Abstract
The advent of nanotechnology has bolstered a variety of nanoparticles based platforms for different biomedical applications. A better understanding for engineering novel nanoparticles for applications in cancer staging and therapy requires careful assessment of the nanoparticle’s physico-chemical properties. Herein we report a facile synthesis method for PEGylated PLGA nanoparticles encapsulating anti-cancer drug doxorubicin for cancer imaging and therapy. The simple nanoprecipitation method reported here resulted in very robust PEGylated PLGA nanoparticles with close to 95% drug encapsulation efficiency. The nanoparticles showed a size of ~110 nm as characterized by TEM and DLS. The nanoparticles were further characterized by optical UV-Visible and fluorescence spectroscopy. The encapsulated doxorubicin showed a sustained release (>80%) from the nanoparticles matrix over a period of 8 days. The drug delivery efficiency of the nanoparticles was confirmed in vitro confocal imaging with PC3 and HeLa cell lines. In vitro quantitative estimation of drug accumulation in PC3 cell line showed a 22 times higher concentration of drug in case of nanoparticles based formulation in comparison to free drug and this was further reflected in the in vitro cytotoxicity assays. Overall the synthesis method reported here provides a simple and robust PLGA based platform for efficient drug delivery and imaging of cancer cells in vitro and in vivo.
Keywords: PEGylation, PLGA nanoparticles, Doxorubicin, Nanoprecipitation, Drug release, Fluorescence imaging
Introduction
Engineered nanomaterials offer tremendous scope in various biomedical applications which include disease diagnosis, imaging, drug and gene delivery [1–4]. A thoughtful design of the nanoparticle characteristics can help overcome several key challenges encountered in conventional biomedical therapies. In chemo-therapy, the use of pharmacologically active cancer drugs is severely limited by poor specificity resulting in minimal tumor accumulation and thus leading to dose limiting toxicities [5–7]. The two major challenges in drug delivery are the poor systemic circulation time of the administered drug and multi drug resistance (MDR) in tumors, which significantly limits the bioavailability of the drugs to the cancer cells [8–12]. For overcoming the MDR induced by the permeability-glycoprotein (Pgp) alternative approaches must be explored to improve drug accumulation and prolong retention of the drugs in resistant cells [13–14]. Both these challenges can be surmounted by designing long circulating ‘stealth’ nanoparticles. Polymeric drug delivery systems are one such class of nanoparticles which address these critical issues and have been studied extensively [15] The biodegradability of the polymers, high payload capacity, and provision of varied surface chemistries provides an ideal platform for engineering nanosized drug delivery vehicles [16–17]
The most widely used and studied class of biodegradable polymers are the polyesters, which include PLA and PLGA. There have been numerous reports on the use of nanoparticulate form of these polymers in drug delivery [18–23]. PLGA nanoparticles encapsulating a myriad of drugs have been formulated using different synthetic strategies [17, 24]. In most cases the nanoparticles are formed by the self- assembly of the polymers in the presence of surfactants. The amphiphilicity of the polymers results in the formation of nanoparticles with a hydrophobic core and hydrophilic shell. The core of these nanoparticles can be used for encapsulating various poorly water soluble drugs. Further the surface of these nanoparticles can be conjugated with PEG for long circulation, as well as other biomolecules for differential specificity towards cancer cells. The addition of PEG on the surface of nanoparticles decreases opsonization by the serum proteins avoiding RES (reticulo-endothelial system) uptake, and subsequent clearance from the systemic circulation [25]. The stealth property imparted by the PEG chains results in a long circulation time and thus results in a higher rate of accumulation in the tumor by the passive uptake via the EPR (Enhanced permeability and retentivity) effect. Thus, for any nanoparticle formulation the presence of PEG is very critical for efficient passive targeting to the target site [26]. The stealth nanoparticles compared to other long circulating systems shows a better shelf stability and ability to control the release of the encapsulated compounds [27–28]. There have been several attempts for the PEGylation of the PLGA nanoparticles and most of the strategies have indicated a cumbersome chemical conjugation, which resulted in a non-robust and inefficient surface conjugation of the PEG. For most of the PEGylated PLGA nanoparticles formulations encapsulating anti-cancer drugs, PEG is either grafted on the nanoparticles surface via a post synthesis chemical conjugation or PEG is directly conjugated to the PLGA backbone prior to synthesizing nanoparticles [29–32]. In both the cases, the lack of functional groups on the aliphatic polyester backbone of the polymer presents difficulty in modifying the parent polymer [33]. Different PEGylation methods like adsorption, covalent conjugation via the amine or carboxyl groups on PLGA or incorporation of PEGylated copolymers in the PLGA matrix usually results in a low density of PEG on the nanoparticles surface, with less physiological stability [31]. The use of phospholipids in combination of PLGA provides an attractive alternative for PEGylating the PLGA nanoparticles in a single step. Some earlier reports used lipid-PLGA combination to formulate hybrid PLGA nanoparticles [23, 34]. So by using phospholipids where the lipid part of the phospholipids where the lipid part of the phospholipid can interact with the hydrophobic pockets of PLGA whereas PEG provides the aqueous stability to the nanoparticles.
In this communication we report a simple robust one step method for preparation of highly stable PEGylated PLGA nanoparticles encapsulating hydrophobic doxorubicin as a model anticancer drug. The nanoprecipitation method involving the use of single emulsion was exploited to fabricate ~110 nm size PLGA nanoparticles. By combining the two amphiphilic components, PLGA and phospholipid PE-PEG, the nanoparticles formed were composed of a hydrophobic core, in which non aqueous compounds can be solubilized, and the surface decorated with dense PEG, which helps evading the recognition by immune system components imparting long systemic circulation. The additional degree of hydrophobicity added by the phospholipid imparts a robust core, which enhances the drug encapsulation efficiency and a slow sustained release of the encapsulated drug from the nanoparticles. We have evaluated in vitro efficacy and stability of the Dox loaded nanoparticles. Also, we have demonstrated the enhanced uptake of the PEGylated nanoparticles, compared with free Dox in HeLa and PC3 cell lines, indicating the nanoparticle formulation is able to bypass the Pgp pumps. The use of biodegradable components like PLGA and phospholipid for synthesis of highly stable nanoparticles, with a sustained release of the encapsulated drug and associated properties like long systemic circulation, makes these PEGylated nanoparticles a potential candidate as efficient probes for drug delivery in vivo.
Materials and Methods
Materials
Poly(lactic-coglycolic acid) (PLGA, Mw: 7–17 kDa, acid terminated with 50:50 LA/GA ratio), Doxorubicin hydrochloride, Polyvinyl alcohol (PVA, Mw: 9–10 kDa, 80% hydrolysed), triethylamine and HPLC grade water were purchased from Sigma Aldrich. All the solvents dimethyl sulfoxide (DMSO), dichloromethane, chloroform were procured from Sigma and used without further purifications. DPPE-PEG (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (ammonium salt)) was purchased from Avanti Polar lipids (Alabaster, AL). Cell culture reagents like media DMEM and F-12, Penstrep (antibiotic) and fetal bovine serum were purchased from Invitrogen (Carlsbad, CA). The cancer cell lines HeLa and PC3 were obtained from ATCC, VA and cultured according to instructions supplied by the vendor. For cell viability assay CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) reagent was procured from Promega.
Synthesis of PEGylated PLGA nanoparticles
The synthesis procedure for the PEGylated PLGA nanoparticles encapsulating doxorubicin involved simple nanoprecipitation method using the single emulsion technique and was adapted from previous reports with several modifications for generating the PEGylated nanoparticles in a single step [21]. Briefly, PLGA and DPPE-PEG were mixed in 1:1 (wt. ratio) in chloroform. The solvent was evaporated under a gentle stream of air and the obtained film was redispersed in 250 μl of dichloromethane. The conversion percentage of the Dox.HCl to the base form was >95%. A known amount of Dox.HCl was dissolved in 12.5% v/v methanol in chloroform mixture followed by addition of 0.54 mmol triethylamine. After overnight stirring at r.t. the base form of the drug was extracted by evaporating the solvent under vacuum followed by lyophilization to obtain powdered Dox base. The powdered Dox was stored at −20°C until further use. 0.2 μmol of Dox (the hydrophilic form of the drug doxorubicin HCl was converted to its hydrophobic form by neutralizing the hydrochloride salt with the base triethylamine [35]) dissolved in 100 μl of DMSO was added to the clear transparent PLGA/DPPE-PEG solution and sonicated for 15 sec. 0.4 ml of 2.5 % aq. PVA solution was added to the reaction mixture while vortexing. The obtained viscous emulsion was further sonicated for 30 sec and added to the 10 ml of 0.3 % aq. PVA solution with vigorous stirring. The solution was stirred overnight to evaporate the dichloromethane. The optically transparent appearance of the reaction mixture results in the formation of PEGylated PLGA nanoparticles. Following the synthesis of doxorubicin loaded nanoparticles, the surfactant, and other unreacted molecules were removed by dialysis against distilled water, using a cellulose membrane with a cutoff size of 12–14 kDa. Following dialysis, the nanoparticles were sterile filtered and stored at 4 °C for future use. The nanoparticles can be easily lyophilized and stored in powdered form at 4 °C.
Characterization of PEGylated PLGA nanoparticles
The absorption spectra were collected using Agilent 8453 UV-Vis spectrophotometer over a wavelength range from 300 to 800 nm. The samples were measured against water as reference. All samples were used as prepared and loaded into a quartz cell for measurements. Photoluminescence (PL) studies were carried out using a Fluoromax 4 Spectrofluorometer (Jobin Yvon, NY). All the samples were diluted in water and loaded into a quartz cell for measurements. TEM images were obtained using a JEOL JEM-1000 microscope operating at 60 kV. Dynamic light scattering (DLS) measurements were done by using 90Plus zeta sizer (Brookhaven Inc, NY) for measuring the hydrodynamic diameter of the PLGA nanoparticles by taking a diluted sample in 3 ml cuvette. Zeta potential measurements were also done using the same instrument. Stability of the nanoparticles was also tested in different medium by diluting and incubating a specified volume of nanoparticles in PBS buffer and DMEM cell culture media with 10 % FBS for 24 hrs. Confocal Microscopy images were obtained using a Zeiss LSM700 confocal microscope (Carl Zeiss, Europe) with laser excitation at 488 nm. All images were taken under exact same conditions of laser power, aperture, gain, offset, scanning speed, and scanning area. The Dox encapsulation efficiency (EE) was calculated as the ratio between the un-encapsulated Dox over the initial drug amount used for synthesis. For the EE determination, a fixed volume of as prepared Dox loaded PEGylated PLGA nanoparticles were taken in Pall’s 100 kDa spin filters (in triplicates) and the samples were centrifuged at 9000 rpm for 30 min at 25 °C. The fluorescence of the flow-through obtained after the centrifuge containing un-encapsulated Dox was measured with an excitation wavelength 488 nm using a spectrofluorometer. Dox concentration was calculated from the fluorescence using a calibration curve established previously.
Drug release Studies
The release of doxorubicin from PEGylated PLGA nanoparticles was investigated using buffered 1 % tween-80 solution as dissolution media. For the release kinetics studies infinite sink conditions were maintained by taking 2 ml of as prepared nanoparticles suspension in dialysis tubing (membrane cutoff size 12–14 kDa) and placing the dialysis tubing in 100 ml of dissolution media. The solution was gently stirred at room temperature. At predetermined time points, 3 ml of dissolution media was sampled and the amount of released doxorubicin was quantified using the standard curve prepared for the free doxorubicin. The standard curve (r2=0.983) was prepared by measuring the fluorescence emission intensity (λex: 488 nm; λem: 590 nm) of different concentrations of doxorubicin solutions.
Cell staining studies
For in vitro imaging with PEGylated PLGA nanoparticles encapsulating Dox, the human prostate cancer cell line PC3 and cervical cancer cell line HeLa were cultured in F-12 and DMEM respectively, with 10% fetal bovine serum (FBS), 1% penicillin, and 1% amphotericin B. The day before nanoparticles treatment, cells were seeded in 35 mm culture dishes. On the treatment day, the cells, at a confluency of 70–80 % in serum-supplemented media, were treated with the nanoparticles at a specific concentration (100 μl/1ml media) for two hours at 37 °C. The excess of nanoparticles present in the culture plates were washed using fresh medium prior to confocal imaging.
Cell viability assay
The PC3 and HeLa cells were dispensed into a 96-well flat-bottom microtiter plate (~10,000 cells/well) and allowed to attach overnight using the respective F-12 and DMEM medium with 10 % FBS. The MTS assay was carried out as per manufacturer’s instructions (PROMEGA). It is based on the absorbance of formazan (produced by the cleavage of MTS by dehydrogenases in living cells), the amount of which is directly proportional to the number of live cells. In brief, after 24 hrs and 48 hrs treatments with the Dox loaded PLGA nanoparticles, media was changed and 150 μL of MTS reagent was added to each well. The absorbance of the mixtures at 490 nm was measured after 4 hrs incubation with the MTS reagent. The cell viability was calculated as the ratio of the absorbance of the sample well to that of the control well and expressed as a percentage. Tests were performed in quadruplicate. Each point represents the mean ±SD (bars) of replicates from one representative experiment.
Quantitative estimation of nanoparticle accumulation in vitro
To quantitate the amount of drug internalized in the cells, fluorescence spectroscopy and bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL) were performed whereby the amount of the doxorubicin in the cell was normalized to the total amount of proteins in the cell. For estimating the total protein content a BSA standard curve was prepared using the BCA assay as per manufacturer’s instructions. A standard curve for the doxorubicin was also prepared by measuring the fluorescence intensity of different concentrations of free doxorubicin.
For quantitative estimation of drug accumulation in the cells, about 80,000 cells were seeded in the 6-well plate and were allowed to adhere by incubating the plate overnight. The media was replaced with 400 μL of nanoparticles (drug content 40 μg/mL) and 400 μL of free doxorubicin (400 μL diluted with 2 mL of growth medium) and incubated at 37 °C for 6 hrs. After incubation, cells were washed twice with 1X PBS solution to ensure complete removal of the non-internalized nanoparticles. About 400 μL of the lysis buffer was added to each well and the plate was kept on shaker at 4 °C until a white film appeared at the bottom which indicates complete lysis. The lysate was collected and centrifuged at 13,000 rpm for 10 min at 4 °C. The supernatant layer was collected and evaluated for the total amount of proteins using the BCA assay kit (Pierce, Rockford, IL). To determine the amount of doxorubicin, the supernatant was taken and the fluorescence intensity was measured (λex: 470 nm; λem: 590 nm). The amount of total proteins and doxorubicin in the lysate was determined by extrapolating the readings obtained from the standard curve.
Results and discussion
The nanoprecipitation method employed here yields PLGA nanoparticles with surface decorated with PEG. The incorporation of phospholipid provides additional stability to the nanoparticles in the hydrophilic environment. The surfactant used in the single emulsion method like polyvinyl alcohol renders a high negative charge on the nanoparticles which when administered systemically is rapidly opsonized by the RES system. During the emulsion formation the hydrophobic segments of PVA penetrate into the organic phase and remain entrapped into the polymeric matrix of the nanoparticles which ultimately binds on the particle surface when the organic solvent was removed from the interface [36–37]. The presence of PEG not only neutralizes the negative charge but also evades the RES system due to its stealth properties [38]. Based on the hydrophobic and hydrophilic interactions in the reaction mixture, scheme 1 represents the structure of PEGylated nanoparticles.
Scheme 1.
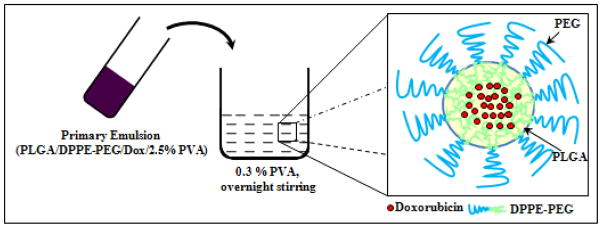
Schematic presentation of the synthetic strategy showing the final structure of the PEGylated PLGA nanoparticles encapsulating Dox.
Since the core of the PLGA is hydrophobic we assume the addition of amphiphilic phospholipid like DPPE-PEG would result in such an alignment that the hydrophobic lipid part incorporates inside the hydrophobic pockets of the PLGA matrix with hydrophilic PEG protruding out for interaction with the aqueous environment. Also the presence of PEG on the surface will embed the excess PVA which remains at the nanoparticles surface. The surface charge of the PEGylated PLGA as measured by the zeta potential studies was −14.17±0.54.
Also, this structure will be more compact and rigid in comparison with the other PLGA nanoparticle formulation grafted with PEG. Zhang et al have reported a similar lipid-polymer hybrid nanoparticle where the PLGA core is basically surrounded by an additional lipid layer followed by further stabilization by PEG [23]. In such a system there is a high possibility of drug (hydrophobic) partitioning between the hydrophobic core of the PLGA and the hydrophobic lipid layer. The hydrophobicity of the drug results in the entrapment in the hydrophobic core of the nanoparticles which we have further confirmed with the encapsulation of an extremely hydrophobic cyanine dye (Cy7.5 derivative) in the PLGA nanoparticles (see supporting data). Cy7.5 is extremely hydrophobic and loses fluorescence in aqueous environment due to self-aggregation but when encapsulated in the PLGA nanoparticles, the dye remained highly fluorescent. Furthermore, PLGA-PEG block copolymers show quite different properties when compared to each constituting polymer. Various kinds of block copolymers have been developed to date and can be classified according to their block structure as AB (A is hydrophobic monomer, B is hydrophilic and usually PEG) diblock, ABA, or BAB triblock, multi-block, and graft block copolymers [39–43]. Moreover, there are very limited commercial vendors supplying block copolymers consisting of hydrophobic blocks of PLA, PGA, or PLGA and hydrophilic PEG blocks [44]. These block copolymers have unique properties that the individual homopolymers cannot provide, and are ideal for formulating controlled drug delivery systems and for making scaffolds for tissue engineering. However, in order to exploit the advantages offered by these block copolymers, one has to first synthesize them by optimizing several parameters in the multi-step synthesis process. The synthetic approach used here avoids these cumbersome synthetic routes for block copolymers synthesis and utilizes a simple versatile method for making nanoparticles.
Dox encapsulated PEGylated PLGA nanoparticles showed an EE of 94.7±1.4%. The effective concentration of Dox encapsulated in PLGA nanoparticles was approximately 0.95 mg of Dox per 0.01 g of PLGA polymer. The EE values obtained for these nanoparticles were higher when compared to the EE of other PLGA based formulations prepared by precipitation method [45]. Moreover, this hybrid approach resulted in robust structure which showed a similar EE values previously reported for nanoparticles formulated from DOX–PLGA conjugate [46]. The partitioning of the drug in the lipid and PLGA part is assumed to be relatively higher as compared to only PLGA backbone leading to a increase in encapsulation efficiency. Also, this can also be attributed to the fact that neutralization of the hydrochloride salt of the Dox with a base, resulted in increased affinity of the neutralized Dox to the organic phase which reduced the loss of drug in external aqueous phase [46]. PEGylated PLGA nanoparticles we report here have a robust hydrophobic core and the surface covered by PEG which provides an excellent stability in varied in vitro and in vivo environment.
Figure 1 shows the nanoparticles characterization data of the PEGylated PLGA nanoparticles encapsulating doxorubicin. Figure 1a shows the TEM images of Dox encapsulated PEGylated PLGA nanoparticles, clearly demonstrating a spherical geometry, with an average diameter around 105 nm. A higher magnification image of the Dox encapsulated PEGylated PLGA nanoparticles showed a central dark core representing the hydrophobic phase and a lighter shell as hydrophilic PEG shell (Inset figure 1a). We have further correlated the size of the nanoparticles by dynamic light scattering (DLS). Figure 1b shows a particle size distribution with mean size of 110 nm.
Figure 1.
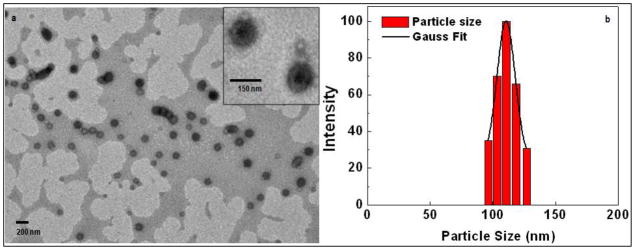
Showing the basic characterization of the PEGylated PLGA nanoparticles encapsulating doxorubicin: Nanoparticles morphology and size measurement by (a) representative TEM image, Inset: high magnification image of individual nanoparticles, (b) distribution profile as measured by DLS.
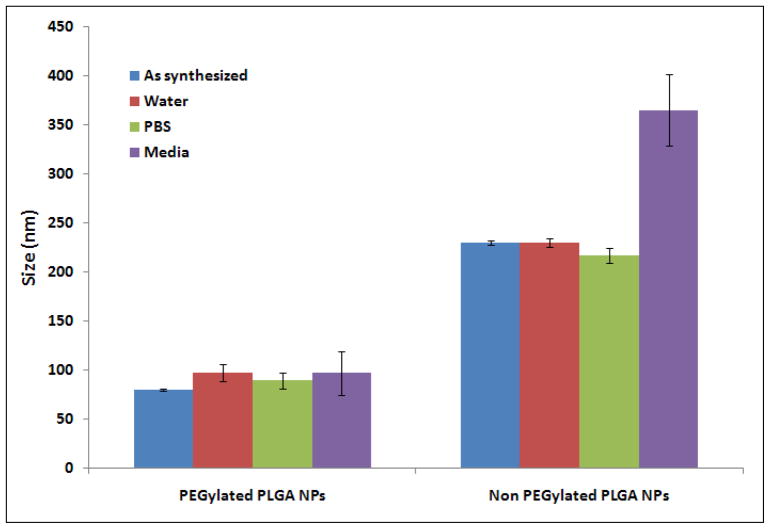
Also, the stability of nanoparticles was studied in presence of different media. Two different nanoparticles formulations both encapsulating Dox: PEGylated and non PEGylated PLGA nanoparticles were incubated with PBS buffer and DMEM media with 10% serum. As can be visualized from the figure 2, after 24 hrs of incubation the size of PEGylated PLGA nanoparticles remains the same in both buffer and cell culture medium (~100 nm). However, for the non PEGylated PLGA nanoparticles, the size of nanoparticles increased by 120 nm when incubated with DMEM which can be attributed to the adsorption of the serum proteins from the cell culture medium onto the nanoparticles surface. A negatively charged surface due to PVA, in absence of PEG results in adsorption of positively charged proteins on nanoparticles resulting in increased size of nanoparticles. The increase in particles size is because of the binding of the serum proteins to the non PEGylated PLGA nanoparticles. Upon serum binding the nanoparticles may agglomerate together because of the hydrophobic-hydrophobic interactions. The fabrication of PEGylated nanoparticles however prevents the serum proteins binding because of the highly hydrophilic nature of the PEG and thus minimal aggregation and particles size increase in serum conditions.
Figure 2.
Size distribution of different PLGA nanoparticles formulations in various environments as measured by Dynamic Light Scattering (DLS). Same volume of nanoparticles was incubated with different solvent medium for 24 hrs.
It is also worth noting that the size of the ‘as synthesized’ non PEGylated PLGA nanoparticles (synthesized by keeping all the parameters same as for PEGylated PLGA nanoparticles but without adding the DDPE-PEG) was much higher than the PEGylated PLGA nanoparticles which clearly suggested that DPPE-PEG played a critical role not only in stabilizing the nanoparticles but also imparted more compactness as well as higher robustness. Also, from the figure 2, it can be concluded that the presence of PEG on the nanoparticles surface provide higher stability by preventing the adsorption of serum proteins on the nanoparticles. This observation provides an indication that these PEGylated PLGA nanoparticles will provide a higher degree of stability in systemic circulation in vivo and thus will provide a longer circulation of the nanoparticles without being cleared out by the macrophages.
Release Kinetics Studies
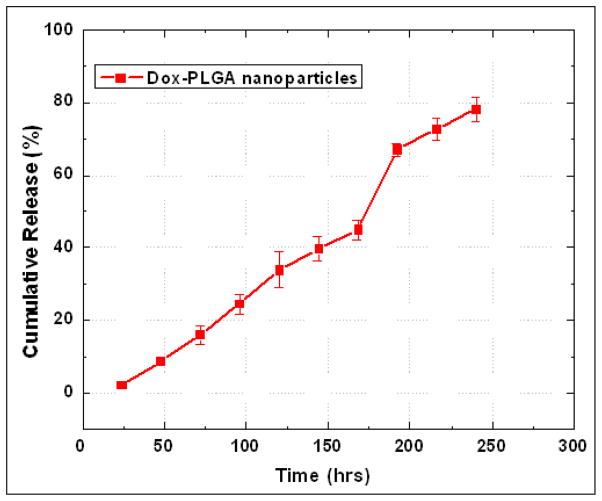
The release of the encapsulated Dox from the PLGA nanoparticles was studied by fluorescence spectroscopic measurements. Figure 4 presents the release kinetic profile of the encapsulated Dox from the PEGylated PLGA nanoparticles. The results showed a sustained release of the Dox from the nanoparticles over a period of 10 days. Almost 80 % of the cumulative drug release was observed from the nanoparticles in 10 days. A burst release typically observed during initial stages is notably absent with these nanoparticles. The release of the drug from the drug can be modulated, if needed, by changing the molecular weight of the PEG as well as by using a higher molecular weight PLGA in the nanoparticles synthesis which imparts higher diffusional constraint as well robustness to the nanoparticle structure and thus slowing down the release of the encapsulated drug. The rate of release of Dox from the PLGA nanoparticles follows the same qualitative as well as quantitative pattern as reported earlier for other PEGylated PLGA nanoparticles formulations via the diffusion of the drug in the polymer matrix and by degradation of the polymer matrix [48]. The presence of PEG on the nanoparticles surface makes them long circulating in the blood in vivo and the uptake of the nanoparticles is determined by the half-life of the nanoparticles in systemic circulations via the well-known EPR (enhanced permeability and retentivity) effect.
Figure 4.
Release kinetics study of doxorubicin from PEGylated PLGA nanoparticles. The robust nanoparticles platform showed a slow and sustained release of the drug over a period of 10 days.
In vitro cellular uptake studies
Following the release kinetic studies, we have done the in vitro imaging studies with the Dox loaded nanoparticles to visualize the accumulation of the nanoparticles in PC3 as well as HeLa cell line. The two different cell lines were used to see if there is any unusual behaviour of uptake pattern is observed with different cell lines. Figure 5 shows the confocal fluorescence images of the PC 3 and HeLa cell lines treated with Dox encapsulated nanoparticles incubated for 2 hrs and 24 hrs. Also, we have done a comparative study to show the uptake behaviour of the molecular Dox in both the cell lines. Both the cell lines had shown a robust uptake of the nanoparticles as can be visualized by the strong cellular staining outside the nucleus in the ER region. As molecular Dox is known to accumulate into the nucleus of cells, the contrasting uptake pattern for the nanoparticulate formulation suggests the in vitro stability and uptake of the intact nanoparticles into the cells without undergoing degradation in the media. It is worth mentioning here that we had observed a slight toxicity after the incubation period which can be attributed to the Dox cytotoxicity which was released from the nanoparticles.
Figure 5.
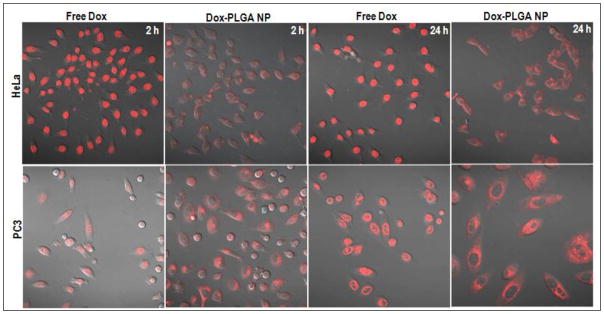
Confocal fluorescence images HeLa and PC3 cell lines showing a marked difference in pattern of uptake in the cells treated with free doxorubicin and doxorubicin loaded PLGA nanoparticles. Images were acquired after 2 and 24 h incubation of nanoparticles
In vitro cytotoxicity assays
To estimate the efficacy of the encapsulated Dox, we investigated the standard MTS assay with two different cell lines which have different response to the Dox (PC3 cell line is known for Dox resistance). Figure 6 shows a comparative cytotoxicity data for the Dox encapsulated PLGA nanoparticles and blank PLGA nanoparticles in PC3 and HeLa cell lines. The cytotoxicity of Dox released from PLGA nanoparticles was comparable to that of free molecular Dox. The concentrations of Dox in nanoparticles were calculated from the drug loading adjusted by the encapsulation efficiency. The relative IC50 value (1.8 μg/ml for PLGA nanoparticles) of doxorubicin from each formulation suggested that the activity of drug encapsulated in nanoparticles was not affected by the encapsulation inside the nanoparticles.
Figure 6.
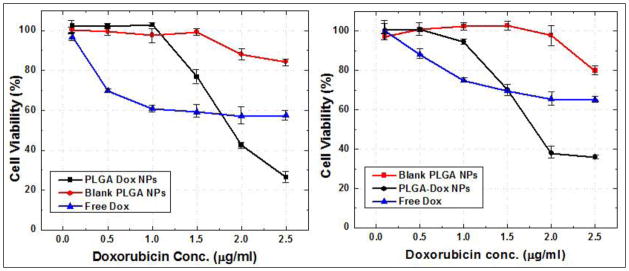
Cell viability assay (MTS assay) with Dox-PLGA nanoparticles and blank PLGA nanoparticles to show the inherent therapeutic efficacy of the Dox from the nanoparticles. The assay was done with HeLa and PC3 cell lines after 24h incubation time with both nanoparticle formulations.
Blank nanoparticles without Dox were also evaluated for any inherent toxicity due to the polymeric nanoparticles. A same volume of blank nanoparticles as Dox-PLGA nanoparticles containing approximately the same number of nanoparticles were added to the cells. Blank PLGA nanoparticles did not showed any considerable cytotoxicity effects as compared to the Dox-PLGA nanoparticles. The higher cytotoxicity of the Dox-PLGA nanoparticles can be attributed to the fact that: (a) the amount of Dox uptake is higher in case of nanoparticles and (b) the reduced efflux of the drug in nanoparticulate form as compared to the free Dox. At lower concentrations even in nanoparticulate form, the released drug from the nanoparticles might be lower and thus the cytotoxicity is comparable with the free Dox whereas at higher nanoparticles concentration the released drug inside the cells will be much higher as compared to free Dox uptake leading to higher cytotoxic effect. No apparent toxicity from the blank PLGA nanoparticles suggests a slow and sustained release of the Dox from the non-cytotoxic Dox-PLGA nanoparticles.
Quantitative retention studies in vitro
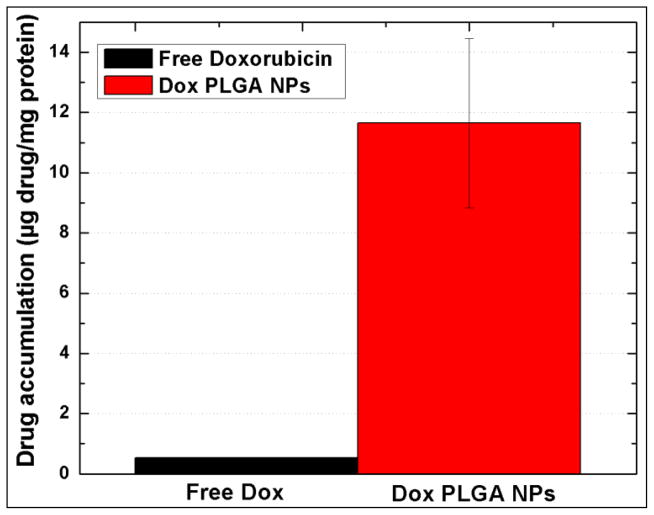
For quantitative estimation of the Dox accumulated within the PC3 cells, when free Dox or Dox-PLGA nanoparticles were added to the cells, the concentration of the Dox present in the cells was calculated by measuring the fluorescence of Dox from the lysed cells and expressed as Dox concentration per mg of protein from the cells. The protein concentration was calculated from BCA assay. Figure 7 shows the differential accumulation of the Dox when used in free form and nanoparticulate form. The differential uptake of the free Dox and nanoparticulate Dox can also be correlated with the results observed in the cytotoxicity assays. This data clearly suggests that the PLGA Dox nanoparticles formulation enhances the delivery of the drug inside the cells. The results in the figure shows that Dox accumulated in the cells at a higher rate when formulated in nanoparticulate form. PC 3 cells are known to overexpress Pgp in presence of molecular Dox, which results in the efflux of the molecular Dox from the cytoplasm to outside the plasma membrane lowering the effective drug concentration within the cells [49]. However, from the quantitative estimation it is quite clear that PLGA nanoparticles encapsulating the Dox enter the cells very efficiently by a combination of simple diffusion and phagocytosis. Once inside the cells Dox loaded nanoparticles cannot be easily removed by the Pgp efflux and serves as intracellular drug depot which releases the Dox in a sustained manner from the nanoparticles and leads to a chronic suppression of the drug resistant cancer cells. Confocal imaging of the cells treated with Dox-PLGA nanoparticles also confirms the presence of nanoparticles in the cytoplasm of the cells. As can be visualized from the figure, the uptake of drug when encapsulated in PLGA nanoparticles is 22 times higher than drug administered in free form.
Figure 7.
Cellular retention of doxorubicin in PC3 cell lines showing a comparison of differential and enhanced accumulation of dox when formulated in nanoparticluate form.
The total accumulated drug 0.549 μg/mg of protein in case of free Dox as opposed to the 11.648 μg/mg in Dox-PLGA clearly depicts that it is more difficult to remove for Pgp to remove the Dox molecules from the cells when these molecules are encapsulated inside nanoparticles. Wong et al have reported this as the principal mechanism responsible for enhanced cellular drug retention and uptake [50]. They also reported that this mechanism is applicable for a variety of Pgp substrates encapsulated in nanoparticulate form. It has been hypothesized that the MDR reversal activities depends on the type of drug carrier and vary with different nanoparticles. The most favoured mechanism involves the inhibition of Pgp by the polymers used for synthesizing drug loaded nanoparticles and increased endocytosis of the drug loaded nanoparticles [51–53]. The exact mechanism of interaction between the nanoparticles and the Pgp requires further investigation.
Conclusions
We have demonstrated a one pot synthesis of a robust PEGylated PLGA nanoparticle formulation encapsulating doxorubicin using a simple nanoprecipitation method. The synthesized nanoparticles showed a uniform size distribution and a sustained release of the encapsulated drug over a period of 10 days. The cytotoxicity studies with the nanoparticles showed efficient cell growth suppression in both HeLa and PC3 cell lines. The in vitro confocal imaging supplemented the cytotoxicity assays. The cellular retention studies with PC3 cell line showed enhanced cellular uptake of PLGA-Dox nanoparticles as opposed to free Dox. The sustained release of the doxorubicin from the PEGylated nanoparticles formulated with the clinical accepted PLGA polymer offers tremendous potential in delivering the drugs efficiently to different disease models like prostate, pancreatic, breast and cervical cancer models.
Supplementary Material
Supporting Figure 1. Fluorescence spectra of hydrophobic fluorophore Cy7.5 in different solvent environments. There is no fluorescence observed for free Cy7.5 in water (Red curve), free Cy7.5 in DMSO showed typical fluorescence spectra of Cy7.5 with maxima at 825 nm (Blue curve). The encapsulation of Cy7.5 in PEGylated PLGA nanoparticles maintained the fluorescent properties of the fluorophore with maximum fluorescence at 821 nm.
Figure 3.
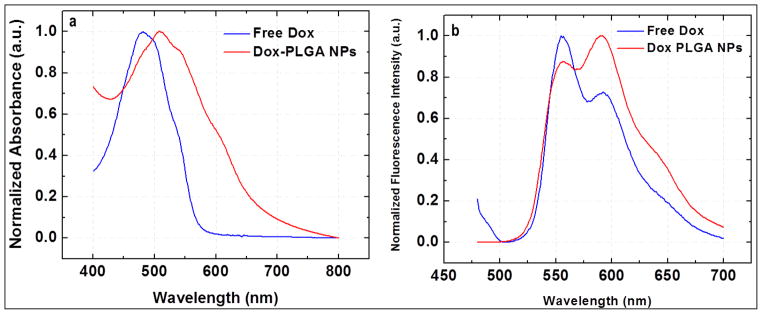
Photophysical characterization by (a) Absorption spectrum, (b) fluorescence emission spectrum of the doxorubicin in free as well as encapsulated form inside the nanoparticles.
Acknowledgments
We acknowledge partial support from NSF DGE 0965843, HHS/5U54CA151881-02, the Electronics Materials Research Institute at Northeastern University, and Brigham and Women’s Hospital.
References
- 1.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–34. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Kumar R, Ohulchanskyy TY, Turowski SG, Thompson ME, Seshadri M, Prasad PN. Combined magnetic resonance and optical imaging of head and neck tumor xenografts using Gadolinium-labelled phosphorescent polymeric nanomicelles. Head & Neck Oncology. 2010;2:35–44. doi: 10.1186/1758-3284-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonoiu AC, Bergey EJ, Ding H, Hu R, Kumar R, Yong KT, Prasad PN, Mahajan S, Picchione KE, Bhattacharjee A, Ignatowski TA. Gold nanorod–siRNA induces efficient in vivo gene silencing in the rat hippocampus. Nanomedicine (Lond) 2011;6:617–30. doi: 10.2217/nnm.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosi NL, Giljohann DA, Thaxon CS, Lytton-Jean AKR, Han MS, Mirkin CA. Oligonucleotide-Modified Gold Nanoparticles for Intracellular Gene Regulation. Science. 2006;312:1027–30. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–71. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 6.Allen TM, Cullis PR. Drug Delivery Systems: Entering the Mainstream. Science. 2004;303:1818–22. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 7.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 8.Merisko-Liversidge EM, Liversidge GG. Drug Nanoparticles: Formulating Poorly Water-Soluble Compounds. Toxicol Pathol. 2008;36:43–8. doi: 10.1177/0192623307310946. [DOI] [PubMed] [Google Scholar]
- 9.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Got-tesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discovery. 2006;5:219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 10.Ouar Z, Lacave R, Bens M, Vandewalle A. Mechanisms of altered sequestration and efflux of chemotherapeutic drugs by multidrug-resistant cells. Cell Biol Toxicol. 1999;15:91–100. doi: 10.1023/a:1007521430236. [DOI] [PubMed] [Google Scholar]
- 11.Narasaki F, Matsuo I, Ikuno N, Fukuka M, Soda H, Oka M. Multidrug resistance-associated protein (MRP) gene expression in human lung cancer. Anticancer Res. 1996;16:2079–82. [PubMed] [Google Scholar]
- 12.Krishnamachary N, Center MS. The MRP Gene Associated with a Non-P-glycoprotein Multidrug Resistance Encodes a 190-kDa Membrane Bound Glycoprotein. Cancer Res. 1993;53:3658–61. [PubMed] [Google Scholar]
- 13.Bellamy WT, Dalton WS. Multidrug resistance in the laboratory and clinic. Adv Clin Chem. 1994;31:1–61. doi: 10.1016/s0065-2423(08)60332-7. [DOI] [PubMed] [Google Scholar]
- 14.Molinari A, Calcabrini A, Meschini S, Stringaro A, Crateri P, Toccacieli L, Marra M, Colone M, Cianfriglia M, Arancia G. Subcellular detection and localization of the drug transporter P-glycoprotein in cultured tumor cells. Curr Protein Pept Sci. 2002;3:653–70. doi: 10.2174/1389203023380413. [DOI] [PubMed] [Google Scholar]
- 15.Lee ES, Na K, Bae YH. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J Control Release. 2005;103:405–18. doi: 10.1016/j.jconrel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Kreuter J. Nanoparticles in Colloidal Drug Delivery Systems. New York: Marcel Dekker; 1994. p. 219. [Google Scholar]
- 17.Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci. 2002;6:319–27. [Google Scholar]
- 18.Langer R. Tissue engineering: a new field and its challenges. Pharm Res. 1997;14:840–41. doi: 10.1023/a:1012131329148. [DOI] [PubMed] [Google Scholar]
- 19.Panyama J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Del Rev. 2003;55:329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 20.Garrec DL, Gori S, Luo L, Lessard D, Smith DC, Yessine MA, Ranger M, Leroux JC. Poly(N-vinylpyrrolidone)-block-poly(d,l-lactide) as a new polymeric solubilizer for hydrophobic anticancer drugs: in vitro and in vivo evaluation. J Control Release. 2004;99:83–101. doi: 10.1016/j.jconrel.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Park J, Fong PM, Lu J, Russell KS, Booth CJ, Saltzman WM, Fahmy TM. PEGylated PLGA nanoparticles for the improved delivery of doxorubicin. Nanomedicine: Nanotechnology, Biology, and Medicine. 2009;5:410–18. doi: 10.1016/j.nano.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musumecia T, Ventura CA, Giannone I, Ruozi B, Mon-tenegro L, Pignatello R, Puglisi G. PLA/PLGA nanoparticles for sustained release of docetaxel. Int J Pharm. 2006;325:172–9. doi: 10.1016/j.ijpharm.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Chan JM, Gu FX, Rhee JW, Wang AZ, Ra-dovic-Moreno AF, Alexis F, Langer R, Farokhzad OC. Self-assembled lipid-polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano. 2008;2:1696–702. doi: 10.1021/nn800275r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–90. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 25.Esmaeili F, Ghahremani MH, Esmaeili B, Khoshayand MS, Atyabi F, Dinarvand R. PLGA nanoparticles of different surface properties: Preparation and evaluation of their body distribution. Int J Pharm. 2008;349:249–55. doi: 10.1016/j.ijpharm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 26.Gref R, Minamitake Y, Percocchia MT, Trubetskoy V, Torchillin VP, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–3. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 27.Stolnik S, Dunn SE, Garnett MC, Davies MC, Coombes AGA, Taylor DC, Irving MP, Purkiss SC, Tadros TF, Davis S, Illum L. Surface modification of poly(lactide-co-glycolide) nanospheres by biodegradable poly(lactide)-poly(ethylene glycol) copolymers. Pharm Res. 1994;11:1800–8. doi: 10.1023/a:1018931820564. [DOI] [PubMed] [Google Scholar]
- 28.Tobio M, Gref R, Sanchez A, Langer R, Alonso MJ. Stealth PLA-PEG nanoparticles as protein carriers for nasal administration. Pharm Res. 1998;15:270–5. doi: 10.1023/a:1011922819926. [DOI] [PubMed] [Google Scholar]
- 29.Faraasen S, Voros J, Csucs G, Textor M, Merkle HP, Walter E. Ligand-specific targeting of microspheres to phagocytes by surface modification with poly(L-lysine)-grafted poly(ethylene glycol) conjugate. Pharm Res. 2003;20:237–46. doi: 10.1023/a:1022366921298. [DOI] [PubMed] [Google Scholar]
- 30.Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Müller RH. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B–Biointerfaces. 2000;18:301–13. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 31.Kocbek P, Obermajer N, Cegna M, Kos J, Kristl J. Targeting cancer cells using PLGA nanoparticles surface modified with monoclonal antibody. J Control Release. 2007;120:18–26. doi: 10.1016/j.jconrel.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Gu F, Zhang L, Teply BA, Mann N, Wang A, Radovic-Moreno AF, Langer R, Farokhzad OC. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc Natl Acad Sci USA. 2008;105:2586–91. doi: 10.1073/pnas.0711714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 2003;42:463–78. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 34.Vij N, Min T, Marasigan R, Belcher CN, Mazur S, Ding H, Yong K-T, Roy I. Development of PEGylated PLGA nanoparticle for controlled and sustained drug delivery in cystic fibrosis. J Nanobiotec. 2010;8:22. doi: 10.1186/1477-3155-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim D, Gao ZG, Lee ES, Bae YH. In vivo evaluation of doxorubicin-loaded polymeric micelles targeting folate receptors and early endosomal pH in drug-resistant ovarian cancer. Mol Pharm. 2009;6:1353–62. doi: 10.1021/mp900021q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahoo SK, Panyama J, Prabhaa S, Labhasetwar V. Residual polyvinyl alcohol associated with poly (D, L-lactide-coglycolide) nanoparticles affects their physical properties and cellular uptake. J Control Release. 2002;82:105–14. doi: 10.1016/s0168-3659(02)00127-x. [DOI] [PubMed] [Google Scholar]
- 37.Boury F, Ivanova T, Panaiotov I, Proust J, Bois EA, Richou J. Dynamic Properties of Poly(DL-lactide) and Polyvinyl Alcohol Monolayers at the Air/Water and Dichloromethane/Water Interfaces. J Colloid Interface Sci. 1995;169:380–92. [Google Scholar]
- 38.Peracchia MT, Fattal E, Desmaële D, Besnard M, Noël JP, Gomis JM, Appel M, d’Angelo J, Couvreur P. Stealth® PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. J Control Release. 1999;60:121–28. doi: 10.1016/s0168-3659(99)00063-2. [DOI] [PubMed] [Google Scholar]
- 39.Beletsi A, Leontiadis L, Klepetsanis P, Ithakissios DS, Avgoustakis K. Effect of preparative variables on the properties of poly(dl-lactide-co-glycolide)-methoxypoly(ethyleneglycol) copolymers related to their application in controlled drug delivery. Int J Pharm. 1999;182:187–97. doi: 10.1016/s0378-5173(99)00058-7. [DOI] [PubMed] [Google Scholar]
- 40.Kissel T, Li Y, Unger F. ABA-triblock copolymers from biodegradable polyester A-blocks and hydrophilic poly(ethylene oxide) B-blocks as a candidate for in situ forming hydrogel delivery systems for proteins. Adv Drug Delivery Rev. 2002;54:99–134. doi: 10.1016/s0169-409x(01)00244-7. [DOI] [PubMed] [Google Scholar]
- 41.Jeong B, Bae YH, Kim SW. Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymer. J Control Rel. 2000;63:155–67. doi: 10.1016/s0168-3659(99)00194-7. [DOI] [PubMed] [Google Scholar]
- 42.Bae YH, Huh KM, Kim Y, Park KH. Biodegradable amphiphilic multiblock copolymers and their implication to biomedical applications. J Control Rel. 2000;64:3–13. doi: 10.1016/s0168-3659(99)00126-1. [DOI] [PubMed] [Google Scholar]
- 43.Jeong B, Kibbey MR, Birnbaum JC, Won YY, Gutowska A. Thermogelling Biodegradable Polymers with Hydrophilic Backbones: PEG-g-PLGA. Macromolecules. 2000;33:8317–22. [Google Scholar]
- 44.Huh KM, Cho YW, Park K. PLGA-PEG Block Copolymers for Drug Formulations. Drug Development & Delivery. 2003;3:5. [Google Scholar]
- 45.Némati F, Dubernet C, Fessi H, Colin de Verdiere A, Poupon MF, Puisieux F, Couvreur P. Reversion of multidrug resistance using nanoparticles in vitro: Influence of the nature of the polymer. Int J Pharm. 1996;138:237–46. [Google Scholar]
- 46.Yoo HS, Lee KH, Oh JE, Park TG. In vitro and in vivo anti-tumor activities of nanoparticles based on doxorubicin-PLGA conjugates. J Control Release. 2000;68:419–31. doi: 10.1016/s0168-3659(00)00280-7. [DOI] [PubMed] [Google Scholar]
- 47.Tewes F, Munnier E, Antoon B, Ngaboni Okassa L, Cohen-Jonathan S, Marchais H, Douziech-Eyrolles L, Soucé M, Dubois P, Chourpa I. Comparative study of doxorubicin-loaded poly (lactide-co-glycolide) nanoparticles prepared by single and double emulsion methods. Eur J Pharm Biopharm. 2007;66:488–92. doi: 10.1016/j.ejpb.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 49.Endicott JA, Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–71. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- 50.Wong HL, Bendayan R, Rauth AM, Xue HY, Babakhanian K, Wu XY. A mechanistic study of enhanced doxorubicin uptake and retention in multidrug resistant breast cancer cells using a polymer-lipid hybrid nanoparticle system. J Pharmacol Exp Ther. 2006;317:1372–1381. doi: 10.1124/jpet.106.101154. [DOI] [PubMed] [Google Scholar]
- 51.Moghimi SM, Hunter AC. Poloxamers and poloxamines in nanoparticle engineering and experimental medicine. Trend Biotechnol. 2000;18:412–20. doi: 10.1016/s0167-7799(00)01485-2. [DOI] [PubMed] [Google Scholar]
- 52.Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers for overcoming drug resistance in cancer. Adv Drug Del Rev. 2002;54:759–79. doi: 10.1016/s0169-409x(02)00047-9. [DOI] [PubMed] [Google Scholar]
- 53.Nori A, Jensen KD, Tijerina M, Kopeckova P, Kopecek J. Subcellular trafficking of HPMA copolymer-Tat conjugates in human ovarian carcinoma cells. J Control Release. 2003;91:53–9. doi: 10.1016/s0168-3659(03)00213-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure 1. Fluorescence spectra of hydrophobic fluorophore Cy7.5 in different solvent environments. There is no fluorescence observed for free Cy7.5 in water (Red curve), free Cy7.5 in DMSO showed typical fluorescence spectra of Cy7.5 with maxima at 825 nm (Blue curve). The encapsulation of Cy7.5 in PEGylated PLGA nanoparticles maintained the fluorescent properties of the fluorophore with maximum fluorescence at 821 nm.