Abstract
Polyhydroxyalkanoates (PHAs), of which polyhydroxybutyrate (PHB) is the most abundant, are bacterial carbon and energy reserve materials of widespread occurrence. They are composed of 3-hydroxyacid monomer units and exist as a small number of cytoplasmic granules per cell. The properties of the C4 homopolymer PHB as a biodegradable thermoplastic first attracted industrial attention more than 20 years ago. Copolymers of C4 (3-hydroxybutyrate [3HB]) and C5 (3-hydroxyvalerate [3HV]) monomer units have modified physical properties; e.g., the plastic is less brittle than PHB, whereas PHAs containing C8 to C12 monomers behave as elastomers. This family of materials is the centre of considerable commercial interest, and 3HB-co-3HV copolymers have been marketed by ICI plc as Biopol. The known polymers exist as 2(1) helices with the fiber repeat decreasing from 0.596 nm for PHB to about 0.45 nm for C8 to C10 polymers. Novel copolymers with a backbone of 3HB and 4HB have been obtained. The native granules contain noncrystalline polymer, and water may possibly act as a plasticizer. Although the biosynthesis and regulation of PHB are generally well understood, the corresponding information for the synthesis of long-side-chain PHAs from alkanes, alcohols, and organic acids is still incomplete. The precise mechanisms of action of the polymerizing and depolymerizing enzymes also remain to be established. The structural genes for the three key enzymes of PHB synthesis from acetyl coenzyme A in Alcaligenes eutrophus have been cloned, sequenced, and expressed in Escherichia coli. Polymer molecular weights appear to be species specific. The factors influencing the commercial choice of organism, substrate, and isolation process are discussed. The physiological functions of PHB as a reserve material and in symbiotic nitrogen fixation and its presence in bacterial plasma membranes and putative role in transformability and calcium signaling are also considered.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrell B. A., Erickson S. K., Jones C. W. The respiratory-chain NADPH dehydrogenase of Azotobacter vinelandii. Eur J Biochem. 1972 Apr 11;26(3):387–392. doi: 10.1111/j.1432-1033.1972.tb01778.x. [DOI] [PubMed] [Google Scholar]
- Anderson A. J., Haywood G. W., Dawes E. A. Biosynthesis and composition of bacterial poly(hydroxyalkanoates). Int J Biol Macromol. 1990 Apr;12(2):102–105. doi: 10.1016/0141-8130(90)90060-n. [DOI] [PubMed] [Google Scholar]
- Barnard G. N., Sanders J. K. The poly-beta-hydroxybutyrate granule in vivo. A new insight based on NMR spectroscopy of whole cells. J Biol Chem. 1989 Feb 25;264(6):3286–3291. [PubMed] [Google Scholar]
- Bayer-Berger M. M., Ravussin P., Fankhauser H., Freeman J. Effect of three pretreatment techniques on hemodynamic and CSFP responses to skull-pin head-holder application during thiopentone/isoflurane or propofol anesthesia. J Neurosurg Anesthesiol. 1989 Sep;1(3):227–232. doi: 10.1097/00008506-198909000-00004. [DOI] [PubMed] [Google Scholar]
- Bloomfield G., Sandhu G., Carr N. G. Activation by Hg(2+) of acetoacetyl-CoA reductase in extracts of Rhodopseudomonas spheroides and Rhodomicrobium vannielii. FEBS Lett. 1969 Nov 29;5(4):246–248. doi: 10.1016/0014-5793(69)80360-1. [DOI] [PubMed] [Google Scholar]
- Brandl H., Gross R. A., Lenz R. W., Fuller R. C. Pseudomonas oleovorans as a Source of Poly(beta-Hydroxyalkanoates) for Potential Applications as Biodegradable Polyesters. Appl Environ Microbiol. 1988 Aug;54(8):1977–1982. doi: 10.1128/aem.54.8.1977-1982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl H., Knee E. J., Jr, Fuller R. C., Gross R. A., Lenz R. W. Ability of the phototrophic bacterium Rhodospirillum rubrum to produce various poly (beta-hydroxyalkanoates): potential sources for biodegradable polyesters. Int J Biol Macromol. 1989 Feb;11(1):49–55. doi: 10.1016/0141-8130(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan Nambiar P. T., Shethna Y. I. Purification and properties of an NADP+-specific isocitrate dehydrogenase from Rhizobium meliloti. Antonie Van Leeuwenhoek. 1976;42(4):471–482. doi: 10.1007/BF00410178. [DOI] [PubMed] [Google Scholar]
- Chung A. E. Pyridine nucleotide transhydrogenase from Azotobacter vinelandii. J Bacteriol. 1970 May;102(2):438–447. doi: 10.1128/jb.102.2.438-447.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau Y., Hall K. J., Oldham W. K. Determination of Poly-beta-Hydroxybutyrate and Poly-beta-Hydroxyvalerate in Activated Sludge by Gas-Liquid Chromatography. Appl Environ Microbiol. 1988 Sep;54(9):2325–2327. doi: 10.1128/aem.54.9.2325-2327.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornibert J., Marchessault R. H. Physical properties of poly- -hydroxybutyrate. IV. Conformational analysis and crystalline structure. J Mol Biol. 1972 Nov 28;71(3):735–756. doi: 10.1016/s0022-2836(72)80035-4. [DOI] [PubMed] [Google Scholar]
- Da Prada M., Keller H. H. Baclofen and gamma-hydroxybutyrate: similar effects on cerebral dopamine neurones. Life Sci. 1976 Oct 15;19(8):1253–1263. doi: 10.1016/0024-3205(76)90261-7. [DOI] [PubMed] [Google Scholar]
- Davis J. T., Chen H. H., Moore R., Nishitani Y., Masamune S., Sinskey A. J., Walsh C. T. Biosynthetic thiolase from Zoogloea ramigera. II. Inactivation with haloacetyl CoA analogs. J Biol Chem. 1987 Jan 5;262(1):90–96. [PubMed] [Google Scholar]
- Davis J. T., Moore R. N., Imperiali B., Pratt A. J., Kobayashi K., Masamune S., Sinskey A. J., Walsh C. T., Fukui T., Tomita K. Biosynthetic thiolase from zoogloea ramigera. I. Preliminary characterization and analysis of proton transfer reaction. J Biol Chem. 1987 Jan 5;262(1):82–89. [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Doi Y., Kawaguchi Y., Nakamura Y., Kunioka M. Nuclear Magnetic Resonance Studies of Poly(3-Hydroxybutyrate) and Polyphosphate Metabolism in Alcaligenes eutrophus. Appl Environ Microbiol. 1989 Nov;55(11):2932–2938. doi: 10.1128/aem.55.11.2932-2938.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi Y., Segawa A., Kawaguchi Y., Kunioka M. Cyclic nature of poly(3-hydroxyalkanoate) metabolism in Alcaligenes eutrophus. FEMS Microbiol Lett. 1990 Jan 15;55(1-2):165–169. doi: 10.1016/0378-1097(90)90188-v. [DOI] [PubMed] [Google Scholar]
- Doi Y., Segawa A., Kunioka M. Biosynthesis and characterization of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in Alcaligenes eutrophus. Int J Biol Macromol. 1990 Apr;12(2):106–111. doi: 10.1016/0141-8130(90)90061-e. [DOI] [PubMed] [Google Scholar]
- Dunlop W. F., Robards A. W. Ultrastructural study of poly- -hydroxybutyrate granules from Bacillus cereus. J Bacteriol. 1973 Jun;114(3):1271–1280. doi: 10.1128/jb.114.3.1271-1280.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellar D., Lundgren D. G., Okamura K., Marchessault R. H. Morphology of poly-beta-hydroxybutyrate granules. J Mol Biol. 1968 Aug 14;35(3):489–502. doi: 10.1016/s0022-2836(68)80009-9. [DOI] [PubMed] [Google Scholar]
- Fernandez-Castillo R., Rodriguez-Valera F., Gonzalez-Ramos J., Ruiz-Berraquero F. Accumulation of Poly (beta-Hydroxybutyrate) by Halobacteria. Appl Environ Microbiol. 1986 Jan;51(1):214–216. doi: 10.1128/aem.51.1.214-216.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay R. H., White D. C. In situ determination of metabolic activity in aquatic environments. Microbiol Sci. 1984 Jul;1(4):90-2,95. [PubMed] [Google Scholar]
- Findlay R. H., White D. C. Polymeric Beta-Hydroxyalkanoates from Environmental Samples and Bacillus megaterium. Appl Environ Microbiol. 1983 Jan;45(1):71–78. doi: 10.1128/aem.45.1.71-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsche K., Lenz R. W., Fuller R. C. Bacterial polyesters containing branched poly(beta-hydroxyalkanoate) units. Int J Biol Macromol. 1990 Apr;12(2):92–101. doi: 10.1016/0141-8130(90)90059-j. [DOI] [PubMed] [Google Scholar]
- Fritzsche K., Lenz R. W., Fuller R. C. Production of unsaturated polyesters by Pseudomonas oleovorans. Int J Biol Macromol. 1990 Apr;12(2):85–91. doi: 10.1016/0141-8130(90)90058-i. [DOI] [PubMed] [Google Scholar]
- Fukui T., Ito M., Saito T., Tomita K. Purification and characterization of NADP-linked acetoacetyl-CoA reductase from Zoogloea ramigera I-16-M. Biochim Biophys Acta. 1987 Feb 23;917(3):365–371. [PubMed] [Google Scholar]
- Fukui T., Narikawa T., Miwa K., Shirakura Y., Saito T., Tomita K. Effect of limited tryptic modification of a bacterial poly(3-hydroxybutyrate) depolymerase on its catalytic activity. Biochim Biophys Acta. 1988 Jan 29;952(2):164–171. doi: 10.1016/0167-4838(88)90112-4. [DOI] [PubMed] [Google Scholar]
- Fukui T., Yoshimoto A., Matsumoto M., Hosokawa S., Saito T. Enzymatic synthesis of poly-beta-hydroxybutyrate in Zoogloea ramigera. Arch Microbiol. 1976 Nov 2;110(23):149–156. doi: 10.1007/BF00690222. [DOI] [PubMed] [Google Scholar]
- Fuller R. W., Hemrick-Luecke S. K. A high dose of MPTP overcomes the protective effect of selegiline against dopaminergic neurotoxicity. J Pharm Pharmacol. 1989 Jul;41(7):492–493. doi: 10.1111/j.2042-7158.1989.tb06509.x. [DOI] [PubMed] [Google Scholar]
- Gavard R., Raynaud C., Hauttecoeur B., Dahinger A. Dégradation du lipide beta-hydroxybutyrique par un extrait enzymatique de Bacillus Megatherium 3 dépolymérase B. C R Acad Sci Hebd Seances Acad Sci D. 1967 Nov 13;265(20):1557–1559. [PubMed] [Google Scholar]
- Griebel R. J., Merrick J. M. Metabolism of poly- -hydroxybutyrate: effect of mild alkaline extraction on native poly- -hydroxybutyrate granules. J Bacteriol. 1971 Nov;108(2):782–789. doi: 10.1128/jb.108.2.782-789.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel R., Smith Z., Merrick J. M. Metabolism of poly-beta-hydroxybutyrate. I. Purification, composition, and properties of native poly-beta-hydroxybutyrate granules from Bacillus megaterium. Biochemistry. 1968 Oct;7(10):3676–3681. doi: 10.1021/bi00850a047. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood G. W., Anderson A. J., Ewing D. F., Dawes E. A. Accumulation of a Polyhydroxyalkanoate Containing Primarily 3-Hydroxydecanoate from Simple Carbohydrate Substrates by Pseudomonas sp. Strain NCIMB 40135. Appl Environ Microbiol. 1990 Nov;56(11):3354–3359. doi: 10.1128/aem.56.11.3354-3359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron J. S., King J. D., White D. C. Recovery of Poly-beta-Hydroxybutyrate from Estuarine Microflora. Appl Environ Microbiol. 1978 Feb;35(2):251–257. doi: 10.1128/aem.35.2.251-257.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippe H., Schlegel H. G. Hydrolyse von PHBS durch intracelluläre Depolymerase von Hydrogenomonas H 16. Arch Mikrobiol. 1967 Mar 29;56(3):278–299. [PubMed] [Google Scholar]
- Huisman G. W., de Leeuw O., Eggink G., Witholt B. Synthesis of poly-3-hydroxyalkanoates is a common feature of fluorescent pseudomonads. Appl Environ Microbiol. 1989 Aug;55(8):1949–1954. doi: 10.1128/aem.55.8.1949-1954.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson F. A., Dawes E. A. Regulation of the tricarboxylic acid cycle and poly-beta-hydroxybutyrate metabolism in Azotobacter beijerinckii grown under nitrogen or oxygen limitation. J Gen Microbiol. 1976 Dec;97(2):303–312. doi: 10.1099/00221287-97-2-303. [DOI] [PubMed] [Google Scholar]
- Jacob G. S., Garbow J. R., Schaefer J. Direct measurement of poly(beta-hydroxybutyrate) in a pseudomonad by solid-state 13C NMR. J Biol Chem. 1986 Dec 25;261(36):16785–16787. [PubMed] [Google Scholar]
- Karr D. B., Waters J. K., Emerich D. W. Analysis of Poly-beta-Hydroxybutyrate in Rhizobium japonicum Bacteroids by Ion-Exclusion High-Pressure Liquid Chromatography and UV Detection. Appl Environ Microbiol. 1983 Dec;46(6):1339–1344. doi: 10.1128/aem.46.6.1339-1344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr D. B., Waters J. K., Suzuki F., Emerich D. W. Enzymes of the Poly-beta-Hydroxybutyrate and Citric Acid Cycles of Rhizobium japonicum Bacteroids. Plant Physiol. 1984 Aug;75(4):1158–1162. doi: 10.1104/pp.75.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDGREN D. G., ALPER R., SCHNAITMAN C., MARCHESSAULT R. H. CHARACTERIZATION OF POLY-BETA-HYDROXYBUTYRATE EXTRACTED FROM DIFFERENT BACTERIA. J Bacteriol. 1965 Jan;89:245–251. doi: 10.1128/jb.89.1.245-251.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDGREN D. G., PFISTER R. M., MERRICK J. M. STRUCTURE OF POLY-BETA-HYDROXYBUTYRIC ACID GRANULES. J Gen Microbiol. 1964 Mar;34:441–446. doi: 10.1099/00221287-34-3-441. [DOI] [PubMed] [Google Scholar]
- Lageveen R. G., Huisman G. W., Preusting H., Ketelaar P., Eggink G., Witholt B. Formation of Polyesters by Pseudomonas oleovorans: Effect of Substrates on Formation and Composition of Poly-(R)-3-Hydroxyalkanoates and Poly-(R)-3-Hydroxyalkenoates. Appl Environ Microbiol. 1988 Dec;54(12):2924–2932. doi: 10.1128/aem.54.12.2924-2932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusty C. J., Doudoroff M. Poly-beta-hydroxybutyrate depolymerases of Pseudomonas lemoignei. Proc Natl Acad Sci U S A. 1966 Sep;56(3):960–965. doi: 10.1073/pnas.56.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERRICK J. M., DOUDOROFF M. DEPOLYMERIZATION OF POLY-BETA-HYDROXYBUTYRATE BY INTRACELLULAR ENZYME SYSTEM. J Bacteriol. 1964 Jul;88:60–71. doi: 10.1128/jb.88.1.60-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERRICK J. M., DOUDOROFF M. Enzymatic synthesis of poly-beta-hydroxybutyric acid in bacteria. Nature. 1961 Mar 18;189:890–892. doi: 10.1038/189890a0. [DOI] [PubMed] [Google Scholar]
- Marchessault R. H., Monasterios C. J., Morin F. G., Sundararajan P. R. Chiral poly(beta-hydroxyalkanoates): an adaptable helix influenced by the alkane side-chain. Int J Biol Macromol. 1990 Apr;12(2):158–165. doi: 10.1016/0141-8130(90)90068-l. [DOI] [PubMed] [Google Scholar]
- Mas J., Pedrós-Alió C., Guerrero R. Mathematical model for determining the effects of intracytoplasmic inclusions on volume and density of microorganisms. J Bacteriol. 1985 Nov;164(2):749–756. doi: 10.1128/jb.164.2.749-756.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick J. M., Yu C. I. Purification and properties of a D(--)-beta-hydroxybutyric dimer hydrolase from Rhodospirillum rubrum. Biochemistry. 1966 Nov;5(11):3563–3568. doi: 10.1021/bi00875a026. [DOI] [PubMed] [Google Scholar]
- Miller N. D., Williams D. F. On the biodegradation of poly-beta-hydroxybutyrate (PHB) homopolymer and poly-beta-hydroxybutyrate-hydroxyvalerate copolymers. Biomaterials. 1987 Mar;8(2):129–137. doi: 10.1016/0142-9612(87)90102-5. [DOI] [PubMed] [Google Scholar]
- Moskowitz G. J., Merrick J. M. Metabolism of poly-beta-hydroxybutyrate. II. Enzymatic synthesis of D-(-)-beta hydroxybutyryl coenzyme A by an enoyl hydrase from Rhodospirillum rubrum. Biochemistry. 1969 Jul;8(7):2748–2755. doi: 10.1021/bi00835a009. [DOI] [PubMed] [Google Scholar]
- Moustafa E., Leong C. K. Effect of adenine nucleotides on NAD-dependent isocitrate dehydrogenases in rhizobia and bacteroids of legume root nodules. Biochim Biophys Acta. 1975 May 23;391(1):9–14. doi: 10.1016/0005-2744(75)90146-1. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Saito T., Fukui T., Shirakura Y., Tomita K. Purification and properties of extracellular poly(3-hydroxybutyrate) depolymerases from Pseudomonas lemoignei. Biochim Biophys Acta. 1985 Jan 21;827(1):63–72. doi: 10.1016/0167-4838(85)90101-3. [DOI] [PubMed] [Google Scholar]
- Nickels J. S., King J. D., White D. C. Poly-beta-Hydroxybutyrate Accumulation as a Measure of Unbalanced Growth of the Estuarine Detrital Microbiota. Appl Environ Microbiol. 1979 Mar;37(3):459–465. doi: 10.1128/aem.37.3.459-465.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson K. W. Purification of Poly-beta-Hydroxybutyrate by Density Gradient Centrifugation in Sodium Bromide. Appl Environ Microbiol. 1982 May;43(5):1208–1209. doi: 10.1128/aem.43.5.1208-1209.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Saito T., Tomita K. Purification and properties of beta-ketothiolase from Zoogloea ramigera. Arch Microbiol. 1978 Jan 23;116(1):21–27. doi: 10.1007/BF00408729. [DOI] [PubMed] [Google Scholar]
- Odham G., Tunlid A., Westerdahl G., Mårdén P. Combined Determination of Poly-beta-Hydroxyalkanoic and Cellular Fatty Acids in Starved Marine Bacteria and Sewage Sludge by Gas Chromatography with Flame Ionization or Mass Spectrometry Detection. Appl Environ Microbiol. 1986 Oct;52(4):905–910. doi: 10.1128/aem.52.4.905-910.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeding V., Schlegel H. G. Beta-ketothiolase from Hydrogenomonas eutropha H16 and its significance in the regulation of poly-beta-hydroxybutyrate metabolism. Biochem J. 1973 May;134(1):239–248. doi: 10.1042/bj1340239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostle A. G., Holt J. G. Nile blue A as a fluorescent stain for poly-beta-hydroxybutyrate. Appl Environ Microbiol. 1982 Jul;44(1):238–241. doi: 10.1128/aem.44.1.238-241.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Knosp O. Hyperproduction of Poly-beta-Hydroxybutyrate during Exponential Growth of Azotobacter vinelandii UWD. Appl Environ Microbiol. 1989 Jun;55(6):1334–1339. doi: 10.1128/aem.55.6.1334-1339.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples O. P., Masamune S., Walsh C. T., Sinskey A. J. Biosynthetic thiolase from Zoogloea ramigera. III. Isolation and characterization of the structural gene. J Biol Chem. 1987 Jan 5;262(1):97–102. [PubMed] [Google Scholar]
- Peoples O. P., Sinskey A. J. Poly-beta-hydroxybutyrate (PHB) biosynthesis in Alcaligenes eutrophus H16. Identification and characterization of the PHB polymerase gene (phbC). J Biol Chem. 1989 Sep 15;264(26):15298–15303. [PubMed] [Google Scholar]
- Peoples O. P., Sinskey A. J. Poly-beta-hydroxybutyrate biosynthesis in Alcaligenes eutrophus H16. Characterization of the genes encoding beta-ketothiolase and acetoacetyl-CoA reductase. J Biol Chem. 1989 Sep 15;264(26):15293–15297. [PubMed] [Google Scholar]
- Ploux O., Masamune S., Walsh C. T. The NADPH-linked acetoacetyl-CoA reductase from Zoogloea ramigera. Characterization and mechanistic studies of the cloned enzyme over-produced in Escherichia coli. Eur J Biochem. 1988 May 16;174(1):177–182. doi: 10.1111/j.1432-1033.1988.tb14079.x. [DOI] [PubMed] [Google Scholar]
- Pool R. In Search of the Plastic Potato: Scientists in the emerging field of biopolymer engineering are aiming to produce bacteria and, eventually, food crops that are genetically tailored to yield a whole new breed of plastics. Science. 1989 Sep 15;245(4923):1187–1189. doi: 10.1126/science.245.4923.1187. [DOI] [PubMed] [Google Scholar]
- Ramsay B. A., Ramsay J. A., Cooper D. G. Production of Poly-beta-Hydroxyalkanoic Acid by Pseudomonas cepacia. Appl Environ Microbiol. 1989 Mar;55(3):584–589. doi: 10.1128/aem.55.3.584-589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
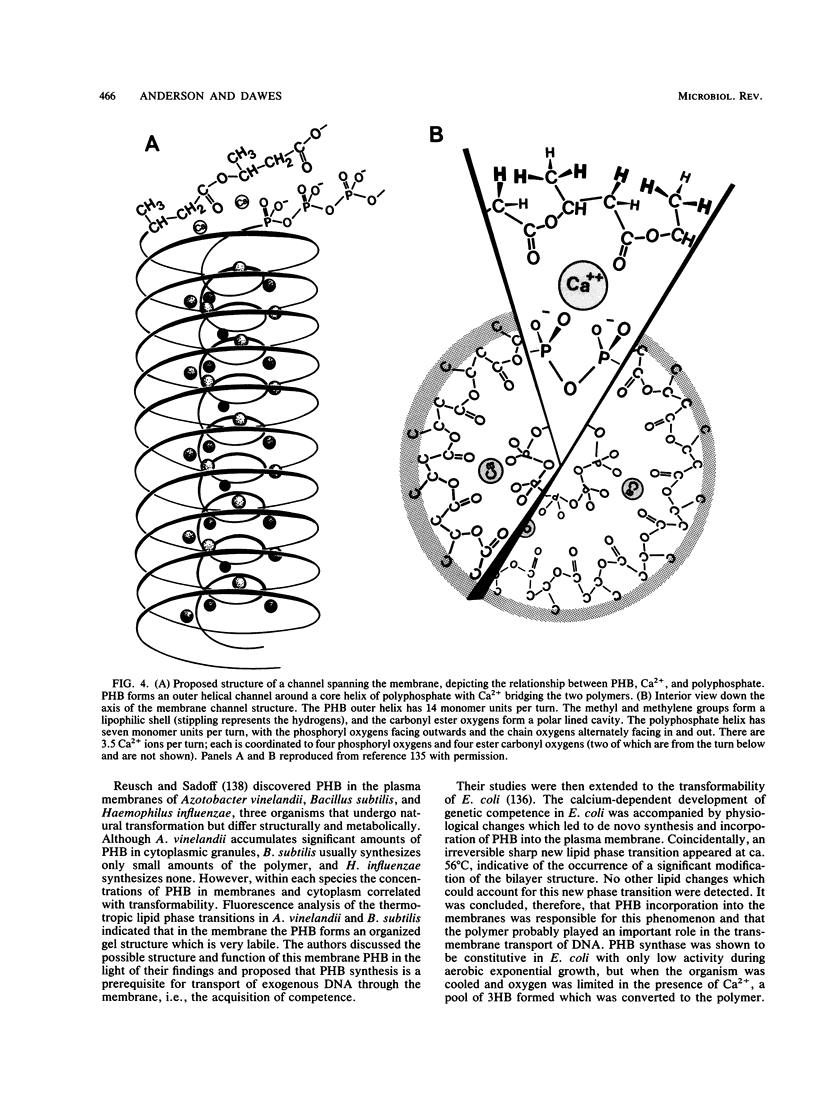
- Reusch R. N., Hiske T. W., Sadoff H. L. Poly-beta-hydroxybutyrate membrane structure and its relationship to genetic transformability in Escherichia coli. J Bacteriol. 1986 Nov;168(2):553–562. doi: 10.1128/jb.168.2.553-562.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch R. N. Poly-beta-hydroxybutyrate/calcium polyphosphate complexes in eukaryotic membranes. Proc Soc Exp Biol Med. 1989 Sep;191(4):377–381. doi: 10.3181/00379727-191-42936. [DOI] [PubMed] [Google Scholar]
- Reusch R. N., Sadoff H. L. D-(-)-poly-beta-hydroxybutyrate in membranes of genetically competent bacteria. J Bacteriol. 1983 Nov;156(2):778–788. doi: 10.1128/jb.156.2.778-788.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch R. N., Sadoff H. L. Putative structure and functions of a poly-beta-hydroxybutyrate/calcium polyphosphate channel in bacterial plasma membranes. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4176–4180. doi: 10.1073/pnas.85.12.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch R., Hiske T., Sadoff H., Harris R., Beveridge T. Cellular incorporation of poly-beta-hydroxybutyrate into plasma membranes of Escherichia coli and Azotobacter vinelandii alters native membrane structure. Can J Microbiol. 1987 May;33(5):435–444. doi: 10.1139/m87-073. [DOI] [PubMed] [Google Scholar]
- Ritchie G. A., Dawes E. A. The non-involvement of cyl-carrir protein in poly-beta-hydroxybutyric acid biosynthesis in Azotobacter beijerinckii. Biochem J. 1969 May;112(5):803–805. doi: 10.1042/bj1120803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie G. A., Senior P. J., Dawes E. A. The purification and characterization of acetoacetyl-coenzyme A reductase from Azotobacter beijerinckii. Biochem J. 1971 Jan;121(2):309–316. doi: 10.1042/bj1210309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Fukui T., Ikeda F., Tanaka Y., Tomita K. An NADP-linked acetoacetyl CoA reductase from Zoogloea ramigera. Arch Microbiol. 1977 Sep 28;114(3):211–217. doi: 10.1007/BF00446864. [DOI] [PubMed] [Google Scholar]
- Saito T., Suzuki K., Yamamoto J., Fukui T., Miwa K., Tomita K., Nakanishi S., Odani S., Suzuki J., Ishikawa K. Cloning, nucleotide sequence, and expression in Escherichia coli of the gene for poly(3-hydroxybutyrate) depolymerase from Alcaligenes faecalis. J Bacteriol. 1989 Jan;171(1):184–189. doi: 10.1128/jb.171.1.184-189.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandola M., Ceccorulli G., Doi Y. Viscoelastic relaxations and thermal properties of bacterial poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Int J Biol Macromol. 1990 Apr;12(2):112–117. doi: 10.1016/0141-8130(90)90062-f. [DOI] [PubMed] [Google Scholar]
- Schlegel H. G., Lafferty R., Krauss I. The isolation of mutants not accumulating poly-beta-hydroxybutyric acid. Arch Mikrobiol. 1970;71(3):283–294. doi: 10.1007/BF00410161. [DOI] [PubMed] [Google Scholar]
- Schubert P., Steinbüchel A., Schlegel H. G. Cloning of the Alcaligenes eutrophus genes for synthesis of poly-beta-hydroxybutyric acid (PHB) and synthesis of PHB in Escherichia coli. J Bacteriol. 1988 Dec;170(12):5837–5847. doi: 10.1128/jb.170.12.5837-5847.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior P. J., Beech G. A., Ritchie G. A., Dawes E. A. The role of oxygen limitation in the formation of poly- -hydroxybutyrate during batch and continuous culture of Azotobacter beijerinckii. Biochem J. 1972 Aug;128(5):1193–1201. doi: 10.1042/bj1281193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior P. J., Dawes E. A. Poly- -hydroxybutyrate biosynthesis and the regulation of glucose metabolism in Azotobacter beijerinckii. Biochem J. 1971 Nov;125(1):55–66. doi: 10.1042/bj1250055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior P. J., Dawes E. A. The regulation of poly-beta-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem J. 1973 May;134(1):225–238. doi: 10.1042/bj1340225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakura Y., Fukui T., Saito T., Okamoto Y., Narikawa T., Koide K., Tomita K., Takemasa T., Masamune S. Degradation of poly(3-hydroxybutyrate) by poly(3-hydroxybutyrate) depolymerase from Alcaligenes faecalis T1. Biochim Biophys Acta. 1986 Jan 15;880(1):46–53. doi: 10.1016/0304-4165(86)90118-2. [DOI] [PubMed] [Google Scholar]
- Shirakura Y., Fukui T., Tanio T., Nakayama K., Matsuno R., Tomita K. An extracellular D(-)-3-hydroxybutyrate oligomer hydrolase from Alcaligenes faecalis. Biochim Biophys Acta. 1983 Oct 28;748(2):331–339. doi: 10.1016/0167-4838(83)90310-2. [DOI] [PubMed] [Google Scholar]
- Shuto H., Fukui T., Saito T., Shirakura Y., Tomita K. An NAD-linked acetoacetyl-CoA reductase from Zoogloea ramigera I-16-M. Eur J Biochem. 1981 Aug;118(1):53–59. doi: 10.1111/j.1432-1033.1981.tb05485.x. [DOI] [PubMed] [Google Scholar]
- Slater S. C., Voige W. H., Dennis D. E. Cloning and expression in Escherichia coli of the Alcaligenes eutrophus H16 poly-beta-hydroxybutyrate biosynthetic pathway. J Bacteriol. 1988 Oct;170(10):4431–4436. doi: 10.1128/jb.170.10.4431-4436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stovall I., Cole M. Organic Acid Metabolism by Isolated Rhizobium japonicum Bacteroids. Plant Physiol. 1978 May;61(5):787–790. doi: 10.1104/pp.61.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki F., Zahler W. L., Emerich D. W. Acetoacetyl-CoA thiolase of Bradyrhizobium japonicum bacteroids: purification and properties. Arch Biochem Biophys. 1987 Apr;254(1):272–281. doi: 10.1016/0003-9861(87)90103-2. [DOI] [PubMed] [Google Scholar]
- Tanio T., Fukui T., Shirakura Y., Saito T., Tomita K., Kaiho T., Masamune S. An extracellular poly(3-hydroxybutyrate) depolymerase from Alcaligenes faecalis. Eur J Biochem. 1982 May;124(1):71–77. doi: 10.1111/j.1432-1033.1982.tb05907.x. [DOI] [PubMed] [Google Scholar]
- Timm A., Steinbüchel A. Formation of polyesters consisting of medium-chain-length 3-hydroxyalkanoic acids from gluconate by Pseudomonas aeruginosa and other fluorescent pseudomonads. Appl Environ Microbiol. 1990 Nov;56(11):3360–3367. doi: 10.1128/aem.56.11.3360-3367.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. P., Evans H. J. Poly-beta-hydroxybutyrate Utilization by Soybean (Glycine max Merr.) Nodules and Assessment of Its Role in Maintenance of Nitrogenase Activity. Plant Physiol. 1971 Jun;47(6):750–755. doi: 10.1104/pp.47.6.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smet M. J., Eggink G., Witholt B., Kingma J., Wynberg H. Characterization of intracellular inclusions formed by Pseudomonas oleovorans during growth on octane. J Bacteriol. 1983 May;154(2):870–878. doi: 10.1128/jb.154.2.870-878.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



