Abstract
Acute lung injury (ALI) attributable to sepsis or mechanical ventilation and subacute lung injury because of ionizing radiation (RILI) share profound increases in vascular permeability as a key element and a common pathway driving increased morbidity and mortality. Unfortunately, despite advances in the understanding of lung pathophysiology, specific therapies do not yet exist for the treatment of ALI or RILI, or for the alleviation of unremitting pulmonary leakage, which serves as a defining feature of the illness. A critical need exists for new mechanistic insights that can lead to novel strategies, biomarkers, and therapies to reduce lung injury. Sphingosine 1–phosphate (S1P) is a naturally occurring bioactive sphingolipid that acts extracellularly via its G protein–coupled S1P1–5 as well as intracellularly on various targets. S1P-mediated cellular responses are regulated by the synthesis of S1P, catalyzed by sphingosine kinases 1 and 2, and by the degradation of S1P mediated by lipid phosphate phosphatases, S1P phosphatases, and S1P lyase. We and others have demonstrated that S1P is a potent angiogenic factor that enhances lung endothelial cell integrity and an inhibitor of vascular permeability and alveolar flooding in preclinical animal models of ALI. In addition to S1P, S1P analogues such as 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol (FTY720), FTY720 phosphate, and FTY720 phosphonates offer therapeutic potential in murine models of lung injury. This translational review summarizes the roles of S1P, S1P analogues, S1P-metabolizing enzymes, and S1P receptors in the pathophysiology of lung injury, with particular emphasis on the development of potential novel biomarkers and S1P-based therapies for ALI and RILI.
Keywords: sphingolipids, S1P receptors, sphingosine kinase, S1P lyase, sepsis
Clinical Relevance
Acute lung injury (ALI) attributable to sepsis or mechanical ventilation and subacute lung injury attributable to ionizing radiation (RILI) share profound increases in vascular permeability as a key element and a common pathway driving increased morbidity and mortality. This translational review summarizes the roles of sphingosine 1–phosphate (S1P), S1P analogues, S1P-metabolizing enzymes, and S1P receptors in the pathophysiology of lung injury, with particular emphasis on the development of potential novel biomarkers and S1P-based therapies for ALI and RILI.
Acute and subacute inflammatory lung injuries are common and devastating disorders resulting from insults such as sepsis, ventilator-induced lung injury, ischemia/reperfusion, hyperoxia, and radiation therapy for thoracic malignancies. Unfortunately, despite recent advances in our understanding of the mechanisms and pathophysiology of acute lung injury (ALI), mortality rates remain very high (30–50%) because of the dearth of specific therapies for the treatment of ALI (1, 2). The only therapy for radiation-induced pneumonitis is based on long-term treatment with a high dose of corticosteroids. However, it is encumbered by severe side effects and relatively low efficacy (3). Therefore, an urgent need exists for new mechanistic insights into the pathophysiology of ALI that are likely to reveal new potential therapeutic targets, discover novel biomarkers, and develop highly efficacious targeted therapies that will effectively reduce the morbidity and mortality associated with acute and subacute lung injury.
“Sphingosin,” first described by J. L. W. Thudichum in 1884, derived its name from the Greek word “sphinx” meaning enigmatic, and it encompasses many compounds commonly referred to as “sphingoid bases” (4). Since their initial description, sphingoid bases have been found to be critical structural components of biological membranes and highly essential bioactive lipids that regulate diverse signaling pathways. The aberrant regulation of the sphingoid bases is known to contribute to a variety of pathologies that underlie cancer, inflammation, injury, edema, and infections (5–9). Of the several hundred sphingoid bases described to date, at least six, namely, sphingomyelin (SM), sphingosine (Sph), Sph-1–phosphate (S1P), ceramide, ceramide 1–phosphate (Cer1P), and sphingosylphosphorylcholine (lyso-SM), are considered key signaling and regulatory bioactive lipids (10). Furthermore, the importance of these lipids in human health and disease is underscored by the exponentially increasing number of publications that define key roles for these lipids in the areas of basic biology and translational and clinical research.
Among the various organs, the lung has been intensely investigated to understand better the role of S1P, its receptors, and its metabolizing enzymes in cellular functions under physiological as well as pathological conditions such as acute and subacute lung injury, pulmonary barrier dysfunction/edema, emphysema, and airway inflammation (5, 6, 11). In view of the complexity of the sphingolipid metabolism, the interconversion of bioactive sphingolipids, and the varied expressions and differential functions of their multiple G protein–coupled receptors, we perceived the need for a comprehensive review of the literature that addresses the pathways that regulate the metabolism and mechanisms of action for S1P in ALI. This review addresses the regulatory mechanisms underlying S1P generation and signaling in the context of lung inflammation and injury, especially in conditions such as sepsis-induced and radiation-induced lung injury, with an emphasis on genomic, lipidomic, and metabolomic approaches. In addition to S1P, the roles of Sph and S1P analogues such as 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol (FTY720), (S)-FTY720 phosphate (FTY720-P), and FTY720 phosphonates as novel therapeutic agents for acute lung injury will be discussed.
Sphingolipid Metabolism In Mammalian Cells
SM, the major sphingolipid of biological membranes, is synthesized de novo from serine and palmitoyl coenzyme A (CoA), which undergo condensation catalyzed by serine palmitoyltransferase (SPT) to form 3–keto-dihydroSph, which is reduced to dihydroSph, followed by ceramide synthase–mediated N-acylation to dihydroceramide and subsequent desaturation to ceramide (12). Ceramide is then channeled to complex sphingolipids such as SM or hydrolyzed to Sph through the action of ceramidases, or glycosylated to form glycosphingolipids (Figure 1). Ceramide is also phosphorylated by ceramide kinase to Cer1P (13). Mammalian cells do not convert dihydroSph to Sph. However, Sph is generated from ceramides by ceramidases (14). Sph is phosphorylated by two Sph kinases, SphK1 and SphK2, to S1P (15), and S1P is dephosphorylated to Sph through the action of specific S1P phosphatases 1 and 2 (S1PPases) (16) or through nonspecific lipid phosphate phosphatases (LPPs) (17, 18). Alternatively, S1P is irreversibly cleaved by S1P lyase (S1PL), a pyridoxal phosphate–dependent enzyme, to ethanolamine phosphate and trans–2-hexadecenal. Subsequently, trans–2-hexadecenal is oxidized by fatty aldehyde dehydrogenase to trans–2-hexadecenoic acid, which is recycled into glycerolipid or sphingolipid metabolic pathways, whereas ethanolamine phosphate is used for the biosynthesis of ethanolamine phospholipids (Figure 1) (19–21). Moreover, in response to TNF-α and other agonists, SM is hydrolyzed to ceramides of variable N-acyl chain lengths by one of the three (acid, neutral, or alkaline) sphingomyelinases (SMases) (Figure 2) (22). Ceramide acts intracellularly and functions as a second messenger by modulating ceramide-activated protein phosphatases and kinases (23, 24). Thus, the complexity in the sphingolipid metabolism enables cells to orchestrate cellular responses by regulating the interconversions via the anabolic and catabolic enzymes that regulate their intracellular concentrations and spatiotemporal distributions.
Figure 1.
Metabolism of sphingolipids in mammalian cells. Key enzymatic steps in the biosynthesis and degradation of sphingoid bases and recycling of trans–2-hexadecenal and ethanolamine phosphate from sphingolipids into glycerophospholipids are summarized. SPT, serine palmitoyltransferase; SMase, sphingomyelinase; S1P, sphingosine 1–phosphate; SphK, sphingosine kinase; SPP, S1P phosphatases; LPP, lipid phosphate phosphatase; CoA, coenzyme A; CDP, cytidine 5'-diphosphate.
Figure 2.
Intracellular generation and catabolism of S1P. The ceramide generated by the agonist-dependent hydrolysis of sphingomyelin (SM) by SMase is converted to sphingosine by ceramidases. Sphingosine is phosphorylated by SphK1 and/or SphK2 to S1P, which is transported outside the cell or catabolized to trans–2-hexadecenal and ethanolamine phosphate by S1P lyase. SMase, sphingomyelinase; S1P, sphingosine 1–phosphate.
Sphingosine 1–Phosphate In Vascular Permeability
S1P is present in plasma and tissues, and the concentrations of S1P are 3–4 times higher in serum than in plasma (25). The source of plasma S1P is controversial. The initial notion that platelets are a major source of circulating S1P may be erroneous, because erythrocytes (26), hematopoietic cells (27), and vascular endothelial cells (ECs) (28) are known to contribute to plasma S1P. S1P, initially identified as a mitogen for fibroblasts (29), is a potent angiogenic factor and plays an essential role in vessel maturation, vascular permeability, the trafficking of T-lymphocytes, B-lymphocytes, and dendritic cells, reproduction, and central nervous system development (30, 31). The ability of platelets to decrease endothelial barrier permeability (32) may be mediated by S1P stored within the platelets (33). Pioneering studies by Dr. J. G. N. Garcia and others identified S1P as a major barrier-protective agent responsible for the maintenance of vascular barrier integrity in vitro and in vivo (33–36). The exogenous addition of S1P to human and bovine lung ECs increased transendothelial monolayer resistance. The barrier-enhancing effect of S1P was rapid, dose-dependent, and mediated mostly through S1P1 (34). Agonists of S1P receptors such as 5-[4-phenyl-5-(trifluoromethyl)-2-thienyl]-3-[3-(trifluoromethyl)phenyl]-1,2,4-oxadiazole (SEW2871), FTY720-P, and FTY720 phosphonates were also effective in enhancing endothelial barrier function (37). Interestingly, the intracellular release of S1P by the photolysis of a caged S1P analogue also rapidly and significantly enhanced endothelial barrier function (38). However, this effect of intracellular S1P was independent of S1P1, and instead required Ras-related C3 botulinum toxin substrate 1 (Rac1) (38). That study delineated an important function for intracellular S1P and enzymes involved in the accumulation of S1P in cells in regulating barrier integrity. In fact, the extracellular action of S1P on endothelial barrier enhancement was dependent on intracellular S1P generation, because the blocking or down-regulation of SphK–1 activity or its forced expression attenuated S1P-induced barrier enhancement (Viswanathan Natarajan, unpublished data). In addition to in vitro barrier-protective effects, a barrier-regulatory role for S1P in murine and canine models of ALI was demonstrated. In an isolated perfused lung model, S1P infusion (1 μM) resulted in a significant decrease in the rate of edema formation, without a change in pulmonary artery pressure (39). Similarly, in mice suffering from LPS-induced lung injury or renal injury, pulmonary edema was significantly attenuated by an intravenous administration of S1P (39). The ability of S1P to confer protection against sepsis-induced barrier dysfunction was also confirmed in a canine model, wherein the infusion of S1P reduced bronchoalveolar lavage (BAL) fluid protein accumulation and alveolar edema, as measured by computed tomography (35).
Mechanisms Of S1p-Mediated Barrier Protection
The mechanisms of the S1P-mediated regulation of vascular permeability are yet to be fully defined. S1P binding to S1P1 or other S1P receptors activates Rac, cortactin translocation, peripheral myosin light chain phosphorylation, focal adhesion, adherens junction rearrangement, and recruits these signaling molecules and cytoskeletal effectors to lipid rafts. S1P also induces tight-junction assembly that further strengthens the endothelial barrier (Figure 3) (31, 34, 40). Because caged S1P–mediated barrier enhancement is Rac1-dependent (38), S1P may directly interact or bind with Rac1, and induce the dissociation of the Rho guanosine diphosphate (GDP) dissociation inhibitor (RhoGDI) from Rac1 for activation and redistribution to the cell periphery (41). This suggests that the action of S1P could be similar to that of another acidic phospholipid, phosphatidic acid (PA), generated by the phospholipase D signaling pathway, wherein PA acts as a membrane anchor of Rac1 by interacting with the polybasic motif in the carboxyl-terminal of Rac1, as shown in ovarian carcinoma-3 (OVCAR-3) cells (42). Further, intracellularly generated S1P may bind to S1P receptors located in organelles such as the endoplasmic reticulum and nuclear membranes, and initiate signal transduction or signal “inside-out” via S1P receptors on the cell surface (20). Thus, localized, intracellularly generated S1P can play an important role in modulating signaling pathways and cell functions, and this role requires further investigation.
Figure 3.
Regulation of endothelial barrier function by S1P. S1P binding to G protein–coupled S1P1 activates Rac1 and induces a series of signaling cascades, including cytoskeletal reorganization, the assembly of adherens junction and tight junction proteins, and the formation of focal adhesions that act together to enhance endothelial barrier function. However, cleavage of the protease-activated receptor (PAR-1) by thrombin induces actin stress fiber formation, and disrupts the assembly of adherens junctions and tight junction and focal adhesion proteins, resulting in barrier disruption. LIM, LIM kinase; PAK, p21-activated kinase–1; ZO, zona occludins; JAM, junctional adhesion molecule; PXN, paxillin; GIT, G protein–coupled receptor kinase–interacting protein; FAK, focal adhesion kinase; MLCK, myosin light chain kinase; cat, catenin; Src, Rous sarcoma oncogene cellular honolog; Rac1, Ras related C3 botulinum toxin 1.
Sphingosine Kinases And S1P Lyase In Sepsis-Induced Lung Injury
The S1P-induced protection of endothelial barrier function in LPS-induced lung injury suggests a role for S1P-metabolizing enzymes in lung injury and repair. Circulating and cellular S1P concentrations are regulated by the synthesis and catabolism of S1P (15, 30, 43). The availability of Sph is a key event in the intracellular generation of S1P, and Sph is derived either from ceramides through ceramidases or from circulating plasma S1P through ecto-LPPs (9, 17, 18). Recent studies showed that human lung ECs have the ability to use exogenously added S1P to generate intracellular S1P by lipid phosphate phosphatases (18). In addition to these two pathways, S1P can also be generated in plasma by the lysophospholipase D/autotaxin–mediated hydrolysis of sphingosylphosphorylcholine (44). However, whether this pathway provides a major source of plasma S1P remains unclear. Thus, targeting SphKs, S1PPases, LPPs, and S1PL represent novel therapeutic approaches with the potential to minimize or ameliorate lung inflammation and injury.
Role Of Sph Kinases 1 And 2 In Acute And Subacute Lung Injury
The role of SphKs in lung inflammation and injury is somewhat controversial. The loss of SphK1 or SphK2 expression in mice exerted no significant effect on inflammatory responses, and normal neutrophil function was observed in SphK1 and SphK2 knockout mice. However, accelerated bacterial lung infection in SphK2, but not in SphK1, knockout mice compared with wild-type control mice was observed (45). The inhibition of SphK1 expression using an antisense or a SphK inhibitor such as N,N-dimethyl-Sph attenuated neutrophil activation, chemotaxis, and lung permeability (46), and disruption of the SphK1 gene in mice exerted no effect on lymphocyte trafficking and lymphocyte distribution (47). The LPS challenge of C57BL/6 wild-type mice differentially up-regulated SphK1 and SphK2 expression levels. However, SphK1−/− mice were more susceptible to LPS-induced lung injury compared with wild-type mice, whereas the overexpression of SphK1 (wild-type) delivered by an adenoviral vector to the lungs protected SphK1−/− mice from lung injury and blunted the severity of the LPS response (48). SphK1 was up-regulated in stimulated human phagocytes and in peritoneal phagocytes of patients with severe sepsis, and the inhibition of SphK1 with siRNA protected mice against sepsis-induced proinflammatory responses (49). In that study, LPS challenge or cecal ligation puncture increased the mortality rate in SphK1−/− mice, yet it was suggested that this phenomenon represents an “adaptive compensation during development” that contradicts several earlier studies demonstrating an anti-inflammatory role for Sphk1 in ALI (49). Because Sphk1 regulates S1P production, macrophages with an increased expression of Sphk1 should generate higher concentrations of S1P. However, the reported results in Sphk1 knockdown experiments are quite contradictory (49). Recently, a novel antimycobacterial role for Sphk1 was reported in macrophages wherein SphK1 knockdown increased sensitivity to Mycobacterium smegmatis infection (50). In phagocytes, SphK1 regulated C5L2 and CD88 expression, and dampened inflammatory responses to endotoxin (51). Therefore, additional ALI models are necessary to determine whether SphK1 has a protective or proinflammatory role in lung injury and inflammation. In contrast to the LPS-induced lung injury model, SphK1 deficiency protected mice from hyperoxia-induced lung inflammation and injury (Viswanathan Natarajan and colleagues, unpublished data). Thus, the role of SphK1 in lung inflammation and injury might depend on the type of insult and the degree of oxidative stress, because increased S1P modulates the generation of reactive oxygen species (ROS) via nicotinamide adenine dinucleotide phosphate–reduced oxidase activation.
In addition to ALI, SphK1 seems to play a role in sub-ALI, such as radiation-induced lung injury (RILI). The thoracic radiation of mice (25 Gy) enhanced the expression of SphK1 and SphK2 in mouse lungs after 6 weeks of treatment and increased the ratio of ceramide to S1P and dihydro-S1P in plasma, BAL fluid, and lung tissue (11). SphK1−/− mice exhibited a higher susceptibility to RILI after 6 weeks of treatment, indicating a protective role for SphK1 against RILI (11). However, pretreatment with myriocin, an inhibitor of SPT, decreased inflammation and fibrogenesis at 18 weeks after irradiation (52). The inhibition of SPT also decreased radiation-induced SphK activity in the lung, which modulated the concentrations of S1P/dihydro-S1P in lung tissue and the circulation (52). Interestingly, simvastatin, a 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor, attenuated RILI in the mouse model by modulating the expression of SphK and S1PL proteins and the concentrations of ceramide to S1P/dihydroS1P in lung tissue (53). These results support the notion that S1P and S1P-metabolizing enzymes offer potential therapeutic targets against RILI.
S1pl Deficiency Protects Against Lps-Induced ALI
Current evidence suggests a role for S1PL in normal development, reproduction, cell survival, cancer, and immunity (19). S1PL function seems to be critical for mammalian survival, because S1P lyase knockout (Sgpl1−/−) mice do not survive beyond a couple of weeks after birth, and exhibit vascular abnormalities (54). S1PL deficiency elevated S1P, Sph, ceramide, and SM concentrations in the serum and liver (55), and produced a proinflammatory response by impairing neutrophil trafficking (56). In Sgpl1+/− mice, LPS challenge increased lethality, serum concentrations of TNF-α, monocyte chemotactic protein 1 (MCP-1), and IL-6, and sphingoid bases compared with Sgpl1+/+ mice (57), suggesting a detrimental effect of high-circulating S1P concentrations. The genetic and chemical inhibition of S1PL with the inhibitors 2-acetyl-4 (5)-[1R,2S,3R,4- tetrahydroxybutyl]-imidazole (THI) and LX2931 increased circulating and tissue S1P concentrations, reduced peripheral lymphocytes, and alleviated inflammatory responses in animal models of autoimmunity (58). A complete deficiency of S1PL in mice resulted in lesions in the lung, heart, urinary system, and bone, as well as T-cell depletion in the blood, thymus, spleen, and lymph nodes, which was attributed to very high circulating S1P concentrations (58). However, the partial restoration of S1PL activity in humanized knock-in mice harboring one (Sgpl1H/−) or two (Sgpl1H/H) alleles of human Sgpl1 offered protection from the lethal lymphoid lesions that developed in Sgpl1−/− mice (58).
A novel role for S1PL and intracellularly generated S1P in protecting against LPS-induced ALI was recently demonstrated in vivo and in vitro (59). An intratracheal instillation of LPS (5 mg/kg) to mice enhanced lung S1PL expression, decreased S1P concentrations in lung tissue, and induced lung injury. Sgpl1+/− mice exhibited increased S1P concentrations in lung tissue and BAL fluid, with reduced lung injury and inflammation in response to LPS challenge. Furthermore, the reduction of S1PL activity by an oral administration of THI showed a direct correlation between elevated S1P concentrations in lung tissue and BAL fluid and reduced concentrations of neutrophils and IL-6 in mice receiving LPS intratracheally (59). The in vitro treatment of human lung microvascular ECs with LPS resulted in reduced concentrations of intracellular S1P and increased mRNA and protein expression of S1PL. The down-regulation of S1PL expression by small interfering RNA (siRNA) increased S1P concentrations in the cells and medium, attenuated the LPS-mediated phosphorylation of p38 mitogen-activated protein kinase (MAPK) and inhibitor of κ B (I-κB), and decreased IL-6 secretion, whereas the overexpression of S1PL enhanced the LPS-induced phosphorylation of p38 MAPK and I-κB, and enhanced IL-6 secretion. S1PL siRNA was more effective than exogenous S1P at attenuating LPS-induced IL-6 secretion. Furthermore, S1PL siRNA attenuated LPS-induced endothelial barrier disruption by inducing the activation and redistribution of Rac1 to the cell periphery. Some controversy persists regarding the role of S1PL-generated metabolites, namely, ethanolamine phosphate and trans–2-hexadecenal, in regulating physiological responses such as mitogenesis, inflammation, and apoptosis. In murine F9 embryonic carcinoma and Henrietta Lacks (HeLa) cells, the overexpression of SphK1 enhanced DNA synthesis. However, in Sgpl1−/− null cells or Sgpl1−/− null cells overexpressing SphK1, no effect on mitogenesis was evident, suggesting that the products of the S1PL pathway, and not S1P itself, stimulated mitogenesis (21, 60). The addition of trans–2-hexadecenal to human embryonic kidney293 (HEK293), National Institutes of Health 3-day transfer, inoculum 3 × 10,000 (NIH 3T3), and HeLa cells induced a cytoskeletal reorganization and apoptosis that was dependent on c-Jun N-terminal kinase (JNK) activation and c-Jun phosphorylation (61). In a recent study, trans–2-hexadecenal was shown to react readily with deoxyguanosine and DNA to form aldehyde-derived DNA adducts that may have potentially mutagenic consequences or trigger a previously unrecognized DNA damage response in living cells (62). Because both ethanolamine phosphate and trans–2-hexadecenal were added exogenously, it remains unclear whether intracellularly generated metabolites of S1P by S1PL exhibit similar physiological responses.
Sm Synthase 2 And ALI
Sphingomyelin synthase (SMS) 1 and 2 catalyze the transfer of phosphorylcholine from phosphatidylcholine to ceramide and generate SM, a major component of all mammalian cell membranes. Recent studies demonstrated that SMS2 deficiency attenuates NF-κB signaling, thereby suggesting a role for ceramide in NF-κB signal transduction (63). However, ceramide also inhibits NF-κB activation (64), indicating a differential role for ceramide in NF-κB activation in different cell types. SMS2−/− mice treated with LPS showed decreased inflammation, cytokine induction, and lung injury compared with SMS2+/+ wild-type control mice (65). Furthermore, SMS2 depletion attenuated LPS-induced p38 MAPK and JNK activation and the transcriptional activity of NF-κB in human pulmonary artery endothelial cells, suggesting that blocking SM synthesis regulates endotoxin-induced inflammatory responses in a murine model of ALI. However, the effect of SMS2 knockdown on S1P, sphingosine, and ceramide concentrations in lung tissue and plasma was not determined (65).
Limitations Of S1P Therapy In ALI
Although S1P infusion has proven beneficial against LPS-induced lung injury in animal models (35, 39), it is an endogenous bioactive lipid that exerts pleiotropic effects, and some of these effects are likely to limit the usefulness of S1P in minimizing ALI. For example, an intravascular administration of S1P decreases the severity of ALI. However, intratracheal administrations can produce pulmonary edema through disruption of the epithelial/endothelial barrier via the ligation of S1P1 or S1P3. In human lung ECs, high concentrations of S1P (> 10 μM) can disrupt EC monolayer integrity in vitro through the ligation of S1P3 and subsequent activation of Rho, suggesting a limited therapeutic window for S1P in barrier enhancement (31). S1P also exhibits well-described cardiac toxicity (bradycardia) through the activation of S1P3 in the heart (66), directly stimulates the contraction of human airway and bronchial smooth muscle cells (67), and increases airway hyperresponsiveness in allergen-challenged mice (68), suggesting a potential for S1P to exacerbate airway obstruction in patients with asthma.
FTY720 And FTY720-P As Barrier-Protective And Anti-Inflammatory Agents
Given the limitations of S1P as a therapeutic agent in ALI, considerable interest has arisen in the biologic effects of a structurally similar compound, FTY720 (Figure 4), a synthetic derivative of the fungal metabolite myriocin. FTY720 has attracted a great deal of clinical interest as an immunosuppressive agent, and in fact was approved by the United States Food and Drug Administration in 2010 for the treatment of multiple sclerosis (70). In addition to its immunomodulatory effects, FTY720 also decreased vascular permeability, both in vivo (39, 70) and in vitro (71). For example, a single intraperitoneal injection of FTY720 significantly attenuated murine pulmonary injury after LPS administration (35). Interestingly, although lower doses of FTY720 (0.01–1 μM) enhanced endothelial barrier function in human umbilical vein endothelial cells (HUVECs), higher concentrations of FTY720 (10–100 μM) induced irreversible barrier breakdown and apoptosis (72). Similarly, low concentrations of FTY720 (0.1 mg/kg) reduced lung permeability in mechanically ventilated mice. However, higher concentrations (2 mg/kg) increased pulmonary leakage and apoptosis in ventilated mice, without affecting permeability in nonventilated mice (72). Because nonphosphorylated FTY720 demonstrates a low affinity for S1P receptors (73), current concepts related to mode of action for FTY720 invoke the phosphorylation of FTY720 in situ by SphK2 to produce the S1P analogue (S)-FTY720-P (74), thereby enhancing an affinity for the S1P family of receptors, and particularly S1P1 and S1P3. The phosphorylation of FTY720 to FTY720-P occurs rapidly both in vitro and in vivo (74). However, approximately 25% of FTY720 remains in a nonphosphorylated state in patients (75). Furthermore, FTY720-P reversed vascular endothelial growth factor–induced transcellular permeability in murine embryonic ECs (71). These investigations indicate that the FTY720-mediated protection against EC barrier dysfunction and lung inflammation is complex and dose-sensitive, and appears to involve both nonphosphorylated and phosphorylated forms of FTY720.
Figure 4.
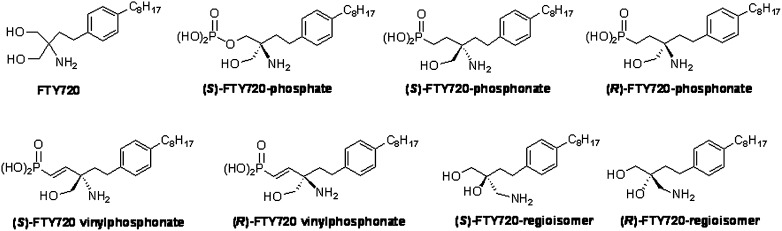
Structures of 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol (FTY720) and FTY720 analogues. The chemical structures of FTY720, the (R) and (S) stereoisomers of FTY720 phosphate, FTY720 phosphonate, and FTY720 vinylphosphonate are illustrated.
Mechanisms Of Barrier Regulation By FTY720 And FTY720-P
The mechanisms of action by FTY720 and FTY720-P in preventing vascular leakage remain unclear. FTY720, unlike S1P, appears to internalize and down-regulate S1P1 signaling, instead of activating this pathway. In contrast to S1P, FTY720 enhances EC barrier function without rapidly increasing intracellular calcium or cortical actin structure, or requiring the expression of proteins integral to the generation of the cortical actin ring (e.g., Rac and cortactin) (70). Furthermore, these studies suggest that FTY720 enhances EC barrier function via a novel S1P1-independent mechanism, because abrogating S1P1 expression using specific siRNA failed to block this effect, whereas embryonic ECs cultured from S1P1−/− mice retained the ability to mount a FTY720-induced barrier-enhancing response (70). Because pertussis toxin completely abolished this effect (70), Gi protein–coupled receptors (GPCRs) appear to play a key role in this FTY720-conferred EC barrier enhancement. GPCRs comprise a large and diverse family of receptors with varying homologies to the S1P receptors that could potentially participate in transducing FTY720 responses (76). For example, FTY720 interacts with the cannabinoid family of GPCRs (77), but the primary cannabinoid receptors, CB1 and CB2, are not involved in the EC barrier–enhancing response (70). In addition, FTY720 (but not S1P) inhibits S1PL (78), cytosolic phospholipase A2 (79), and ceramide synthases (80, 81), and activates protein phosphatase 2A (82). In human lung ECs, FTY720 increased c-Abelson tyrosine kinase (c-Abl) tyrosine kinase activity, and c-Abl siRNA attenuated FTY720-dependent barrier enhancement (Figure 5) (83). However, although FTY720 increased the expression of protein phosphatase 2A, it did not alter FTY720-induced barrier enhancement (83). FTY720 also up-regulated the expression of EC junctional proteins β-catenin and zonula occludens protein 1 (ZO-1) (71), and it promoted adherens junction assembly (84). However, ECs treated with specific siRNAs against claudin-5 or ZO-1/ZO-2 did not alter FTY720-induced barrier enhancement, suggesting that adherens junction or tight junction proteins are not involved in FTY720-induced barrier enhancement (83). A new paradigm has been developed in which FTY720 appears to function as an S1P1 antagonist and exerts its observed inhibitory effects on lymphocyte circulation (85). In contrast to FTY720, FTY720-P increased [Ca2+]i in ECs, and induced cortical actin distribution to the cell periphery and focal adhesion activity via Rac1 (Figure 5). Further evidence in support of the FTY720-induced down-regulation of S1P1 derives from the ability of FTY720-P to induce the ubiquitination and proteosomal degradation of S1P1 in cultured ECs to a greater extent than observed with S1P (86).
Figure 5.
Comparative signaling pathways involved in endothelial cell barrier enhancement by FTY720 and FTY720-phosphate (P). Both FTY720 (left panel) and FTY720-P (right panel) potently increase endothelial cell (EC) barrier function in vitro when concentrations of less than 10 μM are used (higher concentrations or prolonged stimulation over hours to days may disrupt barrier function), but multiple aspects differ in the signaling pathways involved. FTY720-P rapidly induces a series of events similar to the actions of S1P to enhance barrier function, including S1P1 ligation, Gi-coupled signaling, lipid raft membrane platforms, increased intracellular Ca2+, Rac1 activation, and dynamic actin changes, producing increased cortical actin linked to adherens junction and focal adhesion complex formation and stabilization. However, FTY720-P also induces the ubiquitination and subsequent proteosomal degradation of barrier-promoting S1P1, eventually leading to increased permeability after prolonged exposure. EC barrier enhancement by FTY720 is slower in onset and may involve an alternative, but not yet identified, G protein–coupled receptor (GPCR) in addition to S1P1. Similar to FTY720-P, FTY720-induced barrier enhancement requires Gi and lipid raft–coupled signaling. However, no significant Ca2+ increase is observed in pulmonary ECs, nor does dramatic cytoskeletal rearrangement or cortical actin formation occur during the timeframe associated with maximal barrier effects. Tyrosine kinase activity is involved, and recent work indicates that c-Abl and FAK signaling are necessary for optimal barrier enhancement. Focal adhesion complexes also appear to participate in this process after FTY720. EC, endothelial cell; Ub, ubiquitination; PXN, paxillin; FAK, focal adhesion kinase; GIT, G protein–coupled receptor kinase interactor–1; Tyr, tyrosine; c-Abl, Abelson tyrosine kinase.
Limitations Of FTY720 And FTY720-P As Therapeutic Agents In ALI
Similar to S1P, FTY720 exhibits physiological responses that may limit its therapeutic utility in patients with life-threatening inflammatory diseases such as ALI. Its immunosuppressant effects may be detrimental to patients with ALI, many of whom exhibit sepsis as a triggering event (87). FTY720 induces bradycardia through its effects on S1P3, similar to the effects of S1P in animal models, and this induction of bradycardia has been confirmed in patients with ALI (87). Further, in a recent multiple sclerosis clinical trial, FTY720 increased rates of dyspnea and decreased lung function (lowered forced expiratory volume in 1 second) (88), perhaps mediated through mechanisms similar to those seen in the S1P-induced contraction of bronchial smooth muscle in mice (67). Importantly, FTY720-P induced the ubiquitination and proteasomal degradation of S1P1 in cultured ECs (84) and HEK293 cells (89). Prolonged exposure to FTY720 resulted in the down-regulation of the EC surface expression of S1P1 and decreased responses to S1P (90). An administration of FTY720 (0.5–5.0 mg/kg) to wild-type C57BL/6 mice induced a dose-dependent S1P1 degradation and an increase in vascular permeability (91). This in vivo barrier-disruptive effect of FTY720 is in contrast to its barrier-protective effect observed in vitro (37, 70, 83), suggesting differential responses in mouse and human endothelium. However, the in vivo effect in mice points to a direct link between S1P1 degradation and vascular leakage, which may account for the recent report of increased lung injury and mortality in bleomycin-injured mice receiving prolonged FTY720 treatment (91). In summary, these studies suggest that FTY720 itself is unlikely to be an optimal therapeutic agent for ALI.
FTY720 Phosphonates And Endothelial Barrier Function
As a result of these limitations of FTY720 and FTY720-P, significant interest has arisen in FTY720-P analogues and related compounds that may exert fewer side effects. Several groups have synthesized multiple derivatives of FTY720-P, including phosphonates (73, 92), phosphothioates (93), and 4(5)-phenylimidazole–containing (94) and conformationally constrained (95) analogues, primarily for the purposes of characterizing their S1P-receptor affinity and their ability to induce lymphopenia (96). A number of novel analogues of FTY720-P, namely, the (R)-enantiomers and (S)-enantiomers of FTY720 phosphonates (Figure 4) (97), have been partly evaluated in vitro and in vivo for protection against endothelial barrier dysfunction and pulmonary leakage in three murine models of lung injury. The (R)-enantiomers and (S)-enantiomers of FTY720 phosphonate and of their unsaturated derivative, FTY720-vinylphosphonate, in contrast to the (R)-enantiomers and (S)-enantiomers of the FTY720 regioisomers (in which the positions of the amino group and one of the hydroxyl groups are reversed), enhanced endothelial barrier integrity in human lung ECs (37). Consistent with these in vitro responses, in a murine model of lung injury, (S)-FTY720 vinylphosphonate significantly reduced LPS-induced vascular leakage and the infiltration of leukocytes into the alveolar space (37). Similarly, a prolonged administration of (S)-FTY720 vinylphosphonate significantly improved the survival of bleomycin-injured in mice (98). In a preclinical model of RILI, (S)-FTY720 vinylphosphonate, but not FTY720, conferred protection against radiation-induced pulmonary leakage and inflammation (11). In human pulmonary artery smooth muscle, breast cancer, and androgen-independent prostate cancer cells, (S)-FTY720 vinylphosphonate regulated SphK1 activity via the induction of proteasomal degradation of SphK1 (99). Furthermore, (S)-FTY720 vinylphosphonate did not activate S1P1–5, whereas the (R)-enantiomer was an agonist of S1P1 and a partial antagonist of S1P2 and S1P5 (100).
Mechanisms Of Barrier Protection By FTY720 Phosphonates
Compared with S1P and FTY720, very little is known about the potential mechanisms underlying the barrier protection promoted by FTY720 phosphonates. In vitro, (S)-FTY720 phosphonate maintained the basal expression of S1P1, in contrast to the significant reduction (> 50%) induced by S1P or FTY720 treatment (89), suggesting an inhibition of the ubiquitination-mediated degradation of S1P1 by (S)-FTY720-phosphonate (98). Furthermore, the recruitment of β-arrestin to S1P1 by (S)-FTY720 phosphonate was significantly lower than that via S1P or FTY720 treatment, indicating prolonged signaling through S1P1 by (S)-FTY720 phosphonate (98).
Limitations Of FTY720 Phosphonates As Therapeutic Agents In ALI
As already stated, FTY720 is approved by the United States Food and Drug Administration for the oral treatment of multiple sclerosis. In contrast to FTY720, studies with FTY720 phosphonates in preclinical animal models are limited, and its metabolism in vivo is unknown. Further studies on its cytotoxicity, pharmacokinetics, and efficacy in animal models are necessary to advance these phosphonate analogues to a Phase 1 trial in humans.
S1P Receptors In Lung Injury And Barrier Regulation
S1P elicits its cellular effects through a family of G protein–coupled S1P1–5 receptors, formerly known as endothelial differentiation gene receptors, which are expressed in various cell types, including endothelial cells (101). Although these receptors share significant homology and overlap in their biological functions, distinct receptor subtype spatial distributions, coupling to different G proteins, and differences in receptor-complex internalization and recycling may provide specificity for each of the S1P receptors (Figure 6). The deletion of S1P1 in mice is embryonically lethal (on Embryonic Days 12.5 and 14.5) (102), whereas S1P2 and S1P3 deletions appear to exert no discernible effect on normal phenotypes (103). However, S1P1/S1P2 double-knockout and S1P1/S1P2/S1P3 triple-null embryos showed a more severe vascular phenotype than did S1P1 knockout embryos, suggesting that the three S1P receptors cooperate to promote vascular development during embryonic angiogenesis (104). The evidence is overwhelming for the role of S1P/S1P1 in preventing the vascular leakage induced by many edemagenic agents, including LPS in the lung (35, 39, 72, 89, 91, 105, 106). Consistent with the barrier-protective role of S1P1, the pretreatment of wild-type mice with an S1P1 inverse agonist, Smith Kline and Beecham-649146 (SB-649146), or the use of S1P1+/− mice, reduced S1P/SEW2871-induced barrier protection after LPS challenge (106). Similar to the protective role of S1P1, S1P2-null mice and mice with a reduced expression of S1P3 (generated by treating with a specific siRNA against S1P3) also offered significant protection against LPS-induced barrier disruption and leakage, compared with wild-type mice (106). However, S1P2 seems to mediate enhanced vascular permeability in newborn mice exposed to a hypoxia-induced model of retinopathy (107) and a hydrogen peroxide–induced model of barrier dysfunction (103). In RILI, the roles of S1P2 and S1P3 in barrier regulation appear to be conflicting, as observed in a preclinical model of LPS-induced ALI. In a murine RILI model, the knockdown or reduced expression of S1P1, S1P2, and S1P3 increased susceptibility to lung injury (11). These results suggest a differential role for S1P1–3 in these two models of lung injury because of the differential transduction of signals via multimeric G proteins (Figure 7). In addition to the genetic engineering of S1P receptors, S1P-receptor agonists and antagonists could be useful in studying the roles of S1P1–5 in lung inflammation and injury. However, many agonists such as SEW2871 and 3-[[2-[4-phenyl-3-(trifluoromethyl)phenyl]-1-benzothiophen-5-yl]methylamino]propanoic acid (AUY954) for S1P1 exhibit poor water solubility, thereby limiting their use in animal models of lung inflammation and injury. A systematic study of S1P receptor–mediated signaling and the study of intracellular targets that regulate S1P concentrations in vascular cells may provide further insights into the mechanisms underlying the barrier function disruption seen in acute and subacute lung injury.
Figure 6.
Potential role of S1P receptors in cellular and biological processes. Extracellular S1P signals via G protein–coupled S1P receptors, and regulates a number of cellular and biological processes such as barrier integrity, barrier disruption, inflammation, migration, and angiogenesis in mammalian cells. S1PR, S1P receptor.
Figure 7.
Comparative signaling pathways involved in endothelial barrier integrity and dysfunction by S1P via S1P1 and S1P3. S1P enhances endothelial barrier functions that include S1P1 ligation, Gi-coupled signaling, lipid raft membrane platforms, increased intracellular Ca2+, Rac1 activation, Tiam 1, PAK1 and PI3K recruitment to lipid rafts, and dynamic actin changes, producing increased cortical actin, which is linked to adherens junction and focal adhesion complex formation and stabilization. However, the ligation of S1P to S1P3 enhances RhoGEF recruitment to lipid rafts, and Rho activation leads to cytoskeletal reorganization, decreased cortical actin, and barrier dysfunction. S1P, sphingosine 1–phosphate; Tiam 1, T-cell lymphoma invasion and metastasis factor–1; PAK, p21–activated kinase; GEF, guanine nucleotide exchange factor; MYPT1, myosin phosphatase–targeting subunit; PI3K, phosphatidylinositol 3–kinase.
Functional Polymorphism Of S1P Receptors And Sphk1 And Sphk2 And Their Association With ALI
Association studies on the functional consequences of single-nucleotide polymorphisms (SNPs) in S1P receptors, SphKs, S1PL, and sphingomyelinase that are linked to acute and subacute lung injury/inflammation are limited. The DNA sequencing of both African American and European American populations consisting of 378 control subjects and 218 cases of sepsis/ALI revealed that S1P3 promoter SNPs rs7022797 (−1899 T/G) and rs11137480 (−1785 G/C) in European Americans conferred decreased susceptibility to both severe sepsis and sepsis-induced ALI. Furthermore, S1P3 promoter SNPs −1899G and −1785C, singly or together, significantly decreased the luciferase promoter activity triggered by the TNF-α–induced binding of transcriptional factors caudal related homeobox1 (CDX1) and early B cell factor 1 (EBF1) to the S1PR3 promoter (108). Thus, multiple SNPs in S1P3 alter promoter activity and confer susceptibility to sepsis/ALI in multiethnic populations.
In silico analyses provide limited information on polymorphic variants in the genomic sequences of SphK1 and SphK2, and on linking the variations to ALI among various ethnic groups. The genotyping of patients with severe sepsis (African Americans, n = 75; European Americans, n = 143) and healthy control subjects (African Americans, n = 187; European Americans, n = 190) revealed 30 SphK1 SNP variants, including 20 novel SNPs with seven SphK1 tagging SNPs whose minor allele frequencies were equal to or greater than 5%. African American patients with the SNP rs3744037 CC genotype (exon 6, Tyr407Tyr) demonstrated greater odds of developing severe sepsis-induced ALI than did carriers of the T allele (odds ratio, 3.93; 95% confidence interval, 1.05–14.77; P = 0.05). A stronger association was found in European American patients, in whom the rs2247856 AA genotype (exon 2, Ala30Thr) demonstrated significantly greater odds of developing severe sepsis than did carriers of the G allele (odds ratio, 1.94; 95% confidence interval, 1.15–3.25; P = 0.013). The resequencing of the SphK2 gene and flanking sequences revealed 54 polymorphisms, and association data analysis revealed five data base single nucleotide polymorphism (dbSNPs) and one novel SNP, rs12610339, located in the promoter region that was significantly associated with sepsis and sepsis-induced ALI in the African American cohort for sepsis and ALI. The strength of the association with ALI suggests that the SphK2 gene may elicit multiple effects in patients with ALI (109). These initial polymorphism studies associated with the S1P3, SphK1, and SphK2 genes suggest a potential association between the SNPs and susceptibility to sepsis and ALI in African Americans and European Americans that should be validated with larger groups of control and patient populations.
S1P Homeostasis In ALI
S1P concentrations in circulation are higher compared with intracellular concentrations, and are tightly regulated by synthesis, secretion, and uptake by different cell types, including endothelial cells. Most likely, circulating S1P helps maintain endothelial barrier integrity under basal conditions. However, pathological conditions such as sepsis may alter S1P homeostasis and offset the critical balance from tight endothelial junctions to barrier dysregulation. In a murine model of ALI, an intratracheal instillation of LPS (5 mg/kg body weight for 24 hours) reduced S1P concentrations in lung tissue (S1P, fmol/nmol lipid phosphorous, vehicle, 296 ± 24; intratracheal LPS, 138 ± 16) and in plasma (S1P, fmol/nmol lipid phosphorous, vehicle, 1,126 ± 36; intratracheal LPS, 825 ± 29) that paralleled the increased expression of S1PL in lungs and lung inflammation and injury (59). Blocking S1PL activity by the administration of THI in mice increased S1P concentrations in lungs without altering plasma concentrations, and reduced LPS-induced lung inflammation (59). In a radiation model of RILI, the ceramide/S1P ratios in BAL, plasma, and lung tissue were significantly increased and remained elevated as RILI progressed throughout a 12-week period of RILI assessment (11). Intriguingly, plasma concentrations of S1P from patients with ALI and sepsis were significantly decreased compared with control subjects. However, no significant differences were evident between African American and Caucasian populations in terms of the association of ALI or sepsis with circulating plasma S1P concentrations (Joe G. N. Garcia and colleagues, unpublished data). An analysis of S1P concentrations from control, sepsis, and sepsis-induced ALI patients revealed a potential correlation between lung injury/edema and a decrease in circulating plasma S1P concentrations (110). Whether lower plasma S1P concentrations serve as a potential biomarker in sepsis and ALI pathologies remains to be confirmed.
Conclusions And Future Directions
Acute and subacute lung injuries share profound increases in vascular permeability as a key element and a common pathway driving the increased morbidity and mortality in these disorders. Recent studies suggest that the bioactive sphingolipid S1P and its receptors, and enzymes of S1P metabolism, are important modulators of lung injury and inflammation. S1P is a potent angiogenic factor, enhances EC integrity in vitro, and is a robust in vivo modulator of the vascular permeability and alveolar flooding induced by endotoxemia. Concentrations of S1P are regulated by its synthesis, catalyzed by SphKs, and its degradation, mediated by S1PL, S1PPases, and LPPs. In an LPS-induced murine model of lung injury inflammation, the down-regulation of SphK1 potentiated ALI, whereas the knockdown of S1PL offered partial protection, suggesting that S1PL may constitute a potential therapeutic target in ameliorating sepsis-induced pulmonary edema. S1P-mediated endothelial responses are driven via G protein–coupled S1P receptors, and current studies using knockout mice and S1P-receptor antagonists show that S1P1 is the primary S1P receptor effecting EC barrier protection, whereas S1P3 and to a lesser extent S1P2 are barrier-disruptive. Although S1P exerts a potentially beneficial effect in restoring endothelial barrier integrity and suppressing the pulmonary leakage attributable to sepsis, there are limitations to the use of S1P as a therapeutic agent in a clinical setting. Among several S1P analogues evaluated for their efficacy in barrier protection against acute and subacute lung injury animal models, (S)-FTY720 phosphonate produced rapid and increased endothelial barrier function in vitro and decreased LPS-induced and radiation-induced lung permeability in vivo. Association studies in Caucasian and African American ALI cohorts revealed novel SNPs in S1P receptors and S1P-metabolizing enzymes. Future studies on functional polymorphisms in sphingolipid pathway genes should provide new approaches to the development of drugs against ALI.
Although the targeting of S1P receptors and metabolizing enzymes is promising in preclinical animal models of sepsis-induced lung injury, the outcome of S1P targeting to minimize alveolar flooding and pulmonary leakage in patients with ALI needs to be evaluated. The development of specific small-molecule agonists and antagonists of S1P receptors and S1P metabolizing enzymes is critical in modulating endothelial barrier dysfunction. This would require not only the development of selective and potent inhibitors of S1P receptors and metabolizing enzymes with minimal cytotoxicity, but the pinpoint targeting of these agents to specific cell types in the lung. Attempts to target S1P receptors and metabolizing enzymes simultaneously should provide a synergistic approach to conferring protection against ALI. Furthermore, the identification of novel S1P-signaling biomarkers and a systems biology approach will greatly facilitate the development of novel S1P-based therapies for patients with severe inflammatory lung injury.
Acknowledgments
Acknowledgments
The authors thank Dr. Prasad Kanteti for helpful comments and for proofreading the manuscript.
Footnotes
This work was supported by National Institutes of Health grants PPG HL58064 and PPG HL98050 (V.N., J.G.N.G, S.M.D, and J.R.J.).
Originally Published in Press as DOI: 10.1165/rcmb.2012-0411TR on February 8, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Goss CH, Brower RG, Hudson LD, Rubenfeld GD. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31:1607–1611. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 2.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr, Hoffman E, Hubmayr RD, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003;167:1027–1035. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 3.Wynn Ta. Integrating mechanisms in pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merrill AH, Schmelz E-M, Dillehay DL, Spiegel S, Shayman JA, Schroeder JJ, Riley KA, Wang E. Sphingolipids—the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol Appl Pharmacol. 1997;142:208–225. doi: 10.1006/taap.1996.8029. [DOI] [PubMed] [Google Scholar]
- 5.Uhlig S. Gulbins: sphingolipids in the lungs. Am J Respir Crit Care Med. 2008;178:1100–1114. doi: 10.1164/rccm.200804-595SO. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Uhlig S. The role of sphingolipids in respiratory disease. Ther Adv Respir Dis. 2011;5:325–344. doi: 10.1177/1753465811406772. [DOI] [PubMed] [Google Scholar]
- 7.Patwardhan GA, Liu YY. Sphingolipids and expression regulation of genes in cancer. Prog Lipid Res. 2011;50:104–114. doi: 10.1016/j.plipres.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ratajczak MZ, Kim C, Wu W, Shin DM, Bryndza E, Kucia M, Ratajczak J. The role of innate immunity in trafficking of hematopoietic stem cells: an emerging link between activation of complement cascade and chemotactic gradients of bioactive sphingolipids. Adv Exp Med Biol. 2012;946:37–54. doi: 10.1007/978-1-4614-0106-3_3. [DOI] [PubMed] [Google Scholar]
- 9.Bikman BT, Summers SA. Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest. 2011;121:4222–4230. doi: 10.1172/JCI57144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gangoiti P, Camacho L, Arana L, Ouro A, Granado MH, Brizuela L, Casas J, Fabrias G, Abad JL, Delgado A, et al. Control of metabolism and signaling of simple bioactive sphingolipids: implications in disease. Prog Lipid Res. 2010;49:316–334. doi: 10.1016/j.plipres.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Mathew B, Jacobson JR, Berdyshev EV, Huang Y, Sun X, Zhao Y, Gerhold LM, Siegler J, Evanovski C, Wang T, et al. Critical role of sphingolipid pathway components in murine radiation-induced lung injury: protection by sphingosine 1–phosphate analogues. FASEB J. 2011;25:3388–3400. doi: 10.1096/fj.11-183970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merrill AH., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem. 2002;277:25843–25846. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- 13.Sugiura M, Kono K, Liu H, Shimizugawa T, Minekura H, Spiegel S, Kohama T. Ceramide kinase, a novel lipid kinase: molecular cloning and functional characterization. J Biol Chem. 2002;277:23294–23300. doi: 10.1074/jbc.M201535200. [DOI] [PubMed] [Google Scholar]
- 14.Canals D, Perry DM, Jenkins RW, Hannun YA. Drug targeting of sphingolipid metabolism: sphingomyelinases and ceramidases. Br J Pharmacol. 2011;163:694–712. doi: 10.1111/j.1476-5381.2011.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piston SM. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem Sci. 2011;36:97–107. doi: 10.1016/j.tibs.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa C, Kihara A, Gokoh M, Igarashi Y. Identification and characterization of a novel human sphingosine-1–phosphate phosphohydrolase, hSPP2. J Biol Chem. 2003;278:1268–1272. doi: 10.1074/jbc.M209514200. [DOI] [PubMed] [Google Scholar]
- 17.Brindley DN, Pilquil C. Lipid phosphate phosphatases and signaling. J Lipid Res. 2009;50:S225–S230. doi: 10.1194/jlr.R800055-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Usatyuk PV, Gorshkova I, He D, Watkins T, Garcia JGN, Saatian B, Brindley DN, Bittman R, Berdyshev EV, et al. Regulation of intracellular generation of sphingosine-1–phosphate by lipid phosphate phosphatase–1 and sphingosine kinase 1 in human lung endothelial cells. J Biol Chem. 2007;282:14165–14177. doi: 10.1074/jbc.M701279200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandhuvula P, Saba JD. Sphingosine-1–phosphate lyase in immunity and cancer: silencing the siren. Trends Mol Med. 2007;13:210–217. doi: 10.1016/j.molmed.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Berdyshev EV, Gorshkova I, Usatyuk P, Kalari S, Zhao Y, Pyne NJ, Pyne S, Sabbadini RA, Garcia JGN, Natarajan V. Intracellular S1P generation is essential for S1P-induced motility of human lung endothelial cells: role of sphingosine kinase 1 and S1P lyase. PLoS ONE. 2011;6:e16571. doi: 10.1371/journal.pone.0016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kariya Y, Kihara A, Ikeda M, Kikuchi F, Nakamura S, Hashimoto S, Choi CH, Lee YM, Igarashi Y. Products by the sphingosine kinase/sphingosine 1–phosphate (S1P) lyase pathway but not S1P stimulate mitogenesis. Genes Cells. 2005;10:605–615. doi: 10.1111/j.1365-2443.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- 22.Perrotta C, Clementi E. Biological roles of acid and neutral sphingomyelinases and their regulation by nitric oxide. Physiology (Bethesda) 2010;25:64–71. doi: 10.1152/physiol.00048.2009. [DOI] [PubMed] [Google Scholar]
- 23.Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268:15523–15530. [PubMed] [Google Scholar]
- 24.Westwick JK, Bielawska AE, Dbaibo G, Hannun YA, Brenner DA. Ceramide activates the stress-activated protein kinases. J Biol Chem. 1995;270:22689–22692. doi: 10.1074/jbc.270.39.22689. [DOI] [PubMed] [Google Scholar]
- 25.Yatomi Y. Plasma sphingosine-1–phosphate metabolism and analysis. Biochim Biophys Acta. 2008;1780:606–611. doi: 10.1016/j.bbagen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1–phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 27.Tani M, Sano T, Ito M, Igarashi Y. Mechanisms of sphingosine and sphingosine 1–phosphate generation in human platelets. J Lipid Res. 2005;46:2458–2467. doi: 10.1194/jlr.M500268-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1–phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung T, Crilly KS, Anderson WH, Mukherjee JJ, Kiss Z. ATP-dependent choline phosphate–induced mitogenesis in fibroblasts involves activation of pp70 S6 kinase and phosphatidylinositol 3′–kinase through an extracellular site: synergistic mitogenic effects of choline phosphate and sphingosine 1–phosphate. J Biol Chem. 1997;272:3064–3072. doi: 10.1074/jbc.272.5.3064. [DOI] [PubMed] [Google Scholar]
- 30.Pyne S, Pyne NJ. Sphingosine 1–phosphate signalling in mammalian cells. Biochem J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1–phosphate. Microvasc Res. 2009;77:39–45. doi: 10.1016/j.mvr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gimbrone MA, Jr, Aster RH, Cotran RS, Corkerry J, Jandl JH, Folkman J. Preservation of vascular integrity in organs perfused in vitro with a platelet-rich medium. Nature. 1969;222:33–36. doi: 10.1038/222033a0. [DOI] [PubMed] [Google Scholar]
- 33.Schaphorst KL, Chiang E, Jacobs KN, Zaiman A, Natarajan V, Wigley F, Garcia JG. Role of sphingosine 1–phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am J Physiol Lung Cell Mol Physiol. 2003;285:L258–L267. doi: 10.1152/ajplung.00311.2002. [DOI] [PubMed] [Google Scholar]
- 34.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1–phosphate promotes endothelial barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, Garcia JG. Sphingosine 1–phosphate reduces leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med. 2004;170:987–993. doi: 10.1164/rccm.200405-684OC. [DOI] [PubMed] [Google Scholar]
- 36.Okazaki M, Kreisel F, Richardson SB, Kreisel D, Krupnick AS, Patterson GA, Gelman AE. Sphingosine 1–phosphate inhibits ischemia reperfusion injury following experimental lung transplantation. Am J Transplant. 2007;7:751–758. doi: 10.1111/j.1600-6143.2006.01710.x. [DOI] [PubMed] [Google Scholar]
- 37.Camp SM, Bittman R, Chiang ET, Moreno-Vinasco L, Mirzapoiazova T, Sammani S, Lu X, Sun C, Harbeck M, Roe M, et al. Synthetic analogs of FTY720 [2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol] differentially regulate pulmonary vascular permeability in vivo and in vitro. J Pharmacol Exp Ther. 2009;331:54–64. doi: 10.1124/jpet.109.153544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Usatyuk PV, He D, Bindokas V, Berdyshev EV, Garcia JGN, Natarajan V. Photolysis of intracellular caged sphingosine-1–phosphate causes G-protein dependent and independent activation of cell signaling pathways in endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L840–L850. doi: 10.1152/ajplung.00404.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1–phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 40.Jacobson JR, Garcia JG. Novel therapies for microvascular permeability in sepsis. Curr Drug Targets. 2007;8:509–514. doi: 10.2174/138945007780362719. [DOI] [PubMed] [Google Scholar]
- 41.Ugolev Y, Berdichevsky Y, Weinbaum C, Edgar P. Dissociation of Rac1(GDP)oRhoGDI complexes by the cooperative action of anionic liposomes containing phosphatidylinositol 3,4,5-trisphosphate, Rac guanine nucleotide exchange factor, and GTP. J Biol Chem. 2008;283:22257–22271. doi: 10.1074/jbc.M800734200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chae YC, Kim JH, Kim KL, Kim HW, Lee HY, Heo WD, Meyer T, Suh P-G, Ryu SH. Phospholipase D activity regulates integrin-mediated cell spreading and migration by inducing GTP–Rac translocation to the plasma membrane. Mol Biol Cell. 2008;19:3111–3123. doi: 10.1091/mbc.E07-04-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Veldhoven PP. Sphingosine-1–phosphate lyase. Methods Enzymol. 2000;311:244–254. doi: 10.1016/s0076-6879(00)11087-0. [DOI] [PubMed] [Google Scholar]
- 44.Nakanaga K, Hama K, Aoki J. Autotaxin: an LPA producing enzyme with diverse functions. J Biochem. 2010;148:13–24. doi: 10.1093/jb/mvq052. [DOI] [PubMed] [Google Scholar]
- 45.Zemann B, Urtz N, Reuschel R, Mechtcheriakova D, Bornancin F, Badegruber R, Baumruker T, Billich A. Normal neutrophil functions in sphingosine kinase Type 1 and 2 knockout mice. Immunol Lett. 2007;109:56–63. doi: 10.1016/j.imlet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Vlasenko LP, Melendez AJ. A critical role for sphingosine kinase in anaphylatoxin-induced neutropenia, peritonitis, and cytokine production in vivo. J Immunol. 2005;174:6456–6461. doi: 10.4049/jimmunol.174.10.6456. [DOI] [PubMed] [Google Scholar]
- 47.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 48.Wadgaonkar R, Patel V, Grinkina N, Romano C, Liu J, Zhao Y, Sammani S, Garcia JGN, Natarajan V. Differential regulation of sphingosine kinases 1 and 2 in lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;296:L603–L613. doi: 10.1152/ajplung.90357.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puneet P, Yap CT, Wong L, Lam Y, Koh DR, Moochhala S, Pfeilschifter J, Huwiler A, Melendez AJ. SphK1 regulates pro-inflammatory responses associated with endotoxin and polymicrobial sepsis. Science. 2010;328:1290–1294. doi: 10.1126/science.1188635. [DOI] [PubMed] [Google Scholar]
- 50.Prakash H, Luth A, Grinkina N, Holzer D, Wadgaonkar R, Gonzalez AP, Anes E, Kleuser B. Sphingosine kinase–1 (SphK-1) regulates Mycobacterium smegmatis infection in macrophages. PLoS ONE. 2010;5:e10657. doi: 10.1371/journal.pone.0010657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bachmaier K, Guzman E, Kawamura T, Gao X, Malik AB. Sphingosine kinase 1 mediation of expression of the anaphylatoxin receptor C5L2 dampens the inflammatory response to endotoxin. PLoS ONE. 2012;7:e30742. doi: 10.1371/journal.pone.0030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gorshkova I, Zhou T, Mathew B, Jacobson JR, Takekoshi D, Bhattacharya P, Smith B, Aydogan B, Weichselbaum RR, Natarajan V, et al. Inhibition of serine palmitoyltransferase delays the onset of radiation-induced pulmonary fibrosis through the negative regulation of sphingosine kinase–1 expression. J Lipid Res. 2011;53:1553–1568. doi: 10.1194/jlr.M026039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathew B, Huang Y, Jacobson JR, Berdyshev E, Gerhold LM, Wang T, Moreno-Vinasco L, Lang G, Zhao Y, Chen CT, et al. Simvastatin attenuates radiation-induced murine lung injury and dysregulated lung gene expression. Am J Respir Cell Mol Biol. 2011;44:415–422. doi: 10.1165/rcmb.2010-0122OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmahl J, Raymond CS, Soriano P. PDGF signaling specificity is mediated through multiple immediate early genes. Nat Genet. 2007;39:52–60. doi: 10.1038/ng1922. [DOI] [PubMed] [Google Scholar]
- 55.Bektas M, Allende ML, Lee BG, Chen W, Amar MJ, Remaley AT, Saba JD, Proia RL. Sphingosine 1–phosphate lyase deficiency disrupts lipid homeostasis in liver. J Biol Chem. 2010;285:10880–10889. doi: 10.1074/jbc.M109.081489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allende ML, Bektas M, Lee BG, Bonifacino E, Kang J, Tuymetova G, Chen W, Saba JD, Proia RL. Sphingosine 1–phosphate deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J Biol Chem. 2011;286:7348–7358. doi: 10.1074/jbc.M110.171819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oravecz T, Donoviel MS, Anderson SJ, Carson K, Sun W, Swaffield J, Liu Q, Kimball SD, Piggott JR, Zambrowicz BP, et al. Genetic and chemical inhibition of sphingosine 1–phosphate lyase results in peripheral lymphopenia and alleviates disease development in animal models of inflammation and autoimmunity [abstract] Blood. 2007;110:2292. [Google Scholar]
- 58.Vogel P, Donoviel MS, Read R, Hansen GM, Hazlewood J, Anderson SJ, Sun W, Swaffield J, Oravecz T. Incomplete inhibition of sphingosine 1–phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PLoS ONE. 2009;4:e4112. doi: 10.1371/journal.pone.0004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Y, Gorshkova IA, Berdyshev E, Ma W, He D, Su Y, Usatyuk PV, Pendyala S, Fu P, Oskouian B, et al. Protection of LPS-induced murine acute lung injury and inflammation by sphingosine-1–phosphate lyase suppression. Am J Respir Cell Mol Biol. 2012;45:426–435. doi: 10.1165/rcmb.2010-0422OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kano-Sueoka T, Cohen DM, Yamaizumi Z, Nishimura S, Mori M, Fujiki H. Phosphoethanolamine as a growth factor of mammary carcinoma cell line of rat. Proc Natl Acad Sci USA. 1979;76:5741–5744. doi: 10.1073/pnas.76.11.5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar A, Byun H-S, Bittman R, Saba JD. The sphingolipid degradation product trans–2-hexadecenal induces cytoskeletal reorganization and apoptosis in a JNK-dependent manner. Cell Signal. 2011;23:1144–1152. doi: 10.1016/j.cellsig.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Upadhyaya P, Kumar A, Byun H-S, Bittman R, Saba JD. The sphingolipid degradation product trans–2-hexadecenal forms adducts with DNA. Biochem Biophys Res Commun. 2012;424:18–21. doi: 10.1016/j.bbrc.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hailemariam TK, Huan C, Li J, Roman C, Kalbfeisch M, Bui HH, Peake DA, Kuo M-S, Cao G, Wadgaonkar R, et al. Sphingomyelin synthase 2 deficiency attenuates NF-κB activation. Arterioscler Thromb Vasc Biol. 2008;28:1519–1526. doi: 10.1161/ATVBAHA.108.168682. [DOI] [PubMed] [Google Scholar]
- 64.Gamard CJ, Dbaibo GS, Liu B, Obeid LM, Hannun YA. Selective involvement of ceramide in cytokine-induced apoptosis: ceramide inhibits phorbol ester activation of nuclear factor kappaB. J Biol Chem. 1997;272:16474–16481. doi: 10.1074/jbc.272.26.16474. [DOI] [PubMed] [Google Scholar]
- 65.Gowda S, Yeang C, Wadgaonkar S, Anjum F, Grinkina N, Cutaia M, Jiang X-C, Wadgaonkar R. Sphingomyelin synthase 2 (SMS2) deficiency attenuates LPS-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;300:L430–L440. doi: 10.1152/ajplung.00208.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Forrest M, Sun SY, Hajdu R, Bergstrom J, Card D, Doherty G, Hale J, Keohane C, Meyers C, Milligan J, et al. Immune cell regulation and cardiovascular effects of sphingosine 1–phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J Pharmacol Exp Ther. 2004;309:758–768. doi: 10.1124/jpet.103.062828. [DOI] [PubMed] [Google Scholar]
- 67.Rosenfeldt HM, Amrani Y, Watterson KR, Murthy KS, Panettieri RA, Jr, Spiegel S. Sphingosine-1–phosphate stimulates contraction of human airway smooth muscle cells. FASEB J. 2003;17:1789–1799. doi: 10.1096/fj.02-0836com. [DOI] [PubMed] [Google Scholar]
- 68.Roviezzo F, Lorenzo A, Bucci M, Brancaleone V, Vellecco V, De Nardo M, Orlotti D, De Palma R, Rossi F, D’Agostino B, et al. Sphingosine-1–phosphate/sphingosine kinase pathway is involved in mouse airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2007;36:757–762. doi: 10.1165/rcmb.2006-0383OC. [DOI] [PubMed] [Google Scholar]
- 69.Strader CR, Pearce CJ, Oberlies NH. Fingolimod (FTY720): a recently approved multiple sclerosis drug based on a fungal secondary metabolite. J Nat Prod. 2011;74:900–907. doi: 10.1021/np2000528. [DOI] [PubMed] [Google Scholar]
- 70.Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, Brinkmann V, Claffey K, Hla T. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor–induced vascular permeability. J Biol Chem. 2003;278:47281–47290. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 71.Dudek SM, Camp SM, Chiang ET, Singleton PA, Usatyuk PV, Zhao Y, Natarajan V, Garcia JG. Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor. Cell Signal. 2007;19:1754–1764. doi: 10.1016/j.cellsig.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muller HC, Hocke AC, Hellwig K, Gutbier B, Peters H, Schnorock SM, Tschernig T, Schmiedl A, Hippenstiel S, N’Guessan PD, et al. The sphingosine-1–phosphate receptor agonist FTY720 dose dependently affected endothelial integrity in vitro and aggravated ventilator-induced lung injury in mice. Pulm Pharmacol Ther. 2011;24:377–385. doi: 10.1016/j.pupt.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 73.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, et al. Alteration of lymphocyte trafficking by sphingosine-1–phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 74.Billich A, Bornancin F, Devay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- 75.Kovarik JM, Schmouder RL, Slade AJ. Overview of FTY720 clinical pharmacokinetics and pharmacology. Ther Drug Monit. 2004;26:585–587. doi: 10.1097/00007691-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 76.Takeda S, Kadowaki S, Haga T, Takaesu H, Mitaku S. Identification of G protein–coupled receptor genes from the human genome sequence. FEBS Lett. 2002;520:97–101. doi: 10.1016/s0014-5793(02)02775-8. [DOI] [PubMed] [Google Scholar]
- 77.Paugh SW, Cassidy MP, He H, Milstien S, Sim-Selley LJ, Spiegel S, Selley DE. Sphingosine and its analog, the immunosuppressant 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol, interact with the CB1 cannabinoid receptor. Mol Pharmacol. 2006;70:41–50. doi: 10.1124/mol.105.020552. [DOI] [PubMed] [Google Scholar]
- 78.Bandhuvula P, Tam YY, Oskouian B, Saba JD. The immune modulator FTY720 inhibits sphingosine-1–phosphate lyase activity. J Biol Chem. 2005;280:33697–33700. doi: 10.1074/jbc.C500294200. [DOI] [PubMed] [Google Scholar]
- 79.Payne SG, Oskeritzian CA, Griffiths R, Subramanian P, Barbour SE, Chalfant CE, Milstien S, Spiegel S. The immunosuppressant drug FTY720 inhibits cytosolic phospholipase A2 independently of sphingosine-1–phosphate receptors. Blood. 2007;109:1077–1085. doi: 10.1182/blood-2006-03-011437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berdyshev EV, Gorshkova I, Skobeleva A, Bittman R, Lu X, Dudek SM, Mirzapoiazova T, Garcia JG, Natarajan V. FTY720 inhibits ceramide synthases and upregulates dihydrosphingosine-1–phosphate formation in human lung endothelial cells. J Biol Chem. 2009;284:5467–5477. doi: 10.1074/jbc.M805186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lahiri S, Park H, Laviad EL, Lu X, Bittman R, Futerman AH. Ceramide synthesis is modulated by the sphingosine analog FTY720 via a mixture of uncompetitive and noncompetitive inhibition in an acyl-CoA chain length–dependent manner. J Biol Chem. 2009;284:16090–16098. doi: 10.1074/jbc.M807438200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roberts KG, Smith AM, McDougal F, Carpenter H, Horan M, Neviani P, Powell JA, Thomas D, Guthridge MA, Perrotti D, et al. Essential requirement for PP2A inhibition by the oncogenic receptor c-KIT suggests PP2A reactivation as a strategy to treat c-KIT+ cancers. Cancer Res. 2010;70:5438–5447. doi: 10.1158/0008-5472.CAN-09-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang L, Chiang ET, Simmons JT, Garcia JGN, Dudek SM. FTY720-induced human pulmonary endothelial barrier enhancement is mediated by c-Abl. Eur Respir J. 2011;38:78–88. doi: 10.1183/09031936.00047810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singer II, Tian M, Wickham LA, Lin J, Matheravidathu SS, Forrest MJ, Mandala S, Quackenbush EJ. Sphingosine-1–phosphate agonists increase macrophage homing, lymphocyte contacts, and endothelial junctional complex formation in murine lymph nodes. J Immunol. 2005;175:7151–7161. doi: 10.4049/jimmunol.175.11.7151. [DOI] [PubMed] [Google Scholar]
- 85.Wei SH, Rosen H, Matheu MP, Sanna MG, Wang S-K, Jo E, Wong C-H, Parker I, Cahalan MD. Sphingosine 1–phosphate Type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol. 2005;6:1228–1235. doi: 10.1038/ni1269. [DOI] [PubMed] [Google Scholar]
- 86.Oo ML, Thangada S, Wu M-T, Liu CH, Macdonald TL, Lynch KR, Lin C-Y, Hla T. Immunosuppressive and anti-angiogenic sphingosine 1–phosphate receptor–1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 87.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 88.Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 89.Oo ML, Chang S-H, Thangada S, Wu M-T, Rezaul K, Blaho V, Hwang S-I, Han DK, Hla T. Engagement of S1P1-degradative mechanisms leads to vascular leak in mice. J Clin Invest. 2011;121:2290–2300. doi: 10.1172/JCI45403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krump-Konvalinkova V, Chwalla I, Siess W. FTY720 inhibits S1P-mediated endothelial healing: relationship to S1P1-receptor surface expression. Biochem Biophys Res Commun. 2008;370:603–608. doi: 10.1016/j.bbrc.2008.03.144. [DOI] [PubMed] [Google Scholar]
- 91.Shea BS, Brooks SF, Fontaine BA, Chun J, Luster AD, Tager AM. Prolonged S1P1 agonist exposure exacerbates vascular leak, fibrosis, and mortality after lung injury. Am J Respir Cell Mol Biol. 2010;43:662–673. doi: 10.1165/rcmb.2009-0345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hale JJ, Neway W, Mills SG, Hajdu R, Keohane CA, Rosenbach M, Milligan J, Shei G-J, Chrebt G, Bergstrom J, et al. Potent S1P receptor agonists replicate the pharmacologic actions of the novel immune modulator FTY720. Bioorg Med Chem Lett. 2004;14:3351–3355. doi: 10.1016/j.bmcl.2004.02.106. [DOI] [PubMed] [Google Scholar]
- 93.Foss FW, Clemens JJ, Davis MD, Snyder AH, Zigler MA, Lynch KR, Macdonald TL. Synthesis, stability, and implications of phosphothioate agonists of sphingosine-1–phosphate receptors. Bioorg Med Chem Lett. 2005;15:4470–4474. doi: 10.1016/j.bmcl.2005.07.057. [DOI] [PubMed] [Google Scholar]
- 94.Clemens JJ, Davis MD, Lynch KR, Macdonald TL. Synthesis of 4(5)-phenylimidazole–based analogues of sphingosine-1–phosphate and FTY720: discovery of potent S1P1 receptor agonists. Bioorg Med Chem Lett. 2005;15:3568–3572. doi: 10.1016/j.bmcl.2005.05.097. [DOI] [PubMed] [Google Scholar]
- 95.Zhu R, Snyder AH, Kharel Y, Schaffter L, Sun Q, Kennedy PC, Lynch KR, Macdonald TL. Asymmetric synthesis of conformationally constrained fingolimod analogues: discovery of an orally active sphingosine 1–phosphate receptor Type–1 agonist and receptor Type–3 antagonist. J Med Chem. 2007;50:6428–6435. doi: 10.1021/jm7010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tarrason G, Auli M, Mustafa S, Dolgachev MT, Prats N, Dominguez M, Lopez R, Aguilar N, Calbet M, Pont M, et al. The sphingosine-1–phosphate receptor–1 antagonist, W146, causes early and short-lasting peripheral blood lymphopenia in mice. Int Immunopharmacol. 2011;11:1773–1779. doi: 10.1016/j.intimp.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 97.Lu X, Sun C, Valentine WJ, Shuyu E, Liu J, Tigyi G, Bittman R. Chiral vinylphosphonate and phosphonate analogues of the immunosuppressive agent FTY720. J Org Chem. 2009;74:3192–3195. doi: 10.1021/jo900023u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang L, Sammani S, Moreno-Vinasco L, Camp SM, Bittman R, Garcia JGN, Dudek SM. FTY720 S-phosphonate exhibits superior barrier promoting properties to S1P and FTY720 in vitro and in vivo. Am J Respir Crit Care Med. 2011;183:A1954. doi: 10.1097/CCM.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tonelli F, Lim KG, Loveridge C, Long J, Piston SM, Tigyi G, Bittman R, Pyne S, Pyne NJ. FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteosomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer. Cell Signal. 2010;22:1536–1542. doi: 10.1016/j.cellsig.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Valentine WJ, Kiss GN, Liu J, Shuyu E, Gotoh M, Murakami-Murofushi K, Pham TC, Baker DL, Parrill AL, Lu X, et al. (S)-FTY720–vinylphosphonate, an analogue of the immunosuppressive agent FTY720, is a pan-antagonist of sphingosine 1–phosphate GPCR signaling and inhibits autotaxin activity. Cell Signal. 2010;22:1543–1553. doi: 10.1016/j.cellsig.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hla T. Sphingosine 1–phosphate receptors. Prostaglandins Other Lipid Mediat. 2001;64:135–142. doi: 10.1016/s0090-6980(01)00109-5. [DOI] [PubMed] [Google Scholar]
- 102.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, et al. Edg-1, the G protein–coupled receptor for sphingosine 1–phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kono M, Allende ML, Proia RL. Sphingosine 1–phosphate regulation of mammalian development. Biochim Biophys Acta. 2008;1781:435–441. doi: 10.1016/j.bbalip.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu Y-P, Yamashita T, Proia RL. The sphingosine-1–phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 105.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1–phosphate receptor–2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007;27:1312–1318. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 106.Sammani S, Moreno-Vinasco L, Mirzapoiazova T, Singleton PA, Chiang ET, Evenoski CL, Wang T, Mathew B, Husain A, Moitra J, et al. Differential effects of sphingosine 1–phosphate receptors on airway and vascular barrier function in murine lung. Am J Respir Cell Mol Biol. 2010;43:394–402. doi: 10.1165/rcmb.2009-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1–phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest. 2007;117:2506–2516. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun X, Ma S-F, Wade MS, Moitra J, Garcia JGN. Two functional promoter variants of sphingosine-1–phosphate receptor 3 gene decrease human susceptibility to severe sepsis-associated acute lung injury. Am J Respir Cell Mol Biol. 2011;183:A1345. [Google Scholar]
- 109.Ma S-F, Wade MS, Hysi P, Yates CR, Meduri GU, Zhao Y, Garcia JG, Natarajan V. Sphingosine kinase 1 gene polymorphisms are associated with acute lung injury in African and European Americans. Am J Respir Cell Mol Biol. 2011;183:A3875. [Google Scholar]
- 110.Petrache I, Goya J, Rush NI, Van DeMark BS, Kamocki K, Petrusca DN, Twigg HL, III, Garcia JGN, Ma S, Natarajan V, et al. Ceramide and sphingosine 1 phosphate (S1P) levels in patients with COPD. Am J Respir Cell Mol Biol. 2010;181:A1539. [Google Scholar]