Abstract
Background
The aim of the study was to assess temporal changes in plaque size and components following heart transplantation (HTx), and to evaluate the differences in treatment effects on plaque progression between sirolimus and calcineurin inhibitors (CNIs).
Methods
The study comprised 146 HTx recipients who were converted from CNIs to sirolimus as primary immunosuppressant (sirolimus group, n=61), and those who were maintained on CNIs (CNI group, n=85). A retrospective compositional analysis of serial virtual histology-intravascular ultrasound was performed.
Results
During a median follow-up of 2.8 years, there was a significant difference in plaque volume in favor of sirolimus between groups (P=0.004). When subjects were subclassified according to the time interval between HTx and study inclusion, those in the early group (≤2 years after HTx) had a greater increase in plaque volume (P=0.006) characterized by a higher progression rate of fibrous plaque volume (P=0.01). The treatment difference between groups in plaque volume was identified in the early group in favor of sirolimus with attenuating effects on the progression of fibrous plaque component (both P=0.03 for interaction). By contrast, there were significant differences of necrotic core and dense calcium volume (both P<0.05 for interaction) in favor of CNIs in the late group (≥6 years after HTx).
Conclusions
Compared with a continued CNI therapy, sirolimus attenuated plaque progression in recipients with early conversion, but contributed to increases in necrotic core and dense calcium volume in those with late conversion. The current study supports the early initiation of sirolimus offers greater benefits on the development of CAV.
Keywords: Cardiac allograft vasculopathy, Sirolimus, Intravascular ultrasound, Virtual histology
Although major improvements have been made in the prevention and treatment of acute transplant rejection, cardiac allograft vasculopathy (CAV) remains one of the most important factors limiting long-term survival in heart transplantation (HTx). Calcineurin inhibitors (CNIs) such as cyclosporine and tacrolimus, which were pivotal in reducing acute rejection and improving early survival, are widely used for primary immunosuppressants of de novo HTx1. Recently, the use of proliferation signal inhibitors (PSIs), such as sirolimus and its derivative everolimus, in combination with a reduction or withdrawal of CNIs has emerged as an alternative therapeutic option for maintenance therapy in patients with CNI intolerance, CNI-induced nephropathy, or CAV development. In an effort to minimize the potential of renal dysfunction due to the combined use of sirolimus and CNI,2, 3 much attention has been directed toward a CNI-free regimen.
Previous IVUS studies from our group demonstrated that conversion to a CNI-free sirolimus based immunosuppression was associated with attenuation of the development of CAV4, 5 and reduction of the incidence of future cardiac events6 even in patients initially receiving a CNI following HTx.
Little is known about the effects of sirolimus on plaque morphology in CAV over time, as well as natural history of CAV lesions in terms of plaque characteristics. The aim of this study was to quantify changes in plaque size and components over time following HTx, and to evaluate the differences in treatment effects on plaque progression between sirolimus and CNIs.
Methods
Study Protocol
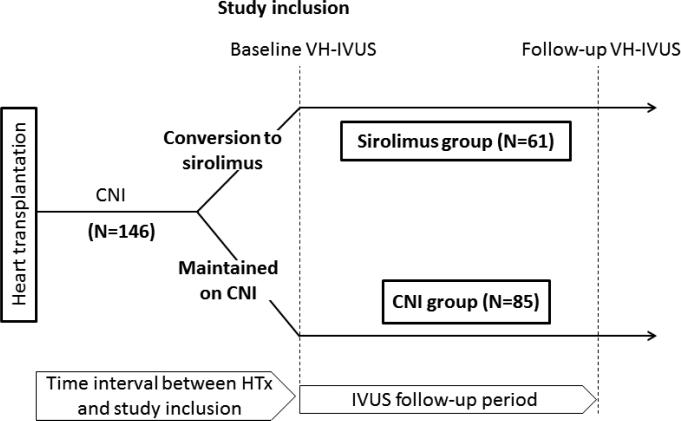
In the Mayo HTx program, VH-IVUS in the left anterior descending coronary artery (LAD) has been performed since 2005 in HTx recipients in conjunction with routine annual coronary angiography for surveillance of CAV. We selected for conversion from CNI to sirolimus as primary immunosuppressant patients with impaired renal function likely secondary to CNIs (glomerular filtration rate ≤50 mL/min without any other identifiable cause of renal dysfunction), CAV (any epicardial coronary artery stenosis ≥50% in any major branch and/or distal pruning of secondary side branches) detected on annual coronary angiography, severe CNI-related side effects, and those interested in conversion 3 to 6 months after transplantation based on our routine conversion protocol, as previously described.4, 5, 6 In this retrospective study of a clinical practice change, a total of 214 HTx recipients were initially screened for inclusion who underwent VH-IVUS examination within 1 year after conversion from CNI to sirolimus (sirolimus group), or who underwent VH-IVUS for those maintained on CNI (CNI group) between June 2005 and December 2010 (Figure 1). Of 214 patients, 41 without follow-up VH-IVUS (n=23, renal dysfunction; n=7, discretion of treating physicians; n=6, not completed one-year follow-up; n=3, died before the second IVUS; n=1, limited arterial access; n=1, followed-up elsewhere) were not included in the study. The following subjects were excluded from analysis: (1) patients converted back to CNI before follow-up IVUS in sirolimus group (n=9), (2) patients treated with both sirolimus and CNI for prior episodes of rejection (n=6), (3) patients undergoing coronary intervention in the LAD (n=8), and (4) patients with inadequate IVUS images limiting quantitative analysis (n=4). Thus, a total of 146 patients were included in the analyses. For patients with more than two IVUS evaluations during the study period, the first (baseline) and the last (follow-up) IVUS examinations were used for analysis. In a further subanalysis, one-year follow-up IVUS data which were available in 99 of 146 patients were used to assess changes in plaque volume during one-year observation period between two treatment groups. All transplant recipients received induction therapy with low-dose OKT3 or antithymocyte globulin as part of a standard induction protocol. Sirolimus, cyclosporine, tacrolimus, prednisone, azathioprine, and mycophenolate mofetil were managed and dosed as previously described5. Second immunosuppressive agents, mycophenolate mofetil or azathioprine, were left unchanged during follow-up, as was the existing dose of prednisone. In order to focus on the optimal timing of conversion to sirolimus, the study subjects were subclassified into three groups according to the time interval between HTx and study inclusion (baseline IVUS): early (≤2 years; n=52 [sirolimus; n=16 and CNI; n=36]), intermediate (between 3 to 5 years; n=44 [sirolimus; n=24 and CNI; n=20]), and late (≥6 years; n=50 [sirolimus; n=21 and CNI; n=29]). Data on patient characteristics were collected before each IVUS examination. The study was approved by Mayo Clinic institutional review board and all patients gave written informed consent.
Figure 1.
Timeline of the study. In this retrospective study of a clinical practice change, sirolimus group consisted of patients undergoing IVUS examination within 1 year after conversion from CNI to sirolimus. CNI group comprised those who were maintained on CNI and underwent IVUS examinations during the study period. The first (baseline) and the last (follow-up) IVUS examinations were used for analysis. Study patients were subclassified into three groups according to the time interval between HTx and study inclusion (baseline IVUS): early (≤2 years), intermediate (between 3 to 5 years), or late (≥6 years). CNI indicates calcineurin inhibitor; HTx, heart transplantation; and IVUS, intravascular ultrasound.
Coronary angiography
Based on the International Society of Heart and Lung Transplantation (ISHLT) guidelines7, CAV was classified by coronary angiography as ISHLT CAV0 (not significant), CAV1 (mild), CAV2 (moderate), and CAV3 (severe).
Grayscale and VH-IVUS Image Acquisition and Analysis
IVUS images were acquired after administration of 100-200 μg intracoronary nitroglycerin with a phased-array, 20 MHz, 3.2 Fr Eagle Eye Gold IVUS imaging catheter (Volcano Therapeutics Inc, Rancho Cordova, CA). The IVUS catheter was placed distally by using a fiduciary side branch as the starting point, and a motorized pull-back was performed at 0.5 mm/s up to the LAD ostium. During pull-back, the ECG-gated grayscale IVUS images were acquired and radiofrequency data were captured at the top of the R-wave.
Off-line volumetric reconstruction was performed using pcVH version 2.2 or Volcano Image Analysis Software V3.1 (Volcano Corporation) by two experienced observers blinded to baseline patient characteristics. Three to four matched coronary segments of the LAD were determined from the images acquired at baseline and follow-up studies on the basis of the fiducial location of distal and proximal major side branches. The length of the segment was assessed as the distance between these two side branches. For each coronary segment, vessel (external elastic membrane) and lumen borders were contoured for all recorded frames. Quantitative IVUS measurements included vessel volume, lumen volume, and plaque (vessel - lumen) volume, and plaque burden (plaque volume/vessel volume×100). Radiofrequency IVUS plaque components were color coded and reported as absolute plaque volume of VH-IVUS parameters (fibrous [dark green], fibrofatty tissue [light green], necrotic core [red], and dense calcium [white]) 8. All volumetric data were divided by segment length to compensate for the different segment length of each examined artery and were shown as a volume index (mm3/mm). The segment with the largest change in plaque volume index from baseline to follow-up in any matched site was used for the analysis in each patient. We previously reported interobserver variability for VH compositional data in transplant recipients.9
Statistical Analysis
Variables were expressed as mean±SD, median (interquartile range [IQR]), or counts (percentage). Differences in baseline parameters were analyzed with Student's t, Mann–Whitney U, chi-square test, or 1-way analysis of variance (ANOVA) followed by Dunnett's multiple comparisons test where appropriate. Within-treatment-group changes from baseline were evaluated by paired t tests. Changes from baseline in IVUS parameters which were divided by follow-up periods (years) to compensate for the different time interval between two IVUS examinations were analyzed using analysis of covariance (ANCOVA) model with treatment as a major factor and baseline value as a covariate. Treatment differences were summarized as the difference between treatment groups (sirolimus minus CNI) in adjusted mean, 95% confidence interval (CI) and P-value. In subgroup analysis, the interaction between subgroup and treatment (sirolimus or CNI) was tested with the interaction test of 2-way ANCOVA and treatment effects were assessed by ANCOVA model including a subgroup-allocated treatment interaction term. Likewise, treatment differences were assessed between two IVUS examinations performed at baseline and at one-year follow-up by ANCOVA model. To account for confounding due to differences in time since heart transplant between two treatment groups at baseline for changes in plaque volume (mm3/mm/year), multivariable linear regression analyses adjusting for treatment groups, time since heart transplant and baseline plaque volume index were performed. All statistical analyses were performed with JMP version 9.0 (SAS Institute Inc., Cary, North Carolina). A P value less than 0.05 was deemed significant.
Results
Baseline Characteristics
Of 146 recipients, 61 were converted to sirolimus at a median of 2.5 (1.2, 7.0) years after HTx. The reasons for conversion were CNI-induced nephropathy in 35, CAV detected on annual angiography in 5, and CIN-related side effects in 3, and our routine conversion protocol in 18. Baseline characteristics were comparable between the two groups (Table 1). Cholesterol and triglyceride levels were slightly but non-significantly higher in patients treated with sirolimus. Concurrent medications were administered to patients in the sirolimus and CNI groups. On coronary angiography, 43 (70%) subjects were graded as CAV 0, 16 (26%) as CAV 1, 1 (2%) as CAV 2, and 1 (2%) as CAV 3 in sirolimus group, whereas 48 (56%) as CAV 0, 33 (39%) as CAV 1, 4 (5%) as CAV 2, and none (0%) as CAV 3 in CNI group (p=0.16).
Table 1.
Patient characteristics
| Sirolimus (N=61) | CNI (N=85) | P | |
|---|---|---|---|
| Recipient age, yrs | 56±11 | 53±14 | 0.16 |
| Male sex, n (%) | 43 (70) | 65 (76) | 0.42 |
| Time since heart transplant, yrs | 2.5 (1.2, 7.0) | 2.9 (0.2, 8.5) | 0.38 |
| Donor age, yrs | 29±13 | 30±13 | 0.82 |
| Previous CMV infection, n (%) | 17 (28) | 22 (26) | 0.79 |
| Treatment episodes of rejection, n (%) | |||
| Baseline | 10 (16) | 13 (15) | 0.86 |
| During follow-up period | 2 (3) | 7 (8) | 0.22 |
| Comorbidities | |||
| BMI, kg/m2 | |||
| Baseline | 27.5±5.1 | 26.9±5.5 | 0.52 |
| Follow-up | 28.3±5.8* | 27.6±5.3* | 0.45 |
| Diabetes, n (%) | |||
| Baseline | 14 (23) | 19 (22) | 0.93 |
| Follow-up | 14 (23) | 19 (22) | 0.93 |
| Hypertension, n (%) | |||
| Baseline | 48 (79) | 58 (68) | 0.16 |
| Follow-up | 47 (77) | 57 (67) | 0.19 |
| Total cholesterol, mg/dl | |||
| Baseline | 198±46 | 184±47 | 0.07 |
| Follow-up | 194±57 | 177±48 | 0.06 |
| Triglyceride, mg/dl | |||
| Baseline | 148 (114, 222) | 128 (87, 193) | 0.06 |
| Follow-up | 168 (120, 226) | 135 (86, 191) | 0.01 |
| HDL cholesterol. mg/dl | |||
| Baseline | 55±17 | 54±18 | 0.59 |
| Follow-up | 50±16‡ | 52±14 | 0.36 |
| LDL cholesterol, mg/dl | |||
| Baseline | 109±34 | 100±35 | 0.12 |
| Follow-up | 100±30 | 93±37 | 0.26 |
| Serum creatinine, mg/dl | |||
| Baseline | 1.4±0.6 | 1.4±0.3 | 0.94 |
| Follow-up | 1.2±0.4* | 1.4±0.6 | 0.04 |
| Medication | |||
| Aspirin, n (%) | |||
| Baseline | 17 (28) | 23 (27) | 0.91 |
| Follow-up | 19 (31) | 25 (29) | 0.82 |
| ACE inhibitor, n (%) | |||
| Baseline | 25 (41) | 29 (34) | 0.40 |
| Follow-up | 24 (39) | 30 (35) | 0.61 |
| CCB, n (%) | |||
| Baseline | 13 (21) | 26 (31) | 0.21 |
| Follow-up | 14 (23) | 28 (33) | 0.19 |
| Statin, n (%) | |||
| Baseline | 56 (92) | 72 (85) | 0.19 |
| Follow-up | 57 (93) | 75 (88) | 0.29 |
| Immunosuppressants | |||
| Sirolimus, n (%) | 61 (100) | - | |
| Cyclosporin, n (%) | - | 61 (72) | |
| Tacrolimus, n (%) | - | 24 (28) | |
| Azathioprine, n (%) | 25 (41) | 25 (29) | 0.15 |
| MMF, n (%) | 35 (57) | 57 (67) | 0.23 |
Data are expressed as mean±SD, median (interquartile range) or n (%).
P<0.05
†P<0.01
P<0.001 vs. baseline.
ACE indicates angiotensin-converting enzyme; BMI, Body mass index; CCB, calcium channel blocker; CMV, cytomegalovirus; CNI, calcineurin inhibitors; HDL, high-density lipoprotein; IVUS, intravascular ultrasound; LDL, low-density lipoprotein; and MMF, mycophenolate mofetil.
IVUS Measurements
Table 2 shows baseline and follow-up results of IVUS analyses in all study subjects. When the change in plaque volume index from baseline was compared between treatment groups, there was a significant treatment effect in favor of sirolimus (least-squares means [sirolimus minus CNI], −0.36 mm3/mm/year; 95% CI, −0.60 to −0.12; P=0.004). In the VH-IVUS analyses, the comparison of the change from baseline between treatment groups did not show any significant differences.
Table 2.
IVUS findings
| Sirolimus (N=61) | CNI (N=85) | P | |
|---|---|---|---|
| IVUS follow-up period, yrs | 2.9 (2.0, 3.6) | 2.3 (1.7, 3.2) | 0.11 |
| Vessel volume index | |||
| Baseline, mm3/mm | 16.7±4.7 | 16.8±4.8 | 0.91 |
| Follow-up, mm3/mm | 16.7±5.1 | 17.2±4.3 | 0.58 |
| Change from baseline, mm3/mm | 0.0±3.6 | 0.3±3.0 | 0.10 |
| Lumen volume index | |||
| Baseline, mm3/mm | 12.2±4.1 | 12.1±4.2 | 0.91 |
| Follow-up, mm3/mm | 11.2±4.1 | 11.2±3.6 | 0.95 |
| Change from baseline, mm3/mm | −0.9±3.6* | −0.9±3.2* | 0.98 |
| Plaque volume index | |||
| Baseline, mm3/mm | 4.6±2.4 | 4.8±2.5 | 0.76 |
| Follow-up, mm3/mm | 5.4±2.8 | 6.0±3.0 | 0.25 |
| Change from baseline, mm3/mm | 0.8±1.5‡ | 1.2±1.6‡ | 0.004 |
| Plaque components | |||
| Fibrous | |||
| Baseline, mm3/mm | 0.5 (0.0, 1.4) | 0.6 (0.0, 1.8) | 0.37 |
| Follow-up, mm3/mm | 0.9 (0.2, 2.2) | 1.1 (0.2, 2.4) | 0.50 |
| Change from baseline, mm3/mm | 0.4±1.0† | 0.4±1.2‡ | 0.42 |
| Fibrofatty | |||
| Baseline, mm3/mm | 0.1 (0.0, 0.2) | 0.1 (0.0, 0.3) | 0.30 |
| Follow-up, mm3/mm | 0.1 (0.0, 0.2) | 0.1 (0.0, 0.3) | 0.16 |
| Change from baseline, mm3/mm | 0.0±0.3 | 0.0±0.6 | 0.96 |
| Necrotic core | |||
| Baseline, mm3/mm | 0.1 (0.0, 0.4) | 0.1 (0.0, 0.4) | 0.63 |
| Follow-up, mm3/mm | 0.3 (0.0, 1.1) | 0.3 (0.0, 0.9) | 0.96 |
| Change from baseline, mm3/mm | 0.3±0.6‡ | 0.3±0.6‡ | 0.92 |
| Dense calcium | |||
| Baseline, mm3/mm | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.2) | 0.12 |
| Follow-up, mm3/mm | 0.0 (0.0, 0.3) | 0.0 (0.0, 0.4) | 0.89 |
| Change from baseline, mm3/mm | 0.2±0.4‡ | 0.1±0.3‡ | 0.25 |
Data are expressed as mean±SD, or median (interquartile range).
P<0.05
P<0.01
P<0.001 vs. baseline.
CNI indicates calcineurin inhibitors; and IVUS, intravascular ultrasound.
Time Course of Plaque Development Following HTx
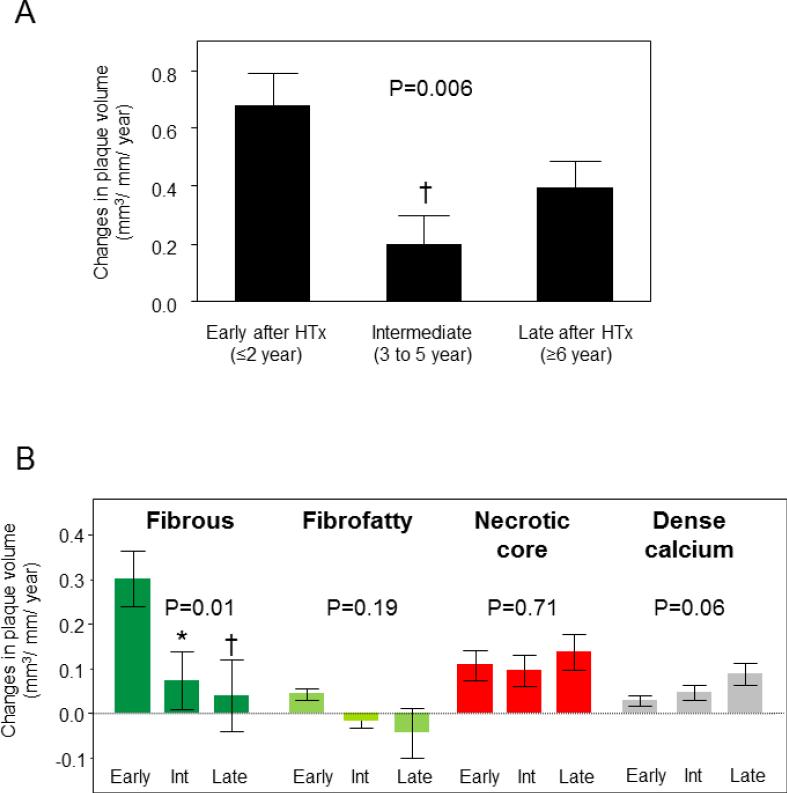
Figure 2 shows differences in plaque progression rate among the three groups stratified according to the time interval between HTx and study inclusion in all subjects. Patient characteristics among three study arms were shown in Table 3. The average annual changes in plaque volume were different over time among three study arms (P=0.006, Figure 2A). Recipients in the early group had a greater rate of increase in plaque volume than those in the intermediate group (P<0.01). VH-IVUS analyses (Figure 2B) demonstrated a higher progression rate of fibrous plaque volume in the early group compared with the intermediate and the late group (P<0.05 and P<0.01, respectively).
Figure 2.
Changes (plaque volume index per year [mm3/mm/year], mean±SE) in coronary plaque volume (A) and plaque volume of each component (B) from baseline among three groups classified according to the time interval between heart transplantation (HTx) and study inclusion. Early (≤2 year after HTx, n=52), Intermediate (Int) (3 to 5 years since HTx, n=44), and Late (≥6 years after HTx, n=50). *P<0.05, †P<0.01 vs. early group.
Table 3.
Sirolimus vs. CNI within three study arms
| Early | Intermediate | Late | ||||
|---|---|---|---|---|---|---|
| Sirolimus (N=16) | CNI (N=36) | Sirolimus (N=24) | CNI (N=20) | Sirolimus (N=21) | CNI (N=29) | |
| Recipient age, yrs | 54±9 | 54±11 | 56±11 | 50±17 | 56±13 | 53±16 |
| Male sex, n (%) | 9 (56) | 28 (78) | 17 (71) | 13 (65) | 17 (81) | 24 (83) |
| Time since heart transplant, yrs | 1.1 (1.0, 1.1)† | 0.2 (0.2, 1.0) | 2.2 (2.0, 3.0)† | 3.1 (2.3, 5.0) | 9.2 (6.5, 11.7) | 10.9 (8.1, 16.6) |
| Donor age, yrs | 27±12 | 33±13 | 33±14 | 29±13 | 26±11 | 26±12 |
| Previous CMV infection, n (%) | 8 (50) | 13 (36) | 7 (29) | 2 (10) | 2 (10) | 7 (24) |
| Treatment episodes of rejection, n (%) | 2 (13) | 6 (17) | 4 (17) | 1 (5) | 4 (19) | 6 (21) |
| Reason for conversion to sirolimus | ||||||
| CNI-induced nephropathy | 4 (25) | - | 16 (67) | - | 15 (71) | - |
| CAV | 1 (6) | - | 1 (5) | - | 3 (14) | - |
| CNI-related side effects | 0 (0) | - | 2 (9) | - | 1 (5) | - |
| Routine conversion protocol | 11 (69) | - | 5 (23) | - | 2 (10) | - |
| Comorbidities | ||||||
| Body mass index, kg/m2 | 27.2±5.9 | 25.8±4.4 | 27±5 | 28±6 | 28±5 | 27±6 |
| Diabetes, n (%) | 3 (19) | 11 (31) | 8 (33) | 3 (15) | 3 (14) | 5 (17) |
| Hypertension, n (%) | 12 (75) | 24 (67) | 19 (79) | 13 (65) | 17 (81) | 21 (72) |
| Total cholesterol, mg/dl | 228±48 | 199±48 | 195±46 | 188±40 | 179±31 | 163±43 |
| Triglyceride, mg/dl | 142 (116, 250) | 169 (107, 210) | 148 (107, 233) | 115 (81, 169) | 147 (122, 194) | 125 (78, 176) |
| HDL cholesterol. mg/dl | 54±13 | 57±22 | 60±18 | 53±14 | 51±18 | 50±13 |
| LDL cholesterol, mg/dl | 138±34† | 106±34 | 101±32 | 108±33 | 95±22 | 86±34 |
| Serum creatinine, mg/dl | 1.1±0.3* | 1.4±0.3 | 1.5±0.8 | 1.4±0.3 | 1.4±0.3 | 1.3±0.3 |
Data are expressed as mean±SD, median (interquartile range) or n (%).
P<0.05
P<0.01 vs. CNI.
CAV indicates cardiac allograft vasculopathy; CMV, cytomegalovirus; CNI, calcineurin inhibitors; HDL, high-density lipoprotein; and LDL, low-density lipoprotein.
Treatment Differences in Plaque Volume and Composition between Sirolimus and CNI
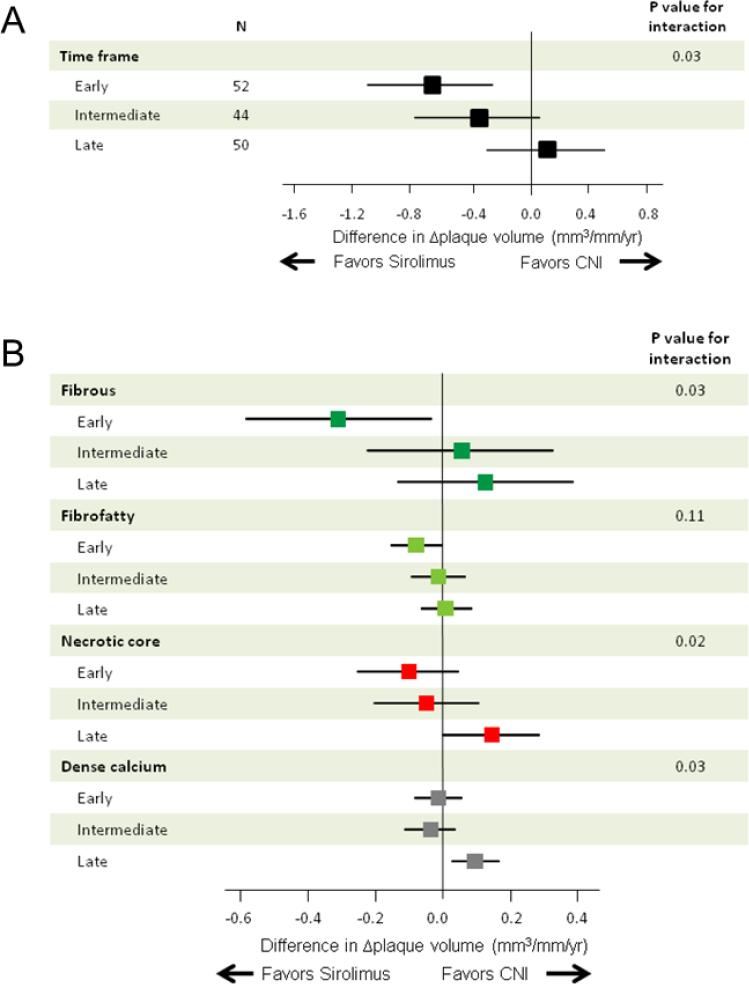
There was significant heterogeneity among the three study arms with respect to changes in plaque volume induced by the different treatments (P=0.03 for interaction, Figure 3A). There was a significantly less plaque volume progression in favor of sirolimus in patients with the early group (−0.67 mm3/mm/year; 95% CI, −1.10 to −0.25), but not in either the intermediate or the late group. After multivariable adjustment for differences in time since heart transplantation, treatment with sirolimus was independently associated with changes in plaque volume in the early group (Table 4). Plaque components analyses (Figure 3B) demonstrated a significant difference of fibrous plaque volume progression in favor of sirolimus in the early group (−0.31 mm3/mm/year; 95% CI, −0.58 to −0.03) (P=0.03 for interaction). On the other hand, there were significant differences of necrotic core (P=0.02 for interaction) and dense calcium volume progression (P=0.03 for interaction) in favor of CNI in the late group (0.15 mm3/mm/year, 95% CI: 0.00 to 0.29; and 0.10 mm3/mm/year, 95% CI: 0.03 to 0.17, respectively).
Figure 3.
Treatment differences in total plaque volume changes (Δplaque volume) (A) and in Δplaque volume of each plaque component (B) according to the time interval between heart transplantation (HTx) and study inclusion: A median follow-up period of 2.8 years. The results are shown as adjusted mean treatment difference (sirolimus minus CNIs) and 95% confidence interval. Changes in plaque volume were divided by follow-up periods (years) to compensate for the different time interval between two IVUS examinations. CNI indicates calcineurin inhibitor.
Table 4.
Multivariable predictors for change in plaque volume index in the early and intermediate group
| Variable | Standardized regression coefficient, P Value |
|||
|---|---|---|---|---|
| Early | Intermediate | |||
| Plaque volume index at baseline | 0.20 | 0.20 | −0.08 | 0.87 |
| Treatment with sirolimus | −0.45 | 0.004 | −0.22 | 0.20 |
| Time since heart transplant | 0.20 | 0.19 | 0.15 | 0.39 |
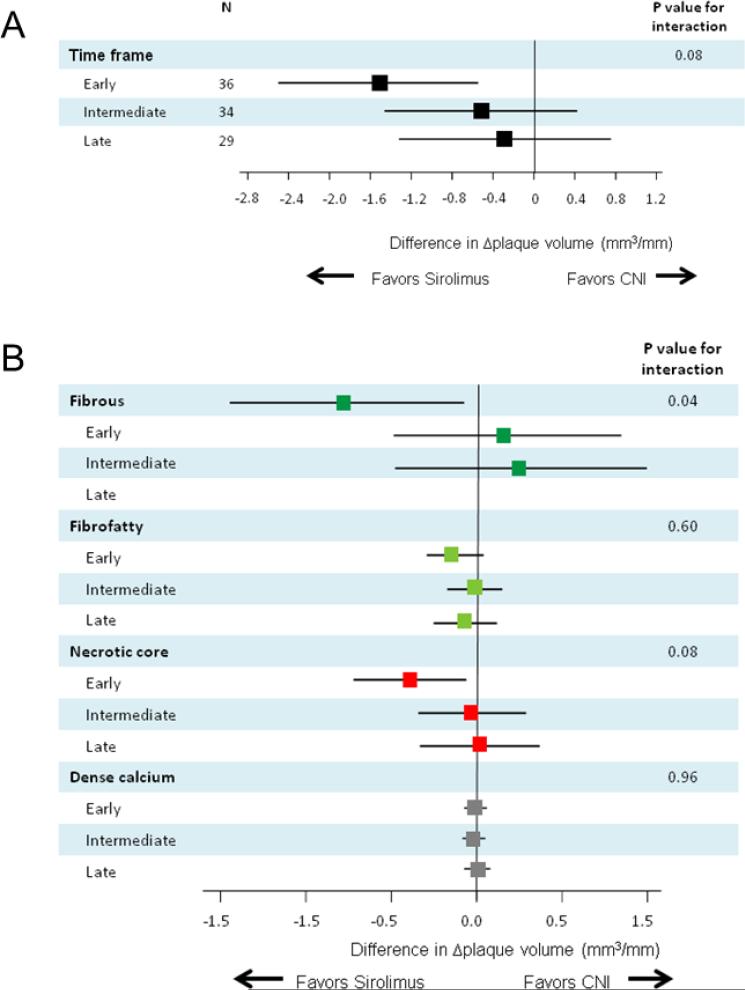
Subsequently, changes in plaque volume and components during the one-year follow-up period after study inclusion were compared between two treatment groups among the three study arms in a subpopulation of 99 patients. A marginally significant interaction (P=0.08 for interaction) was observed favoring sirolimus in the early group (Figure 4A). Plaque components analyses (Figure 4B) showed a significant difference of fibrous plaque volume progression in favor of sirolimus in the early group (−0.78 mm3/mm/year; 95% CI, −1.46 to −0.09) (P=0.03 for interaction).
Figure 4.
Treatment differences in total plaque volume changes (Δplaque volume) (A) and in Δplaque volume of each plaque component (B) according to the time interval between heart transplantation (HTx) and study inclusion: One-year follow-up in a subpopulation of 99 patients. The results are shown as adjusted mean treatment difference (sirolimus minus CNIs) and 95% confidence interval. CNI indicates calcineurin inhibitor.
Discussion
The current study demonstrates heterogeneity of CAV development in terms of plaque size and components over time, and suggests significant differential effects on CAV by the timing of switching to sirolimus-based immunosuppression after HTx.
The current serial VH-IVUS revealed an early rapid progression of plaque volume characterized by the development of fibrous plaque component followed by late accelerated calcification after HTx. These results extend previous IVUS observations at a single time point demonstrating that the degree of intimal thickness was most prominent during the first 2 years after HTx10 and compositionally, the fibrotic and fibrofatty tissue were predominant early after HTx whereas dense calcium and necrotic core were observed only in patients with a long transplant evolution time. 11, 12 The non-uniformity of chronological changes in each plaque component following HTx suggests the contribution of different pathophysiological mechanisms to the development of CAV over time.
Early conversion to sirolimus
The early conversion to sirolimus had beneficial effects on CAV progression, as was evidenced by the preventive effects on progression of fibrous plaque components. Previous studies have demonstrated that sirolimus3 or everolimus2 in combination with cyclosporine slowed plaque progression in de novo HTx recipients, as well as sirolimus-based immunosuppression with CNI-free regimen in subjects in the early period after HTx.4, 5, 6 Our in vivo observations on plaque composition may provide the potential mechanism to this effect and are consistent with previous experimental findings that sirolimus inhibits activated T-cell proliferation induced by alloantigens, regulates the proliferation and migration of vascular smooth muscle cells, and inhibits extracellular matrix accumulation and fibrotic tissue13.
Late conversion to sirolimus
Our observations further suggest that preventive effects on plaque progression may be attenuated when sirolimus is initiated late after HTx. Moreover, late conversion to sirolimus may influence plaque composition, in particular the development of a necrotic core and dense calcium components. Previous studies of PSIs in subjects with a long transplant evolution time have yielded conflicting results. A single-center study demonstrated that sirolimus together with cyclosporine slowed angiographic disease progression14, whereas a multicenter study demonstrated that the addition of everolimus to low-doses of CNI did not influence plaque volume15, but may have shifted plaque composition towards calcified and necrotic plaque components,16 consistent with our findings.
The mechanisms and clinical significance of an increase in necrotic core and calcium components in subjects with later sirolimus conversion are highly speculative at present. Sirolimus has a systemic effect of destabilizing the metabolic milieu with the development of hyperlipidemia17 which may exacerbate CAV. Although earlier studies have demonstrated that hyperlipidemia is associated with CAV, it is unclear whether hyperlipidemia in the face of sirolimus based immunosuppression has any negative impact because of the powerful anti-proliferative effects of PSI agents. Clinical observations in native atherosclerosis suggest that culprit lesions responsible for acute coronary syndrome are less calcified compared with those in stable angina, indicating that calcium provides plaque stability18. In addition, given a potential misclassification of areas surrounding dense calcium as necrotic core, inherent to VH-IVUS technology19, there is a possibility that a parallel increase in necrotic core and dense calcium might be associated with plaque stabilization. In fact, recent VH-IVUS studies of native atherosclerosis showed increases in necrotic core and dense calcium components after statin therapy,20, 21 better known for its plaque stabilizing effects. Given heterogeneous plaque morphology in CAV, 22 additional researches using other imaging techniques are warranted to better characterize plaques in CAV.
Clinical implications
The current study has potential implications for clinical practice. Regarding natural history of CAV lesions, chronological heterogeneity of plaque characteristics over time after HTx may raise the possibility that optimal therapeutic approaches should take into consideration the time frame following HTx. Furthermore, the initiation of sirolimus early after HTx could offer greater benefits in terms of CAV prevention. Further studies are needed to confirm whether specific plaque types characterized by VH-IVUS could be a major determinant of future cardiovascular events in CAV, as was shown in native atherosclerosis.23
Limitations
There are several limitations to be addressed. First, the current study is limited by its observational design with a small number of study patients. Second, there is an inherent selection bias as recipients with extremely progressive CAV, less severe CAV, or renal dysfunction may not have follow-up examination. Thus, the findings in the current study may not be extrapolated to these subjects. Third, we analyzed CAV only in the LAD. This could result in underdiagnosis of CAV, although a previous study demonstrated good correlation between LAD imaging and the incidence of CAV2. Forth, although comparisons were made using changes in IVUS parameters divided by IVUS follow-up period, this formula does not fully compensate for the different time intervals. This may partially account for variations in the findings between two different IVUS follow-up periods. Fifth, changes in several parameters regarding patient characteristics during follow-up period may be a source of bias in the IVUS analysis. Sixth, the possibility of residual confounding cannot be ruled out, although time interval between HTx and study inclusion in the early group was not significant for changes in plaque volume in multivariable analysis. Finally, as this study did not include subjects undergoing IVUS many years after conversion to sirolimus, any long-term effects of sirolimus on CAV were not determined.
Conclusions
Sirolimus attenuated plaque progression in recipients with early conversion to sirolimus, and induced substantial modification of plaque composition in those with late conversion compared with a continued CNI therapy. These observations demonstrated a heterogeneous response of CAV to switching to sirolimus, a process that appeared to be dependent on the timing of switching to sirolimus.
Acknowledgments
This work is supported by the National Institute of Health (NIH Grant HL-92954 and AG-31750 to A.L., and NIH Grants DK-73608, HL-77131, and HL-085307 to L.O.L.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors report no conflicts of interest to disclose.
References
- 1.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report--2011. J Heart Lung Transplant. 2011;30:1078–94. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Eisen HJ, Tuzcu EM, Dorent R, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349:847–58. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- 3.Keogh A, Richardson M, Ruygrok P, et al. Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: a randomized clinical trial. Circulation. 2004;110:2694–700. doi: 10.1161/01.CIR.0000136812.90177.94. [DOI] [PubMed] [Google Scholar]
- 4.Kushwaha SS, Khalpey Z, Frantz RP, et al. Sirolimus in cardiac transplantation: use as a primary immunosuppressant in calcineurin inhibitor-induced nephrotoxicity. J Heart Lung Transplant. 2005;24:2129–36. doi: 10.1016/j.healun.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Raichlin E, Bae JH, Khalpey Z, et al. Conversion to sirolimus as primary immunosuppression attenuates the progression of allograft vasculopathy after cardiac transplantation. Circulation. 2007;116:2726–33. doi: 10.1161/CIRCULATIONAHA.107.692996. [DOI] [PubMed] [Google Scholar]
- 6.Topilsky Y, Hasin T, Raichlin E, et al. Sirolimus as primary immunosuppression attenuates allograft vasculopathy with improved late survival and decreased cardiac events after cardiac transplantation. Circulation. 2012;125:708–20. doi: 10.1161/CIRCULATIONAHA.111.040360. [DOI] [PubMed] [Google Scholar]
- 7.Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010;29:717–27. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 8.García-García HM, Mintz GS, Lerman A, et al. Tissue characterisation using intravascular radiofrequency data analysis: recommendations for acquisition, analysis, interpretation and reporting. EuroIntervention. 2009;5:177–89. doi: 10.4244/eijv5i2a29. [DOI] [PubMed] [Google Scholar]
- 9.Raichlin E, Bae JH, Kushwaha SS, et al. Inflammatory burden of cardiac allograft coronary atherosclerotic plaque is associated with early recurrent cellular rejection and predicts a higher risk of vasculopathy progression. J Am Coll Cardiol. 2009;53:1279–86. doi: 10.1016/j.jacc.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 10.Rickenbacher PR, Pinto FJ, Chenzbraun A, et al. Incidence and severity of transplant coronary artery disease early and up to 15 years after transplantation as detected by intravascular ultrasound. J Am Coll Cardiol. 1995;25:171–7. doi: 10.1016/0735-1097(94)00323-i. [DOI] [PubMed] [Google Scholar]
- 11.König A, Kilian E, Sohn HY, et al. Assessment and characterization of time-related differences in plaque composition by intravascular ultrasound-derived radiofrequency analysis in heart transplant recipients. J Heart Lung Transplant. 2008;27:302–9. doi: 10.1016/j.healun.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Sarno G, Lerman A, Bae JH, et al. Multicenter assessment of coronary allograft vasculopathy by intravascular ultrasound-derived analysis of plaque composition. Nat Clin Pract Cardiovasc Med. 2009;6:61–9. doi: 10.1038/ncpcardio1410. [DOI] [PubMed] [Google Scholar]
- 13.Murphy GJ, Bicknell GR, Nicholson ML. Rapamycin inhibits vascular remodeling in an experimental model of allograft vasculopathy and attenuates associated changes in fibrosis-associated gene expression. J Heart Lung Transplant. 2003;22:533–41. doi: 10.1016/s1053-2498(02)00571-5. [DOI] [PubMed] [Google Scholar]
- 14.Mancini D, Pinney S, Burkhoff D, et al. Use of rapamycin slows progression of cardiac transplantation vasculopathy. Circulation. 2003;108:48–53. doi: 10.1161/01.CIR.0000070421.38604.2B. [DOI] [PubMed] [Google Scholar]
- 15.Arora S, Ueland T, Wennerblom B, et al. Effect of everolimus introduction on cardiac allograft vasculopathy--results of a randomized, multicenter trial. Transplantation. 2011;92:235–43. doi: 10.1097/TP.0b013e31822057f1. [DOI] [PubMed] [Google Scholar]
- 16.Arora S, Erikstad I, Ueland T, et al. Virtual Histology Assessment of Cardiac Allograft Vasculopathy Following Introduction of Everolimus-Results of a Multicenter Trial. Am J Transplant. 2012;12:2700–9. doi: 10.1111/j.1600-6143.2012.04234.x. [DOI] [PubMed] [Google Scholar]
- 17.Hoogeveen RC, Ballantyne CM, Pownall HJ, et al. Effect of sirolimus on the metabolism of apoB100- containing lipoproteins in renal transplant patients. Transplantation. 2001;72:1244–50. doi: 10.1097/00007890-200110150-00011. [DOI] [PubMed] [Google Scholar]
- 18.Beckman JA, Ganz J, Creager MA, Ganz P, Kinlay S. Relationship of clinical presentation and calcification of culprit coronary artery stenoses. Arterioscler Thromb Vasc Biol. 2001;21:1618–22. doi: 10.1161/hq0901.095554. [DOI] [PubMed] [Google Scholar]
- 19.Sales FJ, Falcão BA, Falcão JL, et al. Evaluation of plaque composition by intravascular ultrasound “virtual histology”: the impact of dense calcium on the measurement of necrotic tissue. EuroIntervention. 2010;6:394–9. doi: 10.4244/EIJV6I3A65. [DOI] [PubMed] [Google Scholar]
- 20.Nozue T, Yamamoto S, Tohyama S, et al. Statin treatment for coronary artery plaque composition based on intravascular ultrasound radiofrequency data analysis. Am Heart J. 2012;163:191–9. e1. doi: 10.1016/j.ahj.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Lee SW, Hau WK, Kong SL, et al. Virtual histology findings and effects of varying doses of atorvastatin on coronary plaque volume and composition in statin-naive patients: the VENUS study. Circ J. 2012;76:2662–72. doi: 10.1253/circj.cj-12-0325. [DOI] [PubMed] [Google Scholar]
- 22.Mehra MR, Ventura HO, Jain SP, Ramireddy K, Ali A, Stapleton DD, Smart FW, Ramee SR, Collins TJ, White CJ. Heterogeneity of cardiac allograft vasculopathy: clinical insights from coronary angioscopy. J Am Coll Cardiol. 1997;29:1339–44. doi: 10.1016/s0735-1097(97)00059-4. [DOI] [PubMed] [Google Scholar]
- 23.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]