Abstract
The accumulation of apoptosis-resistant fibroblasts within fibroblastic foci is a characteristic feature of idiopathic pulmonary fibrosis (IPF), but the mechanisms underlying apoptosis resistance remain unclear. A role for the inhibitor of apoptosis (IAP) protein family member X-linked inhibitor of apoptosis (XIAP) has been suggested by prior studies showing that (1) XIAP is localized to fibroblastic foci in IPF tissue and (2) prostaglandin E2 suppresses XIAP expression while increasing fibroblast susceptibility to apoptosis. Based on these observations, we hypothesized that XIAP would be regulated by the profibrotic mediators transforming growth factor (TGF)β-1 and endothelin (ET)-1 and that increased XIAP would contribute to apoptosis resistance in IPF fibroblasts. To address these hypotheses, we examined XIAP expression in normal and IPF fibroblasts at baseline and in normal fibroblasts after treatment with TGF-β1 or ET-1. The role of XIAP in the regulation of fibroblast susceptibility to Fas-mediated apoptosis was examined using functional XIAP antagonists and siRNA silencing. In concordance with prior reports, fibroblasts from IPF lung tissue had increased resistance to apoptosis compared with normal lung fibroblasts. Compared with normal fibroblasts, IPF fibroblasts had significantly but heterogeneously increased basal XIAP expression. Additionally, TGF-β1 and ET-1 induced XIAP protein expression in normal fibroblasts. Inhibition or silencing of XIAP enhanced the sensitivity of lung fibroblasts to Fas-mediated apoptosis without causing apoptosis in the absence of Fas activation. Collectively, these findings support a mechanistic role for XIAP in the apoptosis-resistant phenotype of IPF fibroblasts.
Keywords: myofibroblast, idiopathic pulmonary fibrosis, inhibitor of apoptosis, lung fibrosis, apoptosis
Clinical Relevance
Resistance to apoptotic stimuli is thought to contribute to the accumulation of fibroblasts in fibrotic lung diseases, including idiopathic pulmonary fibrosis. This study shows that a subset of idiopathic pulmonary fibrosis fibroblasts has increased expression of X-linked inhibitor of apoptosis (XIAP), a member of the Inhibitor of Apoptosis family of proteins. Moreover, profibrotic mediators increase expression of XIAP in normal fibroblasts and functional inhibition of XIAP enhances the susceptibility of normal and fibrotic lung fibroblasts to apoptosis. Thus, XIAP represents a novel potential target of antifibrotic therapy.
Mesenchymal cells display a dynamic spectrum of phenotypes ranging between an undifferentiated state (fibroblast) and a differentiated state (myofibroblast) (1, 2). Although these effector cells are essential for the homeostatic reestablishment of normal architecture and function after tissue injury, the persistence of these cells is associated with fibrosis (3, 4). The resolution phase of normal wound repair coincides with the reduction of fibroblast numbers by apoptosis, but the triggers of apoptosis in normal wound repair and the mechanisms underlying the persistence of fibroblasts during fibrotic wound repair remain poorly understood (5, 6). The profibrotic mediators transforming growth factor (TGF)β-1 and endothelin (ET)-1 have been found to promote resistance to apoptotic stimuli in fibroblasts, and the antifibrotic lipid mediator prostaglandin (PG)E2 has been shown to increase the responsiveness of these cells to apoptotic stimuli, supporting a role for dysregulation of fibroblast apoptosis in the pathogenesis of fibrosis (7–12).
Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease of unknown etiology that is characterized by progressive alveolar fibrosis (13, 14). The overall prognosis of patients with IPF is dismal, although there is substantial variability in the clinical course (15). Accordingly, it is reasonable to postulate that the clinical variability observed in IPF reflects biologically heterogeneous mechanisms that ultimately converge on the common end-point of parenchymal fibrosis. IPF is characterized by the accumulation of fibroblasts and myofibroblasts, which aggregate in clusters termed “fibroblastic foci,” the number of which correlates with patient mortality (13, 15). Although several studies have shown that fibroblasts within fibroblastic foci lack evidence of apoptosis, the mechanisms responsible for this apparent resistance to apoptosis are not clear (12, 16, 17).
Parallels have been made between the biologic mechanisms observed in IPF and cancer (18, 19). The inhibitor of apoptosis (IAP) protein family contains eight different members that contain at least one baculovirus IAP repeat (BIR) domain, which is critical for the ability of these proteins to interact with caspases and prevent apoptosis in vitro (20). Increased expression of IAPs is associated with a variety of malignancies, and the inhibition of these proteins can enhance the susceptibility of cancer cells to apoptotic stimuli without detriment to normal cells. Thus, IAPs represent attractive targets for cancer treatment, motivating the development of IAP antagonists (21–25). Although their role in cancer is well established, few studies have examined IAPs in the context of fibrosis. We previously showed that one IAP family member, survivin (also known as baculoviral inhibitor of apoptosis repeat–containing 5 [BIRC5]), is induced in normal lung fibroblasts by ET-1 and is suppressed in normal lung fibroblasts by PGE2 (7, 10). Moreover, induction of survivin promoted resistance to apoptosis, whereas suppression of survivin was associated with enhanced fibroblast apoptosis. Additionally, we recently reported that survivin expression is variably increased in IPF fibroblasts in situ within fibroblastic foci and in cultures of explanted lung fibroblasts. Inhibition of survivin could enhance susceptibility to apoptosis in a subset of fibroblasts isolated from IPF lung tissue (26). Another recent report similarly showed that X-linked inhibitor of apoptosis (XIAP or BIRC4) is highly expressed within the mesenchymal cells of fibroblastic foci but not in the overlying epithelium (12). PGE2 treatment suppressed XIAP and enhanced Fas-mediated apoptosis in lung fibroblasts, supporting a potential mechanistic role for XIAP in fibroblast survival (12).
Based on these studies and on the fact that XIAP is the only IAP believed to function as a direct apoptosis inhibitor in vivo, we hypothesized that XIAP would contribute to the apoptosis resistance of IPF fibroblasts (20, 21). To address this hypothesis, we examined XIAP levels in primary explanted lung fibroblasts from normal and IPF tissue, assessed the impact of soluble profibrotic mediators on XIAP expression in normal lung fibroblasts, and determined how XIAP inhibition modulated the sensitivity of normal and IPF fibroblasts to Fas-mediated apoptosis. Portions of this work have been previously published in abstract form (27).
Materials and Methods
Cells and Cell Culture
Normal primary human fetal (IMR-90) and adult (CCL-210) lung fibroblasts were obtained from ATCC (Manassas, VA). Cells between passages 8 and 16 were cultured as previously described (7). Patient-derived primary human lung fibroblasts were cultured from the lungs of patients with IPF or from normal areas of lung from patients undergoing thoracic surgery for nonfibrotic disease as described previously (28). Written informed consent was obtained from all subjects in accordance with the University of Michigan Institutional Review Board.
Reagents
Porcine TGF-β1 was from R&D Systems (Minneapolis, MN). ET-1 and cycloheximide were from Sigma (St. Louis, MO). The activating anti-Fas antibody (clone CH11, designated as Fas-Ab) was from Millipore (Billerica, MA). Antibodies to XIAP, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and poly-(ADP-ribose) polymerase (PARP) were from Cell Signaling (Danvers, MA). The Cell Death Detection ELISA Kit detecting histone-associated DNA fragments was from Roche Applied Science (Indianapolis, IN). Horseradish peroxidase–conjugated secondary antibodies were from Pierce (Rockford, IL).
AT406, a monovalent Smac/DIABLO (second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI) mimetic that binds the BIR3 domain of XIAP, cIAP1, and cIAP2 and SM164, a bivalent Smac/DIABLO mimetic that binds to the BIR3 domain and to the BIR2 domain and its preceding linker region on XIAP, were kind gifts from Shaomeng Wang, Ph.D., University of Michigan (22, 29–32). Preliminary dose-response studies established an impact on apoptosis at concentrations of SM164 (100 nM) and AT406 (1.0 μM); these concentrations have been shown to effectively antagonize XIAP, cIAP1, and cIAP2 (29–32).
siRNA Silencing
IMR-90 fibroblasts were transfected with siRNA targeting XIAP or with a control, nontargeting siRNA (Ambion; Life Technologies). The siRNA was incubated with Oligofectamine (Invitrogen, Grand Island, NY) in Opti-MEM for 30 minutes, after which the siRNA-Oligofectamine complexes in Opti-MEM were added to cells in serum-free DMEM in a 1:3 ratio to achieve a final siRNA concentration of 10 nM. Twenty-four hours later, the transfection medium was replaced with serum-free DMEM for another 24 hours before experimentation.
SDS-PAGE Electrophoresis and Western Blotting
Whole cell lysates were collected and subjected to SDS-PAGE and Western blotting as previously described (7, 11). All Western blots were stripped and reprobed for GAPDH.
Densitometry
Western blots were analyzed using the public domain NIH ImageJ program available at http://rsbweb.nih.gov/ij/ as previously described (7). The ratio of band density for the target protein and the corresponding GAPDH was determined on each blot, and this ratio was normalized such that the untreated controls (for expression of XIAP) or Fas-Ab–treated cells (for PARP) were expressed as 1.0.
Apoptosis Assessments
Apoptosis was assessed by Western blotting for cleaved PARP and by ELISA-based detection of histone-associated DNA fragments using the Cell Death Detection ELISA Kit (Roche Applied Science, Indianapolis, IN) as previously described (7). For each ELISA, samples were run in triplicate along with a manufacturer-supplied positive control.
Quantitative Real-Time RT-PCR
Quantitative real-time PCR was performed on an Applied Biosystems 7300 (Foster City, CA) real-time PCR machine as we have reported previously (28). Relative quantitation was based on the ΔΔCT method and used primers obtained from Applied Biosystems TaqMan Gene Expression Assays for XIAP (BIRC4 [Hs00236913], BIRC2 [Hs00357350], and BIRC3 [Hs00154109]).
Statistical Analyses
Statistical analyses were completed using ANOVA with Tukey posttest (Graphpad Prism software version 5.01) for experiments involving three or more conditions simultaneously. Comparisons between primary patient-derived normal and IPF fibroblasts were made using Student’s t test. Spearman correlations (r) were determined using Graphpad Prism software.
Results
IPF Lung Fibroblasts Have Decreased Susceptibility to Fas-Mediated Apoptosis
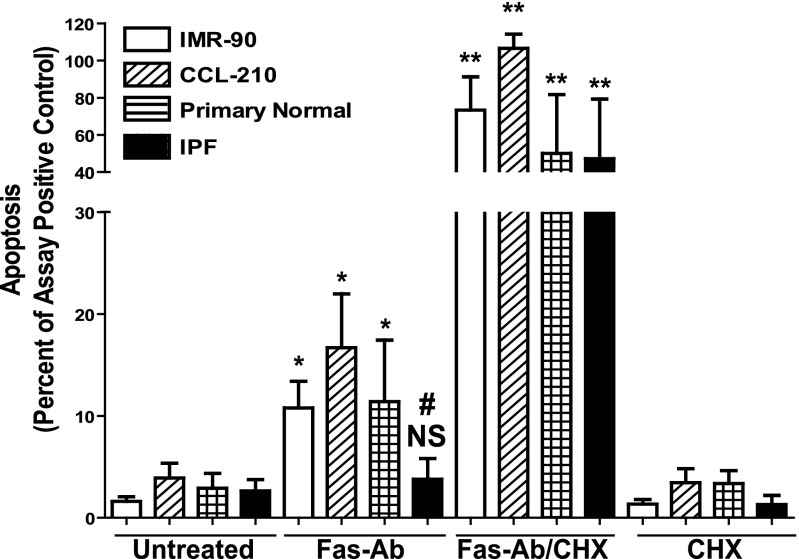
Fibroblasts have an inherent resistance to Fas-mediated apoptosis in vitro, although the response to Fas activation can be enhanced by (1) treatment with the protein translation inhibitor cycloheximide, (2) treatment with the antifibrotic mediator PGE2, or (3) treatment with proinflammatory cytokines (10, 11, 33–36). We assessed apoptosis in normal lung fibroblast cell lines (IMR-90 and CCL-210) and patient-derived primary fibroblasts from normal and IPF tissue after exposure to a Fas-activating antibody (Fas-Ab) with and without cycloheximide. At baseline, there were no significant differences detected between these four cell populations (1.6% of the assay positive control in IMR-90, 3.9% in CCL-210, 2.89% in normal, and 2.63% in IPF fibroblasts) (Figure 1). After Fas activation, there was significantly increased apoptosis in IMR-90 (10.78% of the assay positive control), CCL-210 (16.68%), and primary normal lung fibroblasts (11.40%). There were no significant differences in the apoptotic responses between these different normal lung fibroblast populations. In contrast, Fas-activation failed to increase apoptosis in the IPF fibroblasts (2.63% in untreated versus 3.77% in Fas-Ab–treated cells), supporting an apoptosis-resistant phenotype in the IPF cells.
Figure 1.
Growth-arrested IMR-90 (white bar; n = 9), CCL-210 (diagonal cross-hatch bars; n = 3), and primary normal (horizontal cross-hatch bars; n = 3) and idiopathic pulmonary fibrosis (IPF) lung fibroblasts (black bars; n = 9) were treated with or without Fas-activating antibodies (Fas-Ab) (250 ng/ml) in the presence or absence of cycloheximide (CHX) (500 ng/ml) for 16 hours, and apoptosis was assessed by ELISA detection of histone-associated DNA fragments. Relative apoptosis is expressed as a percentage of the assay-positive control that was run on the ELISA plate for each experiment. All samples were run in triplicate for each ELISA. The CHX-alone bars represent a subset of experiments for each fibroblast population, including three IMR-90 experiments, two CCL-210 experiments, two primary normal cell lines, and three of the IPF fibroblast cell lines. *P < 0.05 compared with untreated cells of the same cell line. **P < 0.01 compared with untreated cells of the same cell type. #P < 0.05 compared with Fas-Ab–treated IMR-90, CCL-210, and primary normal lung fibroblasts. NS = no statistically significant difference.
Treatment with Fas-Ab in combination with cycloheximide induced robust apoptotic responses in the normal fibroblast cell lines (IMR-90 and CCL-210), the patient-derived normal lung fibroblasts, and the IPF lung fibroblasts (mean 73.35, 106.5, 50.00, and 47.15% of assay positive control, respectively). There was no statistically significant difference between the IPF fibroblasts and any of the normal lung fibroblast lines after this combined exposure. Previous studies have shown that cycloheximide alone is insufficient to induce fibroblast apoptosis (11, 35), a finding that was confirmed in a subset of experiments for each of the different fibroblast populations studied. Together, these findings are consistent with prior studies showing that normal fibroblasts are relatively resistant to Fas-mediated apoptosis, that IPF lung fibroblasts have increased resistance to Fas-mediated apoptosis compared with normal lung fibroblasts, and that cycloheximide “sensitizes” normal and IPF fibroblasts to undergo robust apoptosis upon Fas activation (8, 10, 12, 33–35, 37–39). Additionally, these findings demonstrate that IMR-90 and CCL-210 fibroblasts exhibit Fas-induced apoptotic responses that are indistinguishable from primary normal lung fibroblasts isolated from lung explants.
IPF Lung Fibroblasts Have Increased Expression of XIAP
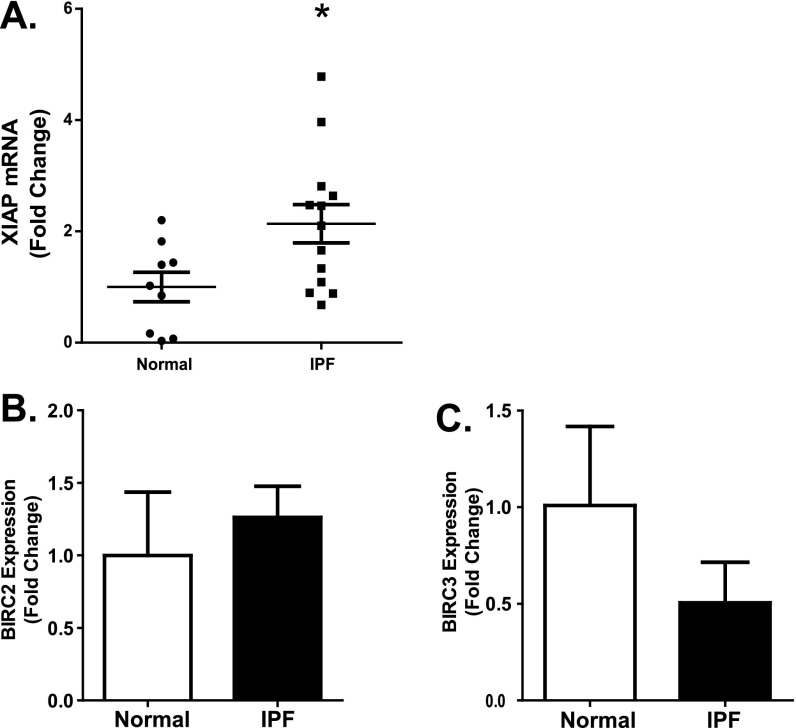
XIAP expression has been shown within the fibroblastic foci of IPF biopsies, suggesting induction of this protein in IPF fibroblasts (12, 35). To examine XIAP expression in fibrotic and normal lung fibroblasts, we first assessed XIAP mRNA expression in nine patient-derived normal lung fibroblast cell lines and in 13 patient-derived IPF fibroblast cell lines by quantitative real-time RT-PCR (Figure 2). Overall, XIAP expression was more than 2-fold higher in the IPF cells compared with the normal cells, although there was variability in expression among the IPF cell lines (mean normal, 1.0 versus mean IPF, 2.14; P = 0.025) (Figure 2A). Having shown that survivin (BIRC5) is also expressed at increased levels in a subset of IPF fibroblasts (26), we compared the expression of XIAP and survivin in these cell lines and found no significant correlation (see Figure E1A in the online supplement). We also compared the expression of BIRC2 and BIRC3 (cIAP1 and cIAP2, respectively), IAPs that are closely related to XIAP, in a subset of the normal and IPF fibroblasts (Figures 2B and 2C). Although we found no significant difference between the relative expression of these IAPs in the normal and IPF fibroblasts, there was a correlation identified between the cIAPs and XIAP expression levels in the IPF fibroblasts, suggesting that they share regulatory mechanisms (Figure E2).
Figure 2.
RNA isolated from nine different patient-derived normal lung fibroblast lines and 13 different IPF fibroblast lines was reverse transcribed and examined by quantitative real-time PCR for (A) X-linked inhibitor of apoptosis (XIAP), (B) baculoviral inhibitor of apoptosis repeat–containing (BIRC)2, and (C) BIRC3. Each sample was run in triplicate and indexed to the average expression level in the normal lung fibroblasts. *P = 0.025 compared with normal lung fibroblasts.
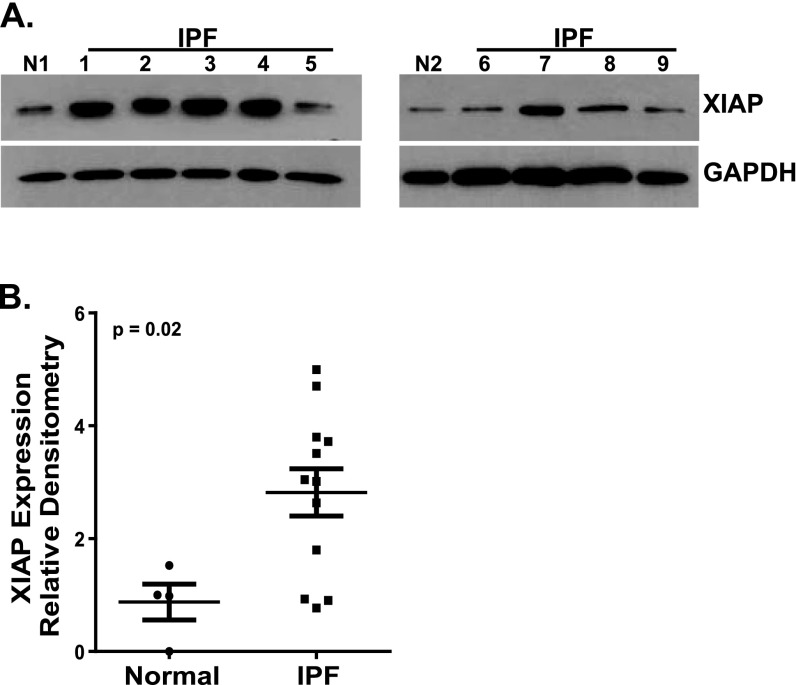
Next, we compared XIAP protein levels in four patient-derived normal and 12 IPF fibroblast cell lines (Figure 3). As with the RNA expression, XIAP protein expression was significantly increased in the IPF fibroblasts. However, this was not a uniform finding because 3 of the 12 IPF cell lines had XIAP levels that were no different from those seen in the normal lung fibroblasts. Consistent with RNA data, we found no significant correlation between XIAP and survivin protein levels in the IPF fibroblast cell lines (Figure E1B).
Figure 3.
Patient-derived normal lung fibroblasts (n = 4) and IPF fibroblasts (n = 12) were cultured to 60% confluence and growth arrested for 24 hours. XIAP expression was assessed by Western blotting, and all blots were stripped and reprobed for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Each Western blot contained one normal lung fibroblast line and several IPF lines. (A) Two representative blots demonstrating two different normal fibroblast lines (N1 and N2) and nine different IPF lines (1–9). (B) Densitometric analysis was done for each normal and IPF band by determining the XIAP/GAPDH ratio. These ratios were then indexed to the average of the four normals expressed as the fold-change in the IPF cell lines. On the scatterplot, each point represents the average relative density for a different cell line. P = 0.02 for normal versus IPF fibroblasts.
ET-1 Induces XIAP Expression in Normal Lung Fibroblasts
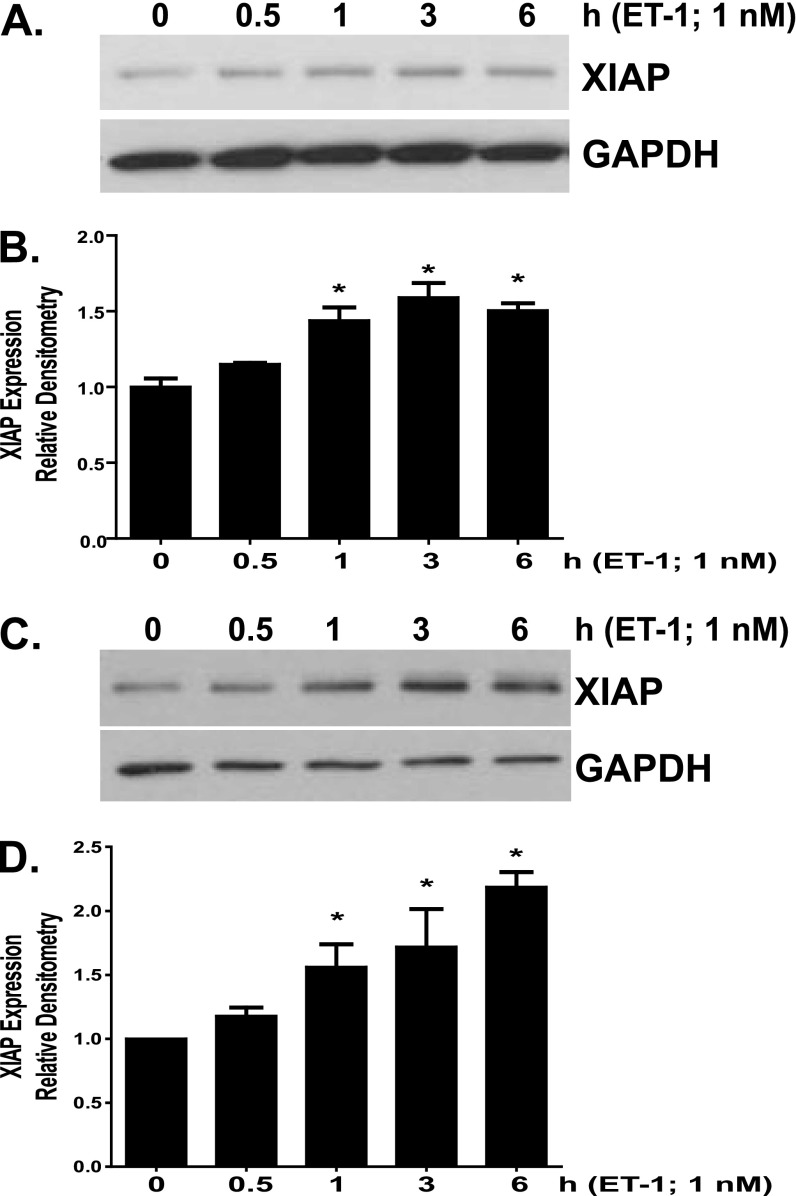
ET-1 has been implicated in the pathogenesis of pulmonary fibrosis and has been shown to promote myofibroblast differentiation, activation, and survival (7, 11, 40–42). In addition, we recently reported that ET-1 induced expression of the IAP protein survivin in IMR-90 fibroblasts (7). Our findings of increased XIAP expression in IPF fibroblasts and the recent demonstration of XIAP expression in fibroblastic foci prompted us to question if ET-1 would induce XIAP in normal lung fibroblasts (12, 35). Indeed, we found that ET-1 treatment does increase XIAP expression in IMR-90 (Figures 4A and 4B) and CCL-210 (Figures 4C and 4D) lung fibroblasts. In each cell line, a significant increase in XIAP expression was observed within 1 hour of ET-1 treatment. XIAP expression levels reached a plateau within 6 hours of treatment and were maintained for 16 to 24 hours (Figure 4 and data not shown).
Figure 4.
IMR-90 (A and B) and CCL-210 (C and D) fibroblasts were treated with endothelin (ET)-1 (1.0 nM). (A and C) Cell lysates were analyzed for XIAP expression by Western blotting, and each blot was stripped and reprobed for GAPDH. (B and D) Each experiment was completed in triplicate; XIAP/GAPDH ratios were indexed to untreated cells for each blot. *P < 0.01 compared with untreated controls.
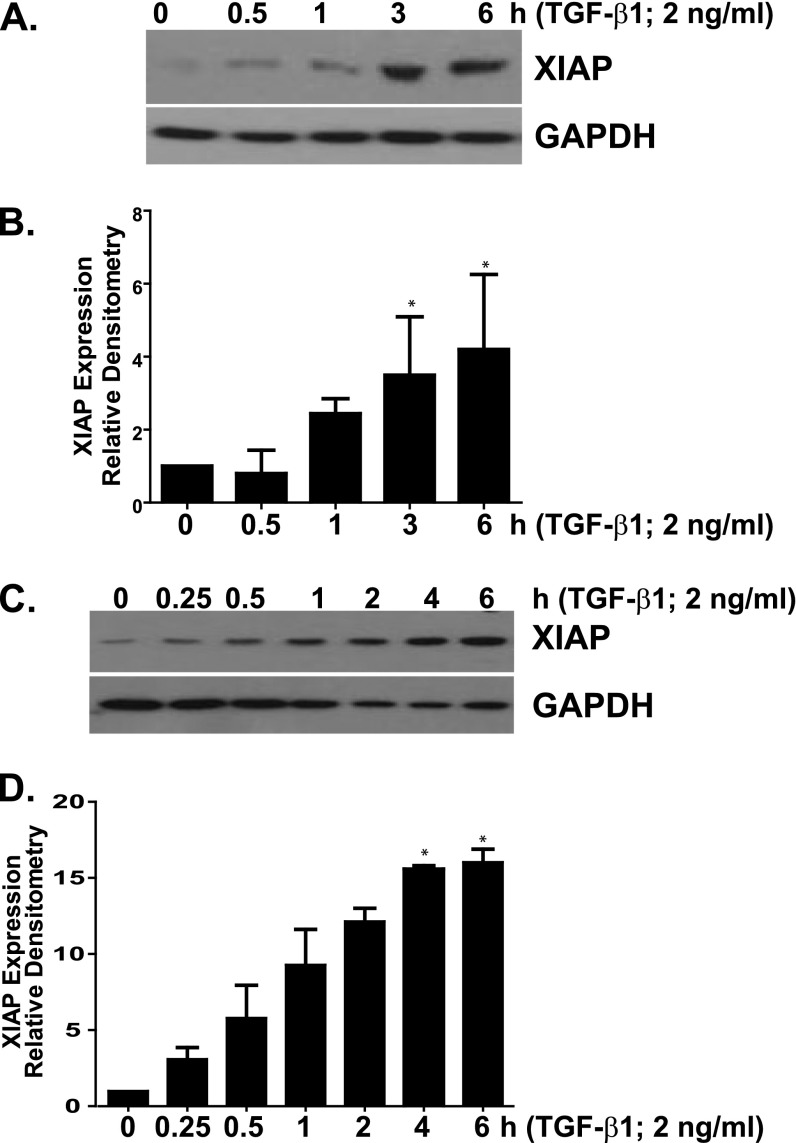
TGF-β1 Induces XIAP Expression in Normal Lung Fibroblasts
TGF-β1 is a potent fibrogenic mediator that promotes myofibroblast differentiation and resistance to apoptosis (3, 4, 9, 43). To determine if up-regulation of XIAP in fibroblasts represents a common mechanism of apoptosis resistance shared by different soluble profibrotic mediators, we treated IMR-90 (Figures 5A and 5B) and CCL-210 (Figures 5C and 5D) lung fibroblasts with TGF-β1 (2 ng/ml) and assessed changes in XIAP over time. With a time course that is consistent with a prior report of XIAP induction (44), XIAP expression increased significantly between 1 and 4 hours of treatment and plateaued between 3 and 6 hours after TGF-β1 exposure (Figures 5A–5D).
Figure 5.
IMR-90 (A and B) and CCL-210 (C and D) fibroblasts were treated with transforming growth factor (TGF)-β1 (2 ng/ml). (A and C) Cell lysates were analyzed for XIAP expression by Western blotting, and each blot was stripped and reprobed for GAPDH. (B and D) Each experiment was completed in triplicate; XIAP/GAPDH ratios were indexed to untreated cells for each blot. *P < 0.01 compared with untreated controls.
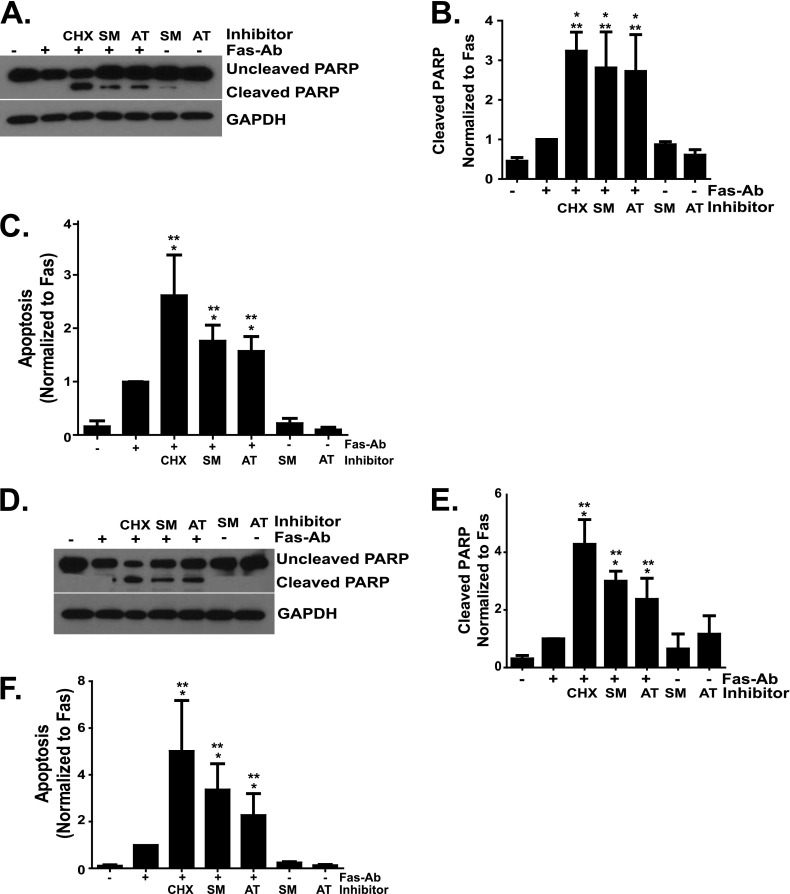
XIAP Antagonists Sensitize Normal Lung Fibroblasts to Fas-Mediated Apoptosis
The robust Fas-mediated apoptosis of lung fibroblasts in the presence of cycloheximide suggests that synthesis of one or more endogenous apoptosis inhibitors may contribute to the relative resistance of these cells to apoptosis induced by Fas activation. To determine if XIAP may contribute to the relative resistance of normal lung fibroblasts to Fas-mediated apoptosis, we used two different Smac/DIABLO mimetics (SM164 and AT406) that functionally antagonize XIAP, cIAP1, and cIAP2 (22, 29, 31, 32). IMR-90 and CCL-210 fibroblasts were treated with or without Fas-Ab in the presence or absence of SM164 or AT406 for 16 hours, and apoptosis was assessed by Western blotting for cleaved PARP and by ELISA for histone-associated DNA fragments. Similar results were obtained with these two assays of apoptosis (Figure 6). The combination of Fas-Ab and cycloheximide was included in each experiment as a positive control for apoptosis. In IMR-90 (Figures 6A–6C) and CCL-210 (Figures 6D–6F) fibroblasts, each IAP antagonist significantly enhanced Fas-mediated apoptosis at concentrations previously shown to functionally inhibit IAPs (29, 32). Neither compound significantly increased fibroblast apoptosis in the absence of Fas-activating antibody, suggesting that inhibition of XIAP function alone is not sufficient to induce fibroblast apoptosis.
Figure 6.
IMR-90 (A–C) and CCL-210 (D–F) fibroblasts were treated with or without Fas-Ab (250 ng/ml) in the presence or absence of CHX (500 ng/ml), the Smac/DIABLO mimetic SM164 (100 nM), the Smac/DIABLO mimetic AT406 (1.0 μM), or the Smac/DIABLO mimetics alone for 16 hours. Apoptosis was assessed by Western blotting for cleaved poly-(ADP-ribose) polymerase (PARP) (A and D) and by ELISA detection of histone-associated DNA fragments (C and F). The Western blots were stripped and probed for GAPDH. Relative densitometry was determined by indexing the cleaved PARP/GAPDH ratio for each band to the Fas-Ab treatment (B and E). Densitometry and ELISA data represent at least three independent experiments. *P < 0.01 compared with untreated controls. **P < 0.05 compared with fibroblasts treated with Fas-Ab.
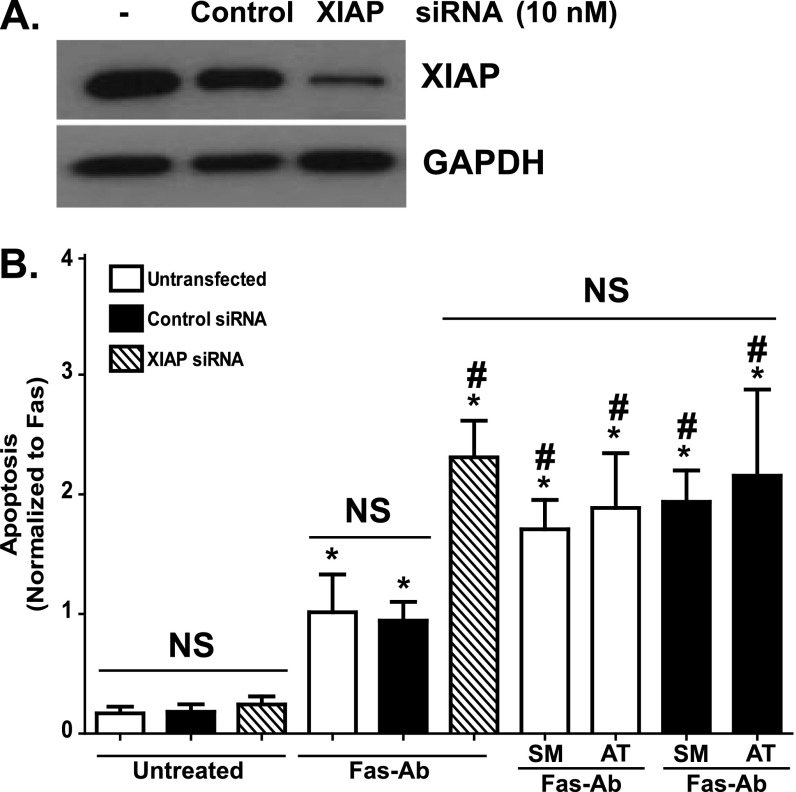
siRNA Knockdown of XIAP Enhances Fas-Mediated Apoptosis
To determine if the enhanced apoptosis conferred by the Smac/Diablo mimetics was due to functional antagonism of XIAP and to exclude the potential for off-target effects of these compounds, we next examined how siRNA-mediated knockdown of XIAP affected the susceptibility of normal fibroblasts to Fas-mediated apoptosis. Using untransfected IMR-90 fibroblasts, IMR-90 fibroblasts transfected with a nontargeting (control) siRNA, and IMR-90 fibroblasts transfected with siRNA targeting XIAP, we confirmed a significant reduction in XIAP protein levels in the cells transfected with the XIAP siRNA (Figure 7A). Next, we compared Fas-induced apoptosis in IMR-90 cells transfected with XIAP siRNA with control siRNA–transfected and untransfected cells (Figure 7B). Basal rates of apoptosis in the XIAP siRNA–transfected cells were not significantly different from the untransfected or control siRNA–transfected cells, confirming that the loss of XIAP is not sufficient to induce apoptosis in these cells. In response to Fas activation, the XIAP siRNA–transfected cells demonstrated more than a 2-fold increase in apoptosis in comparison with the untransfected and control siRNA–transfected cells. The increase in apoptosis in XIAP siRNA–transfected cells was indistinguishable from the increased apoptosis seen in untransfected and control siRNA–transfected cells treated with Fas-Ab in combination with either of the Smac/Diablo mimetics. Moreover, treatment with either of the Smac/Diablo mimetics had no additional effect on Fas-induced apoptosis in the XIAP siRNA–transfected cells (Figure E3A), further supporting a specific role for XIAP in fibroblast resistance to apoptosis. Treatment with cycloheximide alone or with either Smac/Diablo mimetic alone had no significant impact on any of the cells in the absence of Fas activation (Figure E3B). Moreover, the addition of cycloheximide to XIAP siRNA–transfected cells treated with Fas-Ab had no additional effect on apoptosis when compared with untransfected or control siRNA–transfected cells treated with Fas-Ab and cycloheximide (Figure E3B).
Figure 7.
(A) XIAP expression was assessed by Western blotting of untransfected IMR-90 fibroblasts and IMR-90 fibroblasts transfected for 24 hours with 10 nM of a nontargeting (control) siRNA or a siRNA-targeting XIAP. The representative blot was stripped and probed for GAPDH. (B) Untransfected IMR-90 fibroblasts and IMR-90 fibroblasts transfected with the control or with XIAP siRNA were treated with or without Fas-Ab (250 ng/ml) with or without the Smac/Diablo mimetics SM164 (100 nM) or AT406 (1.0 μM) for 16 hours, and apoptosis was assessed using ELISA for detection of histone-associated DNA fragments. Data are indexed to the average of the untransfected Fas-Ab–treated cells to allow relative comparisons. Data shown are from five independent experiments with each condition measured in triplicate. *P < 0.01 compared with untransfected cells without treatment. #P < 0.01 compared with untransfected cells treated with Fas-Ab. Additional control conditions (Fas-Ab with cycloheximide, cycloheximide alone, SM164 alone, and AT406 alone) are shown in Figure E3. NS, not significantly different from untreated cells.
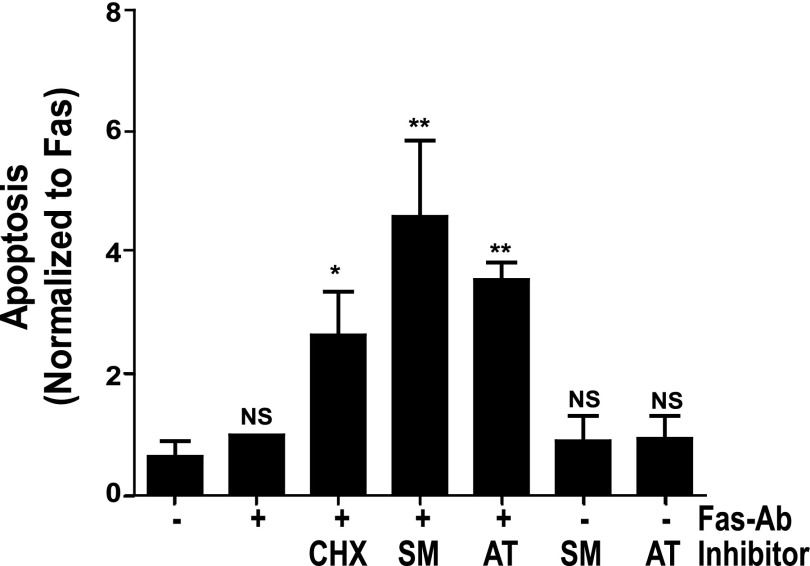
XIAP Antagonists Sensitize IPF Fibroblasts to Fas-Mediated Apoptosis
After finding that inhibition or knock-down of XIAP enhanced susceptibility to Fas-mediated apoptosis in normal lung fibroblasts, we asked if inhibition of XIAP function would enhance Fas-induced apoptosis in IPF fibroblasts. To address this question, seven different IPF fibroblast lines were treated with or without Fas-Ab in the presence or absence of cycloheximide or a Smac/DIABLO mimetic (SM164 or AT406) or with the Smac/DIABLO mimetic alone. Apoptosis was assessed after 16 hours by ELISA for histone-associated DNA fragments. As with the normal lung fibroblast lines, each of the Smac/DIABLO mimetics significantly enhanced the apoptotic response to Fas activation in the IPF fibroblasts, but neither mimetic induced apoptosis in the absence of Fas-Ab (Figure 8).
Figure 8.
IPF fibroblasts (n = 7) were treated with or without Fas-Ab in the presence or absence of CHX (250 ng/ml), with the Smac/DIABLO mimetics (SM164, 100 nM or AT406, 1.0 μM), or with the Smac/DIABLO mimetics alone for 16 hours, and apoptosis was assessed by ELISA detection of histone-associated DNA fragments. Each experiment was indexed to Fas-Ab–treated cells in the absence of any inhibitor. *P < 0.01 versus untreated or Fas-Ab–treated cells. **P < 0.001 versus untreated or Fas-Ab–treated cells. NS = not significantly different from untreated cells.
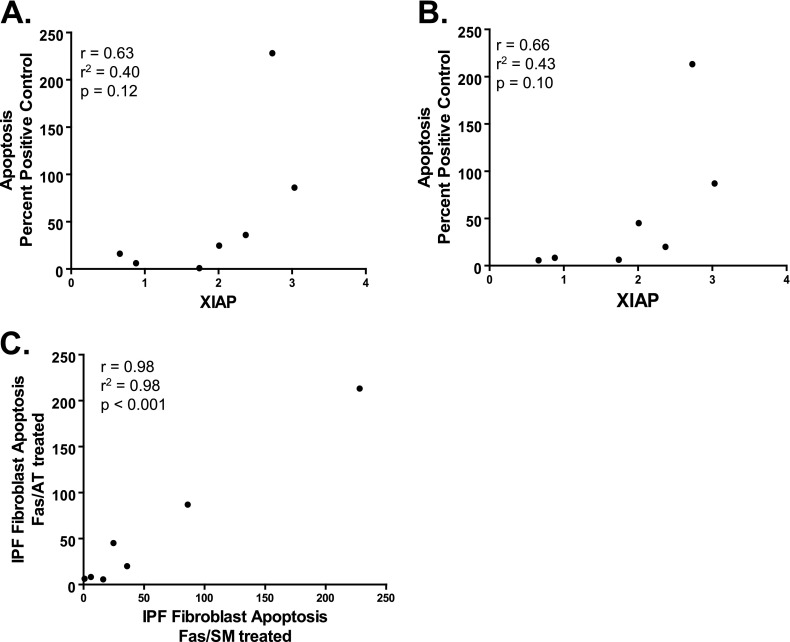
Because of the heterogeneity seen in XIAP protein expression among IPF fibroblast lines (Figure 3), we asked if there was a correlation between the basal expression of XIAP and the extent of apoptosis induced by Fas activation in the presence of the XIAP antagonists. Using seven IPF cell lines for which we had data on apoptosis and protein expression, we observed a trend between increased XIAP and the ability of SM164 (r = 0.63; P = 0.12) (Figure 9A) and AT406 (r = 0.66; P = 0.10) (Figure 9B) to enhance apoptosis. Finally, we examined the relationship between apoptosis of IPF fibroblasts in response to Fas activation with SM164 and AT406 and found a very strong and statistically significant correlation between the effects of these two compounds, further supporting their specificity as functional antagonists of XIAP (r = 0.98; P < 0.001) (Figure 9C).
Figure 9.
Correlation between XIAP protein expression in IPF fibroblasts, as determined by densitometric analysis indexed to the average expression of XIAP in normal lung fibroblasts, and apoptosis (shown as the percent of assay positive control) induced by Fas-Ab combined with (A) SM164 (100 nM; Fas/SM) or (B) AT406 (1.0 μM; Fas/AT). (C) Correlation between apoptosis in IPF fibroblasts treated with Fas-Ab in combination with SM164 (Fas/SM) or AT406 (Fas/AT). For A–C, each point represents a distinct IPF fibroblast cell line.
Discussion
The persistence of activated fibroblasts within fibroblastic foci is a hallmark of the dysregulated wound repair that underlies IPF pathogenesis (13, 45). Although homeostatic wound repair requires the clearance of myofibroblasts by apoptosis, several studies demonstrate the lack of apoptosis within fibroblastic foci (3, 5, 12, 16, 17, 46). This absence of apoptosis suggests that resistance to programmed cell death serves as a mechanism contributing to the inappropriate persistence of fibroblasts in IPF. In the current study, we used patient-derived normal and IPF lung fibroblasts and two commercially available primary normal lung fibroblast cell lines to explore the role of XIAP in the regulation of apoptosis resistance. We demonstrate that XIAP expression is increased in fibroblasts from IPF lungs and that the soluble fibrogenic mediators TGF-β1 and ET-1 significantly increase XIAP expression in normal lung fibroblasts. Furthermore, the loss of XIAP function mediated by siRNA knockdown or by functional antagonism with two distinct Smac/DIABLO mimetics enhances fibroblast susceptibility to Fas-mediated apoptosis without directly stimulating the death of these cells. Collectively, these findings support increased XIAP expression as a mechanism by which fibroblasts display increased survival and accumulation in IPF.
In concordance with other studies, we found that normal lung fibroblasts are relatively resistant to Fas-mediated apoptosis and that this resistance is accentuated in IPF fibroblasts (10, 33–35, 38, 39). The mechanisms accounting for the increased resistance to apoptosis in IPF fibroblasts have not been completely defined. Studies show that decreased cell surface expression of the Fas receptor is likely to contribute because increasing Fas receptor expression is sufficient to sensitize these cells to Fas-induced apoptosis (33, 34, 36). Consistently, we have shown that PGE2 enhances Fas-mediated apoptosis in lung fibroblasts while increasing expression of the Fas receptor (10).
Additional studies show that Fas-induced apoptosis can be enhanced through inhibition of new protein synthesis with cycloheximide (10, 11, 35). Because cycloheximide inhibits new protein synthesis and would, therefore, not be expected to increase Fas receptor expression, these studies suggest an alternative mechanism for the resistance to Fas-mediated apoptosis in which the synthesis of an endogenous protein impedes the postreceptor transduction of death signals. Candidate proteins associated with the Fas receptor include FLIP (Fas-associated death-domain-like IL-1β–converting enzyme inhibitory protein), which prevents caspase-8 activation after assembly of the Fas-associated death domain (47). Indeed, one recent study showed that FLIP induction prevents embryonic fibroblast apoptosis induced by TNF-α (48). Another report recently showed c-FLIP expression in the fibroblastic foci of IPF biopsies while demonstrating increased c-FLIP expression in IPF fibroblasts (49). Moreover, loss of c-FLIP increased the sensitivity of fibroblasts to apoptosis (49). In contrast, another study argued against a role for c-FLIP as a mechanism of apoptosis resistance in IPF fibroblasts by demonstrating that c-FLIP was expressed in the epithelium overlying fibroblastic foci but not in the fibroblasts themselves (46). The reasons for these discordant findings in IPF samples are unclear, although it is possible that they reflect the heterogeneity of biologic mechanisms that can result in tissue fibrosis.
The IAP proteins XIAP and survivin are endogenously expressed in fibroblasts and are suppressed by PGE2 in association with enhanced Fas-mediated apoptosis (10, 12). We recently reported that survivin, an IAP protein that is regulated by profibrotic mediators and is involved in inhibition of apoptosis and in cell proliferation, is expressed in mesenchymal cells within fibroblastic foci and in the epithelium overlying fibroblastic foci in IPF lung tissue (7, 26). Supporting the concept of mechanistic heterogeneity in IPF, we found that a subset of IPF fibroblasts had increased expression of survivin and that inhibition of survivin could sensitize those cells to Fas-mediated apoptosis (26).
The contribution of survivin to apoptosis-resistance prompted us to hypothesize that other apoptosis inhibitor proteins may similarly participate in the apoptosis resistance of fibroblasts in fibrotic lung disease. In the current study, we focused our investigation on XIAP for several reasons. First, two studies have shown that XIAP is expressed within fibroblastic foci of IPF tissue (12, 35). Second, the study by Maher and colleagues showed that XIAP, which is the only IAP that is thought to function as a direct apoptosis inhibitor in vivo, was not expressed in the epithelium overlying the fibroblastic foci, suggesting cell specificity that could be exploited for therapeutic benefit (12, 21). Third, small-molecule Smac/Diablo mimetics that specifically antagonize XIAP and the structurally related cIAP1 and cIAP2 but have no effect on survivin have been developed as clinical therapeutics (22). Although other IAP family members have been described, data on their role in disease are limited (20).
To examine a potential regulatory role in the antiapoptotic phenotype of IPF fibroblasts, we first compared XIAP expression levels in primary normal lung fibroblasts and IPF lung fibroblasts, finding significantly, but variably, increased levels in the IPF lung fibroblasts. We next determined whether TGF-β1 and ET-1, soluble mediators mechanistically implicated in fibrogenesis and fibroblast resistance to apoptosis, could increase XIAP expression levels in normal lung fibroblasts (7, 9, 11, 43). Supporting a role for XIAP in fibrogenesis, each of these mediators rapidly induced expression of this protein in IMR-90 and CCL-210 fibroblasts.
Having shown that XIAP is regulated by profibrotic mediators in normal lung fibroblasts, we sought to establish a mechanistic link between XIAP and fibroblast susceptibility to apoptosis. To do this, we used two structurally distinct Smac/DIABLO mimetics (SM164 and AT406, which act as functional antagonists of XIAP, cIAP1, and cIAP2 but not of survivin) and assessed their effect on susceptibility to Fas-mediated apoptosis in normal lung fibroblasts. In IMR-90 and CCL-210 fibroblasts, these functional antagonists significantly enhanced the apoptotic response to Fas activation. IAP antagonism alone was not sufficient to induce fibroblast apoptosis, a finding consistent with the notion that functional inhibition of IAPs is not detrimental to cell survival in the absence of an apoptotic stimulus (21). The Smac/Diablo mimetics used to antagonize XIAP also antagonize cIAP1 and cIAP2 (22). However, siRNA knockdown of XIAP enhanced Fas-mediated apoptosis to the same degree as treatment of control fibroblasts with the Smac/Diablo mimetics. The fact that no additional increase in apoptosis susceptibility was seen when the XIAP knockdown fibroblasts were treated with the Smac/Diablo mimetics (Figure 7; Figure E3) suggests that the enhanced apoptosis observed in cells treated with the Smac/Diablo mimetics is attributable to inhibition of XIAP and that XIAP inhibition is sufficient to regulate the apoptosis susceptibility of these cells. Although there was no significant difference in the expression of cIAP1 and cIAP2 between normal and IPF lung fibroblasts, the relative expression of these IAPs did correlate with XIAP in the IPF fibroblasts, suggesting that they are similarly regulated and may have a cooperative function with XIAP (50). However, these studies also suggest that any contribution to apoptosis resistance mediated by cIAP1 or cIAP2 was dependent on the presence of functionally intact XIAP.
After validating a role for XIAP in the regulation of Fas-mediated apoptosis in normal fibroblasts, we questioned if functional antagonism of XIAP would affect the resistance to Fas-mediated apoptosis observed in IPF fibroblasts. Treatment of the IPF fibroblasts with the Smac/Diablo mimetics confirmed that the increased resistance to Fas-mediated apoptosis in IPF fibroblasts was reversible and supported a mechanistic link between XIAP expression and apoptosis resistance in these cells. As with the normal lung fibroblasts, the XIAP antagonists had no impact on apoptosis in the absence of Fas activation.
Finally, we explored the correlation between XIAP protein expression and the ability of XIAP antagonists to enhance Fas-mediated apoptosis in IPF fibroblasts. Despite the small sample size, we observed a trend between XIAP expression and the induction of apoptosis in response to Fas-Ab in combination with either of the Smac/Diablo mimetics. There was a highly significant correlation between the Fas-mediated apoptotic responses of individual IPF fibroblast lines to treatment with SM164 versus AT406, further supporting the mechanistic specificity of these compounds. Accordingly, we conclude that IPF fibroblasts have a reversible resistance to Fas-mediated apoptosis that is, at least in part, regulated by XIAP expression.
IPF is characterized by biologic and clinical heterogeneity, although the basis for this heterogeneity is poorly understood. In this study, we have identified XIAP as a protein that is inducible by soluble fibrogenic mediators and contributes to fibroblast resistance to apoptosis. However, in 25% of the IPF fibroblast lines evaluated, we saw no difference in XIAP protein expression between normal and IPF fibroblasts. Similarly, we recently reported that inhibition of survivin enhanced apoptosis in approximately half of the IPF cell lines assessed (26). These findings are congruous with another recent report showing that the proapoptotic p14ARF gene is epigenetically silenced through hypermethylation in four out of eight IPF fibroblast cell lines and that hypermethylation is associated with increased resistance to apoptosis (51). Taken together, these studies highlight the concept that IPF fibroblasts can acquire resistance to apoptosis through a variety of distinct mechanisms. It remains to be determined if different antiapoptotic mechanisms can combine to function in an additive or synergistic manner and whether their expression correlates with clinical phenotypes in IPF. Our current study demonstrates that XIAP expression represents one mechanism by which IPF fibroblasts may acquire resistance to apoptosis. In future studies, we plan to measure the expression of XIAP and other apoptosis inhibitors in larger data sets to better appreciate their diversity of expression in IPF fibroblasts.
The specific mechanisms by which XIAP regulates fibroblast apoptosis have not been determined. In addition to direct inhibition of caspase activation in vivo, XIAP can modulate apoptosis through alternative mechanisms associated with its function as an E3 ubiquitin ligase (21, 52). Further studies to delineate these mechanisms could provide additional insight into the pathobiology of fibrotic wound repair.
In summary, this is the first study to directly implicate XIAP in the regulation of fibroblast susceptibility to apoptosis and to demonstrate that functional antagonism of an apoptosis inhibitor can restore susceptibility of these cells to Fas-mediated apoptosis. It is not known if restoration of fibroblast apoptosis can mitigate fibrotic lung disease in vivo, but several studies provide proof of principal that modulation of signaling pathways involved in fibroblast resistance to apoptosis can attenuate fibrogenesis in murine models (53–57). Our findings suggest that XIAP antagonists may have therapeutic potential in disease processes characterized by fibroblast accumulation and fibrosis, although it will be critical to determine the impact of these antagonists on other cell populations before translating this strategy to patient care.
Acknowledgments
Acknowledgments
The authors thank Shaomeng Wang, Ph.D., (University of Michigan) for providing SM164 and AT406.
Footnotes
This work was supported by National Institutes of Health/National Heart, Lung and Blood Institute grants HL105489 (J.C.H.), HL R01085083 (E.S.W.), and HL078871 (T.H.S.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0224OC on March 14, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hecker L, Jagirdar R, Jin T, Thannickal VJ. Reversible differentiation of myofibroblasts by MyoD. Exp Cell Res. 2011;317:1914–1921. doi: 10.1016/j.yexcr.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinz B, Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2010;2:78. doi: 10.3410/B2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 5.Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 6.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:350–356. doi: 10.1513/pats.200601-001TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horowitz JC, Ajayi IO, Kulasekaran P, Rogers DS, White JB, Townsend SK, White ES, Nho RS, Higgins PD, Huang SK, et al. Survivin expression induced by endothelin-1 promotes myofibroblast resistance to apoptosis. Int J Biochem Cell Biol. 2012;44:158–169. doi: 10.1016/j.biocel.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horowitz JC, Cui Z, Moore TA, Meier TR, Reddy RC, Toews GB, Standiford TJ, Thannickal VJ. Constitutive activation of prosurvival signaling in alveolar mesenchymal cells isolated from patients with nonresolving acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2006;290:L415–L425. doi: 10.1152/ajplung.00276.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, Cui Z, Thannickal VJ. Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal. 2007;19:761–771. doi: 10.1016/j.cellsig.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang SK, White ES, Wettlaufer SH, Grifka H, Hogaboam CM, Thannickal VJ, Horowitz JC, Peters-Golden M. Prostaglandin E2 induces fibroblast apoptosis by modulating multiple survival pathways. FASEB J. 2009;23:4317–4326. doi: 10.1096/fj.08-128801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulasekaran P, Scavone CA, Rogers DS, Arenberg DA, Thannickal VJ, Horowitz JC. Endothelin-1 and transforming growth factor-beta1 independently induce fibroblast resistance to apoptosis via AKT activation. Am J Respir Cell Mol Biol. 2009;41:484–493. doi: 10.1165/rcmb.2008-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maher TM, Evans IC, Bottoms SE, Mercer PF, Thorley AJ, Nicholson AG, Laurent GJ, Tetley TD, Chambers RC, McAnulty RJ. Diminished prostaglandin E2 contributes to the apoptosis paradox in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;182:73–82. doi: 10.1164/rccm.200905-0674OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horowitz JC, Thannickal VJ. Idiopathic pulmonary fibrosis: new concepts in pathogenesis and implications for drug therapy. Treat Respir Med. 2006;5:325–342. doi: 10.2165/00151829-200605050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 15.King TE, Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA, Jr, Flint A, Thurlbeck W, Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164:1025–1032. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 16.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:838–846. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lepparanta O, Pulkkinen V, Koli K, Vahatalo R, Salmenkivi K, Kinnula VL, Heikinheimo M, Myllarniemi M. Transcription factor gata-6 is expressed in quiescent myofibroblasts in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;42:626–632. doi: 10.1165/rcmb.2009-0021OC. [DOI] [PubMed] [Google Scholar]
- 18.Vancheri C, Failla M, Crimi N, Raghu G. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J. 2010;35:496–504. doi: 10.1183/09031936.00077309. [DOI] [PubMed] [Google Scholar]
- 19.Vancheri C. Idiopathic pulmonary fibrosis: an altered fibroblast proliferation linked to cancer biology. Proc Am Thorac Soc. 2012;9:153–157. doi: 10.1513/pats.201203-025AW. [DOI] [PubMed] [Google Scholar]
- 20.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 21.Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer. 2010;10:561–574. doi: 10.1038/nrc2889. [DOI] [PubMed] [Google Scholar]
- 22.Wang S. Design of small-molecule Smac mimetics as IAP antagonists. Curr Top Microbiol Immunol. 2011;348:89–113. doi: 10.1007/82_2010_111. [DOI] [PubMed] [Google Scholar]
- 23.Wright CW, Duckett CS. Reawakening the cellular death program in neoplasia through the therapeutic blockade of IAP function. J Clin Invest. 2005;115:2673–2678. doi: 10.1172/JCI26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flygare JA, Fairbrother WJ. Small-molecule pan-IAP antagonists: a patent review. Expert Opin Ther Pat. 2010;20:251–267. doi: 10.1517/13543770903567077. [DOI] [PubMed] [Google Scholar]
- 25.Vaux DL. Inhibitor of apoptosis (IAP) proteins as drug targets for the treatment of cancer. F1000 Biol Rep. 2009;1:79. doi: 10.3410/B1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sisson TH, Maher TM, Ajayi IO, King JE, Higgins PDR, Booth AJ, Sagana RL, Huang SK, White ES, Moore BB, et al. Increased survivin expression contributes to apoptosis-resistance in IPF fibroblasts. Adv Biosci Biotechnol. 2012;3:657–664. doi: 10.4236/abb.2012.326085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horowitz JC, Ajayi IO, Sagana RL, White ES, Moore BB, Huang SK, Sisson TH. Expression of inhibitor of apoptosis proteins in normal and fibrotic lung fibroblasts [abstract] Am J Respir Crit Care Med. 2012;185:A4925. [Google Scholar]
- 28.White ES, Thannickal VJ, Carskadon SL, Dickie EG, Livant DL, Markwart S, Toews GB, Arenberg DA. Integrin alpha4beta1 regulates migration across basement membranes by lung fibroblasts: a role for phosphatase and tensin homologue deleted on chromosome 10. Am J Respir Crit Care Med. 2003;168:436–442. doi: 10.1164/rccm.200301-041OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai Q, Sun H, Peng Y, Lu J, Nikolovska-Coleska Z, McEachern D, Liu L, Qiu S, Yang CY, Miller R, et al. A potent and orally active antagonist (SM-406/AT-406) of multiple inhibitor of apoptosis proteins (IAPS) in clinical development for cancer treatment. J Med Chem. 2011;54:2714–2726. doi: 10.1021/jm101505d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang D, Zhao Y, Li AY, Wang S, Wang G, Sun Y. Smac-mimetic compound SM-164 induces radiosensitization in breast cancer cells through activation of caspases and induction of apoptosis. Breast Cancer Res Treat. 2012;133:189–199. doi: 10.1007/s10549-011-1752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu J, McEachern D, Sun H, Bai L, Peng Y, Qiu S, Miller R, Liao J, Yi H, Liu M, et al. Therapeutic potential and molecular mechanism of a novel, potent, nonpeptide, smac mimetic SM-164 in combination with TRAIL for cancer treatment. Mol Cancer Ther. 2011;10:902–914. doi: 10.1158/1535-7163.MCT-10-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu J, Bai L, Sun H, Nikolovska-Coleska Z, McEachern D, Qiu S, Miller RS, Yi H, Shangary S, Sun Y, et al. SM-164: a novel, bivalent smac mimetic that induces apoptosis and tumor regression by concurrent removal of the blockade of cIAP-1/2 and XIAP. Cancer Res. 2008;68:9384–9393. doi: 10.1158/0008-5472.CAN-08-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buhling F, Wille A, Rocken C, Wiesner O, Baier A, Meinecke I, Welte T, Pap T. Altered expression of membrane-bound and soluble CD95/fas contributes to the resistance of fibrotic lung fibroblasts to FasL induced apoptosis. Respir Res. 2005;6:37. doi: 10.1186/1465-9921-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frankel SK, Cosgrove GP, Cha SI, Cool CD, Wynes MW, Edelman BL, Brown KK, Riches DW. TNF-alpha sensitizes normal and fibrotic human lung fibroblasts to Fas-induced apoptosis. Am J Respir Cell Mol Biol. 2006;34:293–304. doi: 10.1165/rcmb.2005-0155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka T, Yoshimi M, Maeyama T, Hagimoto N, Kuwano K, Hara N. Resistance to Fas-mediated apoptosis in human lung fibroblast. Eur Respir J. 2002;20:359–368. doi: 10.1183/09031936.02.00252602. [DOI] [PubMed] [Google Scholar]
- 36.Wynes MW, Edelman BL, Kostyk AG, Edwards MG, Coldren C, Groshong SD, Cosgrove GP, Redente EF, Bamberg A, Brown KK, et al. Increased cell surface Fas expression is necessary and sufficient to sensitize lung fibroblasts to Fas ligation-induced apoptosis: implications for fibroblast accumulation in idiopathic pulmonary fibrosis. J Immunol. 2011;187:527–537. doi: 10.4049/jimmunol.1100447. [DOI] [PubMed] [Google Scholar]
- 37.Chang W, Wei K, Jacobs SS, Upadhyay D, Weill D, Rosen GD. SPARC suppresses apoptosis of idiopathic pulmonary fibrosis fibroblasts through constitutive activation of beta-catenin. J Biol Chem. 2010;285:8196–8206. doi: 10.1074/jbc.M109.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moodley YP, Caterina P, Scaffidi AK, Misso NL, Papadimitriou JM, McAnulty RJ, Laurent GJ, Thompson PJ, Knight DA. Comparison of the morphological and biochemical changes in normal human lung fibroblasts and fibroblasts derived from lungs of patients with idiopathic pulmonary fibrosis during FasL-induced apoptosis. J Pathol. 2004;202:486–495. doi: 10.1002/path.1531. [DOI] [PubMed] [Google Scholar]
- 39.Moodley YP, Misso NL, Scaffidi AK, Fogel-Petrovic M, McAnulty RJ, Laurent GJ, Thompson PJ, Knight DA. Inverse effects of interleukin-6 on apoptosis of fibroblasts from pulmonary fibrosis and normal lungs. Am J Respir Cell Mol Biol. 2003;29:490–498. doi: 10.1165/rcmb.2002-0262OC. [DOI] [PubMed] [Google Scholar]
- 40.Ross B, D'Orleans-Juste P, Giaid A. Potential role of endothelin-1 in pulmonary fibrosis: from the bench to the clinic. Am J Respir Cell Mol Biol. 2010;42:16–20. doi: 10.1165/rcmb.2009-0175TR. [DOI] [PubMed] [Google Scholar]
- 41.Fonseca C, Abraham D, Renzoni EA. Endothelin in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;44:1–10. doi: 10.1165/rcmb.2009-0388TR. [DOI] [PubMed] [Google Scholar]
- 42.Shi-Wen X, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, du Bois RM, Black CM, et al. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a Rac/phosphoinositide 3-kinase/AKT-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell. 2004;15:2707–2719. doi: 10.1091/mbc.E03-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horowitz JC, Lee DY, Waghray M, Keshamouni VG, Thomas PE, Zhang H, Cui Z, Thannickal VJ. Activation of the pro-survival phosphatidylinositol 3-kinase/AKT pathway by transforming growth factor-beta1 in mesenchymal cells is mediated by p38 MAPK-dependent induction of an autocrine growth factor. J Biol Chem. 2004;279:1359–1367. doi: 10.1074/jbc.M306248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaur S, Wang F, Venkatraman M, Arsura M. X-linked inhibitor of apoptosis (XIAP) inhibits c-jun n-terminal kinase 1 (Jnk1) activation by transforming growth factor beta1 (TGF-beta1) through ubiquitin-mediated proteosomal degradation of the TGF-beta1-activated kinase 1 (TAK1) J Biol Chem. 2005;280:38599–38608. doi: 10.1074/jbc.M505671200. [DOI] [PubMed] [Google Scholar]
- 45.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cha SI, Groshong SD, Frankel SK, Edelman BL, Cosgrove GP, Terry-Powers JL, Remigio LK, Curran-Everett D, Brown KK, Cool CD, et al. Compartmentalized expression of c-FLIP in lung tissues of patients with idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;42:140–148. doi: 10.1165/rcmb.2008-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaufmann T, Strasser A, Jost PJ. Fas death receptor signalling: roles of Bid and XIAP. Cell Death Differ. 2012;19:42–50. doi: 10.1038/cdd.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moujalled DM, Cook WD, Lluis JM, Khan NR, Ahmed AU, Callus BA, Vaux DL. In mouse embryonic fibroblasts, neither caspase-8 nor cellular FLICE-inhibitory protein (FLIP) is necessary for TNF to activate NF-kappab, but caspase-8 is required for TNF to cause cell death, and induction of FLIP by NF-kappaB is required to prevent it. Cell Death Differ. 2012;19:808–815. doi: 10.1038/cdd.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Golan-Gerstl R, Wallach-Dayan SB, Zisman P, Cardoso WV, Goldstein RH, Breuer R. Cellular FLICE-like inhibitory protein deviates myofibroblast Fas-induced apoptosis toward proliferation during lung fibrosis. Am J Respir Cell Mol Biol. 2012;47:271–279. doi: 10.1165/rcmb.2010-0284RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin HS, Lee DH, Kim DH, Chung JH, Lee SJ, Lee TH. cIAP1, cIAP2, and XIAP act cooperatively via nonredundant pathways to regulate genotoxic stress-induced nuclear factor-kappaB activation. Cancer Res. 2009;69:1782–1791. doi: 10.1158/0008-5472.CAN-08-2256. [DOI] [PubMed] [Google Scholar]
- 51.Cisneros J, Hagood J, Checa M, Ortiz-Quintero B, Negreros M, Herrera I, Ramos C, Pardo A, Selman M. Hypermethylation-mediated silencing of p14ARF in fibroblasts from idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303:L295–L303. doi: 10.1152/ajplung.00332.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Themsche C, Leblanc V, Parent S, Asselin E. X-linked inhibitor of apoptosis protein (XIAP) regulates PTEN ubiquitination, content, and compartmentalization. J Biol Chem. 2009;284:20462–20466. doi: 10.1074/jbc.C109.009522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang HR, Lee CG, Homer RJ, Elias JA. Semaphorin 7a plays a critical role in TGF-beta1-induced pulmonary fibrosis. J Exp Med. 2007;204:1083–1093. doi: 10.1084/jem.20061273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Russo RC, Garcia CC, Barcelos LS, Rachid MA, Guabiraba R, Roffe E, Souza AL, Sousa LP, Mirolo M, Doni A, et al. Phosphoinositide 3-kinase gamma plays a critical role in bleomycin-induced pulmonary inflammation and fibrosis in mice. J Leukoc Biol. 2011;89:269–282. doi: 10.1189/jlb.0610346. [DOI] [PubMed] [Google Scholar]
- 55.Vittal R, Horowitz JC, Moore BB, Zhang H, Martinez FJ, Toews GB, Standiford TJ, Thannickal VJ. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol. 2005;166:367–375. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei X, Han J, Chen ZZ, Qi BW, Wang GC, Ma YH, Zheng H, Luo YF, Wei YQ, Chen LJ. A phosphoinositide 3-kinase-gamma inhibitor, AS605240 prevents bleomycin-induced pulmonary fibrosis in rats. Biochem Biophys Res Commun. 2010;397:311–317. doi: 10.1016/j.bbrc.2010.05.109. [DOI] [PubMed] [Google Scholar]
- 57.Lagares D, Busnadiego O, Garcia-Fernandez RA, Kapoor M, Liu S, Carter DE, Abraham D, Shi-Wen X, Carreira P, Fontaine BA, et al. Inhibition of focal adhesion kinase prevents experimental lung fibrosis and myofibroblast formation. Arthritis Rheum. 2012;64:1653–1664. doi: 10.1002/art.33482. [DOI] [PMC free article] [PubMed] [Google Scholar]