Abstract
Environmental enrichment (EE) has been shown to exert various behavioral and mood effects in rodents including emotionality, which has a high propensity to be influenced by sex. However, there are only a few comparative studies evaluating the effect of EE and their results are both inconsistent and inconclusive. In the present study, male and female C57BL/6J adolescent mice were housed in either physical enrichment or standard conditions for four weeks with analysis of affective behaviors in the open field, elevated T-maze and forced swim tests. Hippocampal gene expression was characterized in an additional group of mice. In the open field test, exploration was similarly inhibited by EE in male and female mice. Both sex and housing condition influenced the time mice spent in the center of the arena. In the elevated T-maze, anxiety-like behavior was increased in female and decreased in male mice following EE. We observed a trend for EE-induced inhibition of glucocorticoid receptor (GR) mRNA expression in male but not in female mice. In contrast, mineralocorticoid receptor (MR) expression was unaffected by 10 days of physical enrichment but was lower in female mice compared to male mice. Our data suggest that the balance between hippocampal GR and MR may contribute to the observed sex-specific effect of physical enrichment on emotionality-related behavior.
Keywords: environmental enrichment, C57BL/6J, behavior, anxiety, glucocorticoid receptor, mineralocorticoid receptor, hippocampus, emotionality
Introduction
Manipulation of the physical and social macroenvironment can lead to significant alterations in brain structure and function including behavior [17,44]. Environmental enrichment (EE) is a well-studied paradigm of such a manipulation that has received much interest and is widely used to investigate gene environment interactions. Living in an enriched environment, which consists of increased space for exploration filled with a variety of inanimate objects that facilitates enhanced sensory, cognitive and motor stimulation, has been shown to induce many beneficial effects in both rodents and primates [17,44,53]. Enrichment has been shown to improve cognition, reduce memory decline in aged animals, decrease anxiety and facilitate recovery from a brain lesion [37,38,44,63]. At the cellular and molecular level, EE has been shown to increase cortical weight and thickness [26,51], increase dendritic branching and length, the number of dendritic spines and the size of synapses on some neuronal populations [25,29,30,38]. Moreover, EE increases hippocampal neurogenesis and the integration of these newly born cells into functional circuits [30]. Environmental enrichment also leads to a plethora of changes in gene expression [50]. Many of these changes are associated with neuronal structure, synaptic plasticity and transmission. However, the roles of the majority of these molecular changes remain to be determined.
Although there are a number of studies examining the effects of EE on behavior, the majority of these studies used only subjects of the same sex. However, certain effects of EE may be sex-specific. For examples, EE improves motor coordination in a genetic model of Rett’s syndrome (heterozygous Mecp2+/−) only in females and not in males [33]. In partially trisomic Ts65Dn mice, a genetic model for Down syndrome, EE reduces circadian activity in females but enhances activity in males. Furthermore, EE improved performance of female Ts65Dn mice but deteriorated performance in Ts65Dn male mice in a spatial learning task [41]. In another study, EE increased social exploratory behavior in male but not in female rats [47].
Affective behaviors have a high propensity to be influenced by sex. Stress-related disorders such as anxiety and depression are much more prevalent in women compared to men [7,34]. Female animals appear to be more susceptible to negative emotional responses when exposed to stress [2,62]. While there are a few studies that characterized the effects of EE on emotional behaviors using both males and females, the findings have been inconsistent [1,4,31,67]. One study of 7 weeks EE reported no difference between male and females in the plus-maze test, locomotor activity test, hot plate test, forced swim test and resident intruder test [1]. On the other hand, other studies have shown that EE reduced freezing behavior following predator exposure only in females [31] and decreased adrenocorticotropic hormone (ACTH) in females but not in males, following a mild stressor [4]. In contrast, others reported increase in anxiety-like behavior in the elevated plus maze in mice subjected to 4 months of enriched housing, with a more prominent effect on female mice [67].
The present study therefore sought to clarify these discrepancies and to compare the effect of EE on emotional responses in both sexes. In addition, we examined changes in hippocampal gene expression in male and female mice with 10 days EE, as these early molecular changes may be involved in the observed behavioral effects.
Materials and Methods
Animals
Male and female C57BL/6J mice (Charles River Laboratories, Wilmington, MA, USA) were housed in either standard or enriched housing from 3 weeks of age (n=25 per sex per housing condition). Mice were housed in groups of five per cage under a 12h light/dark cycle (lights off at 1800 hr), with food and water provided ad libitum. Animals assigned to the enriched housing condition were housed in big plastic tubs (70 cm × 45 cm × 43 cm) containing a running wheel, habitrail, a standard cage for food and water access (31 cm × 17 cm × 14 cm),and various plastic objects (Fig. 1). The objects were re-arranged once per week to increase novelty in the environment. All use of animals was approved by the Ohio State University Animal Care and Use Committee, and was in accordance with the NIH guidelines.
Fig. 1.
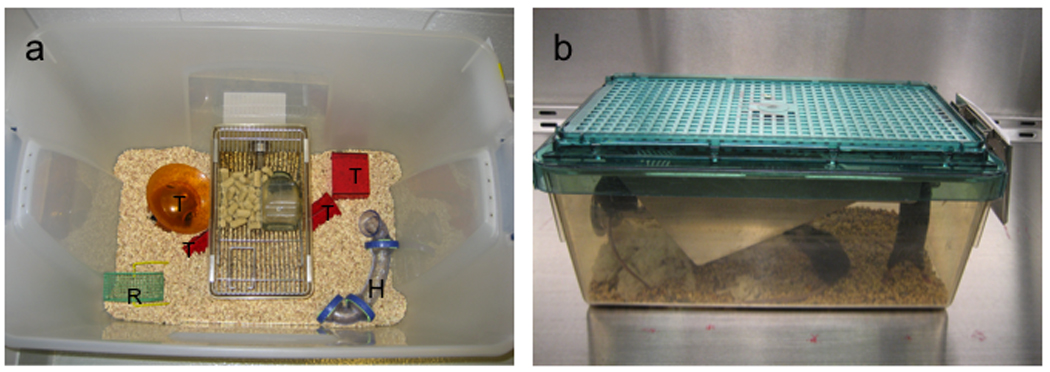
(a) Environmental enrichment housing. (b) Standard control housing. H, habitrail; R, running wheel; T, plastic toys.
Behavioral analysis
After 4 weeks of housing in the different conditions, mice were subjected to behavioral testing in the following order: (1) open field test, (2) elevated T-maze and (3) forced swim test. Different tests were conducted at least 2 days apart and all tests were conducted between 1300 and 1730 hr.
Open field test (OF)
To assess exploration and general motor activity, mice were placed individually into the center of an open square arena (60 cm × 60 cm, enclosed by walls of 48 cm). Each mouse was allowed 10 minutes in the arena, during which time its activity was recorded and analyzed by TopScan (Clever Sys Inc, Vienna, VA, USA). Specifically, the parameters measured include distance traveled in the periphery and the center of the arena (36 cm × 36 cm), the total distance traveled, and the time spent in the center of the arena. The total distance traveled provides a measure of exploratory activity while the time and distance ratio of arena center exploration provide indication of anxiety level. The arena was cleaned with 30% ethanol between trials to remove any odor cues.
Elevated T-maze
The elevated T-maze is an ethologically-based approach-avoidance conflict test targeting the natural conflict between the tendency of mice to explore a novel environment and the tendency to avoid a brightly lit open area [43]. The T-maze consists of two open arms (30.5 cm × 15.5 cm) and an enclosed arm (46 cm × 10 cm) positioned in the shape of a ‘T’, with the enclosed arm as the stem of the T. The whole apparatus was elevated 88cm above the floor. The open and enclosed arms of the T-maze generate exploratory behavior and the avoidance of elevated open arms is an indication of the intensity of anxiety. Each mouse was placed at the end of the closed arm facing toward the open arms and was allowed to explore the maze for 5 minutes. The behavior and movement of each mouse was recorded by a video camera and subsequently scored by a blinded experimenter. Anxiety was indicated by the time spent on the open arms as well as the number of open arm entries. In addition, the latency to enter the open arms was used as a further indication of anxiety level. After each test, mouse was returned to its home cage and the maze was cleaned with 30% ethanol.
Forced swim test
Forced swim test (FST) is one of the most commonly used rodent behavioral tests for screening antidepressant drugs [13]. Mice were placed individually in a transparent cylinder (21 cm diameter, 24 cm height) containing water (25 ± 2°C) to a depth of 15 cm for 6 minutes. At the end of each trial, mice were dried and returned to their home cage. Trials were video-recorded and the amount of time mice remained immobile was scored by a blinded experimenter as a measure of depressive-like behavior.
Short-term enrichment study
A separate group of animals (n=5 per sex per housing condition) was examined for early molecular changes following enriched housing. Male and female mice were housed under different conditions as described above. Ten days later, mice were culled by decapitation under deep isoflurane anaesthesia. Brain was quickly removed and the dorsal hippocampus was dissected on ice. Tissue samples were frozen on dry ice and stored in −80°C until subsequent analysis.
Quantitative RT-PCR
Total RNA from hippocampal tissue was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instruction. First-strand cDNA was generated using TaqMan Reverse Transcription Reagent (Applied Biosystems, Roche, Branchburg, NJ, USA) and quantitative PCR was carried out using a LightCycler Sequence Detection System (Roche, Indianapolis, IN, USA) with the Power SYBR Green PCR Master Mix (Applied Biosystems). We designed primers to detect the following mouse mRNA: Gapdh (Glyceraldehyde 3-phosphate dehydrogenase), C-fos, Bdnf (brain-derived neurotrophic factor), Npy (neuropeptide Y), Vegf (Vascular endothelial growth factor), Nr3c1 (nuclear receptor subfamily 3, group C, member 1, also known as glucocorticoid receptor) and Nr3c2 (also known as mineralocorticoid receptor). Primer sequences are listed in Table 1. PCR data analysis was performed using the comparative 2−ΔΔCT method with Gapdh as an endogenous reference [55].
Table 1.
Primers used in quantitative RT-PCR
| Forward | Reverse | |
|---|---|---|
| Gapdh | TCCCACTCTTCCACCTTCGA | TGCTGTAGCCGTATTCATTGTCA |
| C-fos | GACAGCCTTTCCTACTACCATTCC | CGCAAAAGTCCTGTGTGTTGA |
| Bdnf | CCATAAGGACGCGGACTTGT | AGGCTCCAAAGGCACTTGACT |
| Npy | CTCCGCTCTGCGACACTACA | AGTGTCTCAGGGCTGGATCTCT |
| Vegf | TACCTCCACCATGCCAAGTG | CATGGGACTTCTGCTCTCCTTCT |
| Nr3c1 | CAGCATGCCGCTATCGAAA | CGCGGCAGGAACTATTGTTTT |
| Nr3c2 | TGTCTCAGACCTTGGAGCGTTC | TTGTTCGGAGTAGCACCGGAA |
For graphical and statistical analysis, the mRNA level for each mouse was expressed as fold change over the mean value of standard housing male control group.
Statistical analysis
Statistical analysis was performed using JMP software (SAS Institute Inc., Cary, NC, USA). Two-way analysis of variance (ANOVA) was used to assess the main effects ‘housing’ and ‘sex’ and their interaction. When the interaction effect did not reach statistical signficance, the main effects were analysed independently of the interaction effect. Planned contrast analysis was used to test the locus of significant main effects and interaction when present. Statistical significance was set at P < 0.05. All data are presented as means ± standard error of the mean (S.E.M). Results of P values for the main effects and the interaction effect are presented in Table 2 (for behavioral tests) and Table 3 (for quantitative RT-PCR results).
Table 2.
Statistical analysis of behavioral test results
| Parameter | Sex x Housing Interaction |
Sex Effect | Housing Effect |
|---|---|---|---|
| Open Field | |||
| Total Distance | P = 0.5113 | P = 0.0023 | P < 0.0001 |
| Center Distance | P = 0.2966 | P = 0.0854 | P < 0.0001 |
| Periphery Distance | P = 0.7791 | P < 0.0001 | P = 0.0023 |
| Center Time | P = 0.5204 | P = 0.0018 | P = 0.0373 |
| Center/Total Distance Ratio | P = 0.1772 | P = 0.2797 | P = 0.0528 |
| T-maze | |||
| Open Arm Latency | P = 0.0368 | P = 0.1306 | P = 0.0147 |
| Open Arm Entries | P = 0.0767 | P = 0.3510 | P = 0.0118 |
| Open Arm Time | P = 0.0084 | P = 0.4795 | P = 0.2496 |
| Forced Swim Test | |||
| Latency | P = 0.0649 | P = 0.3978 | P = 0.0314 |
| Immobility Time | P = 0.4821 | P = 0.0381 | P = 0.1439 |
Table 3.
Statistical analysis of quantitative RT-PCR results
| Gene transcript | Sex x Housing Interaction |
Sex Effect | Housing Effect |
|---|---|---|---|
| C-fos | P = 0.3875 | P = 0.0243 | P = 0.0010 |
| Bdnf | P = 0.2062 | P = 0.8968 | P = 0.8418 |
| Vegf | P = 0.1022 | P = 0.5861 | P = 0.5947 |
| Npy | P = 0.3938 | P = 0.7945 | P = 0.3600 |
| Nr3c1 | P = 0.2407 | P = 0.7770 | P = 0.0701 |
| Nr3c2 | P = 0.5966 | P = 0.0117 | P = 0.9315 |
Results
Open field test
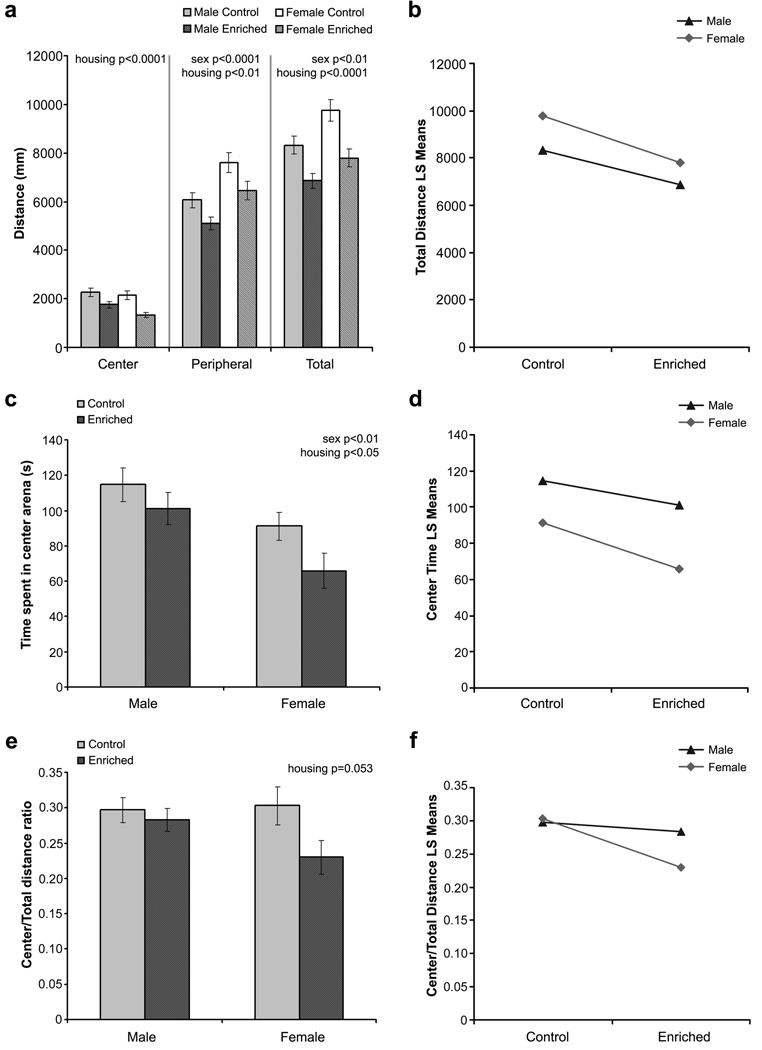
Three animals (1 standard housing female and 2 enriched housing females) were excluded from analysis because of incomplete recording. For total distance traveled in the open field, there was no significant interaction effect between sex and housing as determined by two-way ANOVA (F1,96 = 0.435, P = 0.51). Analysis of the main effects showed a significant sex effect (F1,96 = 9.86, P < 0.01, Fig. 2a & 2b), due to a greater locomotor activity exhibited by the female mice. A strong housing effect was also observed (F1,96 = 20.47, P < 0.0001, Fig. 2a & 2b), with enriched mice exhibiting reduced locomotion irrespective of sex. When analyzing the distance traveled by zones, similar effects were observed for locomotor activity in the periphery of the open field (sex x housing interaction: F1,96 = 0.079, P = 0.78; sex effect: F1,96 = 18.54, P < 0.0001; housing effect: F1,96 = 9.82, P < 0.01; Fig. 2a). Female mice exhibited more locomotor activity in the periphery compared to male mice, whereas enriched mice traveled less in the periphery compared to standard housing mice. Exploratory activity in the center zone was significantly different between standard and enriched housing mice (housing effect: F1,96 = 19.85, P < 0.0001; Fig. 2a), with enriched mice exhibiting less exploratory activity in the center zone compared to standard housing controls. There was no significant interaction effect between sex and housing (sex x housing interaction: F1,96 = 1.10, P = 0.30) and no main sex effect (F1,96 = 3.02, P = 0.085) for this parameter.
Fig. 2.
Mice performance in the open field test. (a) Distance traveled in the different regions of the open field and total distance traveled. (b) Interaction plot for total distance traveled. (c) Time spent in the center of the open field. (d) Interaction plot for time spent in the center zone. (e) Ratio of distance traveled in the center zone of the open field. (f) Interaction plot for center to total distance ratio. All data are presented as mean ± standard error of the mean (S.E.M).
In addition to its utility in evaluating the general motor activity of animals, this test also draws on the natural conflict in mice between the tendency to explore a novel environment and to avoid an exposed open area [12,15] and is now commonly used as a test to screen for changes in anxiety level [5,27]. An increase in time spent in the center of the open field is often considered to reflect reduced anxiety. There was no significant interaction between sex and housing conditions (F1,96 = 0.416, P = 0.520). Both housing and sex appear to affect the time spent in the center arena (sex effect: F1,96 = 10.31, P < 0.01; housing effect: F1,96 = 4.46, P < 0.05; Fig. 2c & 2d). Male mice spent more time in the center of the open field compared to female mice. On the other hand, enriched mice exhibited reduced center time.
Another measure used to examine anxious behaviors in the OF is the proportion of distance that the mouse traveled in the center zone (center/total distance), which allows for the adjustment of difference in locomotor activity between groups. In this parameter, we did not observe a significant interaction effect (F1,96 = 1.85, P = 0.18). Main effect analysis revealed an almost significant housing effect (F1,96 = 3.84, P = 0.053), but no significant sex effect (F1,96 = 1.18, P = 0.28). This housing effect was due to reduced center/total distance ratio in the enrichment group (P = 0.053), which was most likely due to the female mice as shown in Fig. 2e and Fig. 2f.
Elevated T-maze
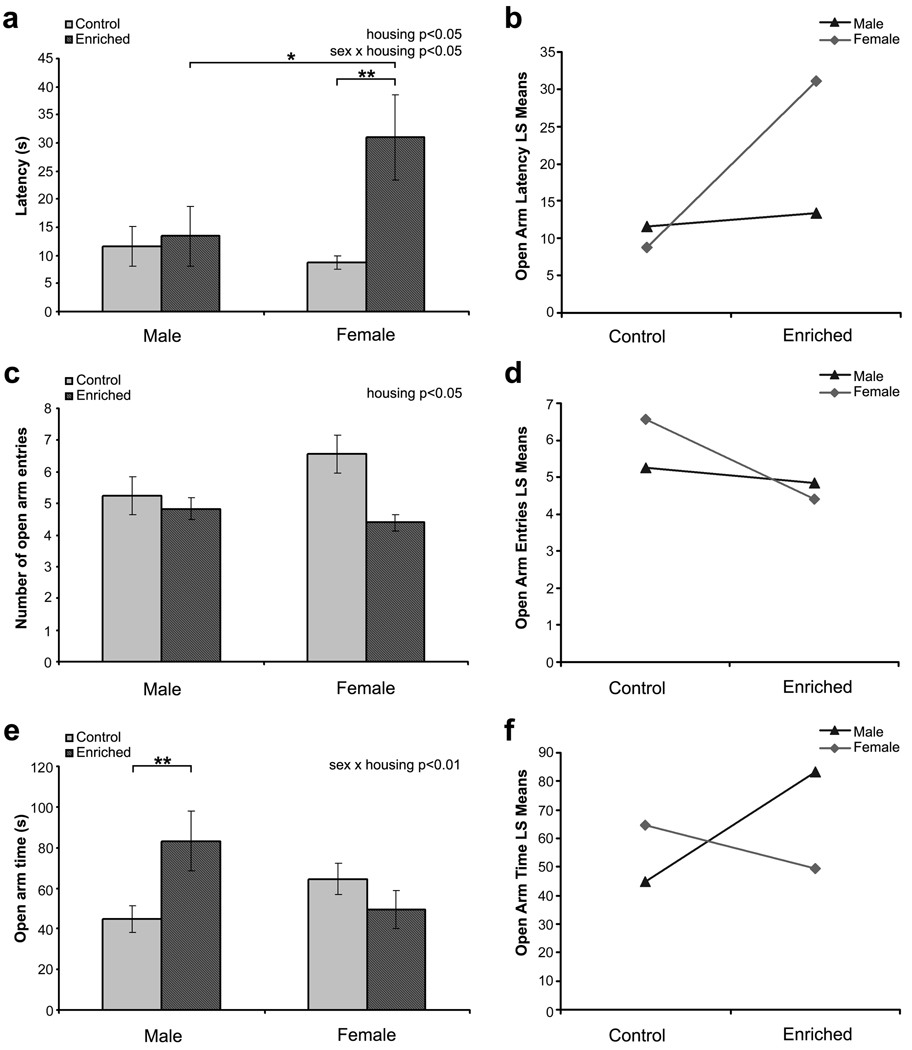
Three animals (2 enriched housing females and 1 enriched housing male) were excluded from analysis because of problems in video recording. Two-way ANOVA revealed a significant interaction between sex and housing effects on the latency to open arm entry (sex x housing interaction: F1,96 = 4.49, P < 0.05; sex effect: F1,96 = 2.33, P = 0.13; housing effect: F1,96 = 6.18, P < 0.05). This interaction was due to the differential effect of enrichment on the latency to open arm entry in male and female mice (Fig. 3a & 3b). While enrichment increased the latency by 3.5 fold in female mice (P < 0.01), it had no effect on the male mice (Fig. 3a). Latency to open arm was comparable between male and female control mice housed in standard condition.
Fig. 3.
Mice performance in the elevated T-maze test. (a) Latency to enter the open arms. (b) Interaction plot for latency to enter the open arms. (c) Number of entries made into the open arms. (d) Interaction plot for open arm entries. (e) Time spent in the open arms. (f) Interaction plot for open arm time. All data are presented as mean ± S.E.M. * P < 0.05, ** P < 0.01.
The number of open arm entries was only significantly influenced by housing condition (housing effect: F1,96 = 6.59, P < 0.05; Fig. 3c). LS Means Plot showed that this was due to the fewer entries made by enriched mice compared to standard housing controls (P < 0.05). However, it is interesting to note that environmental enrichment caused a greater reduction in open arm entries in females compared to males (Fig. 3d).
A significant interaction effect between sex and housing was observed for the total time spent on open arm (F1,96 = 7.26, P < 0.01, Fig. 3e & 3f). This was due a two-fold increase in the time spent on the open arm by the enriched male mice compared to male controls (P < 0.01; Fig. 3e). In this parameter, no significant difference was detected for female mice of the two housing groups.
Forced swim test (FST)
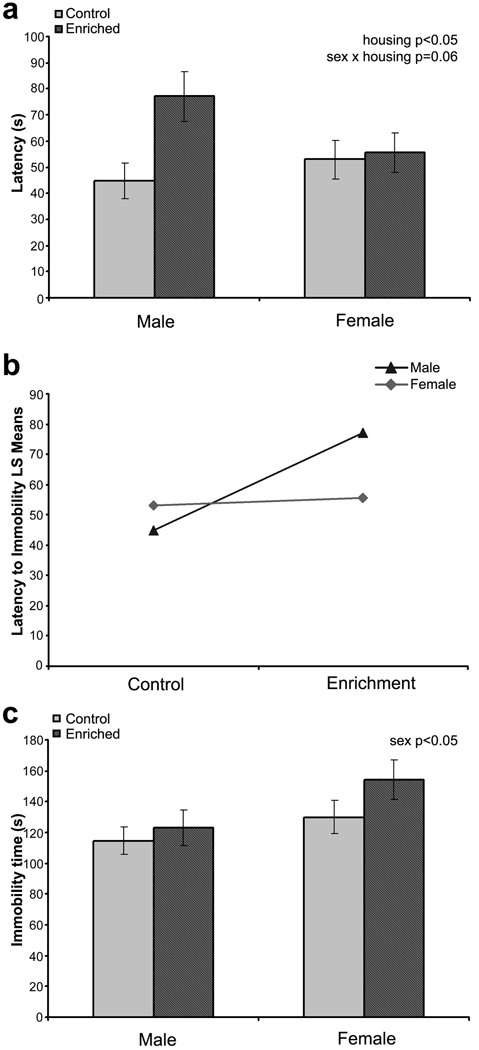
Three female mice and two male mice were excluded from this study because of problems in video recording. A trend for interaction effect between sex and housing was observed (F1,94 = 3.49, P = 0.06). Considering the main effects alone, only housing significantly influenced this parameter (F1,94 = 4.77, P < 0.05), with enriched mice exhibiting delayed latency to immobility (Fig. 4a & 4b). Interaction plot showed that the effect of enrichment in delaying latency to immobility was mainly observed in male mice rather than in female mice (Fig. 4b).
Fig. 4.
Mice performance in the forced swim test. (a) Latency to immobility. (b) Interaction plot for latency to immobility. (c) Total immobility time. All data are presented as mean ± S.E.M.
No significant interaction between sex and housing was observed in the total immobility time in the FST. However, this parameter was significantly different between male and female mice (sex effect: F1,94 = 4.43, P < 0.05), with female mice exhibiting longer immobility time (P < 0.05; Fig. 4c).
Hippocampal gene expression
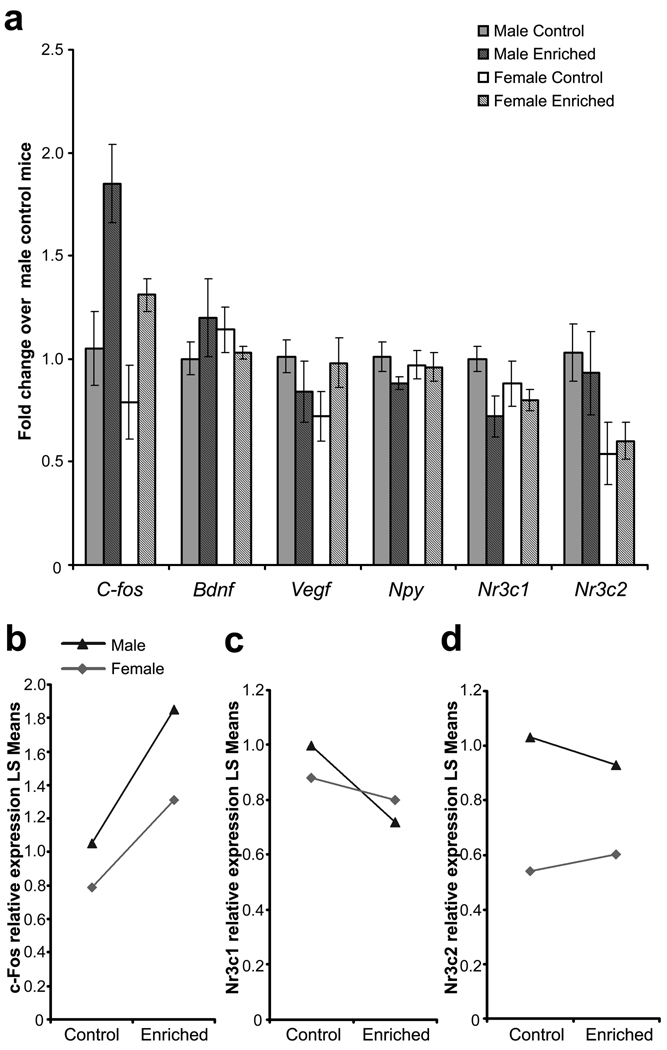
Using quantitative RT-PCR, we assessed the relative expression of six genes implicated in hippocampal dependent behaviors and neuronal plasticity in the dorsal hippocampus ten days after enrichment housing. This time point was chosen because it allowed enough time for the animals to acclimatize to the new housing environment, thereby avoiding the potential confounding stress effect due to the initial cage change. However, the time was relatively short to allow us to assess the early effects of environmental enrichment on gene expression. Of the six genes assessed, there was no significant difference in hippocampal Bdnf, Vegf and Npy expression due to housing or sex (Fig. 5a). For C-fos, significant effects for housing (F1,17 = 16.50, P = 0.001) and sex (F1,17 = 6.27, P < 0.05; Fig. 5a) were detected. This was due to the upregulation of C-fos expression by environmental enrichment and a greater C-fos expression in the male mice (Fig. 5b). It was interesting to note that there was a trend for housing effect in hippocampal glucocorticoid receptor Nr3c1 mRNA expression (F1,17 = 3.80, P = 0.07; Fig. 5c), with enrichment groups exhibiting lower Nr3c1 mRNA expression. As shown in Fig. 5a and 5c, this effect was more evident in the males compared to females. Unlike glucocorticoid receptor, gene expression of the other steroid receptor, mineralocorticoid receptor Nr3c2, was affected by sex and not housing (sex effect: F1,17 = 8.23, P < 0.05), with female mice showing lower hippocampal Nr3c2 expression independent of housing conditions (Fig. 5a & 5d).
Fig. 5.
(a) Hippocampal mRNA expression 10 days after differential housing. Data are presented as fold change over the mean value of male standard housing control group. All data are presented as means ± S.E.M. (b) Interaction plot for C-fos mRNA expression. Housing effect, P = 0.001; Sex effect, P < 0.05. (c) Interaction plot for Nr3c1 mRNA expression. (d) Interaction plot for Nr3c2 mRNA expression. Sex effect, P < 0.05.
Discussion
Environmental enrichment has previously been shown to have a number of behavioral effects including changes in emotionality. Here, we show that EE produced sex-specific effects on certain affective behaviors in C57BL/6J mice, one of the most commonly used strains and the basis for many transgenic lines. Of interest, our data suggest that four weeks of physical enrichment during the adolescence phase is anxiolytic in male mice but anxiogenic in female mice. This sex-specific effect may in part be caused by a differential effect of physical enrichment on hippocampal steroid receptor expression.
Our study showed that EE reduced the distance traveled in the open field test in both male and female mice. This inhibitory effect on locomotion has been reported previously [32,49,67], although to date, existing literature on the effect of EE on locomotion and exploration in mice remains inconsistent. Even in studies using the same mouse strain, both increases and decreases of locomotion caused by EE have been reported [1,32,49,57,67]. The reason for these conflicting results is still unclear and cannot be explained simply by sex, age of animals, duration of enrichment, social grouping or time of behavioral testing, since none of these variables consistently produced the same locomotor effects. It is likely that a combination of these factors, in addition to other factors such as handling, testing sequence and experiences, culminates in the final observed behavioral effect. Here we show that physical enrichment exerted similar inhibitory effects on locomotion in male and female mice. This effect has been suggested to be the result of enhanced habituation in the enriched animals [9,56,60]. Reduced locomotor activity in a novel environment over time is an indication of acclimation and has been used as an index of simple information procession [60]. The accelerated habituation in the open field, a novel environment, might be a reflection of more efficient information processing by the enrichment-reared mice, likely a consequence of their greater experience with a novel and changing environment.
Early studies on the effect of EE on emotionality tended to reveal an anxiolytic action [6,17,19]. Indeed, it was shown that EE could even protect against or reverse the negative effects of stress on emotionality-related measures [18,36,54]. However, there is increasing evidence for a more complex function of EE on emotionality, with several studies reporting an anxiogenic-like effect [24,32,67]. Results from our study lend further support to the view that the effects exerted by EE are not simply positive as generally perceived, and may be influenced by various factors including sex. We have shown here that while four weeks of physical enrichment exerted a mild anxiolytic effect on male mice, as indicated by an increase in the open arm time in the T-maze, it had the opposite effect on female mice. Female mice showed a considerable delay in latency to enter the open arms of the T-maze, suggesting an increase in their anxiety level. Although the interaction effects between sex and housing of the other behavioral parameters examined did not reach statistical significance, it is interesting to note that the overall trend of these parameters is consistent with a possibly greater anxiogenic effect of EE on female mice. These trends were revealed in the LS Means plots. In the open field test, reductions in the center time and the center to total distance ratio by EE were mainly due to the effect on female mice. In the T-maze, the reduction in the number of open arm entries by EE was also mainly due to EE’s effect on female mice, as EE male mice showed comparable open arm entries as their standard housing controls.
The results of our study are in partial agreement with the study by Zhu et al. (2006), which also examined male and female C57BL/6J mice housed in physical enrichment from postnatal day 21 [67]. Similar to our study, the same number of animals was housed in both the standard and enriched condition, eliminating the additional social stimulation component as used in some enrichment paradigms. In their study, Zhu and colleagues also observed an increase in anxiety-like behavior in the enriched female mice in the elevated plus maze as well as food neophobia. Interestingly, in the elevated plus maze, they also observed a significant interaction between sex and housing, although for different parameters of the test. While EE reduced the percentage of open arm time in female mice, it did not affect male mice. Zhu et al. also reported a reduction in the percentage of total entries into open arms for the enriched male mice, which may be a sign of increased anxiety. However, in light of the increase in total arm entries, it is possible that the reduction in the percentage of open arm entries was a result of enhanced exploration in the male mice. Our data is however in disagreement with the recent report by Sztainberg et al. (2010), which showed an anxiolytic effect by EE in female C57BL/6J mice after 6 weeks of enrichment [57]. One important difference between this study and ours is the larger social group in their enrichment paradigm. While we used the same number of mice for each group, thereby studying only the physical aspect of enrichment, they used a larger group (12 mice per cage) in the EE condition compared to standard conditions (4 mice per cage). The additional social stimulation may have significant impact on emotionality and contributed to the observed differences between the studies. Indeed, there are studies that demonstrate differential effects of physical and social enrichment on various parameters, such as exploration, information processing and recovery from inflammatory pain [16,20,48]. It is interesting to note that the majority of studies showing an anxiolytic response following EE in females used a paradigm with increased social stimulation [21,47], highlighting the possibility that the social component may be critical in conferring a positive effect on emotionality by EE in females. Future studies comparing the physical and social enrichment effect on emotionality-related behaviors in male and female mice will yield insight into the specific contribution and interaction of these different aspects of enrichment. Another important difference between the present study and the studies of Zhu et al. (2006) and Sztainberg et al. (2010) is the different length of EE exposure and hence their behaviors were tested at different stages of sexual maturity. Both basic and clinical findings suggest an involvement of gonadal hormones in shaping brain plasticity in key emotional centers and that they may be important in modulating stress responsiveness [22,23]. Therefore, it is possible that the different behavioral outcomes observed in these studies were partially contributed by the different time exposures to EE after sexual maturation and the effect of EE acting upon the different levels of sex steroids.
Physical enrichment exerted a subtle effect on depressive-like behavior and delayed the latency to immobility in enriched male mice. Although the increase in total immobility time in the female enriched mice was not significant compared to female standard housing controls, it was significantly higher compared to male mice housed in either standard housing or enriched condition. The delayed latency to immobility may be considered a delayed emergence of despair behavior, and is consistent with other studies demonstrating EE’s antidepressive-like effect [9,24,39,40]. The lack of a significant difference in the total immobility time may have been due in part to the difference in species (the antidepressive effect appears to be more readily detectable in rats compared to mice) [1,9,24,40], duration of the EE paradigm [39] and possibly the presence of additional social stimulation [24]. The significant sex effect in total immobility time and the difference between male and female mice under enriched conditions but not standard housing conditions is consistent with the notion that EE may produce sex-dependent differential effects on emotionality.
The underlying basis for the sex-specific effects of EE on emotionality-related behavior is largely unexplored. One rationale may be that EE represents a mild stress situation, supported by previously reported increases in serum corticosterone [11,42], and males and females have different threshold for stress tolerance. This is supported by previous studies showing female mice are more sensitive to mild stress compared to male mice [2,31]. Hence with males, the mild form of stress associated with EE (due to increased novelty and physical stimulation) leads to stress inoculation and confers a protective effect, to females, this may represent a chronic stress situation.
To further understand the mechanism underlying the observed sex-dependent effects by EE, we analyzed gene expression in the dorsal hippocampus of mice that were housed in either enriched or standard housing without exposure to behavioral testing. Hippocampal C-fos expression was significantly up-regulated following physical enrichment in both male and female mice indicating hippocampal activation, which is consistent with numerous reports showing that the hippocampus is a key brain region influenced by EE [30,59]. Interestingly, we observed a trend for EE to down-regulate hippocampal GR expression and the effect was prominent in male mice but not in female mice. While our observation is in disagreement with some early reports of an EE-induced up-regulation of GR [46], it is consistent with the observed differential effect on anxiety-like behavior observed following EE. Recently, it was shown in C57BL/6J mice that lower trait anxiety and resistance to acute stressors is associated with reduced levels of GR mRNA under basal conditions [28]. Moreover, mice with deficient GR expression in the brain exhibit reduced anxiety [58]. In contrast, transgenic overexpression of GR in the forebrain enhances anxiety-like behavior in mice [61]. Polymorphisms in the GR gene have been found to correlate with the corticosteroid response to stress in both mice and human [64,65] and GR antagonists were shown to improve the mood of patients affected by either psychotic depression or bipolar disorder [3,66].
Interestingly, we observed a significant sexual dimorphism in the expression of hippocampal MR, with female mice expressing less MR compared to male mice. This difference has not been well-documented previously [8], although the MR mRNA expression analysis in the recent report by Rozeboom et al. (2007) seemed to reveal a slight decrease in the female mice compared to males. The same study demonstrated that transgenic overexpression of MR in forebrain area reduced anxiety-like behavior in mice, indicating a potentially anxiolytic role by MR [52]. On the other hand, forebrain-specific inactivation of the MR gene enhanced emotional arousal and impaired adaptive behavior in relation to stress [10]. It has been proposed that the hippocampal GR:MR ratio may be critical for stress responsiveness and behavioral adaptation [14,45]. Therefore, it is plausible that the baseline difference in GR:MR ratio (females have a much higher GR:MR ratio due to lower MR expression) may contribute to the heightened sensitivity to mild stress presented in EE condition and also a poorer adaptive response. Also, although it is yet unclear why EE induced a reduction in hippocampal GR specifically in male and not in female mice, this change further accentuates the GR:MR ratio difference favoring better stress adaptation in male mice. Thus, the difference in steroid receptor expression may be one of the mechanisms by which EE exerts different effects on male and female mice. In addition, brain regions involved in stress regulation and emotional affects such as the amygdala and hippocampus, continue to mature well into adolescence [35]. Therefore, it is likely that some of the observed sex differences in behavioral stress responsiveness were related to developmental differences in these brain structures and that experiences and steroid hormones affect the plasticity of these brain regions.
In conclusion, we have shown a sex-specific effect of physical enrichment on emotionality in adolescent C57BL/6J mice. Long-term physical enrichment increased anxious behaviors in female mice but had a mild anxiolytic effect in male mice. This result is consistent with a difference in stress-sensitivity and adaptive ability due to an altered hippocampal GR:MR ratio. Our study highlights the importance of considering sexual dimorphism in studies of experience-dependent effects on emotionality-related systems. In particular, paradigms involving mild forms of stress, such as environmental enrichment or novelty-induced stimulation, may lead to significantly different behavioral responses depending on the sex of the test subjects.
Research Highlights.
Environmental enrichment affects affective behavior in C57BL/6J adolescent mice.
Physical environmental enrichment reduces anxiety in male mice.
Physical environmental enrichment increases anxious behavior in female mice.
Hippocampal steroid receptor ratio may contribute to this sex-specific effect of EE.
Acknowledgement
The project was supported in part by US National Institutes of Health grant NS44576. E. D. L. is supported by the New Zealand Foundation for Research, Science and Technology. The authors thank Dr. Xiaobai Li for assistance in statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abramov U, Puussaar T, Raud S, Kurrikoff K, Vasar E. Behavioural differences between C57BL/6 and 129S6/SvEv strains are reinforced by environmental enrichment. Neurosci Lett. 2008;443:223–227. doi: 10.1016/j.neulet.2008.07.075. [DOI] [PubMed] [Google Scholar]
- 2.Adamec R, Head D, Blundell J, Burton P, Berton O. Lasting anxiogenic effects of feline predator stress in mice: sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiol Behav. 2006;88:12–29. doi: 10.1016/j.physbeh.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Belanoff JK, Rothschild AJ, Cassidy F, DeBattista C, Baulieu EE, Schold C, et al. An open label trial of C-1073 (mifepristone)for psychotic major depression. Biol Psychiatry. 2002;52:386–392. doi: 10.1016/s0006-3223(02)01432-4. [DOI] [PubMed] [Google Scholar]
- 4.Belz EE, Kennell JS, Czambel RK, Rubin RT, Rhodes ME. Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol Biochem Behav. 2003;76:481–486. doi: 10.1016/j.pbb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 6.Benaroya-Milshtein N, Hollander N, Apter A, Kukulansky T, Raz N, Wilf A, et al. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. Eur J Neurosci. 2004;20:1341–1347. doi: 10.1111/j.1460-9568.2004.03587.x. [DOI] [PubMed] [Google Scholar]
- 7.Blehar MC. Gender differences in risk factors for mood and anxiety disorders: implications for clinical treatment research. Psychopharmacol Bull. 1995;31:687–691. [PubMed] [Google Scholar]
- 8.Bohn MC, Dean D, Hussain S, Giuliano R. Development of mRNAs for glucocorticoid and mineralocorticoid receptors in rat hippocampus. Brain Res Dev Brain Res. 1994;77:157–162. doi: 10.1016/0165-3806(94)90192-9. [DOI] [PubMed] [Google Scholar]
- 9.Brenes JC, Padilla M, Fornaguera J. A detailed analysis of open-field habituation and behavioral and neurochemical antidepressant-like effects in postweaning enriched rats. Behav Brain Res. 2009;197:125–137. doi: 10.1016/j.bbr.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Brinks V, Berger S, Gass P, de Kloet ER, Oitzl MS. Mineralocorticoid receptors in control of emotional arousal and fear memory. Horm Behav. 2009;56:232–238. doi: 10.1016/j.yhbeh.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Cao L, Liu X, Lin EJ, Wang C, Choi EY, Riban V, et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142:52–64. doi: 10.1016/j.cell.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 13.Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 14.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 15.DeFries JC, Hegmann JP, Weir MW. Open-field behavior in mice: evidence for a major gene effect mediated by the visual system. Science. 1966;154:1577–1579. doi: 10.1126/science.154.3756.1577. [DOI] [PubMed] [Google Scholar]
- 16.Elliott BM, Grunberg NE. Effects of social and physical enrichment on open field activity differ in male and female Sprague-Dawley rats. Behav Brain Res. 2005;165:187–196. doi: 10.1016/j.bbr.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 17.Fox C, Merali Z, Harrison C. Therapeutic and protective effect of environmental enrichment against psychogenic and neurogenic stress. Behav Brain Res. 2006;175:1–8. doi: 10.1016/j.bbr.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friske JE, Gammie SC. Environmental enrichment alters plus maze, but not maternal defense performance in mice. Physiol Behav. 2005;85:187–194. doi: 10.1016/j.physbeh.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Gabriel AF, Paoletti G, Della Seta D, Panelli R, Marcus MA, Farabollini F, et al. Enriched environment and the recovery from inflammatory pain: Social versus physical aspects and their interaction. Behav Brain Res. 2010;208:90–95. doi: 10.1016/j.bbr.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Galani R, Berthel MC, Lazarus C, Majchrzak M, Barbelivien A, Kelche C, et al. The behavioral effects of enriched housing are not altered by serotonin depletion but enrichment alters hippocampal neurochemistry. Neurobiol Learn Mem. 2007;88:1–10. doi: 10.1016/j.nlm.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Goel N, Bale TL. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J Neuroendocrinol. 2009;21:415–420. doi: 10.1111/j.1365-2826.2009.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goel N, Bale TL. Sex differences in the serotonergic influence on the hypothalamic-pituitary-adrenal stress axis. Endocrinology. 2010;151:1784–1794. doi: 10.1210/en.2009-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DE, Birnbaum SG, et al. Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol Psychiatry. 2010;67:28–35. doi: 10.1016/j.biopsych.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenough WT, Volkmar FR. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp Neurol. 1973;40:491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- 26.Henderson ND. Brain weight increases resulting from environmental enrichment: a directional dominance in mice. Science. 1970;169:776–778. doi: 10.1126/science.169.3947.776. [DOI] [PubMed] [Google Scholar]
- 27.Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav. 2002;1:55–69. doi: 10.1046/j.1601-1848.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- 28.Jakovcevski M, Schachner M, Morellini F. Individual variability in the stress response of C57BL/6J male mice correlates with trait anxiety. Genes Brain Behav. 2008;7:235–243. doi: 10.1111/j.1601-183X.2007.00345.x. [DOI] [PubMed] [Google Scholar]
- 29.Johansson BB, Belichenko PV. Neuronal plasticity and dendritic spines: effect of environmental enrichment on intact and postischemic rat brain. J Cereb Blood Flow Metab. 2002;22:89–96. doi: 10.1097/00004647-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 31.Klein SL, Lambert KG, Durr D, Schaefer T, Waring RE. Influence of environmental enrichment and sex on predator stress response in rats. Physiol Behav. 1994;56:291–297. doi: 10.1016/0031-9384(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi K, Ikeda Y, Suzuki H. Locomotor activity correlates with modifications of hippocampal mossy fibre synaptic transmission. Eur J Neurosci. 2006;24:1867–1873. doi: 10.1111/j.1460-9568.2006.05079.x. [DOI] [PubMed] [Google Scholar]
- 33.Kondo M, Gray LJ, Pelka GJ, Christodoulou J, Tam PP, Hannan AJ. Environmental enrichment ameliorates a motor coordination deficit in a mouse model of Rett syndrome--Mecp2 gene dosage effects and BDNF expression. Eur J Neurosci. 2008;27:3342–3350. doi: 10.1111/j.1460-9568.2008.06305.x. [DOI] [PubMed] [Google Scholar]
- 34.Kornstein SG. Gender differences in depression: implications for treatment. J Clin Psychiatry. 1997;58(Suppl 15):12–18. [PubMed] [Google Scholar]
- 35.Koshibu K, Levitt P, Ahrens ET. Sex-specific, postpuberty changes in mouse brain structures revealed by three-dimensional magnetic resonance microscopy. Neuroimage. 2004;22:1636–1645. doi: 10.1016/j.neuroimage.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 36.Larsson F, Winblad B, Mohammed AH. Psychological stress and environmental adaptation in enriched vs. impoverished housed rats. Pharmacol Biochem Behav. 2002;73:193–207. doi: 10.1016/s0091-3057(02)00782-7. [DOI] [PubMed] [Google Scholar]
- 37.Leal-Galicia P, Castaneda-Bueno M, Quiroz-Baez R, Arias C. Long- term exposure to environmental enrichment since youth prevents recognition memory decline and increases synaptic plasticity markers in aging. Neurobiol Learn Mem. 2008;90:511–518. doi: 10.1016/j.nlm.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, et al. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav Brain Res. 2005;163:78–90. doi: 10.1016/j.bbr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Llorens-Martin MV, Rueda N, Martinez-Cue C, Torres-Aleman I, Florez J, Trejo JL. Both increases in immature dentate neuron number and decreases of immobility time in the forced swim test occurred in parallel after environmental enrichment of mice. Neuroscience. 2007;147:631–638. doi: 10.1016/j.neuroscience.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 40.Magalhaes A, Summavielle T, Tavares MA, de Sousa L. Effects of postnatal cocaine exposure and environmental enrichment on rat behavior in a forced swim test. Ann N Y Acad Sci. 2004;1025:619–629. doi: 10.1196/annals.1316.077. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Cue C, Baamonde C, Lumbreras M, Paz J, Davisson MT, Schmidt C, et al. Differential effects of environmental enrichment on behavior and learning of male and female Ts65Dn mice, a model for Down syndrome. Behav Brain Res. 2002;134:185–200. doi: 10.1016/s0166-4328(02)00026-8. [DOI] [PubMed] [Google Scholar]
- 42.Moncek F, Duncko R, Johansson BB, Jezova D. Effect of environmental enrichment on stress related systems in rats. J Neuroendocrinol. 2004;16:423–431. doi: 10.1111/j.1365-2826.2004.01173.x. [DOI] [PubMed] [Google Scholar]
- 43.Montgomery KC. The relation between fear induced by novel stimulation and exploratory behavior. J Comp Physiol Psychol. 1955;48:254–260. doi: 10.1037/h0043788. [DOI] [PubMed] [Google Scholar]
- 44.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 45.Oitzl MS, Champagne DL, van der Veen R, de Kloet ER. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci Biobehav Rev. 2010;34:853–866. doi: 10.1016/j.neubiorev.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Olsson T, Mohammed AH, Donaldson LF, Henriksson BG, Seckl JR. Glucocorticoid receptor and NGFI-A gene expression are induced in the hippocampus after environmental enrichment in adult rats. Brain Res Mol Brain Res. 1994;23:349–353. doi: 10.1016/0169-328x(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 47.Pena Y, Prunell M, Dimitsantos V, Nadal R, Escorihuela RM. Environmental enrichment effects in social investigation in rats are gender dependent. Behav Brain Res. 2006;174:181–187. doi: 10.1016/j.bbr.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Pietropaolo S, Branchi I, Cirulli F, Chiarotti F, Aloe L, Alleva E. Long-term effects of the periadolescent environment on exploratory activity and aggressive behaviour in mice: social versus physical enrichment. Physiol Behav. 2004;81:443–453. doi: 10.1016/j.physbeh.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 49.Pietropaolo S, Feldon J, Alleva E, Cirulli F, Yee BK. The role of voluntary exercise in enriched rearing: a behavioral analysis. Behav Neurosci. 2006;120:787–803. doi: 10.1037/0735-7044.120.4.787. [DOI] [PubMed] [Google Scholar]
- 50.Rampon C, Jiang CH, Dong H, Tang YP, Lockhart DJ, Schultz PG, et al. Effects of environmental enrichment on gene expression in the brain. Proc Natl Acad Sci U S A. 2000;97:12880–12884. doi: 10.1073/pnas.97.23.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenzweig MR, Bennett EL. Cerebral changes in rats exposed individually to an enriched environment. J Comp Physiol Psychol. 1972;80:304–313. doi: 10.1037/h0032978. [DOI] [PubMed] [Google Scholar]
- 52.Rozeboom AM, Akil H, Seasholtz AF. Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc Natl Acad Sci U S A. 2007;104:4688–4693. doi: 10.1073/pnas.0606067104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sale A, Berardi N, Maffei L. Enrich the environment to empower the brain. Trends Neurosci. 2009;32:233–239. doi: 10.1016/j.tins.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 56.Schrijver NC, Bahr NI, Weiss IC, Wurbel H. Dissociable effects of isolation rearing and environmental enrichment on exploration, spatial learning and HPA activity in adult rats. Pharmacol Biochem Behav. 2002;73:209–224. doi: 10.1016/s0091-3057(02)00790-6. [DOI] [PubMed] [Google Scholar]
- 57.Sztainberg Y, Kuperman Y, Tsoory M, Lebow M, Chen A. The anxiolytic effect of environmental enrichment is mediated via amygdalar CRF receptor type 1. Mol Psychiatry. 2010 doi: 10.1038/mp.2009.151. [DOI] [PubMed] [Google Scholar]
- 58.Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 59.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 60.Varty GB, Paulus MP, Braff DL, Geyer MA. Environmental enrichment and isolation rearing in the rat: effects on locomotor behavior and startle response plasticity. Biol Psychiatry. 2000;47:864–873. doi: 10.1016/s0006-3223(99)00269-3. [DOI] [PubMed] [Google Scholar]
- 61.Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S, et al. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci U S A. 2004;101:11851–11856. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem Res. 2007;32:1730–1740. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- 63.Will BE, Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Relatively brief environmental enrichment aids recovery of learning capacity and alters brain measures after postweaning brain lesions in rats. J Comp Physiol Psychol. 1977;91:33–50. doi: 10.1037/h0077306. [DOI] [PubMed] [Google Scholar]
- 64.Wust S, Van Rossum EF, Federenko IS, Koper JW, Kumsta R, Hellhammer DH. Common polymorphisms in the glucocorticoid receptor gene are associated with adrenocortical responses to psychosocial stress. J Clin Endocrinol Metab. 2004;89:565–573. doi: 10.1210/jc.2003-031148. [DOI] [PubMed] [Google Scholar]
- 65.Xu D, Buehner A, Xu J, Lambert T, Nekl C, Nielsen MK, et al. A polymorphic glucocorticoid receptor in a mouse population may explain inherited altered stress response and increased anxiety-type behaviors. FASEB J. 2006;20:2414–2416. doi: 10.1096/fj.06-5926fje. [DOI] [PubMed] [Google Scholar]
- 66.Young AH, Gallagher P, Watson S, Del-Estal D, Owen BM, Ferrier IN. Improvements in neurocognitive function and mood following adjunctive treatment with mifepristone (RU-486) in bipolar disorder. Neuropsychopharmacology. 2004;29:1538–1545. doi: 10.1038/sj.npp.1300471. [DOI] [PubMed] [Google Scholar]
- 67.Zhu SW, Yee BK, Nyffeler M, Winblad B, Feldon J, Mohammed AH. Influence of differential housing on emotional behaviour and neurotrophin levels in mice. Behav Brain Res. 2006;169:10–20. doi: 10.1016/j.bbr.2005.11.024. [DOI] [PubMed] [Google Scholar]