Abstract
Numerous epidemiological studies have linked exposure to particulate matter (PM) air pollution with acute respiratory infection and chronic respiratory and cardiovascular diseases. We have previously shown that soluble nickel (Ni), a common component of PM, alters the release of CXC chemokines from cultured human lung fibroblasts (HLF) in response to microbial stimuli via a pathway dependent on disrupted prostaglandin (PG)E2 signaling. The current study sought to identify the molecular events underlying Ni-induced alterations in PGE2 signaling and its effects on IL-8 production. PGE2 synergistically enhances Ni-induced IL-8 release from HLF in a concentration-dependent manner. The effects of PGE2 were mimicked by butaprost and PGE1-alcohol and inhibited with antagonists AH6809 and L-161,982, indicating PGE2 signals via PGE2 receptors 2 and 4. PGE2 and forskolin stimulated cAMP, but it was only in the presence of Ni-induced hypoxia-inducible factor 1, α subunit (HIF1A) that these agents stimulated IL-8 release. The Ni-induced HIF1A DNA binding was enhanced by PGE2 and mediated, in part, by activation of p38 MAPK. Negation of cAMP-response element binding protein 1 or HIF1A using short interfering RNA blocked the synergistic interactions between Ni and PGE2. The results of the current study provide novel information on the ability of atmospheric hypoxia-mimetic metals to disrupt the release of immune-modulating chemokines by HLF in response to PGE2. Moreover, in the presence of HIF1A, cAMP-mediated signaling pathways may be altered to exacerbate inflammatory-like processes in lung tissue, imparting a susceptibility of PM-exposed populations to adverse respiratory health effects.
Keywords: prostaglandin E2, HIF1A, nickel, IL-8, fibroblast
Clinical Relevance
This manuscript contains novel information regarding the potential for hypoxia-mimetic metals found in particulate matter (PM) pollution to dysregulate eicosanoid signaling in the lung. The current findings show that nickel synergistically interacts with prostaglandin (PG)E2 to promote IL-8 release from human lung fibroblasts via enhanced activation of the transcription factor hypoxia inducible factor 1, alpha subunit (HIF1A) and that activation of HIF1A can switch a normally suppressive role of PGE2/cAMP signaling on IL-8 synthesis to a stimulatory role. These findings suggest that HIF1A disrupts PGE2-mediated signaling pathways to exacerbate inflammatory-like processes in lung tissue, imparting a susceptibility of PM-exposed populations to adverse respiratory health effects.
Numerous epidemiological studies have linked exposure to particulate matter (PM) air pollution, originating from natural and man-made sources, with acute respiratory infection and chronic respiratory and cardiovascular diseases (1–4). It is well recognized that the toxicity of PM is largely determined by surface area, surface-absorbed organic components, and transition metal content. PM with an aerodynamic diameter of less than 2.5 μm (PM2.5) is thought to pose a more significant health hazard than PM with an aerodynamic diameter of less than 10 μm because PM2.5 is more likely to penetrate more distal regions of the lung and be retained (5). PM2.5 also contains a high content of a variety of toxic heavy metals, such as nickel (Ni), vanadium (V), and chromium (Cr), among others (6, 7).
Ni is a common component of PM and has been shown to mediate some of the biological effects of PM in vitro and in vivo. Lippmann and colleagues report that cardiac dysfunction in response to fine particulate matter is largely attributable to Ni (8), which can also contribute to the onset of asthma (9) and pulmonary fibrosis (10) in the lower airways. Inhaled Ni has also been linked with immunodysfunction, chronic active inflammation of the airways, and cancer (11–13). Moreover, in vivo and in vitro studies have implicated initiation of inflammatory cascades within the lung as mediating Ni-induced toxicity (14–16). However, the molecular and cell-specific events that are fundamental in modulating gene expression after Ni exposure are not completely understood.
Lung fibroblasts are thought to play an active role in the response to tissue injury, contributing to cytokine and chemokine release as well as their activation and expansion in fibroproliferative disorders (17, 18). One of the hallmarks of inflammation is increased elaboration of prostaglandins (PGs) through the induction of the cyclooxygenase-2 enzyme (prostaglandin-endoperoxidase synthase 2 [PTGS2]). We have previously shown that NiSO4·6H2O (Ni) alters the pattern of TLR-2–dependent chemokine release from cultured human lung fibroblasts via a PTGS2-dependent pathway (19). Further studies revealed Ni synergistically interacts with PGE2 in the absence of microbial stimuli to promote release of the immune-modulating chemokine IL-8 in HLF (20). This is of interest because PGE2 is thought to have antiinflammatory effects in the lung (21) and has been shown to suppress IL-8 release in response to microbial and bacterial stimuli (19, 20). To gain a better understanding of how Ni may influence PGE2-mediated response to inflammation in the lung, the current study focuses on molecular events underlying stimulation of IL-8 release from HLF after mixed exposures to Ni and PGE2. These studies highlight interactions between hypoxia-inducible factor 1, α subunit (basic helix-loop-helix transcription factor) (HIF1A) and cAMP-response element binding protein 1 (CREB1) as a pivotal step in Ni-induced dysregulation of PGE2 signaling in HLF.
Materials and Methods
Experimental Design
In human lung fibroblasts, IL-8 release was measured after exposure to 200 μM NiSO4·6H2O (Ni), PGE2 (0–10 μM), or the two treatments in combination using specific ELISA. The concentration of Ni used in the current study was chosen based on the concentration–response relationships for IL-8 release in HLF reported previously (19, 20). To determine which PGE2 receptor(s) mediate the synergistic interactions between PGE2 and Ni on IL-8 release from HLF, cells were coexposed to Ni with or without 0 to 1,000 nM of the individual PTGER receptor agonists 17-phenyl trinor PGE2 (PTGER1/PTGER3), Butaprost (PTGER2), Sulprostone (PTGER3), and PGE1-alcohol (PTGER3/PTGER4). In a separate set of experiments, HLF were pretreated with 10 μM of PGE2 receptor antagonists SC-19220 (PTGER1), AH6809 (PTGER1, -2, and -3-III), or L 161,982 (PTGER4) before stimulation with Ni and 10 nM PGE2 for 48 hours. Levels of cAMP in HLF treated with Ni and/or PGE2 were determined using the cAMP EIA kit (Cayman Chemical, Ann Arbor, MI) and normalized to total protein content. Activation of HIF1A after mixed exposures to Ni and PGE2 was measured using a DNA-binding ELISA (TransAM HIF-1; Active Motif, Carlsbad, CA). To determine the role of HIF1A, cAMP, and mitogen-activated protein kinase (MAPK) signaling in IL-8 release, cells were transiently transfected with small interfering RNA (siRNA) to HIF1A and CREB1 or pretreated with p38 MAPK inhibitor (SB 203,580) before stimulation with Ni and PGE2. Data are presented as means ± SE and were considered significant when P < 0.05 as determined by one- or two-way ANOVA, as appropriate, using an all-pairwise, multiple-comparison procedure (GraphPad PRISM, version 5.0; GraphPad Software, San Diego, CA). Addition details are provided in the online supplement.
Results
PGE2 Signals through PTGER2 and PTGER4 to Synergistically Enhance Ni-Induced IL-8 Release from HLF
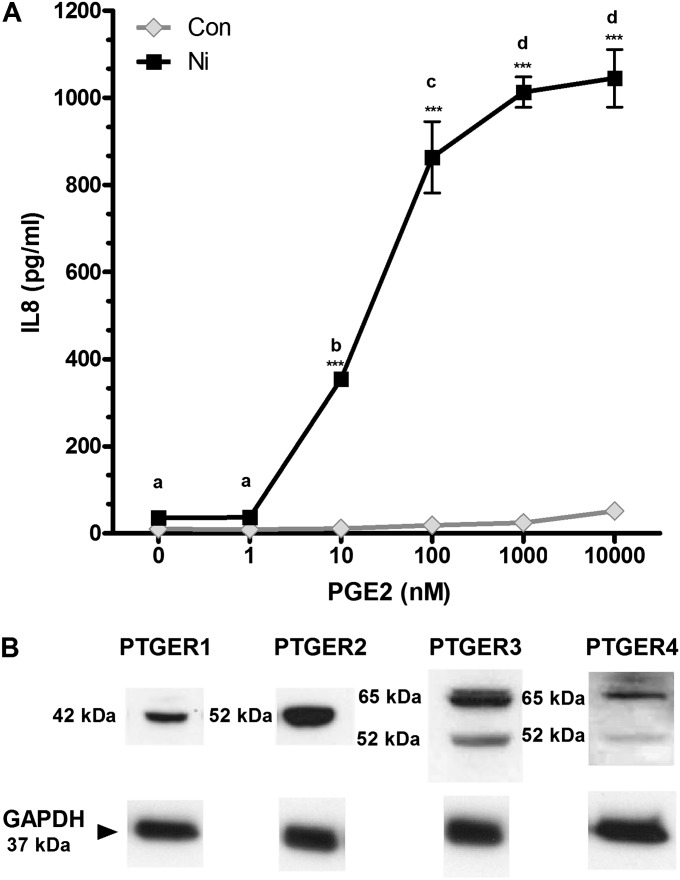
We have previously reported that Ni enhances the TLR2-mediated release of IL-8 from HLF in a mechanism involving induction of PTGS2 and consequent release of PGE2 (19, 20). Here we sought to determine the underlying mechanism by which PGE2 interacts with Ni to stimulate IL-8 release and identify the PGE2 receptor(s) involved in this process. PGE2 alone does not stimulate IL-8 release from HLF, even at concentrations up to 10 μM (Figure 1A, gray symbols). In contrast, in the presence of 200 μM Ni, PGE2 elicits a concentration-dependent release of IL-8 from HLF (Figure 1A, black symbols) to levels significantly greater than what was observed in media from control-treated cells or cells receiving either stimuli alone (P < 0.001). PGE2 enhanced Ni-induced IL-8 release by 10-fold with concentrations as low as 10 nM and enhanced IL-8 release up to 30-fold in cells cotreated with 1 and 10 μM PGE2 compared with cells receiving Ni or PGE2 alone. Additional dose–response analysis indicates that when PGE2 is held constant at 10 nM Ni begins to synergistically enhance IL-8 release at a concentration of 150 μM (see Figure E1 in the online supplement). Figure 1B shows immunoblot analysis indicating the presence of all four PTGER receptor subtypes in unstimulated HLF. No changes in PTGER expression were observed in cells treated with Ni in the presence or absence of PGE2 compared with control-treated cells (data not shown).
Figure 1.
Prostaglandin (PG)E2 enhances Ni-induced IL-8 release from human lung fibroblasts (HLF). (A) Cells were stimulated with 200 μM nickel (Ni) with or without PGE2 for 48 hours as described in Materials and Methods. IL-8 levels in conditioned medium were measured using ELISA. Points with different letters have means that are significantly different within the Ni-treated group; ***different from respective concentration of PGE2 in the absence of Ni (P < 0.01) as determined by two-way ANOVA with Bonferroni multiple comparison test. (B) Western blot analysis indicating expression of PGE2 receptors (PTGER)1–4 in human lung fibroblasts. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is shown as a loading control. Con, control.
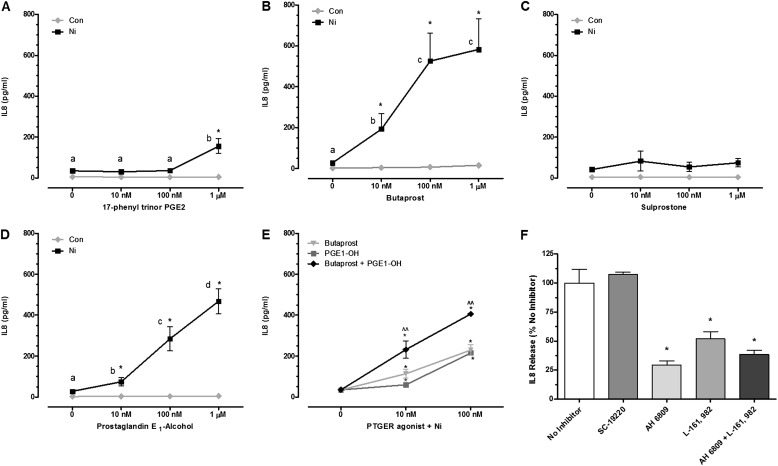
To determine which PTGER receptors mediate PGE2-enhanced IL-8 release by Ni, HLF were cotreated with different PTGER agonists alone or in combination with 200 μM Ni or pretreated with PTGER antagonists before stimulation with Ni and 10 nM PGE2. The PTGER1/3 agonist, 17-phenyl trinor PGE2, stimulated a 4-fold increase in IL-8 release from HLF after exposure at the highest concentration tested (1,000 nM) compared with cells receiving Ni alone (Figure 2A). PTGER2 and PTGER3/4 receptor agonists (butaprost [Figure 2B] and PGE1-alcohol [Figure 2D], respectively) synergistically interact with Ni to promote IL-8 release from HLF, similar to the findings observed with PGE2. Butaprost (10 nM) enhanced Ni-induced IL-8 release approximately 7-fold above that observed in control cells or cells receiving butaprost or Ni alone. The effects of butaprost were further enhanced at 100 and 1,000 nM, with approximately 20-fold more IL-8 released in the presence of Ni compared with cells receiving Ni alone. The PTGER3/4 agonist PGE1-alcohol amplified Ni-induced IL-8 release 3- to 20-fold over a 10 to 1,000 nM concentration range compared with cells receiving Ni alone (Figure 2D). No significant interactions were observed between Ni and the PTGER3 agonist sulprostone on IL-8 release from HLF (Figure 2C).
Figure 2.
Stimulation of PTGER2 and PTGER4 enhances Ni-induced IL-8 release from HLF. Cells were stimulated with 200 μM Ni in the presence or absence of the PGE2 receptor agonists 17-phenyl trinor PGE2 (PTGER1/3) (A), Butaprost (PTGER2) (B), Sulprostone (PTGER3) (C), PGE1-alcohol (PTGER3/4) (D), and Butaprost and PGE1-alchohol in combination for 48 hours (E). Data shown are mean ± SEM and are representative of three independent experiments performed in triplicate. Points with different letters have means that are significantly different within the Ni-treated group; *different from respective concentration of PGE2 in the absence of Ni (P < 0.05); ^^different from either agonist alone in the presence of Ni (P < 0.05) as determined by two-way ANOVA with Bonferroni multiple comparison test. (F) HLF were pretreated with DMSO solvent control (no inhibitor) or 10 μM AH 6809 (PTGER1, PTGER2, and PTGER3-III antagonist), L-161,982 (PTGER4 antagonist), SC-19220 (PTGER1 antagonist), or AH 6809 and L-161,982 for 1 hour before a 48-hour exposure to 200 μM Ni and 10 nM PGE2. Data shown are mean ± SEM and are representative of three independent experiments performed in triplicate. *Different from Ni- and PGE2-treated cells in the absence of inhibitor (P < 0.05) as determined by ANOVA with Tukey’s multiple comparisons test.
Given the findings that stimulation of both Gs-coupled PTGER receptors enhanced IL-8 release by Ni, we next determined the effects of dual receptor stimulation. Figure 2E shows that at concentrations of 10 and 100 nM, butaprost and PGE1-alcohol have an additive effect on Ni-mediated IL-8 release from HLF. Although absolute levels of IL-8 released varied among HLF from different donors, similar observations were made when examining the effects of Ni and PTGER stimulation on IL-8 release. Consistent with PGE2 signaling via PTGER2 and PTGER4 to enhance Ni-induced IL-8 release from HLF, chemokine levels after Ni and PGE2 cotreatment were significantly attenuated by AH 6809 and L-161,982 (Figure 2F). AH 6809 is an antagonist with near equal affinity for PTGER1, PTGER2, and PTGER3-III receptors. When HLF were pretreated with 10 µM AH 6809 for 1 hour before a 48-hour exposure with Ni and 10 nM PGE2, IL-8 release was attenuated by approximately 80%. Given that no inhibition was observed in the presence of the PTGER1 antagonist (SC-19220), combined with the findings that the sulprostone (PTGER3 agonist) had no effect on Ni-induced IL-8 release, the attenuation by AH 6809 is likely mediated by PTGER2 receptors. The PTGER4 antagonist L-161,982 also attenuated IL-8 release by 60% compared with cells receiving Ni and PGE2 in the absence of inhibitor, consistent with the results shown in Figure 2D. Inhibition of Ni and PGE2-induced IL-8 release when HLF were pretreated with both AH 6809 and L-161,982 was comparable to the response observed when cells were pretreated with either inhibitor alone (Figure 2F).
Synergistic Interaction between PGE2 and Ni on IL-8 Involves Activation of cAMP
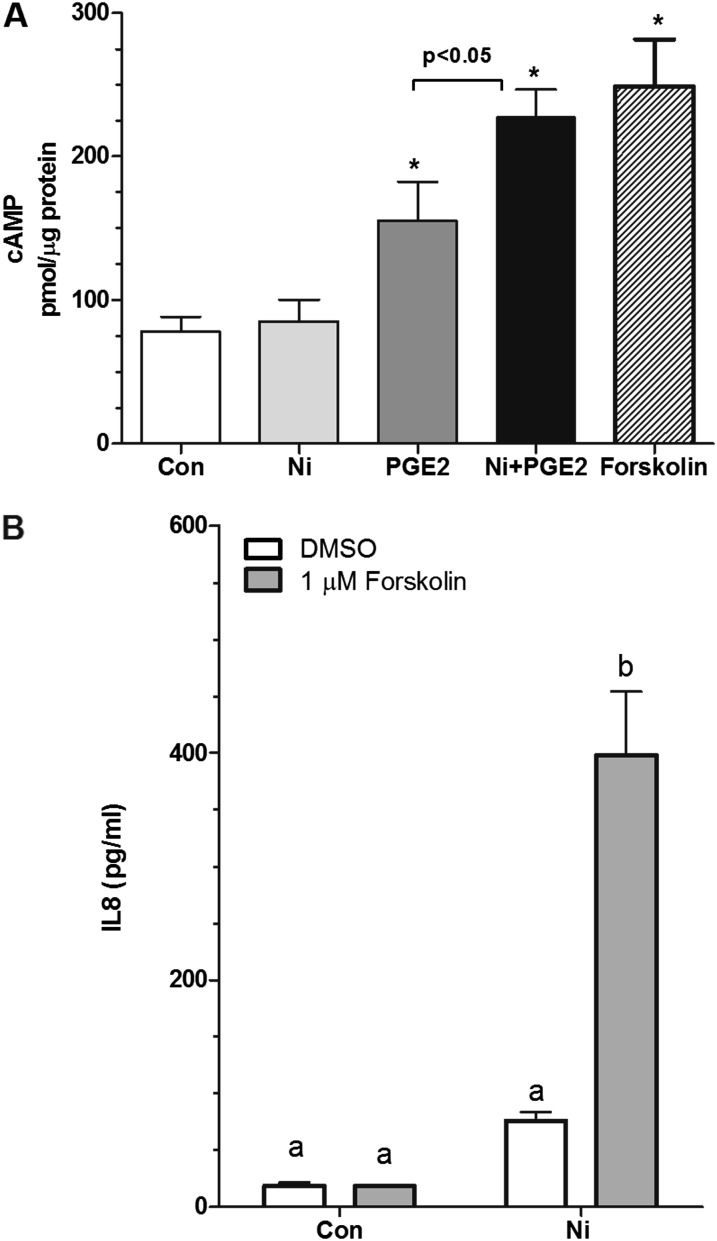
Of the four PGE2 receptor subtypes, PTGER2 and PTGER4 are coupled to Gs, which stimulates cAMP synthesis. Based on our evidence that PGE2 signals through PTGER2 and PTGER4 to enhance Ni-induced IL-8 release from HLF (Figure 2), we sought to determine whether Ni alters cAMP production after exposure to PGE2. Figure 3A shows that levels of cAMP production in unstimulated cells are 78 ± 10 pmol/μg protein. Upon 10-minute stimulation with 10 nM PGE2, levels of cAMP are increased approximately 2-fold compared with control-treated cells, consistent with activation of PTGER2 and -4. Although Ni alone was without effect, when given in combination with PGE2, cAMP production increased to 227 ± 20 pmol/μg protein, levels that were 3-fold greater than control-treated cells and significantly greater than cells treated with PGE2 alone (Figure 3A). Levels of cAMP in cells treated with Ni and PGE2 in combination were similar to those observed with the positive control (forskolin; 1 μM). Forskolin, like PGE2, significantly interacts with Ni to promote IL-8 release from HLF, further supporting that activation of cAMP enhances Ni-induced IL-8 release from HLF (Figure 3B).
Figure 3.
PGE2 increases cAMP levels in HLFs. (A) Cells were seeded into 60-mm dishes at a density of 5.5 × 104 cells/cm2 and allowed to attach overnight before stimulation with 10 nM PGE2 in the presence or absence of 200 μM Ni in serum-free modified Eagle’s medium containing 0.1% BSA for 10 minutes. Forskolin (1 μM) was used as a positive control. Activation of cAMP was determined using an enzyme immunoassay kit. Data shown are mean ± SEM pmol cAMP/μg protein (n = 4–5 dishes). *Different from control-treated cells (P < 0.05) as determined by ANOVA with Tukey’s multiple comparisons test. (B) Cells were stimulated with 200 μM Ni in the presence or absence of forskolin (1 μM) for 48 hours. Data shown are mean ± SEM and are representative of three independent experiments performed in triplicate. Points with different letters have means that are significantly different (P < 0.001) as determined by two-way ANOVA with Bonferroni multiple comparisons test.
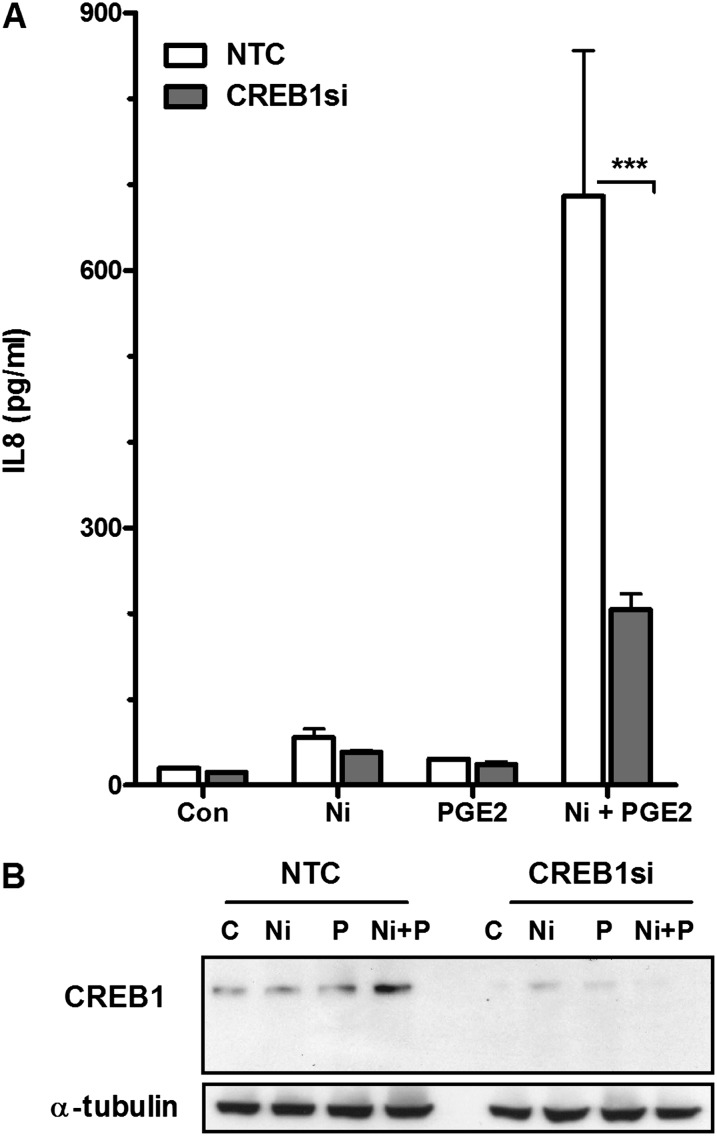
CREB1 has previously been reported to regulate IL-8 expression (22). HLF treated with Ni and PGE2 in combination for 48 hours showed an increase in CREB1 protein expression compared with control-treated cells and cells treated with Ni or PGE2 alone (Figure 4). IL-8 release induced by Ni and PGE2 was significantly attenuated by approximately 65% in HLF transfected with CREB1 siRNA compared with the nontargeting siRNA control (P < 0.001) (Figure 4A).
Figure 4.
cAMP-response element binding protein 1 (CREB1) silencing attenuates Ni- and PGE2-stimulated release of IL-8 from HLF. Cells were transiently transfected with 50 nM of CREB1 small interfering RNA (siRNA) or a nontargeting siRNA control (NTC) before a 48-hour combined Ni and PGE2 exposure. (A) IL-8 levels in conditioned medium from the different treatment groups were analyzed using specific enzyme-linked immunoassay. Data shown are mean ± SEM and are representative of three independent experiments performed in triplicate. ***P < 0.001 compared with NTC as determined by two-way ANOVA with Bonferroni multiple comparisons test. (B) Western blot analysis indicating Ni- and PGE2-induced CREB1 protein expression after a 48-hour exposure and siRNA-mediated knockdown of CREB1 protein expression in HLF. Alpha-tubulin is shown as a loading control.
PGE2 Enhances Activation of HIF1A by Ni
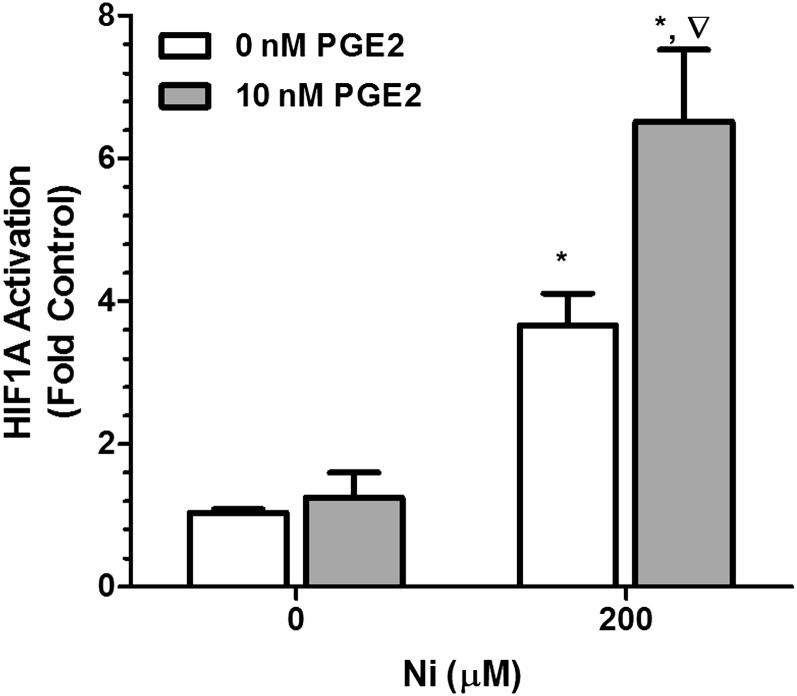
We have previously shown that Ni stabilizes HIF1A in HLF and that this response is enhanced in the presence of TLR2 receptor ligands that promote PGE2 production (20). In the current study, we examined HIF1A activation using a DNA-binding ELISA in which nuclear protein from HLF was incubated in a 96-well plate on which an oligonucleotide containing the hypoxia response element (5′-TACGTGCT-3′) from the erythropoietin gene has been immobilized (HIF TransAM kit; Active Motif). Figure 5 shows that 200 μM Ni stimulated a 3.5-fold increase in HIF1A activation compared with control-treated cells (open bars). Ni-dependent activation of HIF1A was even further enhanced in the presence of 10 nM PGE2. Levels of HIF1A binding activity in nuclear extracts from cells treated with Ni and PGE2 were 6.5-fold greater than control-treated cells and significantly greater than extracts from cells treated with Ni alone, whereas PGE2 alone did not activate HIF1A.
Figure 5.
PGE2 interacts with Ni to promote HIF1A activation in HLF. Cells were stimulated with 200 μM Ni with or without 10 nM PGE2 for 48 hours, and activation of nuclear HIF1A was determined using a DNA-binding ELISA. Data shown are mean ± SEM and are representative of three independent experiments. *Different from 0 μM NiSO4 within PGE2 treatment; ∇different from 200 μM NiSO4 in the absence of PGE2 (P < 0.05) as determined by two-way ANOVA with Bonferroni multiple comparisons test.
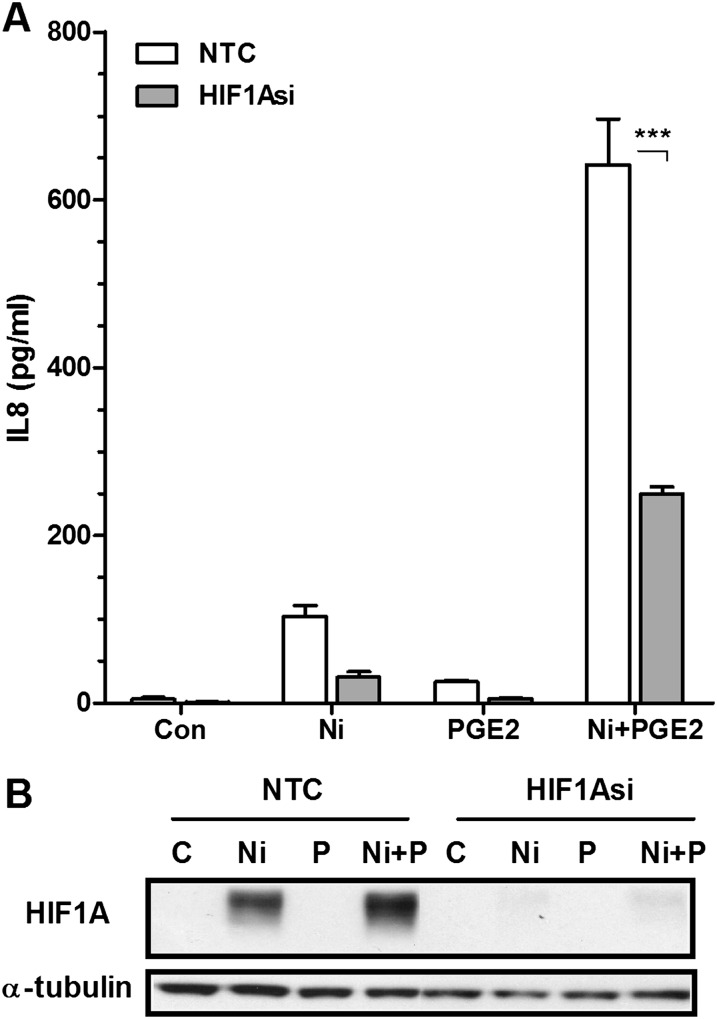
To determine whether PGE2-enhanced activation and stabilization of HIF1A by Ni plays a role in IL-8 release from HLF, cells were transfected with 50 nM HIF1A siRNA or nontargeting siRNA control before stimulation. Figure 6 shows that PGE2 enhances HIF1A stabilization by Ni, consistent with the findings described above. As depicted in the immunoblot, 50 nM HIF1A siRNA was sufficient to prevent HIF1A activation in cells treated with Ni or Ni and PGE2 in combination. Moreover, 50 nM HIF1A siRNA attenuates Ni and PGE2-induced IL-8 release by 60% (Figure 6A). Activation of HIF1A may serve as a common mechanism whereby PM-derived metals interact with PGE2 to promote IL-8 release from lung fibroblasts. Figure E2 indicates that PGE2 synergistically interacts with vanadium to promote IL-8 release from HLF via a HIF1A-mediated pathway.
Figure 6.
Ni- and PGE2-stimulated release of IL-8 from HLF is mediated by HIF1A. Cells were transiently transfected with 50 nM of HIF1A siRNA or a NTC before a 48-hour combined Ni and PGE2 exposure. (A) IL-8 levels in conditioned medium from the different treatment groups were analyzed using specific enzyme-linked immunoassays. Data shown are mean ± SEM and are representative of three independent experiments. ***Different compared with NTC (P < 0.001) as determined by two-way ANOVA with Bonferroni multiple comparisons test. (B) Western blot analysis indicating siRNA-mediated knockdown of HIF-1A protein expression in HLF. Alpha-tubulin is shown as a loading control.
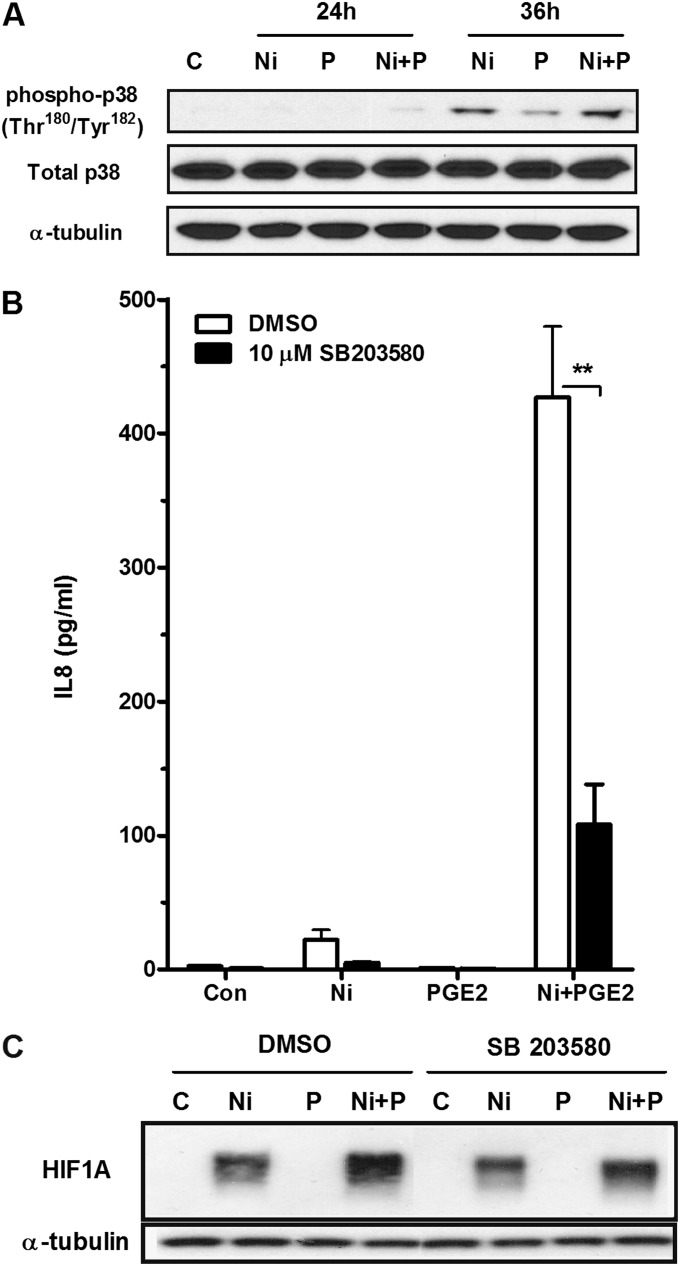
Activation of the p38 MAPK signaling pathway can lead to activation of CREB1 and HIF1A (23, 24). PGE2 has been shown to phosphorylate p38 MAPK, and we have previously reported Ni-dependent phosphorylation of p38 MAPK in HLF after a 24- and 48-hour exposure, which was potentiated by stimulation of toll-like receptor 2 (25). Here we show that Ni and PGE2 interact to promote phosphorylation of p38 MAPK in HLF after prolonged exposures. Figure 7A shows a slight increase in p38 MAPK phosphorylation after a 24-hour mixed exposure to Ni and PGE2, and this effect is further enhanced at 36 hours. To determine if activation of p38 MAPK is involved in the synergistic release of IL-8 from HLF after exposure to Ni and PGE2, cells were pretreated for 1 hour with the p38 MAPK inhibitor SB 203580 (10 μM). Figure 7B shows that SB 203580 attenuated IL-8 release in the presence of Ni and PGE2 by approximately 75% (P < 0.01). Inhibition of p38 MAPK also decreased HIF1A stabilization in response to Ni alone or in combination with 10 nM PGE2 (Figure 7C). Inhibition of additional MAPK pathways (ERK 1/2 and JNK) had no observable effect on IL-8 release, and no interactions between Ni and PGE2 on phosphorylation of ERK1/2 and JNK were observed in HLF (data not shown).
Figure 7.
Activation of p38 MAPK contributes to IL-8 release and HIF1A expression in HLF after stimulation with Ni and PGE2. HLF were cultured in 6-well plates and treated as described in Materials and Methods. (A) Representative Western blot indicating the time-dependent interactions between Ni and PGE2 on p38 MAPK phosphorylation. (B and C) Cells were pretreated with the p38 MAPK inhibitor, SB203580 (10 μM), or DMSO (solvent control, 0.1%) for 1 hour before stimulation with 200 μM Ni in the presence or absence of 10 nM PGE2. IL-8 levels in conditioned medium from the different treatment groups were analyzed using specific enzyme-linked immunoassay. Data shown are mean ± SEM and are representative of three independent experiments. **Different compared with DMSO control (P < 0.01) as determined by two-way ANOVA with Bonferroni multiple comparisons test. Western blot analysis indicates changes in HIF1A expression after Ni + PGE2 with and without SB203580. Alpha-tubulin was included as a loading control. Blot is representative of three independent experiments.
Discussion
Limited information is available regarding the potential for metals found in PM to dysregulate eicosanoid signaling in the lung. The function of lung fibroblasts extends beyond extracellular matrix protein and collagen production. Once thought to be relatively inert, fibroblasts are now considered to play an active role in the response to tissue injury, contributing to cytokine and chemokine release and the development of fibroproliferative disorders. The results provided in the current study provide novel information regarding how exposure to metals found in PM may enhance susceptibility of lung fibroblasts to production of IL-8 through dysregulation of PGE2.
IL-8 plays an important role in host defense by activating and recruiting neutrophils to sites of inflammation and injury. In addition to inducing chemotaxis of neutrophils and T lymphocytes, IL-8 has also been implicated in the regulation of cell adhesion (26), angiogenesis (27), and contraction of airway smooth muscle cells (28). Initially identified as a chemokine released from monocytes (29), IL-8 has since been shown to be synthesized and released by nonimmune cells such as endothelial cells and fibroblasts. Given the considerable evidence that fibroblasts play an active role in the immune response and contribute to the recruitment of inflammatory infiltrate (30, 31), elevated production of IL-8 from fibroblasts in response to Ni and PGE2 could promote wound healing and limit tissue injury. In support of this, levels of IL-8 in the conditioned medium from HLF after Ni and PGE2 exposure reached 400 to 1200 pg/ml, within the range of concentrations shown to stimulate proliferation and migration of endothelial cells (30). However, the balance of immunomodulatory cytokines synthesized, and the inability of fibroblasts to maintain tight control over their production may contribute to the persistence of inflammatory infiltrates, leading to chronic inflammation and fibrosis (32–34).
It is well recognized that Ni stabilizes HIF1A and that Ni has been shown to induce profibrogenic and proinflammatory cytokine gene expression, including IL-8, by hypoxia-like responses in airway epithelial cells (35–38). Here we show that, in contrast to findings in epithelial cells, Ni exposures alone have no effect on IL-8 release, despite an induction in HIF1A. It is only in the presence of PGE2 that HIF1A plays a pivotal role in modulating IL-8 release from human lung fibroblasts. Given our observation that HIF1A has the potential to reprogram fibroblast response to PGE2, this could enhance susceptibility to prolonged inflammation and tissue instability during Ni exposures.
Ambient air concentrations of Ni range between 7 and 12 ng/m3 over different United States geographic areas, with concentrations up to 150 ng/m3 near point sources (39). Moreover, ambient levels approaching 50 ng/m3 are routinely recorded in the winter months in New York City (40). We previously estimated that, assuming normal respiration, 50% deposition, and negligible elimination, breathing 100 ng/m3 over a 24-hour period would produce approximately 1 μM concentration of Ni within the extracellular space of the lung (41). Although this is less than the concentration used in this study for synergistic interactions with PGE2, accumulation of Ni over time, heterogenous distribution, and altered breathing patterns could produce regional concentrations sufficient to recapitulate the effects seen here.
It is widely recognized that PTGS2 plays an important role in the onset of inflammation in the lung (42), yet the role of PGE2 in the immune-inflammatory response is complex. Differences in the cellular response to PGE2 are mediated by cell-specific expression of PGE2 receptor subtypes and differential coupling to signal transduction pathways. Four transmembrane G protein–coupled receptor subtypes mediate the biological actions of PGE2 (PTGER1–4). PTGER1 contributes to calcium mobilization through phosphatidylinositol turnover, and multiple alternate splice forms of PTGER3 offer a broad range of effects, including the modulation of cellular cAMP levels and activation of protein kinase C and phosphatidylinositol 3 kinase (43, 44). PGE2 signaling through PTGER2 and PTGER4 leads to the generation of cAMP. The IL-8 promoter, located between −1,491 and +43 bp of the transcriptional start site, contains multiple putative transcription factor binding sites, including a cAMP response element–like site (22), suggesting a regulatory role for cAMP on IL-8 release. PGE2 has been shown to regulate IL-8 release via PTGER4, albeit with different results depending on cell type. Activation of PTGER4 with subsequent cAMP stimulation induces IL-8 secretion in pulmonary microvascular endothelial cells (45). Conversely, in human macrophages, PGE2 suppresses IL-8 via PTGER4 (46). Our findings indicate that, in lung fibroblasts, PGE2/cAMP-induced IL-8 release depends on the presence of additional environmental factors. Consistent with PTGER2 and PTGER4 signaling, PGE2 and forskolin stimulated a significant increase in cAMP in lung fibroblasts (Figure 4). However, activation of cAMP alone is not enough to stimulate IL-8 release from HLF because no differences were observed after stimulation with PGE2 or forskolin compared with controls. It was only in the presence of HIF1A, induced by the hypoxia-mimetic metal Ni, that PGE2 enhanced the production of IL-8 in lung fibroblasts. This enhanced production also requires CREB1 because negation of CREB1 using siRNA blocked the synergistic interactions by Ni and PGE2. This phenomenon may not be unique to Ni because other hypoxia-mimetic metals present in PM, like vanadium, have similar results (Figure E2). A recent finding by Maybin and colleagues (47) that hypoxia and PGE2 synergistically stimulate the release of IL-8 from human endometrial stromal cells corroborates our results that HIF1A and CREB1 can interact to positively regulate IL-8 release.
The PGE2/cAMP-dependent regulation of pulmonary fibroblast function has focused primarily on inhibition of collagen synthesis and proliferation mediated predominantly via PTGER2 (48–51). For example, fibrotic lung fibroblasts have been shown to exhibit resistance to inhibitory effects of PGE2 through down-regulation of PTGER2 (52). It is possible that chemical and other environmental exposures can modulate PTGER receptor expression or subcellular localization, thereby altering cellular response to PGE2 (53). However, we did not observe changes in PTGER2 or PTGER4 expression in response to Ni exposure (data not shown), and PGE2-induced cAMP production via PTGER2 and PTGER4 is maintained in the presence of Ni (Figure 4). We have previously shown that, in the absence of Ni and HIF1A induction, PGE2 suppresses IL-8 release from HLF in response to microbial and bacterial stimulation (20), and these findings can be recapitulated by forskolin, indicating that suppressive effects of PGE2 in lung fibroblasts are mediated via cAMP. The added presence of HIF1A may therefore facilitate a switch in the role of PGE2 from antiinflammatory to proinflammatory, enhancing IL-8 release (Figure 6; Figure E2), consistent with our previous findings (20). These results indicate that PGE2 may use different receptor subtypes to regulate cytokine production (PTGER2 versus PTGER4) than it does for collagen synthesis and cellular proliferation (PTGER2). In addition, Ni may modulate the coupling efficiency of PTGER2/4 to subsequent cAMP generation in response to PGE2.
The PGE2concentration capable of eliciting a synergistic interaction with Ni on IL-8 release (10 nM) is within the range of PGE2 levels reported in the epithelial lining fluid of the lower respiratory tract of normal individuals (∼ 50 nM) (54). This suggests that Ni can interact with physiologic levels of PGE2 to disrupt chemokine production. The possibility that Ni could interact with additional endogenous mediators to promote chemokine release cannot be ignored. Glista-Baker and colleagues recently reported that Ni synergistically interacts with platelet-derived growth factor to promote increased expression of profibrogenic chemokines in pleural mesothelial cells (55). The synergistic interactions with Ni and PGE2 in the current study and those of Ni with platelet-derived growth factor reported by Glista-Baker and colleagues involve enhanced stabilization of HIF1A.
Recent evidence has indicated that HIF1A plays a significant contribution to various inflammatory-like processes (56). For example, conditional deletions of HIF1A impair chemotaxis and bacteriocidal activity of macrophages in vitro (57). Prolyl hydroxylases regulate HIF1A under normal oxygen conditions by targeting the protein for degradation by the ubiquitin-proteasome pathway via association with the von Hippel-Lindau protein tumor suppressor protein (58). Under hypoxic conditions, prolyl hydroxylase activity is inhibited, and HIF1A accumulates, translocates to the nucleus, and activates gene transcription. Similar to hypoxia, disruption of prolyl hydroxylase activity has been indicated as a mechanism for Ni-induced HIF1A stabilization (59, 60). The current findings show that Ni stabilizes and activates HIF1A, and this process is enhanced in the presence of PGE2. The precise mechanism whereby Ni stabilizes HIF1A in lung fibroblasts is unknown but may result from disruptions in prolyl hydroxylase activity as indicated above. How Ni-induced disruptions in prolyl hydroxylase may be altered by PGE2 warrants further investigation.
The contribution of p38 MAPK in the regulation of inflammatory and innate immune responses has been reported, and activation of p38 MAPK has been implicated in HIF1A stabilization (23). Consistent with these reports, we found that inhibition of p38 MAPK attenuated HIF1A stabilization along with synergistic release of IL-8 by Ni and PGE2 from HLF (Figure 7). We have previously reported that prolonged exposure to Ni (24 or 48 h) stimulates phosphorylation of p38 MAPK (25), consistent with findings reported by others (61, 62). We did not find evidence that PGE2 alone activates p38 MAPK (Figure 7). However, enhanced activation of HIF1A by Ni and PGE2 stimulated PTGS2 expression in HLF (Figure E3). PTGS2 has been shown to be autoamplified in a PGE2-dependent manner, a process that is in part dependent on p38 activation (63, 64). It is likely that once PTGS2 is induced in HLF, subsequent production of PGE2 establishes a positive feedback loop that, in the presence of Ni, further amplifies activation of p38 MAPK signaling and accumulation of HIF1A, thereby maintaining IL-8 release.
Increased IL-8 production has been associated with numerous respiratory disorders, including asthma (65) and pulmonary fibrosis (66). As such, elaborated production of IL-8 in response to chemical stimuli could set up an environment in the lung that would have detrimental effects on respiratory health, promoting prolonged tissue inflammation (67). The current study suggests that PGE2-dependent regulation of human lung fibroblasts is context specific. PGE2 is generally thought to be a protective factor occurring after lung injury, possessing antiinflammatory and immunosuppressive properties that inhibit chemotaxis (68) and reduce collagen deposition and fibroblast proliferation (21). However, the findings described herein indicate that HIF1A-dependent disruptions in PGE2 signaling can enhance IL-8 release. The pathologic consequences of HIF1A-dependent modifications in PGE2 signaling pathways in the lung after prolonged Ni exposures in vivo is unknown and warrants further investigation.
In conclusion, the current study provides novel information on the ability of atmospheric hypoxia-mimetic metals to modulate PGE2-dependent release of immune-modulating chemokines in HLF. Moreover, in the presence of HIF1A, cAMP-mediated signaling pathways may be altered to exacerbate inflammatory-like processes in lung tissue, imparting a susceptibility of PM-exposed populations to adverse respiratory health effects.
Footnotes
This work was supported by National Institutes of Health grant R01-ES-001986 (J.P.F.) and by National Institutes of Health grant F32-ES-015966 (K.A.B.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0297OC on March 22, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Chiaverini L. Asthma, particulates, and diesel exhaust. Med Health R I. 2002;85:140–142. [PubMed] [Google Scholar]
- 2.Englert N. Fine particles and human health: a review of epidemiological studies. Toxicol Lett. 2004;149:235–242. doi: 10.1016/j.toxlet.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 3.Kappos AD, Bruckmann P, Eikmann T, Englert N, Heinrich U, Hoppe P, Koch E, Krause GH, Kreyling WG, Rauchfuss K, et al. Health effects of particles in ambient air. Int J Hyg Environ Health. 2004;207:399–407. doi: 10.1078/1438-4639-00306. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen M, Autrup H, Moller P, Hertel O, Jensen SS, Vinzents P, Knudsen LE, Loft S. Linking exposure to environmental pollutants with biological effects. Mutat Res. 2003;544:255–271. doi: 10.1016/j.mrrev.2003.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Ferin J, Oberdorster G, Penney DP. Pulmonary retention of ultrafine and fine particles in rats. Am J Respir Cell Mol Biol. 1992;6:535–542. doi: 10.1165/ajrcmb/6.5.535. [DOI] [PubMed] [Google Scholar]
- 6.Figueroa DA, Rodriguez-Sierra CJ, Jimenez-Velez BD. Concentrations of ni and v, other heavy metals, arsenic, elemental and organic carbon in atmospheric fine particles (PM2.5) from Puerto Rico. Toxicol Ind Health. 2006;22:87–99. doi: 10.1191/0748233706th247oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gioda A, Fuentes-Mattei E, Jimenez-Velez B. Evaluation of cytokine expression in beas cells exposed to fine particulate matter (PM2.5) from specialized indoor environments. Int J Environ Health Res. 2011;21:106–119. doi: 10.1080/09603123.2010.515668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lippmann M, Ito K, Hwang JS, Maciejczyk P, Chen LC. Cardiovascular effects of nickel in ambient air. Environ Health Perspect. 2006;114:1662–1669. doi: 10.1289/ehp.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz MJ, Costa R, Marquilles E, Morell F, Munoz X. (Occupational asthma caused by chromium and nickel.) Arch Bronconeumol. 2006;42:302–306. doi: 10.1016/s1579-2129(06)60147-x. [DOI] [PubMed] [Google Scholar]
- 10.Oller AR, Costa M, Oberdorster G. Carcinogenicity assessment of selected nickel compounds. Toxicol Appl Pharmacol. 1997;143:152–166. doi: 10.1006/taap.1996.8075. [DOI] [PubMed] [Google Scholar]
- 11.Haley PJ, Shopp GM, Benson JM, Cheng YS, Bice DE, Luster MI, Dunnick JK, Hobbs CH. The immunotoxicity of three nickel compounds following 13-week inhalation exposure in the mouse. Fundam Appl Toxicol. 1990;15:476–487. doi: 10.1016/0272-0590(90)90034-h. [DOI] [PubMed] [Google Scholar]
- 12.Dunnick JK, Elwell MR, Benson JM, Hobbs CH, Hahn FF, Haly PJ, Cheng YS, Eidson AF. Lung toxicity after 13-week inhalation exposure to nickel oxide, nickel subsulfide, or nickel sulfate hexahydrate in F344/N rats and B6C3F1 mice. Fundam Appl Toxicol. 1989;12:584–594. doi: 10.1016/0272-0590(89)90031-6. [DOI] [PubMed] [Google Scholar]
- 13.National Toxicology Programme. NTP toxicology and carcinogenesis studies of nickel oxide (cas no. 1313–99–1) in F344 rats and B6C3F1 mice (inhalation studies) Natl Toxicol Program Tech Rep Ser. 1996;451:1–381. [PubMed] [Google Scholar]
- 14.Bayram H, Devalia JL, Sapsford RJ, Ohtoshi T, Miyabara Y, Sagai M, Davies RJ. The effect of diesel exhaust particles on cell function and release of inflammatory mediators from human bronchial epithelial cells in vitro. Am J Respir Cell Mol Biol. 1998;18:441–448. doi: 10.1165/ajrcmb.18.3.2882. [DOI] [PubMed] [Google Scholar]
- 15.van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R, Hogg JC. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)) Am J Respir Crit Care Med. 2001;164:826–830. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- 16.Morimoto Y, Ogami A, Todoroki M, Yamamoto M, Murakami M, Hirohashi M, Oyabu T, Myojo T, Nishi K, Kadoya C, et al. Expression of inflammation-related cytokines following intratracheal instillation of nickel oxide nanoparticles. Nanotoxicology. 2010;4:161–176. doi: 10.3109/17435390903518479. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman J, Graf BA, Leung EC, Pollock SJ, Koumas L, Reddy SY, Blieden TM, Smith TJ, Phipps RP. Fibroblasts as sentinel cells: role of the CDcd40-CDcd40 ligand system in fibroblast activation and lung inflammation and fibrosis. Chest. 2001;120:53S–55S. doi: 10.1378/chest.120.1_suppl.s53. [DOI] [PubMed] [Google Scholar]
- 18.Smith RS, Fedyk ER, Springer TA, Mukaida N, Iglewski BH, Phipps RP. Il-8 production in human lung fibroblasts and epithelial cells activated by the pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-kappa b and activator protein-2. J Immunol. 2001;167:366–374. doi: 10.4049/jimmunol.167.1.366. [DOI] [PubMed] [Google Scholar]
- 19.Brant KA, Fabisiak JP. Nickel alterations of TLR2-dependent chemokine profiles in lung fibroblasts are mediated by COX-2. Am J Respir Cell Mol Biol. 2008;38:591–599. doi: 10.1165/rcmb.2007-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brant KA, Fabisiak JP. Nickel and the microbial toxin, MALP-2, stimulate proangiogenic mediators from human lung fibroblasts via a HIF-1alpha and COX-2-mediated pathway. Toxicol Sci. 2009;107:227–237. doi: 10.1093/toxsci/kfn208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vancheri C, Mastruzzo C, Sortino MA, Crimi N. The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol. 2004;25:40–46. doi: 10.1016/j.it.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Bezzerri V, Borgatti M, Finotti A, Tamanini A, Gambari R, Cabrini G. Mapping the transcriptional machinery of the IL-8 gene in human bronchial epithelial cells. J Immunol. 2011;187:6069–6081. doi: 10.4049/jimmunol.1100821. [DOI] [PubMed] [Google Scholar]
- 23.Shemirani B, Crowe DL. Hypoxic induction of HIF-1alpha and VEGF expression in head and neck squamous cell carcinoma lines is mediated by stress activated protein kinases. Oral Oncol. 2002;38:251–257. doi: 10.1016/s1368-8375(01)00052-5. [DOI] [PubMed] [Google Scholar]
- 24.Di Giacomo V, Sancilio S, Caravatta L, Rana RA, Di Pietro R, Cataldi A. Regulation of CREB activation by p38 mitogen activated protein kinase during human primary erythroblast differentiation. Int J Immunopathol Pharmacol. 2009;22:679–688. doi: 10.1177/039463200902200313. [DOI] [PubMed] [Google Scholar]
- 25.Gao F, Brant KA, Ward RM, Cattley RT, Barchowsky A, Fabisiak JP. Multiple protein kinase pathways mediate amplified IL-6 release by human lung fibroblasts co-exposed to nickel and TLR-2 agonist, malp-2. Toxicol Appl Pharmacol. 2010;247:146–157. doi: 10.1016/j.taap.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber AR, Kunkel SL, Todd RF, III, Weiss SJ. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991;254:99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- 27.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- 28.Nakamura H, Yoshimura K, Jaffe HA, Crystal RG. Interleukin-8 gene expression in human bronchial epithelial cells. J Biol Chem. 1991;266:19611–19617. [PubMed] [Google Scholar]
- 29.Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, Leonard EJ. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci USA. 1987;84:9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells: synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–322. [PMC free article] [PubMed] [Google Scholar]
- 31.Olgart C, Frossard N. Human lung fibroblasts secrete nerve growth factor: effect of inflammatory cytokines and glucocorticoids. Eur Respir J. 2001;18:115–121. doi: 10.1183/09031936.01.00069901. [DOI] [PubMed] [Google Scholar]
- 32.Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22:199–204. doi: 10.1016/s1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- 33.Hogaboam CM, Bone-Larson CL, Lipinski S, Lukacs NW, Chensue SW, Strieter RM, Kunkel SL. Differential monocyte chemoattractant protein-1 and chemokine receptor 2 expression by murine lung fibroblasts derived from Th1- and Th2-type pulmonary granuloma models. J Immunol. 1999;163:2193–2201. [PubMed] [Google Scholar]
- 34.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salnikow K, An WG, Melillo G, Blagosklonny MV, Costa M. Nickel-induced transformation shifts the balance between HIF-1 and p53 transcription factors. Carcinogenesis. 1999;20:1819–1823. doi: 10.1093/carcin/20.9.1819. [DOI] [PubMed] [Google Scholar]
- 36.Salnikow K, Su W, Blagosklonny MV, Costa M. Carcinogenic metals induce hypoxia-inducible factor-stimulated transcription by reactive oxygen species-independent mechanism. Cancer Res. 2000;60:3375–3378. [PubMed] [Google Scholar]
- 37.Andrew AS, Klei LR, Barchowsky A. Nickel requires hypoxia-inducible factor-1α, not redox signaling, to induce plasminogen activator inhibitor-1. Am J Physiol Lung Cell Mol Physiol. 2001;281:L607–L615. doi: 10.1152/ajplung.2001.281.3.L607. [DOI] [PubMed] [Google Scholar]
- 38.Barchowsky A, Soucy NV, O'Hara KA, Hwa J, Noreault TL, Andrew AS. A novel pathway for nickel-induced interleukin-8 expression. J Biol Chem. 2002;277:24225–24231. doi: 10.1074/jbc.M202941200. [DOI] [PubMed] [Google Scholar]
- 39.Schroeder JM, Dobson M, Kane DM. Toxic trace elements associated with airborne particulate matter: a review. JAPCA. 1987;11:1267–1287. doi: 10.1080/08940630.1987.10466321. [DOI] [PubMed] [Google Scholar]
- 40.Peltier RE, Hsu SI, Lall R, Lippmann M. Residual oil combustion: a major source of airborne nickel in New York City. J Expo Sci Environ Epidemiol. 2009;19:603–612. doi: 10.1038/jes.2008.60. [DOI] [PubMed] [Google Scholar]
- 41.Gao F, Barchowsky A, Nemec AA, Fabisiak JP. Microbial stimulation by mycoplasma fermentans synergistically amplifies IL-6 release by human lung fibroblasts in response to residual oil fly ash (rofa) and nickel. Toxicol Sci. 2004;81:467–479. doi: 10.1093/toxsci/kfh205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park GY, Christman JW. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol. 2006;290:L797–L805. doi: 10.1152/ajplung.00513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol. 2004;36:1187–1205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Chung KF.Evaluation of selective prostaglandin E2 (PGE2) receptor agonists as therapeutic agents for the treatment of asthma Sci STKE 2005. 2005:pe47 [DOI] [PubMed] [Google Scholar]
- 45.Aso H, Ito S, Mori A, Morioka M, Suganuma N, Kondo M, Imaizumi K, Hasegawa Y. Prostaglandin E2 enhances interleukin-8 production via EP4 receptor in human pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2012;302:L266–L273. doi: 10.1152/ajplung.00248.2011. [DOI] [PubMed] [Google Scholar]
- 46.Takayama K, Garcia-Cardena G, Sukhova GK, Comander J, Gimbrone MA, Jr, Libby P. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem. 2002;277:44147–44154. doi: 10.1074/jbc.M204810200. [DOI] [PubMed] [Google Scholar]
- 47.Maybin JA, Hirani N, Jabbour HN, Critchley HO. Novel roles for hypoxia and prostaglandin E2 in the regulation of IL-8 during endometrial repair. Am J Pathol. 2011;178:1245–1256. doi: 10.1016/j.ajpath.2010.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haag S, Warnken M, Juergens UR, Racke K. Role of Epac1 in mediating anti-proliferative effects of prostanoid EP(2) receptors and cAMP in human lung fibroblasts. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:617–630. doi: 10.1007/s00210-008-0334-3. [DOI] [PubMed] [Google Scholar]
- 49.Huang SK, Wettlaufer SH, Chung J, Peters-Golden M. Prostaglandin E2 inhibits specific lung fibroblast functions via selective actions of PKA and Epac-1. Am J Respir Cell Mol Biol. 2008;39:482–489. doi: 10.1165/rcmb.2008-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Insel PA, Murray F, Yokoyama U, Romano S, Yun H, Brown L, Snead A, Lu D, Aroonsakool N. cAMP and Epac in the regulation of tissue fibrosis. Br J Pharmacol. 2012;166:447–456. doi: 10.1111/j.1476-5381.2012.01847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yokoyama U, Patel HH, Lai NC, Aroonsakool N, Roth DM, Insel PA. The cyclic AMP effector epac integrates pro- and anti-fibrotic signals. Proc Natl Acad Sci USA. 2008;105:6386–6391. doi: 10.1073/pnas.0801490105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore BB, Ballinger MN, White ES, Green ME, Herrygers AB, Wilke CA, Toews GB, Peters-Golden M. Bleomycin-induced E prostanoid receptor changes alter fibroblast responses to prostaglandin E2. J Immunol. 2005;174:5644–5649. doi: 10.4049/jimmunol.174.9.5644. [DOI] [PubMed] [Google Scholar]
- 53.Tober KL, Thomas-Ahner JM, Kusewitt DF, Oberyszyn TM. Effects of UVB on E prostanoid receptor expression in murine skin. J Invest Dermatol. 2006;127:214–221. doi: 10.1038/sj.jid.5700502. [DOI] [PubMed] [Google Scholar]
- 54.Ozaki T, Rennard SI, Crystal RG. Cyclooxygenase metabolites are compartmentalized in the human lower respiratory tract. J Appl Physiol. 1987;62:219–222. doi: 10.1152/jappl.1987.62.1.219. [DOI] [PubMed] [Google Scholar]
- 55.Glista-Baker EE, Taylor AJ, Sayers BC, Thompson EA, Bonner JC. Nickel nanoparticles enhance platelet-derived growth factor-induced chemokine expression by mesothelial cells via prolonged mitogen-activated protein kinase activation. Am J Respir Cell Mol Biol. 2012;47:552–561. doi: 10.1165/rcmb.2012-0023OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappab/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 57.Cramer T, Johnson RS. A novel role for the hypoxia inducible transcription factor HIF-1alpha: critical regulation of inflammatory cell function. Cell Cycle. 2003;2:192–193. [PubMed] [Google Scholar]
- 58.Ivan M, Kaelin WG., Jr The von hippel-lindau tumor suppressor protein. Curr Opin Genet Dev. 2001;11:27–34. doi: 10.1016/s0959-437x(00)00152-0. [DOI] [PubMed] [Google Scholar]
- 59.Costa M, Davidson TL, Chen H, Ke Q, Zhang P, Yan Y, Huang C, Kluz T. Nickel carcinogenesis: epigenetics and hypoxia signaling. Mutat Res. 2005;592:79–88. doi: 10.1016/j.mrfmmm.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 60.Salnikow K, Donald SP, Bruick RK, Zhitkovich A, Phang JM, Kasprzak KS. Depletion of intracellular ascorbate by the carcinogenic metals nickel and cobalt results in the induction of hypoxic stress. J Biol Chem. 2004;279:40337–40344. doi: 10.1074/jbc.M403057200. [DOI] [PubMed] [Google Scholar]
- 61.Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, tnf-alpha, and contact sensitizers. J Immunol. 2001;166:3837–3845. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- 62.Boisleve F, Kerdine-Romer S, Rougier-Larzat N, Pallardy M. Nickel and DNCB induce CCR7 expression on human dendritic cells through different signalling pathways: role of TNF-alpha and MAPK. J Invest Dermatol. 2004;123:494–502. doi: 10.1111/j.0022-202X.2004.23229.x. [DOI] [PubMed] [Google Scholar]
- 63.Faour WH, He Y, He QW, de Ladurantaye M, Quintero M, Mancini A, Di Battista JA. Prostaglandin E(2) regulates the level and stability of cyclooxygenase-2 mRNA through activation of p38 mitogen-activated protein kinase in interleukin-1 beta-treated human synovial fibroblasts. J Biol Chem. 2001;276:31720–31731. doi: 10.1074/jbc.M104036200. [DOI] [PubMed] [Google Scholar]
- 64.Suda M, Tanaka K, Yasoda A, Natsui K, Sakuma Y, Tanaka I, Ushikubi F, Narumiya S, Nakao K. Prostaglandin E2 (PGE2) autoamplifies its production through ep1 subtype of pge receptor in mouse osteoblastic MC3T3-E1 cells. Calcif Tissue Int. 1998;62:327–331. doi: 10.1007/s002239900440. [DOI] [PubMed] [Google Scholar]
- 65.Ordonez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma: clinical and biologic significance. Am J Respir Crit Care Med. 2000;161:1185–1190. doi: 10.1164/ajrccm.161.4.9812061. [DOI] [PubMed] [Google Scholar]
- 66.Tsoutsou PG, Gourgoulianis KI, Petinaki E, Germenis A, Tsoutsou AG, Mpaka M, Efremidou S, Molyvdas PA. Cytokine levels in the sera of patients with idiopathic pulmonary fibrosis. Respir Med. 2006;100:938–945. doi: 10.1016/j.rmed.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 67.Gauldie J, Kolb M, Sime PJ. A new direction in the pathogenesis of idiopathic pulmonary fibrosis? Respir Res. 2002;3:1. doi: 10.1186/rr158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kohyama T, Ertl RF, Valenti V, Spurzem J, Kawamoto M, Nakamura Y, Veys T, Allegra L, Romberger D, Rennard SI. Prostaglandin E(2) inhibits fibroblast chemotaxis. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1257–L1263. doi: 10.1152/ajplung.2001.281.5.L1257. [DOI] [PubMed] [Google Scholar]