am J Hum Genet. 2012;91:672–684. doi: 10.1016/j.ajhg.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brody SL, Yan XH, Wuerffel MK, Song S-K, Shapiro SD. Ciliogenesis and left–right axis defects in Forkhead factor HFH-4–null mice. Am J Respir Cell Mol Biol. 2000;23:45–51. doi: 10.1165/ajrcmb.23.1.4070. [DOI] [PubMed] [Google Scholar]
- 21.Ibanez-Tallon I, Gorokhova S, Heintz N. Loss of function of axonemal dynein Mdnah5 causes primary ciliary dyskinesia and hydrocephalus. Hum Mol Genet. 2002;11:715–721. doi: 10.1093/hmg/11.6.715. [DOI] [PubMed] [Google Scholar]
- 22.Lucas JS, Adam EC, Goggin PM, Jackson CL, Powles-Glover N, Patel SH, Humphreys J, Fray MD, Falconnet E, Blouin J-L, et al. Static respiratory cilia associated with mutations in Dnahc11/Dnah11: a mouse model of PCD. Hum Mutat. 2012;33:495–503. doi: 10.1002/humu.22001. [DOI] [PubMed] [Google Scholar]
- 23.Araneda O, Tuesta M. Lung oxidative damage by hypoxia. Oxid Med Cell Longev. 2012;2012:1–18. doi: 10.1155/2012/856918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turgeon B, Meloche S. Interpreting neonatal lethal phenotypes in mouse mutants: insights into gene function and human diseases. Physiol Rev. 2009;89:1–26. doi: 10.1152/physrev.00040.2007. [DOI] [PubMed] [Google Scholar]
- 25.O’Toole E, Giddings T, Porter M, Ostrowski L. Computer-assisted image analysis of human cilia and Chlamydomonas flagella reveals both similarities and differences in axoneme structure. Cytoskeleton (Hoboken) 2012;69:577–590. doi: 10.1002/cm.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goduti D, Smith E. Analyses of functional domains within the PF6 protein of the central apparatus reveal a role for PF6 sub-complex members in regulating flagellar beat frequency. Cytoskeleton (Hoboken) 2012;69:179–194. doi: 10.1002/cm.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castleman V, Romio L, Chodhari R, Hirst R, de Castro S, Parker K, Ybot-Gonzalez P, Emes R, Wilson S, Wallis C, et al. Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular–pair abnormalities. Am J Hum Genet. 2009;84:197–209. doi: 10.1016/j.ajhg.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zietkiewicz E, Bukowy-Bieryllo Z, Voelkel K, Klimek B, Dmenska H, Pogorzelski A, Sulikowska-Rowins A, Rutkiewicz E, Witt M. Mutations in radial spoke head genes and ultrastructural cilia defects in East-European cohort of primary ciliary dyskinesia patients. PLoS ONE. 2012;7:e33667. doi: 10.1371/journal.pone.0033667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, Kostetskii I, Moss SB, Jones BH, Ho C, Wang H, Kishida T, Gerton GL, Radice GL, Strauss JF., III Haploinsufficiency for the murine orthologue of Chlamydomonas PF20 disrupts spermatogenesis. Proc Natl Acad Sci USA. 2004;101:12946–12951. doi: 10.1073/pnas.0404280101. [DOI] [PMC free article] [PubMed] [Google Scholar]