Abstract
Purpose
Transfusion of blood components is common in patients admitted to the intensive care unit (ICU) for gastrointestinal (GI) bleeding, yet the incidence and risk factors for development of transfusion-related acute lung injury (TRALI) in these patients are unknown.
Methods
Patients admitted to a medical ICU for GI bleeding (n = 225) were analyzed for patient-and transfusion-specific risk factors for development of TRALI.
Results
In transfused patients (n = 150), the incidence of TRALI was 15% [95% confidence interval (CI), 10–21%] and accounted for 76% (22/29) of all acute lung injury (ALI) cases. Transfused patients with end-stage liver disease (ESLD) (n = 72) developed TRALI more frequently than those without ESLD (29% versus 1%, p < 0.01). Fresh frozen plasma (FFP) was temporally associated with TRALI in 86% of cases. Transfusion-specific risk factors for development of TRALI included number of transfused units of FFP and nonleukoreduced red blood cells. Patient-specific risk factors included Model for End-Stage Liver Disease (MELD) score, admission serum albumin level, and presence of ALI risk factors.
Conclusions
TRALI is common in critically ill ESLD patients with gastrointestinal bleeding. Nonleukoreduced red blood cells and FFP are significant transfusion-specific risk factors and their use should be re-evaluated in bleeding patients with ESLD.
Keywords: Transfusion-related acute lung injury, Transfusion complications, Acute respiratory distress syndrome, Variceal bleeding, Chronic liver disease, Plasma transfusion
Introduction
Blood transfusion remains a common practice in the intensive care unit (ICU), with 37–44% of admitted patients receiving at least one transfusion of a blood component [1, 2]. The most common admission diagnosis for those medical ICU patients who require transfusion is gastrointestinal bleeding [3]. Approximately 70% of ICU admissions due to gastrointestinal bleeding receive transfusion, with the incidence rising to 85% in patients with end-stage liver disease (ESLD) bleeding from varices [4, 5].
The classically reported risks of blood transfusion include allergic reaction, infectious transmission, and mismatched blood (hemolysis). In critically ill patients, more attention has been recently focused on the development of acute lung injury (ALI) as a risk of blood transfusion. In trauma, medical, and surgical ICU patients, cohort studies have demonstrated a dose-dependent increase in development of ALI associated with transfusion of blood components [3, 6–9].
Even though strong evidence supports an independent temporal association between transfusion and ALI in critically ill patients, the incidence of transfusion-related acute lung injury (TRALI) in patients admitted to the ICU with gastrointestinal bleeding has not been defined [3, 7, 9, 10]. Additionally, the effect of comorbidities on the development of TRALI such as the presence of ESLD and the type of blood component transfused has not been examined in these patients. In this study we sought to determine the incidence of ALI and more specifically TRALI in patients admitted to the ICU with a gastrointestinal bleed, and to identify specific risk factors associated with TRALI in these patients. We hypothesized that comorbidities such as ESLD and transfusion of plasma-containing blood components such as fresh frozen plasma (FFP) and platelets would be independently associated with development of TRALI.
Materials and methods
Design
Using the University of Colorado Hospital (UCH) medical records system, we examined patients with primary diagnosis of gastrointestinal bleeding who were admitted to the medical ICU from January 2002 through July 2008. Inclusion criteria were ICU admission and primary diagnosis of gastrointestinal bleeding. Exclusion criteria were age <18 years, death within 12 h of arrival to the ICU, and transfer from a non-ICU service or outside hospital >12 h after emergency department presentation. The protocol was approved by the Colorado Multiple Institutional Review Board.
Data collection
Data were abstracted from various components of the medical record including: admission and progress notes, ICU flow sheets, laboratory and radiologic data, internal diagnostic coding, and a gastroenterology procedural database.
Transfusion data
The medical ICU does not have a protocol for transfusion of blood components in bleeding patients. The vast majority of the FFP and platelets were pheresed, and transfused plasma was frozen within 6 h of collection. Our main blood supplier adopted universal leukoreduction for packed red blood cells (PRBCs) in 2005 and began TRALI mitigation for FFP using both male-only and never-pregnant females in December 2007. Details regarding collection of transfusion and donor data can be found in the Electronic Supplementary Material (ESM).
ALI risk factors
Standard diagnostic criteria were used to identify risk factors including severe acute pancreatitis, pneumonia, severe sepsis, and septic shock [11]. Aspiration was diagnosed if it was witnessed by nurses, respiratory therapists or treating physicians, or if it was suspected based on a new chest X-ray abnormality and recorded in the medical record as such.
Outcome variables
Our primary outcome measures were ALI and TRALI. We used the American–European Consensus Conference (AECC) definition to define ALI adjusted for altitude [12]. TRALI was defined by the 2004 Consensus Conference definition, requiring the development of ALI within 6 h of initiation of blood transfusion [13]. Detailed information regarding the strict definition of ALI and the timing of both transfusion and onset of ALI can be found in the ESM.
Assessment of intravascular volume status
Diagnosis of ALI was excluded when the physician progress note documented pulmonary edema or volume overload. When available, data from pulmonary artery catheters, central lines, and echocardiograms were examined for evidence of left atrial hypertension. Possible intravascular volume overload was identified by previously accepted techniques: (1) pulmonary artery occlusion pressure ≥18 mmHg at time of ALI onset, (2) central venous pressure ≥15 mmHg at time of ALI onset or (3) an echocardiogram documenting ejection fraction <55% and/or inferior vena caval dilatation without respiratory collapse within 24 h of ALI onset [12, 14, 15].
Statistical analysis
Data that were not normally distributed are reported as median [25–75% interquartile range]. Chi-square test of independence was used to examine categorical variables at baseline, and independent-sample t tests were used to compare continuous characteristics of the two groups. Univariate analysis was performed between transfused patients with ESLD who did and did not develop TRALI to identify significant transfusion and patient-specific risk factors for development of TRALI. Logistic regression models were used to determine the effect of TRALI on mortality. SAS 9.1 was used for all analyses, and p < 0.05 was considered statistically significant. Confidence intervals (95%) for adjusted odds ratios and 25–75% interquartile range for median values are recorded in square brackets.
Results
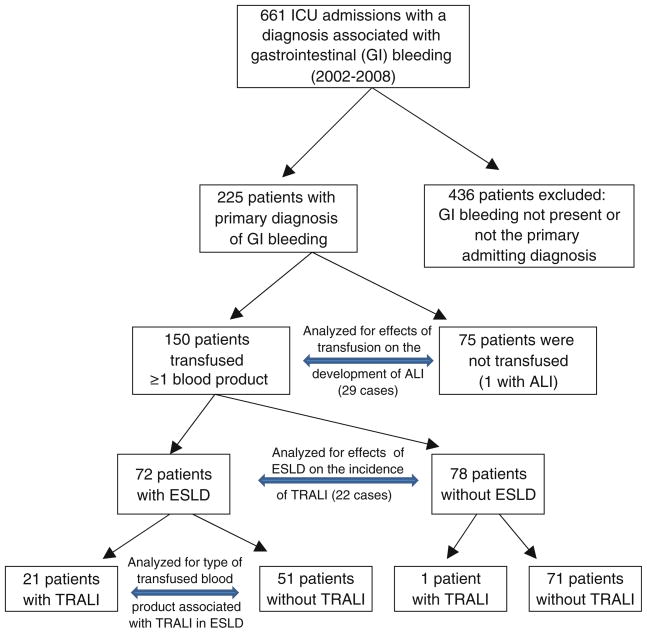
Study enrollment is detailed in Fig. 1. The number of patients included in our final analysis was 225. Their demographics and transfusion characteristics are included in Table 1. ALI developed in 13% (30/225) [95% CI, 10–18%] of all patients with gastrointestinal bleeding who required admission to the ICU. The majority of ALI cases, i.e., 97% (29/30), occurred in patients who received transfusion. The incidence of ALI in patients receiving transfusion was significantly greater than in patients not receiving transfusion (19%, 29/150 versus 1%, 1/75; p < 0.01). Of the cases of ALI in transfused patients, 76% (22/29) met the diagnostic criteria for TRALI and the remaining 24% (7/29) were classified as non-transfusion-related ALI.
Fig. 1.
Flow chart detailing recruitment of subjects subgroup analyses
Table 1.
Baseline characteristics of critically ill patients admitted with gastrointestinal bleeding (n = 225)
| Age, years | 58 [50–70] |
| Hospital length of stay, days | 2 [2–4] |
| End-stage liver disease (ESLD)a | 40% (89/225) |
| Coronary artery disease (CAD) | 16% (35/225) |
| Heart failure (CHF) | 10% (22/225) |
| Chronic kidney disease (stage V) | 2% (4/225) |
| Pulmonary hypertension | 3% (6/225) |
| ALI risk factors | 15% (32/225) |
| Sepsis syndromes | 5% (12/225) |
| Pneumonia | 4% (10/225) |
| Aspiration | 5% (16/225) |
| Severe acute pancreatitis | 1% (2/225) |
| Alcohol use (current) | 35% |
| APACHE II score (median) | 12 [8–16] |
| Hematocrit nadir | 26% [21.5–30%] |
| Coagulopathy | 40% (89/225) |
| Thrombocytopenia | 40% (91/225) |
| Hematemesis presentation | 68% (152/225) |
| Variceal bleed etiology | 31% (70/225) |
| Transfusion incidence | 67% (150/225) |
| PRBCs | 61% (131/225) |
| FFP | 39% (79/225) |
| Platelets | 16% (36/225) |
| Transfusion amount, median no. units | |
| PRBCs [25–75% interquartile range], units (n = 131) | 4 [2–7.3] |
| FFP, median no. units (n = 79) | 6 [3–11] |
| Platelets, median no. units (n = 36) | 2 [1–3.8] |
Only ESLD patients receiving transfusion (n = 72) rather than all patients with ESLD (n = 89) were included for TRALI analysis non-transfused patients (n = 17) are not at risk for TRALI
Characteristics of transfused patients stratified by ESLD
Patients with ESLD were more likely to receive transfusion of any blood component (81% versus 57%, p <0.01) and were more likely to receive transfusion of PRBCs (74% versus 53%, p < 0.01). The most notable difference was the disparity in receipt of plasma-containing blood components among patients with ESLD versus those without ESLD: FFP (61% versus 18%, p < 0.01) and platelets (35% versus 4%, p < 0.01). Patients with ESLD also more commonly received cryoprecipitate (12% versus 2%, p < 0.01). Of patients who received transfusion of PRBCs or FFP, those patients with ESLD received statistically more units of each component than patients without ESLD: PRBCs (6 [2–14] units versus 3 [3–4] units, p < 0.01) and FFP (8 [4–14] units versus 4 [2–6] units, p <0.01). Though patients with ESLD received twice as much blood, they were 29 times more likely to develop TRALI (Table 2).
Table 2.
Incidence of TRALI stratified by ESLD
| (All transfused patients = 150) | ESLD (n = 72) | Non-ESLD (n = 78) | P value |
|---|---|---|---|
| TRALI (within 6 h: n = 150) | 21/72 (29%) | 1/78 (1%) | <0.01 |
| TRALI (no ALI risk factors: n = 124) | 12/54 (22%) | 0/70 (0%) | <0.01 |
| TRALI (presence of ALI risk factors: n = 26) | 9/18 (50%) | 1/8 (13%) | 0.06 |
| Incidence non-transfusion-related ALI (n = 150) | 7/72 (10%) | 0/78 (0%) | <0.01 |
Patient and blood-component-specific risk factors for development of TRALI in patients with ESLD
In an attempt to determine which transfusion and non-transfusion-specific risk factors accounted for the high incidence (29%) of TRALI in patients with ESLD we stratified these patients into TRALI and non-TRALI subgroups and performed univariate analysis of these variables (Table 3). Statistically significant transfusion-specific risk factors included number of units of FFP, platelets, and nonleukoreduced PRBCs received. Significant non-transfusion-related risk factors included presence of ALI risk factors, MELD score, serum albumin, and hematocrit nadir within the first 72 h (Table 3).
Table 3.
Characteristics of transfused GI bleeders with ESLD stratified by TRALI
| Characteristics (n = 72) | TRALI (n = 21) | No TRALI (n = 51) | P value |
|---|---|---|---|
| Age, years | 56 [49–61] | 54 [50–60] | 0.84 |
| Chronic kidney disease | 0% | 0% | |
| Congestive heart failure | 1/21 (5%) | 3/51 (6%) | 0.85 |
| Pulmonary hypertension | 0/21 (0%) | 3/51 (6%) | 0.15 |
| Coronary artery disease | 1/21 (5%) | 3/51 (6%) | 0.85 |
| Current alcohol use | 8/21 (38%) | 26/51 (51%) | 0.32 |
| Acute lung injury risk factors | 9/21 (43%) | 9/51 (18%) | 0.03* |
| Pneumonia | 1/21 (5%) | 2/51 (4%) | 0.87 |
| Aspiration | 3/21 (14%) | 5/51 (10%) | 0.59 |
| Sepsis syndromes | 5/21 (24%) | 5/51 (10%) | 0.13 |
| Hematocrit nadir (first 72 h) | 22 [17–25] | 24 [22–28] | 0.02* |
| Coagulopathy (INR > 1.5) | 18/21 (86%) | 37/51 (73%) | 0.22 |
| Thrombocytopenia (plts < 100 k/μl) | 16/21 (76%) | 42/51 (82%) | 0.55 |
| Serum albumin, g/dl | 2.0 [1.6–2.5] | 2.6 [2.1–3.1] | <0.01* |
| APACHE II score (median) | 17 [14–26] | 15 [12–20] | 0.13 |
| MELD score (median) | 25 [19–31] | 18 [15–24] | <0.01* |
| Etiology due to variceal bleed | 14/21 (67%) | 38/51 (75%) | 0.50 |
| Outcomes | |||
| Length of hospital stay, days | 7 [6–10] | 3 [2–7] | <0.01 |
| In-hospital mortality | 19/21 (90%) | 19/51 (37%) | <0.01 |
| Transfusion amount | |||
| Median PRBC, units | 10 [4–17] | 4 [2–7] | 0.02 |
| Leukoreduced PRBCs, units | 0 [0–4] | 0 [0–4] | 0.55 |
| Nonleukoreduced PRBCs, units | 7 [3–14] | 2 [0–4] | <0.01* |
| Median FFP, units | 14 [8–22] | 2 [0–7] | <0.01* |
| Median platelets, units | 2 [0–3] | 0 [0–1] | 0.04* |
| Incidence of blood component use | |||
| PRBCs | 20/21 (95%) | 46/51 (90%) | 0.46 |
| Leukoreduced PRBCs | 8/21 (38%) | 25/51 (49%) | 0.40 |
| Nonleukoreduced PRBCs | 18/22 (86%) | 35/51 (69%) | 0.12 |
| FFP | 20/21 (95%) | 34/51 (18.4%) | <0.01 |
| Platelets | 12/21 (57%) | 19/32 (37%) | 0.12 |
| Cryoprecipitate | 4/21 (19%) | 7/51 (14%) | 0.58 |
Only ESLD patients receiving transfusion (n = 72) are included in the analysis, as nontransfused patients (n = 17) are not at risk for TRALI
p < 0.05, INR international normalized ratio, plts platelet count, APACHE II severity of illness score, MELD severity of liver disease score
Transfusion characteristics of patients who developed TRALI
In the 21 patients with ESLD who developed TRALI, plasma-containing components (FFP and platelets) were the only blood components temporally associated with its development in 43% (9/21) of cases. Plasma-containing blood components in combination with PRBCs were temporally associated with development of TRALI in an additional 43% (9/21) of cases. In these ESLD patients, each unit of FFP increased the risk of developing TRALI by 11%. In contrast, only 14% (3/21) of TRALI cases were due to PRBCs alone, and all of these units were nonleukoreduced PRBCs. The number of patients receiving nonleukoreduced PRBCs decreased from the first half to the second half of the study (87%, 27/31 versus 63%, 26/41; p = 0.02), while the incidence of TRALI did not decrease significantly (35%, 11/33 versus 24%, 10/41; p = 0.31). We were able to obtain gender of donor for 97% (75/77) of the plasma-containing blood products (FFP and platelets) and age of blood for 100% (50/50) of the PRBC units that were temporally associated with our 21 TRALI cases in patients with ESLD. Of the plasma-containing blood components associated with TRALI, donor gender was female in only 51% (38/75) of the implicated units. There were three cases of TRALI due to FFP only where the donor gender of all implicated units was male. With regard to age of blood, 72% (36/50) of implicated units of PRBCs were >14 days old. The median age of PRBC units temporally associated with TRALI was 19 days [11–26 days].
Physiologic characteristics of patients who developed TRALI
In patients who developed TRALI, the median creatinine immediately preceding the development of TRALI was 1.4 mg/dL [1.2–1.8 mg/dL]. The median urine output was 2.4 l [0.8–3.6 l] over the 24-h period preceding onset of TRALI. Of patients diagnosed with TRALI, one had a pulmonary artery catheter, eight had central venous pressure measurements at time of ALI onset, and seven had echocardiograms within 24 h of onset of ALI. Of these patients, 27% (6/22) had two of the above measurements of intravascular volume status. In the patients (11/22) with objective measurements of volume status, 73% (8/11) had no evidence of intravascular volume overload while 27% (3/11) had evidence of circulatory overload.
Development of TRALI and hospital mortality
Hospital mortality for patients who were admitted to the ICU with gastrointestinal bleeding was 22% (49/225). Patients with ESLD had higher mortality (46%, 41/89) when compared with those patients without ESLD (6%, 8/136; p < 0.01). Development of TRALI was also associated with higher mortality (91%, 20/22) when compared with those patients who received transfusion but did not develop TRALI (20%, 26/128; p < 0.01). The effect of development of TRALI on mortality persisted when we examined those patients (n = 72) with ESLD who received transfusion. The mortality was 90% (19/21) in the ESLD patients who developed TRALI compared with 37% (19/51) in the ESLD patients who did not develop TRALI (p <0.01). The effects of development of TRALI on mortality in transfused patients with ESLD remained significant on multivariate analysis when adjusting for the presence of alternative ALI risk factors, severity of illness (APACHE II) and MELD score (adjusted odds ratio 12.3, CI [2.5–98], p < 0.01).
Discussion
Critically ill patients admitted to the ICU for gastrointestinal bleeding are at significant risk of developing TRALI, especially those with prior history of ESLD. Development of TRALI in patients with ESLD was most commonly associated with administration of FFP and nonleukoreduced units of PRBCs. In this study, we showed a clear temporal relationship (within 6 h) between transfusion and development of ALI. In addition, our data demonstrate that the presence of pre-existing ALI risk factors potentiate the risk of developing TRALI. Because medical ICU patients admitted for gastrointestinal bleeding receive a disproportionate amount of transfused blood components, understanding the risks of TRALI is critical when determining the necessity of transfusion in this critically ill patient population.
The association between TRALI and liver disease has been previously suggested in a subgroup analysis of 901 consecutive medical ICU patients who received transfusion. Among the 8% of patients who developed TRALI, 27% had liver disease, compared with only 15% in matched controls [3]. In a recent prospective study looking at the effects of male-only versus mixed-donor FFP transfusion on the development of pulmonary distress in surgical patients, baseline liver dysfunction was the only significant patient-specific risk factor for development of pulmonary distress on multivariate analysis [16]. In our study, 97% of the patients who developed TRALI had ESLD, though they made up only 48% of the study population.
FFP administration has been identified as an independent risk factor for ALI and TRALI in trauma, medical, and surgical ICU populations [3, 6, 7, 9, 17, 18]. In our study, 61% of patients with ESLD received FFP and 37% of these patients developed TRALI. The majority of TRALI cases (86%) in our cohort were temporally associated with transfusion of FFP. Despite the evidence that correction of prothrombin time using either FFP or factor VIIa has never been reported to improve hemostasis, decrease red cell transfusion needs, or improve outcome, FFP administration remains the standard of care in bleeding patients with ESLD and elevated prothrombin time [19, 20]. Given the risks of TRALI with administration of FFP, it seems prudent to examine the use of a more restrictive plasma transfusion approach in patients with ESLD.
Fresh frozen plasma from multiparous donors has been linked to TRALI in multiple epidemiologic studies [21, 22]. As a result, many countries have transitioned to male-only plasma transfusion. Our data do not show that plasma-containing blood components implicated in TRALI have a donor gender predilection. In fact, three patients received male-only plasma components and developed the clinical syndrome of TRALI. Unfortunately we were unable to compare the gender differences between plasma donors across our whole cohort to compare differences with those units implicated in TRALI.
Only nonleukoreduced PRBCs were associated with TRALI in our cohort. Clinical trials have not demonstrated that leukoreduction is protective against TRALI [23, 24]. In addition, this observation is of unclear clinical importance, as many blood centers in industrialized nations have switched to a policy of universal leukoreduction.
One limitation of this study is a relative inability to accurately differentiate ALI from hydrostatic edema. We conservatively used clinician documentation of ALI as a surrogate of “no clinical evidence of left atrial hypertension,” which is the current standard definition [12]. However, differentiation between TRALI and transfusion-associated circulatory overload (TACO) can be clinically challenging. Unfortunately, we were only able to analyze objective data regarding intravascular volume status on 50% (11/22) of our TRALI patients. In this subgroup, the majority (73%) had no objective evidence of intravascular volume overload. In those with volume overload we cannot rule out TRALI, because we know that hydrostatic edema commonly coexists with ALI [25]. In addition, critically ill patients who develop TACO alone without evidence of TRALI have lower 1-year mortality (7.8%) as compared with those who develop TRALI (43.2%) [26]. We believe that the high mortality in our patients (92%) is more consistent with TRALI as the mechanism of pulmonary edema rather than isolated hydrostatic edema (TACO). We acknowledge that the lower serum albumin in the group that developed TRALI could have also been a coexisting risk factor for development of pulmonary edema independent of transfusion [27]. Another limitation of this study is the inherent difficulty of identifying ALI risk factors in bleeding patients with ESLD [28]. It is possible that some TRALI patients who were classified as without ALI risk factors had occult aspiration or sepsis unidentified by the treating physician. Some experts argue that, in this scenario, ALI risk factors may be the sole cause of ALI, while the blood components transfused just prior to the development of ALI are a mere coincidence [29]. While this scenario is possible, epidemiologic studies in the critically ill suggest that ALI risk factors potentiate the risk of ALI from transfusion, and the pathophysiologic rationale for this observation is supported by the two-event model of TRALI [3, 6–8, 18, 19, 30].
Conclusions
TRALI is common in ESLD patients admitted to the ICU for gastrointestinal bleeding. Higher MELD score, presence of other ALI risk factors, FFP transfusion, and higher number of nonleukoreduced RBCs were associated with development of TRALI.
Supplementary Material
Acknowledgments
National Heart, Lung and Blood Institute, National Institute of Health; K24 HLO89223. National Institute of General Medical Sciences, National Institute of Health; Grant GM49222. American Gastroenterological Association Research Scholar Award.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s00134-010-1954-x) contains supplementary material, which is available to authorized users.
Contributor Information
Alexander B. Benson, Email: Alexander.benson@ucdenver.edu, Division of Pulmonary Sciences and Critical Care, University of Colorado Denver, Anschutz Medical Campus Research 2, Box C272, 9th floor, 12770 East 19th Ave., Aurora, CO 80045, USA, Tel.: +1-303-7246083, Fax: +1-303-7246042
Gregory L. Austin, Division of Gastroenterology and Hepatology, University of Colorado Denver, Aurora, CO 80045, USA
Mary Berg, Department of Pathology, University of Colorado Denver, Aurora, CO 80045, USA.
Kim K. McFann, School of Public Health, University of Colorado Denver, Aurora, CO 80045, USA
Sila Thomas, Bonfils Blood Center, Denver, CO, USA.
Gina Ramirez, Bonfils Blood Center, Denver, CO, USA.
Hugo Rosen, Division of Gastroenterology and Hepatology, University of Colorado Denver, Aurora, CO 80045, USA.
Christopher C. Silliman, Bonfils Blood Center, Denver, CO, USA
Marc Moss, Division of Pulmonary Sciences and Critical Care, University of Colorado Denver, Anschutz Medical Campus Research 2, Box C272, 9th floor, 12770 East 19th Ave., Aurora, CO 80045, USA.
References
- 1.Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, MacIntyre NR, Shabot MM, Duh MS, Shapiro MJ. The CRIT Study: anemia and blood transfusion in the critically ill—current clinical practice in the United States. Crit Care Med. 2004;32:39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D ABC (Anemia and Blood Transfusion in Critical Care) Investigators . Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 3.Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, O’Byrne MM, Evenson LK, Malinchoc M, DeGoey SR, Afessa B, Hubmayr RD, Moore SB. Transfusion-related acute lung injury in the critically ill: prospective nested case–control study. Am J Respir Crit Care Med. 2007;176:886–891. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorbi D, Gostout CJ, Peura D, Johnson D, Lanza F, Foutch PG, Schleck CD, Zinsmeister AR. An assessment of the management of acute bleeding varices: a multicenter prospective member-based study. Am J Gastroenterol. 2003;98:2424–2434. doi: 10.1111/j.1572-0241.2003.t01-1-07705.x. [DOI] [PubMed] [Google Scholar]
- 5.Garrido A, Marquez JL, Guerrero FJ, Pizarro MA, Leo E, Giraldez A. Transfusion requirements in patients with gastrointestinal bleeding: a study in a Blood Unit at a referral hospital. Rev Esp Enferm Dig. 2006;98:760–769. doi: 10.4321/s1130-01082006001000006. [DOI] [PubMed] [Google Scholar]
- 6.Chaiwat O, Lang JD, Vavilala MS, Wang J, MacKenzie EJ, Jurkovich GJ, Rivara FP. Early packed red blood cell transfusion and acute respiratory distress syndrome after trauma. Anesthesiology. 2009;110:351–360. doi: 10.1097/ALN.0b013e3181948a97. [DOI] [PubMed] [Google Scholar]
- 7.Gajic O, Rana R, Mendez JL, Rickman OB, Lymp JF, Hubmayr RD, Moore SB. Acute lung injury after blood transfusion in mechanically ventilated patients. Transfusion. 2004;44:1468–1474. doi: 10.1111/j.1537-2995.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- 8.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33:1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 9.Vlaar AP, Binnekade JM, Prins D, van Stein D, Hofstra JJ, Schultz MJ, Juffermans NP. Risk factors and outcome of transfusion-related acute lung injury in the critically ill: a nested case–control study. Crit Care Med. 2010;38:981–982. doi: 10.1097/CCM.0b013e3181cc4d4b. [DOI] [PubMed] [Google Scholar]
- 10.Marik PE, Corwin HL. Acute lung injury following blood transfusion: expanding the definition. Crit Care Med. 2008;36:3080–3084. doi: 10.1097/CCM.0b013e31818c3801. [DOI] [PubMed] [Google Scholar]
- 11.Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275:50–54. [PubMed] [Google Scholar]
- 12.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 13.Toy P, Popovsky MA, Abraham E, Ambruso DR, Holness LG, Kopko PM, McFarland JG, Nathens AB, Silliman CC, Stroncek D National Heart, Lung and Blood Institute Working Group on TRALI . Transfusion-related acute lung injury: definition and review. Crit Care Med. 2005;33:721–726. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 14.Vieillard-Baron A, Chergui K, Rabiller A, Peyrouset O, Page B, Beauchet A, Jardin F. Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med. 2004;30:1734–1739. doi: 10.1007/s00134-004-2361-y. [DOI] [PubMed] [Google Scholar]
- 15.Barbier C, Loubieres Y, Schmit C, Hayon J, Ricome JL, Jardin F, Vieillard-Baron A. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004;30:1740–1746. doi: 10.1007/s00134-004-2259-8. [DOI] [PubMed] [Google Scholar]
- 16.Nakazawa H, Ohnishi H, Okazaki H, Hashimoto S, Hotta H, Watanabe T, Ohkawa R, Yatomi Y, Nakajima K, Iwao Y, Takamoto S, Shimizu M, Iijima T. Impact of fresh-frozen plasma from male-only donors versus mixed-sex donors on postoperative respiratory function in surgical patients: a prospective case-controlled study. Transfusion. 2009;49:2434–2441. doi: 10.1111/j.1537-2995.2009.02321.x. [DOI] [PubMed] [Google Scholar]
- 17.Gajic O, Dara SI, Mendez JL, Adesanya AO, Festic E, Caples SM, Rana R, St Sauver JL, Lymp JF, Afessa B, Hubmayr RD. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32:1817–1824. doi: 10.1097/01.ccm.0000133019.52531.30. [DOI] [PubMed] [Google Scholar]
- 18.Khan H, Belsher J, Yilmaz M, Afessa B, Winters JL, Moore SB, Hubmayr RD, Gajic O. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. 2007;131:1308–1314. doi: 10.1378/chest.06-3048. [DOI] [PubMed] [Google Scholar]
- 19.Bosch J, Thabut D, Bendtsen F, D’Amico G, Albillos A, Gonzalez Abraldes J, Fabricius S, Erhardtsen E, de Franchis R European Study Group on rFVIIa in UGI Haemorrhage. Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: a randomized, double-blind trial. Gastroenterology. 2004;127:1123–1130. doi: 10.1053/j.gastro.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Tripodi A, Mannucci PM. Abnormalities of hemostasis in chronic liver disease: reappraisal of their clinical significance and need for clinical and laboratory research. J Hepatol. 2007;46:727–733. doi: 10.1016/j.jhep.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Navarrete C, Lucas G, Soni N, Morgan C, Choo L, Cohen H, Williamson LM Serious Hazards of Transfusion Steering Group . Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49:440–452. doi: 10.1111/j.1537-2995.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 22.Eder AF, Herron R, Strupp A, Dy B, Notari EP, Chambers LA, Dodd RY, Benjamin RJ. Transfusion-related acute lung injury surveillance (2003–2005) and the potential impact of the selective use of plasma from male donors in the American Red Cross. Transfusion. 2007;47:599–607. doi: 10.1111/j.1537-2995.2007.01102.x. [DOI] [PubMed] [Google Scholar]
- 23.Dzik WH, Anderson JK, O’Neill EM, Assmann SF, Kalish LA, Stowell CP. A prospective, randomized clinical trial of universal WBC reduction. Transfusion. 2002;42:1114–1122. doi: 10.1046/j.1537-2995.2002.00182.x. [DOI] [PubMed] [Google Scholar]
- 24.Watkins TR, Rubenfeld GD, Martin TR, Nester TA, Caldwell E, Billgren J, Ruzinski J, Nathens AB. Effects of leukoreduced blood on acute lung injury after trauma: a randomized controlled trial. Crit Care Med. 2008;36:1493–1499. doi: 10.1097/CCM.0b013e318170a9ce. [DOI] [PubMed] [Google Scholar]
- 25.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. New Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Kojicic M, Reriani MK, Fernandez Perez ER, Thakur L, Kashyap R, Van Buskirk CM, Gajic O. Long-term survival and quality of life after transfusion associated pulmonary edema in critically ill medical patients. Chest. 2009;137:783–789. doi: 10.1378/chest.09-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangialardi RJ, Martin GS, Bernard GR, Wheeler AP, Christman BW, Dupont WD, Higgins SB, Swindell BB. Hypoproteinemia predicts acute respiratory distress syndrome development, weight gain, and death in patients with sepsis. Crit Care Med. 2000;28:3137–3145. doi: 10.1097/00003246-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Bernard B, Grange JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29:1655–1661. doi: 10.1002/hep.510290608. [DOI] [PubMed] [Google Scholar]
- 29.Kleinman S, Caulfield T, Chan P, Davenport R, McFarland J, McPhedran S, Meade M, Morrison D, Pinsent T, Robillard P, Slinger P. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44:1774–1789. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 30.Silliman CC. The two-event model of transfusion-related acute lung injury. Crit Care Med. 2006;34(5 Suppl):S124–S131. doi: 10.1097/01.CCM.0000214292.62276.8E. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.