Abstract
Increased lung vascular permeability, the consequence of endothelial cell (EC) barrier dysfunction, is a cardinal feature of inflammatory conditions such as acute lung injury and sepsis and leads to lethal physiological dysfunction characterized by alveolar flooding, hypoxemia, and pulmonary edema. We previously demonstrated that the nonmuscle myosin light chain kinase isoform (nmMLCK) plays a key role in agonist-induced pulmonary EC barrier regulation. The present study evaluated posttranscriptional regulation of MYLK expression, the gene encoding nmMLCK, via 3′ untranslated region (UTR) binding by microRNAs (miRNAs) with in silico analysis identifying hsa-miR-374a, hsa-miR-374b, hsa-miR-520c-3p, and hsa-miR-1290 as miRNA candidates. We identified increased MYLK gene transcription induced by TNF-α (24 h; 4.7 ± 0.45 fold increase [FI]), LPS (4 h; 2.85 ± 0.15 [FI]), and 18% cyclic stretch (24 h; 4.6 ± 0.24 FI) that was attenuated by transfection of human lung ECs with mimics of hsa-miR-374a, hsa-miR-374b, hsa-miR-520c-3p, or hsa-miR-1290 (20–80% reductions by each miRNA). TNF-α, LPS, and 18% cyclic stretch each increased the activity of a MYLK 3′UTR luciferase reporter (2.5–7.0 FI) with induction reduced by mimics of each miRNA (30–60% reduction). MiRNA inhibitors (antagomirs) for each MYLK miRNA significantly increased 3′UTR luciferase activity (1.2–2.3 FI) and rescued the decreased MLCK-3′UTR reporter activity produced by miRNA mimics (70–110% increases for each miRNA; P < 0.05). These data demonstrate that increased human lung EC expression of MYLK by bioactive agonists (excessive mechanical stress, LPS, TNF-α) is regulated in part by specific miRNAs (hsa-miR-374a, hsa-miR-374b, hsa-miR-520c-3p, and hsa-miR-1290), representing a novel therapeutic strategy for reducing inflammatory lung injury.
Keywords: miRNA, MLCK, acute lung injury, ventilator-induced lung injury, endothelial cells
Clinical Relevance
These studies extend our knowledge about MYLK as a novel candidate gene in human inflammatory lung injuries such as acute lung injury and asthma. Our findings may lead to the development of novel therapeutic strategies to reducing inflammatory lung injury.
The pulmonary vascular endothelium serves as a semiselective barrier between circulating blood and surrounding tissues, with endothelial cell (EC) integrity being critical to tissue and organ function. Disruption of this vascular barrier induced by inflammatory agonists, such as IL-1β, TNF-α, and LPS, and by excessive mechanical stress, such as produced by shear stress or mechanical ventilation, leads to potentially lethal physiological dysfunction, such as hypoxemia and severe lung edema, which are hallmarks of acute inflammatory lung injury (ALI) (1–3). We previously demonstrated that MYLK, the gene encoding the critical cytoskeletal effector myosin light chain kinase (MLCK), is a compelling candidate gene in inflammatory lung injuries with critical modulation of vascular EC barrier integrity such as ALI and asthma (4–6). We cloned the full-length human MYLK gene encoding a novel nonmuscle MLCK isoform (nmMLCK) (210 kD) (7) and several nmMLCK splice variants that are also highly expressed in endothelium (8) and convincingly demonstrated nmMLCK as a multifunctional enzyme driving cytoskeletal participation in vascular barrier disruption and in barrier-restorative processes (9, 10). Barrier-disrupting edemagenic agonists produce spatially localized myosin light chain phosphorylation (Ser19, Thr18) within cytoplasmic contractile stress fibers, resulting in actomyosin contraction, tension, and formation of paracellular gaps. In contrast, barrier-protective agonists induce nmMLCK translocation to cortical actin networks and into lamellipodial membrane protrusions designed to close paracellular gaps and restore barrier integrity. In addition, nmMLCK regulates lung trafficking of inflammatory cells (11) and is robustly activated by biophysical forces (excessive mechanical stress) that are key to ventilator-induced lung injury (VILI) (5). Levels of nmMLCK gene expression and enzymatic activities contribute to the risk and severity of ALI and VILI, as shown in preclinical models and humans (4–6). Despite the multifunctionality of nmMLCK, the regulatory mechanisms governing nmMLCK gene and protein expression and spatially directed kinase activities are poorly understood.
MicroRNAs (miRNAs) are a novel class of small, endogenous, noncoding RNAs with important participation in posttranscriptional gene regulation in plants and animals. miRNAs act via multiple signaling pathways to regulate transcription, coding messenger ribonucleic acid (mRNA) processing, mRNA stability, and translation and indirectly through global effects on methylation or targeting of transcription factors (12, 13). MicroRNAs predominantly bind to 3′ untranslated regions (UTRs) of target genes and only sporadically bind to 5′UTRs or open reading frames (14, 15) and are critical for biological processes such as cell differentiation, proliferation, apoptosis, and tumorigenesis (16, 17). In addition, miRNAs recently emerged as novel biomarkers and therapeutic strategies because miRNA deregulation is associated with diverse disorders such as cancer, neurodegenerative disease, and cardiovascular and myeloproliferative diseases (reviewed in Refs. 18 and 19) as well as with complex inflammatory lung diseases such as ALI, asthma, pulmonary arterial hypertension, lung cancer, and idiopathic pulmonary fibrosis (20–24). Because the role of miRNAs in posttranscriptional MYLK gene regulation and in the pathogenesis of ALI has not been investigated, we used a MYLK 3′UTR luciferase reporter, combined with overexpression of mature miRNAs and endogenous inhibitory miRNA antagomirs, to explore the potential involvement of miRNAs in MYLK gene expression in human lung ECs.
Materials and Methods
Reagents
LPS from Escherichia coli was obtained from Sigma-Aldrich (St. Louis, MO). Human TNF-α was purchased from Cell Signaling Technology (Danvers, MA). Human MYLK 3′ UTR cloned downstream of a firefly luciferase gene was obtained from SwitchGear Genomics (Menlo Park, CA). Unless specified, biochemical reagents were obtained from Sigma-Aldrich.
Human Lung Endothelial Cell Culture
Human pulmonary artery ECs were obtained from Lonza, Inc. (Allendale, NJ) and cultured in Endothelial Basal Medium-2 with 10% FBS at 37°C in a humidified incubator with 5% CO2 as previously described (9). Passages 5 through 9 were used for the experimentation.
Bioinformatics Analysis and miRNA Prediction
To predict the miRNAs that potentially regulate MYLK mRNA, four computational algorithms were used: TargetScan 6.2 developed by Whitehead Institute for Biomedical Research (Cambridge, MA) (http://www.targetscan.org/) (25, 26), MiRanda developed by Computational and Systems Biology Center, Memorial Sloan-Kettering Cancer Center (New York, NY) (http://www.microrna.org/microrna/home.do) (27), DIANA-microT-CDS developed by DIANA-Lab, Institute of Molecular Oncology, Biomedical Sciences Research Center ‘Alexander Fleming’ (Vari, Greece) (http://diana.cslab.ece. ntua.gr/microT/) (28), and MirTarget2 developed by Department of Radiation Oncology, Washington University School of Medicine (St. Louis, MO) and included in the miRDB database (http://mirdb.org/miRDB/) (29). Further details are provided in the online supplement.
Transient Transfections and Reporter Assays
Transfection of luciferase reporter constructs and miRNA or miRNA inhibitors was performed according to manufacturer’s protocol. Luciferase activity was measured using the Dual Luciferase Assay System (Promega, Fitchburg, WI) following the manufacturer’s protocol. Further details are provided in the online supplement.
Cyclic Stretch Experiments
All cyclic stretch (CS) experiments were performed using the FX-4000T Flexcell Tension Plus system (Flexcell International, Hillsborough, NC) equipped with a 25-mm BioFlex loading station designed to provide uniform radial and circumferential strain across a membrane surface along all radii, as described previously (6). Briefly, ECs were seeded at standard densities (8 × 105 cells/well) onto collagen type I–coated, flexible-bottomed BioFlex plates transfected with MYLK luciferase reporter and/or miRNA mimics/antagomirs and cultured in EGM-2 complete medium containing 2% FBS at 37°C. After 24 hours of culture, the medium was changed in each plate, and confluent EC monolayers were subjected to cyclic stretch (0.5 Hz; 18% linear elongation; 25 cycles/min for 20 min) of desired duration (0–48 h). EC cultures grown on BioFlex plates and maintained without CS stimulation were used as controls. At the end of the experiment, cells were lysed in SDS buffer for gel electrophoresis and Western blot analysis, lysed in PLB (Promega) for luciferase assay, or treated with Trizol for RT and qRT-PCR analysis.
Extraction of RNA, RT-PCR, and qRT-PCR Analyses
Total RNA from endothelial cells was isolated using Trizol and purified using a miRNeasy column (Qiagen, Valencia, CA). All procedures were performed according manufacturer’s recommendations for each assay as described in detail in the online supplement.
Measurement of Transendothelial Electrical Resistance
Measurements of transendothelial electrical resistance (TER) across confluent EC monolayers were performed using an electrical cell substrate impedance-sensing system (ECIS; Applied Biophysics, Troy, NY) as previously described (9, 30).
Statistical Analysis
Results are expressed as mean ± SE of three to six independent experiments. We performed statistical comparison among treatment groups by unpaired Student’s t test or by randomized-design, two-way ANOVA followed by the Newman-Keuls post hoc test for multiple groups. Results with P < 0.05 were considered statistically significant.
Results
Inflammatory Agonists Increase nmMLCK/MYLK Gene Transcription and MYLK 3′UTR Reporter Activity
TNF-α, LPS, and excessive mechanical stress are important contributors to the development and severity of ALI. To explore the effect of these inflammatory agonists on MYLK gene expression in human lung ECs, we measured the level of the MYLK mRNA encoding nmMLCK (0–24 h) and activity of MYLK 3′UTR luciferase reporter after exposure to TNF-α (10 ng/ml), LPS (100 ng/ml), or 18% cyclic stretch (CS). Consistent with epigenetic regulation, each inflammatory agonist independently induced sustained increases in nmMLCK mRNA levels. LPS induced early elevation in nmMLCK mRNA levels in ECs (a maximum 2.85 ± 0.15 FI at 4 h), which gradually declined to nearly basal levels at 24 hours (Figure 1A). In contrast, TNF-α and 18% CS augmented nmMLCK transcript levels, which reached maximum levels at 24 hours (4.7 ± 0.45 FI in TNF-α; 4.6 ± 0.24 FI in 18% CS) (Figure 1A). Agonist-induced MYLK increases were also observed using MYLK 3′UTR luciferase reporter activity: 3.0 ± 0.1 FI in TNF-α, 6.95 ± 0.3 FI in LPS, and 7.0 ± 0.2 FI in 18% CS (24 h) as compared with unstimulated cells (Figure 1B). These data suggest that nmMLCK transcription is altered in ECs by exposure to 18% CS, TNF-α, or LPS.
Figure 1.
Effects of inflammatory agonists on nonmuscle myosin light chain kinase isoform (nmMLCK) expression and MYLK 3′untranslated region reporter activity. (A) Total RNA was isolated from human pulmonary artery endothelial cells (ECs) and treated with control vehicle or LPS (100 ng/ml) or TNF-α (10 ng/ml) or exposed to 18% cyclic stretch (CS) for 0 to 24 hours, and nmMLCK mRNA level was detected via real-time PCR. Data are presented as fold change in mRNA level over vehicle-treated control and expressed as means ± SE from three independent experiments. (B) Human pulmonary artery ECs were cotransfected with MYLK 3′UTR reporter along with phRL-TK, a Renilla luciferase normalization control vector, and treated with LPS or TNF-α or exposed to 18% CS (24 h), and luciferase activity was measured using the Dual Luciferase Assay System (Promega) according the manufacturer’s protocol. The bar graph represents relative luciferase unit (RLU). Data are presented as RLUs over vehicle-treated control and expressed as means ± SE from three independent experiments. *P < 0.05 versus unstimulated control. #P < 0.01 versus unstimulated control.
Specific miRNAs (miR-374a, miR-374b, miR-520c-3p, and miR-1290) Are Down-Regulated in 18% CS–, LPS-, and TNF-α–Challenged ECs
In silico bioinformatic analysis (DIANA-microT-CDS, MiRanda, TargetScan, and MirTarget2) revealed that miR-374a, miR-374b, miR-520c-3p, and miR-1290 are able to potentially regulate MYLK gene expression by binding to the 3′UTR with miR-520c-3p, exhibiting two potential binding sites within the MYLK 3′UTR. Moreover, as predicted by the TargetScan bioinformatic tool, the potential binding sites for miR-520c-3p are conserved binding sites for miRNA families, broadly conserved among vertebrates, and the potential binding site for miR-374a and miR-374b is a conserved binding site for miRNA families, broadly conserved among mammals. To investigate whether these miRNAs have a role in MYLK epigenetic regulation, we first sought to determine whether 18% CS, TNF-α, and LPS regulate miR-374a, miR-374b, miR-520c-3p, and miR-1290 expression in human lung ECs. In these experiments, ECs were treated with 18% CS, TNF-α, or LPS for 0 to 24 hours. Figure 2 demonstrates that mature miRNA expression is significantly decreased by these agonists, which reach minimum levels by 4 to 8 hours (18% CS), 24 hours (TNF-α), and 4 hours (LPS) for miR-374a; 8 hours (18% CS), 24 hours (TNF-α), and 4 hours (LPS) for miR-374b; 8 hours (18% CS), 24 hours (TNF-α), and 16 hours (LPS) for miR-520c-3p; and 8 hours (18% CS), 8 to 16 hours (TNF-α), and 2 to 4 hours (LPS) for miR-1290. These results are highly correlated with augmented nmMLCK transcription (Figure 1A) and 3′UTR reporter activity (Figure 1B). We observed augmented recovered expression of miR-520c-3p at the 8- and 24-hour time points and miR-374b at 24 hours and recovery of miR-1290 to basal level after earlier decreased expression of LPS stimulation (Figure 2C). The nmMLCK mRNA levels at these time points declined to basal levels (Figure 1A), suggesting that these miRNAs may potentially participate in down-regulation of nmMLCK mRNA levels after this bioactive agonist during later stages (8–24 h). Overall, the inverse correlation between expression levels of putative miRNA and mRNA and/or protein increases the likelihood that this interaction is functional in vivo (31).
Figure 2.
Time-dependent effects of inflammatory agonist on miR-374a, miR-374b, miR-520c-3p, and miR-1290 expression in human lung ECs. Total RNA was isolated from human pulmonary artery ECs and treated with control vehicle or 18% CS (A), TNF-α (10 ng/ml) (B), or LPS (100 ng/ml) (C) for 0 to 24 hours, and the levels of miR-374a, miR-374b, miR-520c-3p, and miR-1290 were determined via real-time PCR. Data are presented as fold change in micro RNA (miRNA) level over vehicle-treated control and expressed as means ± SE from three independent experiments. *P < 0.05 versus unstimulated control. #P < 0.01 versus unstimulated control.
Mimics of miRNAs Attenuate LPS-Induced Human Lung EC Permeability
Our previous in vitro studies (5) demonstrated that nmMLCK-specific siRNAs abolish nmMLCK expression and LPS-mediated reduction in TER. To evaluate the functional involvement of miR-374a, miR-374b, miR-1290, and miR-520c-3p, miRNAs predicted to potentially regulate MYLK gene expression, we measured changes in LPS-induced lung EC hyperpermeability reflected by increased TER, a highly sensitive in vitro assay of permeability. ECs treated with single miRNA mimics demonstrate statistically significant attenuation of LPS-induced hyperpermeability in ECs (P < 0.01) (Figures 3A–3E) compared with agonist-stimulated ECs transfected with negative control mimics (43 ± 2.9% for miR-374a, 35 ± 1.4% for miR-374b, 42.5 ± 1 for miR-1290, and 44 ± 1% for miR-520c-3p). EC treatment with a combination of all four miRNAs markedly augmented the reduction in LPS-induced permeability (70 ± 1.7% reduction) compared with ECs treated with the miRNAs individually (Figures 3E and 3F), suggesting that these miRNAs may have additive or synergistic effects in the LPS-induced EC response. These data clearly indicate novel roles for these miRNAs in mediating LPS-induced lung EC barrier hyperpermeability.
Figure 3.
Effect of miRNA mimics on LPS-induced human lung EC permeability. ECs grown in chambers on gold microelectrodes were transfected with miR-374a mimic (A), miR-374b mimic (B), miR-1290 mimic (C), miR-520c-3p (D), or a combination of miR-374a, miR-374b, miR-1290, and miR-520c-3p mimics (4 miRs) (E) or were treated with nonspecific negative control mimic (nc) as described in Materials and Methods and used for transendothelial electrical resistance (TER) measurements. At time = 0, cells were stimulated with LPS (100 ng/ml) or vehicle control. Shown are pooled data of five independent experiments. (F) Pooled TER data (n = 5). Maximal value of permeability in endothelial cells treated with nc reagent (Control) achieved within 10 hours was taken as 100% ± SE. *Significantly different from cells treated with nc reagent with LPS (P < 0.01).
Both miRNA Mimics and Antagomirs Alter Inflammatory Agonist-Induced nmMLCK Expression and MYLK 3′UTR Reporter Activity in Human Lung ECs
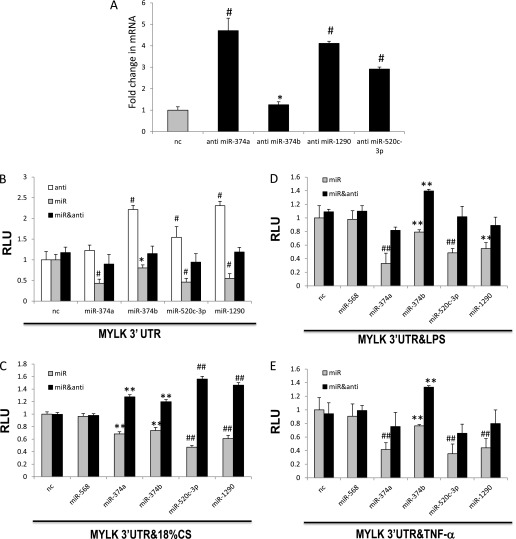
To investigate whether miR-374a, miR-374b, miR-520c-3p, and miR-1290 participate in 18% CS–, LPS-, or TNF-α–induced nmMLCK expression, we transfected ECs with negative control miRNA mimics or synthetic mimics and/or specific miRNA antagomirs for miR-374a, miR-374b, miR-520c-3p, or miR-1290 followed by challenge with LPS, TNF-α, or 18% CS. Figures 4A through 4D depict the significantly decreases in nmMLCK mRNA levels produced by transfection with miR-374a, miR-374b, miR-1290, and miR-520c-3p mimics. Consistent with these results, overexpression of miR-374a, miR-374b, miR-1290, and miR-520c-3p mimics significantly attenuate basal 3′UTR reporter activity (Figure 5A) (31 ± 8%, 25 ± 5%, 53 ± 2%, and 39 ± 4%, respectively) and 3′UTR reporter activity after exposure to 18% CS (Figure 5B), LPS (Figure 5C), and TNF-α (Figure 5D) (compared with stimulated ECs). Overexpression of combination of miR-374a and miR-520c-3p mimics, as well as combination of all four miRNAs mimics, enhances attenuation of 3′UTR reporter activity after EC treatment with LPS (Figure 5C), again suggesting cooperative regulation by these miRNAs. Figure 6A demonstrates that miR-374a, miR-374b, miR-1290, and miR-520c-3p antagomirs enhanced the endogenous nmMLCK mRNA level in ECs compared with ECs transfected with negative antagomir control. Furthermore, these antagomirs enhanced MYLK 3′ UTR reporter activity in EC (1.2 ± 0.13 FI for hsa-miR-374a antagomir, 2.2 ± 0.1 FI for hsa-miR-374b antagomir, 1.55 ± 0.26 FI for hsa-miR-520c-3p antagomir, and 2.3 ± 0.1 FI for hsa-miR-1290 antagomir), compared with negative antagomir controls (Figure 6B). Each antagomir attenuated the decreased reporter activity observed in miRNA mimic–treated ECs (104 ± 4% for hsa-miR-374a, 71 ± 5% for hsa-miR-374b, 102% ± 5 for hsa-miR-520c-3p, and 108 ± 4% for hsa-miR-1290) (Figure 6B) as well as in miRNA mimic–treated ECs exposed to 18% CS (Figure 6C), LPS (Figure 6D), and TNF-α (Figure 6E). Together, these data suggest that miR-374a, miR-374b, miR-1290, and miR-520c-3p strongly influence MYLK and nmMLCK expression.
Figure 4.
Effects of miRNA mimics on inflammatory agonist-induced nmMLCK expression in human lung ECs. Total RNA was isolated from ECs that were transfected with negative control mimic (nc) or with the indicated miRNA mimics and untreated (A) or exposed to 18% CS (B; 24 h), treated with LPS (C; 4 h), or treated with TNF-α (D; 24 h). nmMLCK mRNA level was detected via real-time PCR. Transfection with miR-568 was used as the second negative control. Data are presented as fold change in mRNA level over vehicle-treated control and expressed as means ± SE from four independent experiments. *Significantly different from control cells without stimulation (P < 0.05). #Significantly different from control cells without stimulation (P < 0.01). **Significantly different from control cells stimulated with 18% CS, LPS, or TNF-α (P < 0.05). ##Significantly different from control cells stimulated with 18% CS, LPS, or TNF-α (P < 0.01).
Figure 5.
Effects of miRNA mimics on inflammatory agonist-induced MYLK 3′ untranslated region (UTR) reporter activity in human lung ECs. ECs were cotransfected with MYLK 3′UTR reporter along with negative control mimic or with the indicated miRNA mimics, and phRL-TK, a Renilla luciferase normalization control vector, and untreated (A) exposed to 18% CS (B), treated with LPS (C), or treated with TNF-α (D) (24 h) and luciferase activity was measured according the manufacturer’s protocol. Transfection with miR-568 was used as the second negative control. Data are presented as RLU over vehicle-treated control and expressed as means ± SE from four independent experiments. **Significantly different from control cells without stimulation (P < 0.05). *Significantly different from control cells stimulated with 18% CS, LPS, or TNF-α (P < 0.05). #Significantly different from control cells stimulated with 18% CS, LPS, or TNF-α (P < 0.01).
Figure 6.
Effects of miRNA antagomirs on inflammatory agonist–induced nmMLCK expression and MYLK 3′UTR reporter activity. Total RNA was isolated from ECs that were transfected with negative control antagomir (nc) or with the indicated miRNA anti- and nmMLCK. mRNA level was detected via real-time PCR (A). ECs were cotransfected with the MYLK 3′UTR reporter along with phRL-TK, controls (nc = negative antagomir [anti], negative mimic control, or a combination of negative anti and negative mimic) or with the indicated miRNA anti, miRNA anti, and mimics combined. Cells were untreated (B) exposed to 18% CS (C), treated with LPS (D), or treated with TNF-α (E) (24 h), and luciferase activity was measured according the manufacturer’s protocol. Transfection with miR-568 was used as the second negative control. Data are presented as RLU over vehicle-treated control and expressed as means ± SE from four independent experiments. *Significantly different from control cells without stimulation (P < 0.05). #Significantly different from control cells without stimulation (P < 0.01). **Significantly different from control cells stimulated with 18% CS, LPS, or TNF-α (P < 0.05). ##Significantly different from control cells stimulated with 18% CS, LPS, or TNF-α (P < 0.01).
Discussion
In our previous studies (4–6), MYLK was identified as a novel candidate gene in human inflammatory lung injuries, such as ALI and asthma, with increased MYLK expression in human ALI and preclinical ALI models and induced by proinflammatory cytokines, ROS, and mechanical stress (32–34). However, the exact regulatory mechanisms of nmMLCK expression modulation in the pathogenesis of ALI involving its 3′-UTR are unknown. Prior studies have indicated that genes with increased expression and function variability have longer 3′UTRs that are enriched in miRNA-binding sites (35, 36). The MYLK gene has a relatively long 3′UTR (1,825 base pair), with several splice variants that contribute to the risk and severity of ALI and VILI (4–8). Because miRNAs regulate a variety of pathophysiological processes, nmMLCK expression may be influenced by ALI-modulating miRNAs that bind to the MYLK 3′UTR region, consistent with the recognition that multiple inflammatory processes and IL1β-, LPS-, and TNF-α−induced cell activation (macrophages, neutrophils, monocytes, and epithelium) are regulated by specific miRNAs (miR-21, -9, -25, -27b, -100, -140, -142–3p, -181c-, -194, -223, -224, -16, -199a, -155, -146a, -let-7a, -31, 17–3p, and -125b) (37–43). In the present study, in silico bioinformatic analysis revealed that the 3′UTR of MYLK contains binding sites for miR-374a, miR-374b, miR-520c-3p, and miR-1290. We sought to define the participation of these miRNAs in the regulation of nmMYLK gene expression induced by the inflammatory agonists 18% CS, LPS, and TNF-α. We found that all four miRNAs are expressed in ECs and significantly down-regulated by 18% CS, TNF-α, and LPS (Figure 2). Three miRNAs (miR-374a, miR-374b, and miR-520c-3p) are broadly conserved among animals as the majority of miRNAs with important function are highly conserved among species (44).
We previously demonstrated that nmMLCK plays a critical role in vascular barrier regulatory responses to ALI pathobiology (4–6). In the present study, we found that nmMLCK expression in ECs is markedly up-regulated in response to 18% CS, TNF-α, and LPS (Figure 1). Our data, together with previous findings (31, 45), demonstrate the negative correlation between expression levels of putative miRNA and mRNA/protein and suggest that down-regulation of these miRNAs may contribute to augmented nmMLCK gene expression in ECs treated with these proinflammatory agonists. However, we demonstrated significant elevation of 18% CS–induced nmMLCK transcript levels in ECs from 16 to 24 hours after induction (Figure 1A), whereas no significant modulation of studied miRNA expression for these time points was observed (Figure 2A), indicating potential involvement of other miRNAs and/or regulatory elements. To further evaluate whether miR-374a, miR-374b, miR-1290, and miR-520c-3p specifically regulate the nmMLCK expression in ECs, the transfection with synthetic miRNA mimics and antagomirs was used. Consistent with the key regulatory role of nmMLCK isoforms in inflammatory lung vascular permeability and pathobiology, we previously reported that targeting nmMLCK by specific siRNAs attenuates LPS-mediated EC hyperpermeability (5). Similarly, our data demonstrate that miR-374a, miR-374b, miR-1290, and miR-520c-3p mimics, similarly to nmMLCK-specific siRNAs, attenuate LPS-induced hyperpermeability in ECs (Figure 3). These mRNAs may modulate LPS-induced change in EC permeability by targeting the nmMLCK 3′UTR, thus altering MLCK expression. Furthermore, we found that transfection of ECs with miR-374a, miR-374b, miR-1290, and miR-520c-3p mimics significantly attenuated the 18% CS–, TNF-α–, and LPS-induced augmented nmMLCK mRNA level (Figure 4). Initial miRNA reports suggested that mammalian miRNAs function by translation repression of their target genes (46); however, recent evidence indicates that these miRNAs directly target mRNA down-regulation via cleavage and degradation (47–50) or accelerated deadenylation of mRNAs (51, 52). Our data support these findings.
We further analyzed the role of miRNAs in nmMLCK expression by using a luciferase reporter gene system. Here we show that exposure of cells to these miRNAs significantly attenuates basal MYLK 3′UTR reporter activity (Figure 5A) and that 18% CS–, LPS-, and TNF-α–induced luciferase activity increases (Figures 5B–5D). Overexpression of miRNAs potentially targets genes otherwise not affected in physiologic conditions (i.e., “off-target effects”). Therefore, we used the complementary approach and used “loss-of-function” studies in which we inhibited the function of specific endogenous miRNA and synthetic miRNA mimics by using the antagomirs. We demonstrated that specific miRNA antagomirs increase nmMLCK expression (Figure 6A) and MYLK 3′UTR reporter activity (Figure 6B) and rescue induced by 18% CS, TNF-α, and LPS decreased 3′UTR reporter activity produced by miRNA mimics (Figures 6C–6E). Thus, our studies clearly demonstrate that miRNAs strongly influence MYLK expression. Furthermore, our data indicate that these four miRNAs may cooperatively regulate nmMLCK expression in pulmonary ECs (Figures 3E, 3F, and 5C).
The nmMLCK is a critical barrier regulatory molecule and an important biomarker of diverse inflammatory pathobiologies such as ALI and asthma. However, information is limited concerning the possible regulatory mechanisms of nmMLCK expression involving 5′ and 3′ gene regions. In silico analysis of the 5′ nmMYLK promoter region revealed putative cis regulatory elements; binding sites for transcription factors (TFs); sequences of homology to antioxidant, shear stress, and mechanical stretch response elements (SA #1A); and a novel CpG island in the nmMYLK promoter suggesting epigenetic regulation of nmMYLK expression via DNA methylation and 5′ promoter interacting elements (unpublished data). To our knowledge, this is the first demonstration that multiple miRNAs potentially regulate nmMLCK expression in human pulmonary ECs via 3′UTR modulation. Increasing evidence links miRNAs to transcription factors or complexes, and several bioinformatic and experimental studies have revealed different types of TF–miRNA pair’s genes coregulation (53, 54). In addition, Tu and colleagues (55) reported that there are two-layer regulatory networks in which TFs function as important mediators of miRNA-initiated secondary regulatory effects. Future studies are needed to show whether miRNAs, TFs, and other regulatory elements collaborate in MYLK gene regulation.
In summary, we demonstrated that miR-374a, miR-374b, miR-1290, and miR-520c-3p down-regulated in human pulmonary artery ECs treated with TNF-α or LPS or exposed to 18% CS and regulate nmMLCK expression in pulmonary ECs. Therefore, targeting of nmMLCK, a critical barrier regulatory gene, by these miRNAs is a potentially promising novel strategy in the development of therapeutic treatment to reduce lung vascular permeability/edema in ALI and VILI.
Acknowledgments
Acknowledgments
The authors thank Lakshmi Natarajan for providing help with the endothelial cell cultures.
Footnotes
This work was supported by National Institutes of Health Heart, Lung, and Blood Institute grants P01 HL58064, P01 HL98050, and R01 HL91889.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0397OC on March 14, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 2.Tsushima K, King LS, Aggarwal NR, De Gorordo A, D'Alessio FR, Kubo K. Acute lung injury review. Intern Med. 2009;48:621–630. doi: 10.2169/internalmedicine.48.1741. [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Gao L, Grant A, Halder I, Brower R, Sevransky J, Maloney JP, Moss M, Shanholtz C, Yates CR, Meduri GU, et al. Novel polymorphisms in the myosin light chain kinase gene confer risk for acute lung injury. Am J Respir Cell Mol Biol. 2006;34:487–495. doi: 10.1165/rcmb.2005-0404OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirzapoiazova T, Moitra J, Moreno-Vinasco L, Sammani S, Turner JR, Chiang ET, Evenoski C, Wang T, Singleton PA, Huang Y, et al. Non-muscle myosin light chain kinase isoform is a viable molecular target in acute inflammatory lung injury. Am J Respir Cell Mol Biol. 2011;44:40–52. doi: 10.1165/rcmb.2009-0197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flores C, Ma SF, Maresso K, Ahmed O, Garcia JG. Genomics of acute lung injury. Semin Respir Crit Care Med. 2006;27:389–395. doi: 10.1055/s-2006-948292. [DOI] [PubMed] [Google Scholar]
- 7.Garcia JG, Lazar V, Gilbert-McClain LI, Gallagher PJ, Verin AD. Myosin light chain kinase in endothelium: molecular cloning and regulation. Am J Respir Cell Mol Biol. 1997;16:489–494. doi: 10.1165/ajrcmb.16.5.9160829. [DOI] [PubMed] [Google Scholar]
- 8.Lazar V, Garcia JG. A single human myosin light chain kinase gene (MLCK; MYLK) Genomics. 1999;57:256–267. doi: 10.1006/geno.1999.5774. [DOI] [PubMed] [Google Scholar]
- 9.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamburg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia JG, Verin AD, Schaphorst KL. Regulation of thrombin-mediated endothelial cell contraction and permeability. Semin Thromb Hemost. 1996;22:309–315. doi: 10.1055/s-2007-999025. [DOI] [PubMed] [Google Scholar]
- 11.Garcia JG, Verin AD, Herenyiova M, English D. Adherent neutrophils activate endothelial myosin light chain kinase: role in transendothelial migration. J Appl Physiol. 1998;84:1817–1821. doi: 10.1152/jappl.1998.84.5.1817. [DOI] [PubMed] [Google Scholar]
- 12.Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, Liu S, Alder H, Costinean S, Fernandez-Cymering C, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci USA. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67:11001–11011. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- 14.Lytle JR, Yario TA, Steitz JA. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc Natl Acad Sci USA. 2007;104:9667–9672. doi: 10.1073/pnas.0703820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124–1128. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- 16.Tomankova T, Petrek M, Kriegova E. Involvement of microRNAs in physiological and pathological processes in the lung. Respir Res. 2010;11:159. doi: 10.1186/1465-9921-11-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman JM, Jones PA. MicroRNAs: critical mediators of differentiation, development and disease. Swiss Med Wkly. 2009;139:466–472. doi: 10.4414/smw.2009.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zampetaki A, Mayr M. MicroRNAs in vascular and metabolic disease. Circ Res. 2012;110:508–522. doi: 10.1161/CIRCRESAHA.111.247445. [DOI] [PubMed] [Google Scholar]
- 19.van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circ Res. 2012;110:496–507. doi: 10.1161/CIRCRESAHA.111.247916. [DOI] [PubMed] [Google Scholar]
- 20.Zhou T, Garcia JG, Zhang W. Integrating microRNAs into a system biology approach to acute lung injury. Transl Res. 2011;157:180–190. doi: 10.1016/j.trsl.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang S, Banerjee S, de Freitas A, Sanders YY, Ding Q, Matalon S, Thannickal VJ, Abraham E, Liu G. Participation of miR-200 in pulmonary fibrosis. Am J Pathol. 2012;180:484–493. doi: 10.1016/j.ajpath.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu TX, Munitz A, Rothenberg ME. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J Immunol. 2009;182:4994–5002. doi: 10.4049/jimmunol.0803560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai ZG, Zhang SM, Zhang Y, Zhou YY, Wu HB, Xu XP. MicroRNAs are dynamically regulated and play an important role in LPS-induced lung injury. Can J Physiol Pharmacol. 2012;90:37–43. doi: 10.1139/y11-095. [DOI] [PubMed] [Google Scholar]
- 24.Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208:535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic Acids Res. 2008;36:D149–D153. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maragkakis M, Reczko M, Simossis VA, Alexiou P, Papadopoulos GL, Dalamagas T, Giannopoulos G, Goumas G, Koukis E, Kourtis K, et al. DIANA-microT web server: elucidating microRNA functions through target prediction. Nucleic Acids Res. 2009;37:W273–W276. doi: 10.1093/nar/gkp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X. MiRDB: a microRNA target prediction and functional annotation database with a wiki interface. RNA. 2008;14:1012–1017. doi: 10.1261/rna.965408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adyshev DM, Moldobaeva NK, Elangovan VR, Garcia JG, Dudek SM. Differential involvement of ezrin/radixin/moesin proteins in sphingosine 1-phosphate-induced human pulmonary endothelial cell barrier enhancement. Cell Signal. 2011;23:2086–2096. doi: 10.1016/j.cellsig.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witkos TM, Koscianska E, Krzyzosiak WJ. Practical aspects of microRNA target prediction. Curr Mol Med. 2011;11:93–109. doi: 10.2174/156652411794859250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol. 2003;285:L785–L797. doi: 10.1152/ajplung.00336.2002. [DOI] [PubMed] [Google Scholar]
- 33.Petrache I, Verin AD, Crow MT, Birukova A, Liu F, Garcia JG. Differential effect of MLC kinase in TNF-alpha-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol. 2001;280:L1168–L1178. doi: 10.1152/ajplung.2001.280.6.L1168. [DOI] [PubMed] [Google Scholar]
- 34.Usatyuk PV, Singleton PA, Pendyala S, Kalari SK, He D, Gorshkova IA, Camp SM, Moitra J, Dudek SM, Garcia JG, et al. Novel role for non-muscle miosin light chain kinase (MLCK) in hyperoxia-induced recruitment of cytoskeletal proteins, NADPH oxidase activation and reactive oxygen species generation in lung endothelium. J Biol Chem. 2012;287:9360–9375. doi: 10.1074/jbc.M111.294546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stark A, Brennecke J, Bushati N, Russell RB, Cohen SM. Animal micrornas confer robustness to gene expression and have a significant impact on 3′UTR evolution. Cell. 2005;123:1133–1146. doi: 10.1016/j.cell.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 36.Zhang R, Su B. MicroRNA regulation and the variability of human cortical gene expression. Nucleic Acids Res. 2008;36:4621–4628. doi: 10.1093/nar/gkn431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moschos SA, Williams AE, Perry MM, Birrell MA, Belvisi MG, Lindsay MA. Expression profiling in vivo demonstrates rapid changes in lung microRNA levels following lipopolysaccharide-induced inflammation but not in the anti-inflammatory action of glucocorticoids. BMC Genomics. 2007;8:240. doi: 10.1186/1471-2164-8-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bazzoni F, Rossato M, Fabbri M, Gaudiosi D, Mirolo M, Mori L, Tamassia N, Mantovani A, Cassatella MA, Locati M. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci USA. 2009;106:5282–5287. doi: 10.1073/pnas.0810909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci USA. 2009;106:7113–7118. doi: 10.1073/pnas.0902636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O'Leary JJ, Ruan Q, Johnson DS, Chen Y, O'Neill LA. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 41.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy AJ, Guyre PM, Pioli PA. Estradiol suppresses NF-kappa B activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J Immunol. 2010;184:5029–5037. doi: 10.4049/jimmunol.0903463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greene CM, Branagan P, McElvaney NG. Toll-like receptors as therapeutic targets in cystic fibrosis. Expert Opin Ther Targets. 2008;12:1481–1495. doi: 10.1517/14728220802515293. [DOI] [PubMed] [Google Scholar]
- 44.Zhang R, Su B. Small but influential: the role of microRNAs on gene regulatory network and 3′UTR evolution. J Genet Genomics. 2009;36:1–6. doi: 10.1016/S1673-8527(09)60001-1. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen JA, Lau P, Maric D, Barker JL, Hudson LD. Integrating microRNA and mRNA expression profiles of neuronal progenitors to identify regulatory networks underlying the onset of cortical neurogenesis. BMC Neurosci. 2009;10:98. doi: 10.1186/1471-2202-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Natl Rev. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 47.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 48.Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- 49.Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 50.Liu Z, Wang D, Hu Y, Zhou G, Zhu C, Yu Q, Chi Y, Cao Y, Jia C, Zou Q. MicroRNA-146a negatively regulates PTGS2 expression induced by Helicobacter pylori in human gastric epithelial cells. J Gastroenterol. 2013;48:86–92. doi: 10.1007/s00535-012-0609-9. [DOI] [PubMed] [Google Scholar]
- 51.Giraldez AJ, Mishima Y, Rihel J, Grocock RJ, Van Dongen S, Inoue K, Enright AJ, Schier AF. Zebrafish miR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312:75–79. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- 52.Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci USA. 2006;103:4034–4039. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shalgi R, Lieber D, Oren M, Pilpel Y. Global and local architecture of the mammalian microRNA-transcription factor regulatory network. PLOS Comput Biol. 2007;3:e131. doi: 10.1371/journal.pcbi.0030131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Y, Ferguson J, Chang JT, Kluger Y. Inter- and intra-combinatorial regulation by transcription factors and microRNAs. BMC Genomics. 2007;8:396. doi: 10.1186/1471-2164-8-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tu K, Yu H, Hua YJ, Li YY, Liu L, Xie L, Li YX. Combinatorial network of primary and secondary microRNA-driven regulatory mechanisms. Nucleic Acids Res. 2009;37:5969–5980. doi: 10.1093/nar/gkp638. [DOI] [PMC free article] [PubMed] [Google Scholar]