Abstract
Objectives
The study aimed to assess whether young binge drinkers have impaired macrovascular and microvascular function and cardiovascular (CV) disease risk factors compared to age-matched alcohol abstainers.
Background
Binge drinking rates are highest on college campuses and among 18- to 25-year-olds; however, macrovascular and microvascular endothelial function in young adults with a history of repeated binge drinking (≥5 standard drinks in 2 hrs. in men; ≥4 standard drinks in 2 hrs. in women) has not been investigated
Methods
We evaluated the cardiovascular profile, brachial artery endothelial-dependent flow mediated vasodilation (FMD), and flow independent nitroglycerin (NTG)-mediated dilation and vasoreactivity of resistance arteries (isolated from gluteal fat biopsies) in abstainers and binge drinkers.
Results
Men and women (18–25 years of age, abstainers [A] n = 17, binge drinkers [BD] n = 19) were enrolled. Among the BD group, past-month average number of binge episodes was 6 ± 1, and average duration of binge drinking behavior was 4 ± 0.6 years. FMD and NTG-mediated dilations were significantly lower in the BD (FMD: 8.4% ± 0.7, P = 0.022; NTG: 19.6% ± 2, P = 0.009) than the A group (FMD: 11 ± 0.7%; NTG: 28.6 ± 2%). ACh- and SNP-induced dilation in resistance arteries was not significantly different between the A and BD groups. However, ET-1-induced constriction was significantly enhanced in the BD group compared to the A group (P = 0.032). No differences between groups were found in blood pressure, lipoproteins, and C-reactive protein.
Conclusions
Alterations in the macrocirculation and microcirculation may represent early clinical manifestations of CV risk in otherwise healthy young binge drinkers. This study has important clinical implications for screening young adults for a repeated history of binge drinking.
Keywords: Alcohol, endothelium, vasculature
Regular heavy episodic alcohol use (or “binge drinking”) is one of the most serious public health problems confronting American colleges (1). More than half of college student drinkers engage in regular binge drinking episodes, which is broadly defined as consuming more than 4–5 standard drinks (13 gm alcohol/drink) in a two-hour period (2–4). Results from retrospective studies enrolling adults ranging in age between 40 and 60 years have found that binge drinking is associated with a heightened risk of cardiovascular (CV) events, such as stroke, sudden death, myocardial infarction, and increased mortality after myocardial infarction (5–8). Others have reported that an alcohol binge drinking pattern is associated with progression of carotid atherosclerosis (9). Several mechanisms may underlie the increased risk of adverse CV events; however, one central mechanism may be changes in vascular biology, such as endothelial dysfunction.
The endothelium is a key regulator of vascular function. Endothelial dysfunction is an early indicator of blood vessel damage and atherosclerosis and a strong prognostic factor for future CV events (10,11). Impaired endothelium-independent dilation, reflective of smooth muscle dysfunction, is also linked to the development of atherosclerosis. Flow-mediated dilation (FMD) and nitroglycerin-mediated dilation (NTG) of the brachial artery are commonly used to evaluate endothelial-dependent and -independent function, respectively. To our knowledge, endothelial function in young adults with a repeated history of binge drinking has not been investigated, nor have studies simultaneously evaluated macrovascular and microvascular endothelial function. Endothelial cells can differ in structure and physiologic function, depending on the vasculature bed, making it clinically important to study vascular function in multiple vascular sites. This study was designed to test the hypothesis that young binge drinkers have impaired macrovascular and microvascular function compared to age-matched alcohol abstainers.
Methods
Study subjects and protocol
Thirty-eight nonsmoking healthy subjects were recruited from an urban university setting into this study: alcohol abstainers (A) (male n = 10; female n = 9) and binge drinkers (BD) (male n = 11; female n = 6). Binge drinkers were defined as those that consumed 5 standard drinks (12 oz. beer, 5 oz. table wine, 1.5 oz 80-proof spirits, 8–9 oz. malt liquor) or more in a two-hour period in the last 2 weeks if male, and 4 or more standard drinks in a two-hour period in the last 2 weeks if female (2). Abstainers were defined as those that consumed no more than 1–5 drinks standard drinks in the last year. Exclusion criteria included: history of diabetes, hypertension, pregnancy, CV disease or events, thyroid disease, pituitary tumor, a genetic disease causing disability, gout, illicit drug use, and body mass index (BMI) ≥ 30 kg/m2. The study was approved by the Office of Protection of Research Subjects and Institutional Review Board, and written consent was obtained from all subjects.
In all subjects, the following tests were performed: fasting lipid panel (total cholesterol, high-density lipoprotein [HDL] cholesterol, low-density lipoprotein [LDL] cholesterol, triglycerides), insulin and glucose, complete blood count (CBC) differential, C-reactive protein (CRP), and blood alcohol levels (Alverno Clinical Laboratories, Hammond, IN, USA). Venous samples were drawn into a serum tube and an ethylenediaminetetraacetic acid-containing tube. Also measured were resting blood pressure (BP), heart rate (HR), oxygen saturation, and temperature. Gluteal fat pad biopsies were performed, and resistance arteries were isolated for isolated perfused microvessel experiments.
All subjects completed questionnaires about medical history, diet, and alcohol beverage consumption. The Block Brief 2000 Food Frequency Questionnaire (NutritionQuest: Berkeley, CA, USA) was used to obtain information about diet. Alcohol consumption (pattern and frequency) was estimated using a modified version of the 6-item set of questions on binge drinking (12). Three additional questions were included on binge drinking that addressed the type of alcohol consumed, history of familial alcohol abuse, and duration of binge drinking. For BD subjects, the rate of alcohol consumption (grams/hour) was calculated based upon their most recent BD episode (rate = total grams of alcohol consumed/time spent consuming alcohol).
Measurement of FMD
All imaging studies were performed in the morning after an overnight fast. Similar to previously reported protocols and methods, ultrasound imaging was conducted using the MicroMaxx ultrasound machine (SonoSite; Seattle, WA) (13). Imaging of the brachial artery was performed in a longitudinal plane, at approximately 5 cm proximal to the antecubital fossa of the right arm, abducted approximately 80° from the body, with the forearm supinated. The ultrasound probe (11 MHz) was positioned at a 60° insonation angle to visualize the anterior and posterior lumen-intima interfaces, to measure diameter or central flow velocity (pulsed Doppler). After baseline ultrasound imaging, Doppler readings of peak flow and average flow were performed for at least 5 seconds. A BP cuff was placed on the forearm, distal to the antecubital fossa of the imaged arm and inflated 60 mmHg above baseline systolic BP (SBP) for 5 minutes. Once the cuff was released, BP and HR measurements in the opposite arm were taken, along with Doppler readings of the first 10 seconds after cuff release. The brachial artery was then imaged continuously to capture 30 seconds, 1 min, 2 min, and 3 min post BP cuff release. The response to NTG was used for the determination of endothelium-independent vasodilation. After obtaining a baseline/resting brachial artery image, a sublingual NTG tablet (0.4 mg) was administered, and brachial artery images and measurements were repeated and obtained as detailed above.
Images were digitally recorded using Brachial Imagery (Medical Imaging, Iowa City, IA, USA) and analyzed as previously described (13). Four hundred fifty frames were captured, digitized, and analyzed from the M-line (border between intima and media of brachial artery) of the same location of blood vessel using visible landmarks through edge detection software. Approximately 75 frames (7.5 frames per second for 10 seconds) were analyzed for each baseline and time point measurement through an average of brachial artery diameters over the entire R-R interval. FMD (%) and the response to NTG were calculated using the averaged minimum mean brachial artery diameter at baseline compared with the largest mean values obtained after release of the forearm occlusion or administration of NTG. The coefficient of variation (intra-observer) was 1.5% for brachial artery diameter, 6.3% for FMD %, and 3.2% for NTG-induced dilation. The peak flow velocity was observed from 5 seconds of baseline diameter Doppler readings, and 10 seconds of post-cuff release Doppler readings were recorded for shear rate calculations. Shear rate was calculated as blood velocity (cms) divided by vessel diameter (cm) (15).
Gluteal adipose biopsy and resistance artery function
Microvessels in subcutaneous fat are easily accessible and have been used to investigate microvascular function in humans (16). Approximately 2 ml of fat tissue was removed and transferred to (4°C) HEPES solution. Resistance arteries (~150 μm) were isolated and cannulated in an organ chamber with glass micropipettes filled with KREBS solution (pH = 7.40) (14). Both ends of the vessel were secured, and the vessel was maintained at an intraluminal pressure of 20 mmHg for 30 minutes, after which intraluminal pressure was gradually increased to 60 mmHg for an additional 30 minutes. Preparations were visualized with video cameras and monitors (Boeckeler model VIA-100: Tucson, AZ, USA). Following the 30-minute equilibration period, vessels were constricted (30–50% of baseline diameter) with ET-1 (100–200 pM final concentration), and dose response curves were measured using acetylcholine (ACh; 10−9-10−4 M; A: n = 10; BD: n = 12) and the nitric oxide (NO) donor sodium nitroprusside (SNP; 10−9-10−4 M; A: n = 8; BD: n = 8). Resistance arteries were monitored continuously, and internal diameters were measured at the maximum diameter after each dilator dose. Dose responses to ACh were measured in the absence and presence of the NO synthase inhibitor N-Nitro-L-Arginine Methyl Ester (L-NAME; 10−4 M; A: n = 9; BD: n = 11). Maximal responses to papaverine (10−4 M) were measured at the end of each dose response of ACh. In separate experiments, also determined were constrictor responses to endothelin-1 (ET-1; 10−11-10–7 M; A: n = 9, BD: n = 10).
Materials
Pharmacological compounds L-NAME, ACh, SNP, and ET-1 were obtained from Sigma-Aldrich Corporation (St. Louis, MO, USA). Chemical reagents for buffer solutions were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA) and Fisher Scientific (San Jose, CA, USA).
Statistics
Analyses were conducted using SAS (version 9.3) and SigmaPlot (Version 12.0) (San Jose, CA, USA). All data are reported as mean ± SE, with P < .05 as significant unless otherwise noted. Nutritional questionnaires were sent to NutritionQuest (Berkeley, CA, USA) for calorie and other nutrient analysis. Normality was tested using the Shapiro-Wilk test. The A and BD group differences were determined using independent t tests. Mann-Whitney U tests were used for continuous variables (e.g., demographic, CRP, lipid, FMD %, NTG %) and chi-squared tests for categorical variables. In dose response experiments, data are expressed as a percentage, with 100% representing the change from constricted diameter to the maximal diameter at 60 cmH2O intraluminal pressure. Dose responses to ACh (−/+ L-NAME), SNP, and ET-1 were compared using a two-factor, repeated-measures analysis of variance and using a Bonferroni adjustment. ET-1 EC50 values were computed in SigmaPlot with the Dynamic Fit Wizard and with an equation (Y = min + max-min/ 1+ 10(logEC50-x) Hillslope) for XY pair data.
Analyses of covariance were performed on endothelium-dependent and -independent vasodilation, to examine the relationship of binge drinking, controlling for covariates (age, sex, ethnicity) using a general linear model. Covariates were tested with binge drinking status individually due to the small sample size. Effect sizes and 95% confidence intervals were computed without covariates (17).
Results
Clinical Characteristics
Demographic characteristics were not significantly different between the A and BD group subjects (Table 1); however, the BD group were more likely to be White (P = .004). Nutritional histories (macro- and micronutrient intake) and white and red blood cell values were not significantly different between groups (data not shown). All lipid values and CRP were similar between groups (Table 1). The average time since the last binge episode in the BD group was 65 ± 11 hours. Subjects in the BD group drank alcohol at a rate of 34 ± 7 g. During the month before the study, the average number of binge episodes among the BD group was 6 ± 1. The average duration of binge drinking behavior was 4 ± 0.6 years.
Table 1.
Demographic and Metabolic Characteristics
| Characteristics | Abstainers (n = 19) | Binge Drinkers (n = 17) | P |
|---|---|---|---|
| Age median, y | 24.5 | 23 | 0.526 |
| 25th quartile | 21 | 21.5 | |
| 75th quartile | 29 | 25 | |
| Male, % | 53 | 65 | 0.463 |
| White, % | 21 | 76 | 0.004 |
| Body Mass Index, kg/m2 | 23.7 ± 0.9 | 25.0 ± 1 | 0.370 |
| Systolic Blood Pressure, mm Hg | 114 ± 3 | 120 ± 2 | 0.092 |
| Diastolic Blood Pressure, mm Hg | 67 ± 1 | 70 ± 2 | 0.163 |
| Heart rate, bpm | 64 ± 1 | 65± 2 | 0.800 |
| Total cholesterol, mg/dL | 162.16 ± 8.88 | 166.12 ± 6.18 | 0.689 |
| LDL cholesterol median, mg/dL | 82 | 86 | 0.975 |
| 25th quartile | 67 | 67 | |
| 75th quartile | 118 | 107 | |
| HDL cholesterol, mg/dL | 54.83 ± 3.09 | 61.78 ± 5.02 | 0.21 |
| Triglycerides median, mg/dL, | 61.0 | 78.5 | 0.159 |
| 25th quartile | 44.0 | 62.2 | |
| 75th quartile | 104 | 98.7 | |
| Glucose, mg/dL | 85.05 ± 1.98 | 89.01 ± 1.98 | 0.167 |
| Insulin, μIU/L | 6.48 ± 1.09 | 4.69 ± 0.71 | 0.167 |
| CRP, mg/dL | 1.29 ± 0.59 | 1.60 ± 0.70 | 0.421 |
| Blood alcohol, % | ND | 0.01 ± 0.002 | 0.762 |
| AST, IU/L | 19 ± 1 | 23 ± 2 | 0.058 |
| ALT, IU/L | 9 ± 1 | 11 ± 1 | 0.249 |
Values are mean ± SEM, except for age, triglycerides, and LDL-cholesterol, which are median and 25th and 75th quartiles.
ALT: alanine aminotransferase, AST: aspartate aminotransferase.
Brachial artery endothelium- dependent and -independent dilation
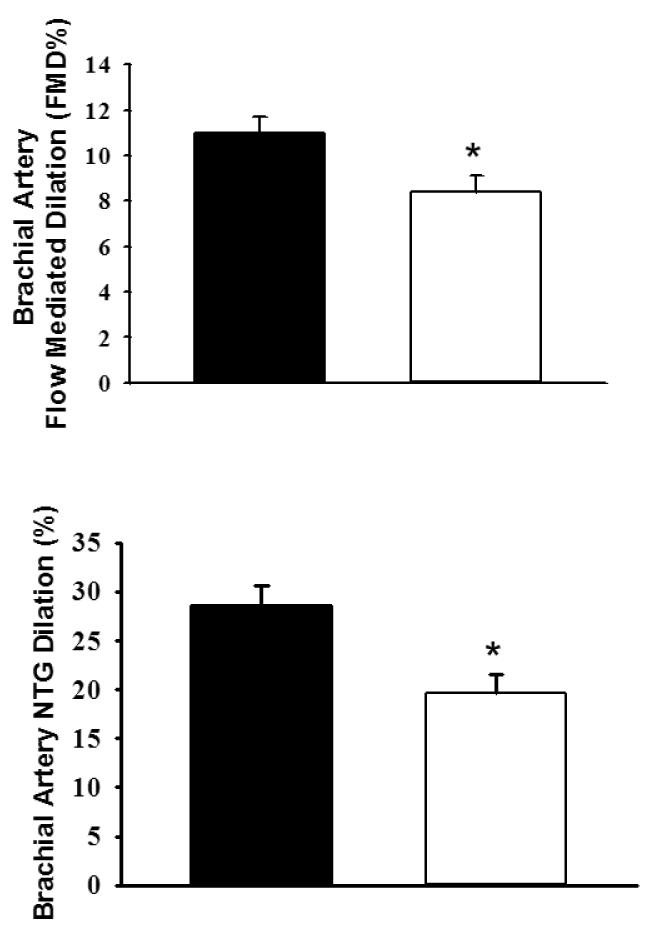
Except for baseline brachial FMD diameter and change in shear rate, blood vessel characteristics were not different between the A and BD groups (Table 2). Unadjusted FMD and NTG-induced dilations were significantly lower in the BD group compared to the A group (P = 0.022, P = 0.009, respectively; Figure 1). When baseline brachial artery diameter was tested as a predictor, there remained a significant difference between groups, though the effect was slightly attenuated. The effect size (eta squared) was 0.25 (95% CI: 0.023, 0.47) for FMD and 0.61 (CI: 0.32, 0.74) for NTG. Also, because the BD group had a higher proportion of Whites, this variable was tested individually as a covariate in a multivariate model, as well as age and sex. No covariates were statistically significant.
Table 2.
Brachial Artery Characteristics
| Abstainers (n = 19) | Binge Drinkers (n = 17) | P | |
|---|---|---|---|
| Baseline brachial FMD diameter, mm | 3.5 ± 0.1 | 3.9 ± 0.2 | 0.049 |
| Maximum brachial FMD diameter, mm | 3.9 ± 0.1 | 4.3 ± 0.2 | 0.053 |
| Δ Flow, cm/s | 98 ± 12 | 80 ± 10 | 0.176 |
| Peak flow, cm/s | 142 ± 7 | 128 ± 9 | 0.142 |
| Δ Shear rate, sec | 98 ± 12 | 95 ± 15 | 0.433 |
| Peak shear rate, sec | 412 ± 18 | 328 ± 20 | <0.005 |
| Baseline brachial NTG diameter, mm | 3.5 ± 0.1 | 4.00 ± 0.2 | 0.017 |
| Maximum brachial NTG diameter, mm | 4.5 ± 0.1 | 4.8 ± 0.2 | 0.153 |
Values are mean ± SEM
Figure 1. Values are mean ±SEM.
Brachial FMD (A) and NTG-induced dilations (B) were significantly lower in the BD group compared to the A group (P = 0.022 and P = 0.009, respectively).
Microvascular resistance artery endothelium-dependent and -independent dilation
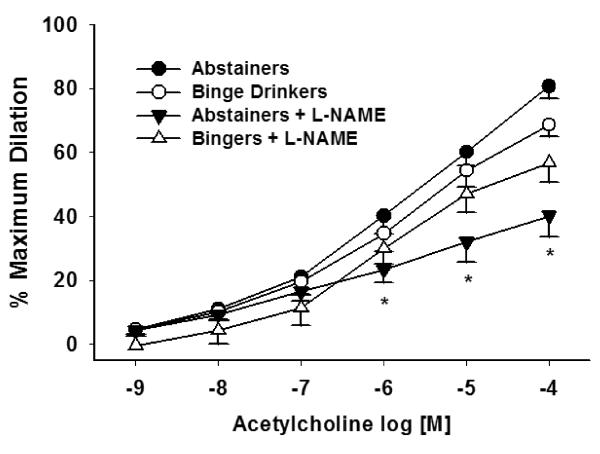
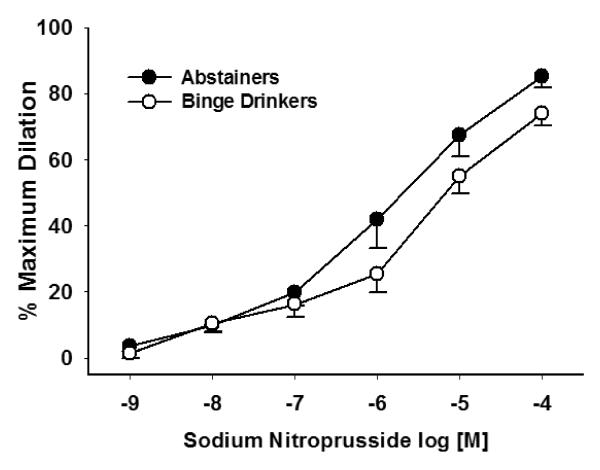
ACh-induced dilation in resistance arteries was not significantly different between the A and BD groups (Figure 2) (P = 0.365). The presence of L-NAME significantly blocked ACh-induced dilation in the A group (P = 0.002), but not in the BD group (P = 0.155). SNP-induced dilation in resistant arteries was not significantly different between groups (P = 0.948) (Figure 3). Because ET-1 was used to pre-constrict vessels prior to ACh dose response experiments, we compared the dose used for preconstriction, and there was no significant difference between groups (A: 1.6 × 10−8, BD: 1.4 × 10−8; P = 0.442).
Figure 2. ACh-induced dilation in resistance arteries were similar between the A group (n = 11) and BD group (n = 10) (P = .365).
L-NAME significantly blocked ACh-induced dilation in the A group (P = 0.007) but not in the BD group (P = 0.155). Values are mean ±SEM. *Denotes significance P ≤ 0.008 between ACh-induced dilation and ACh + L NAMEinduced
dilation in A group.
Figure 3. SNP-induced dilation was similar between the A (n = 5) and BD (n = 6) groups (P = 0.948).
Values are mean ±SEM.
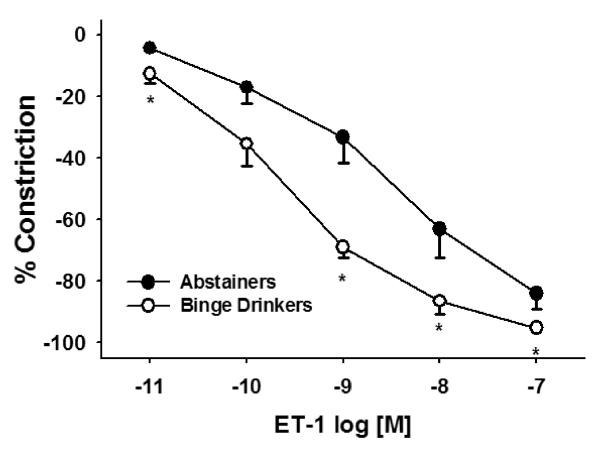
At several concentrations tested, ET-1-induced constriction was significantly enhanced in the BD group compared to the A group (Figure 4; P = 0.002). EC50 for ET-1 was significantly more potent in the BD group than the A group (A: 8.7 ± 0.3 nM/L, BD: 9.4 ± 0.1 nM/L; P = 0.032).
Figure 4. ET-1-induced constriction was significantly greater in the BD group (n = 10) compared to the A group (n = 10) (P = 0.002).
Values are mean ±SEM. *Denotes significant difference P ≤ 0.01.
Discussion
We demonstrate that young adults with normal serum lipoproteins, triglycerides, CRP, glucose, and insulin levels and a history of binge drinking (mean 4 years) had reduced FMD and NTG-induced dilation. This study addressed the importance of binge drinking on macro- and micro-vascular function and found important changes in both vascular beds.
Binge Drinking and Macrovascular Function
Our findings of impairment in brachial artery endothelial-dependent and -independent vasodilation are similar to others who have examined the one-time effect of a single binge episode on endothelial function (18–20). Bau et al. found that FMD and NTG-induced dilations were impaired at 4 hours after alcohol intoxication, returning to baseline levels at 13 hours after alcohol intoxication (19). There are also reports that FMD is impaired in abstinent individuals with a long-term history of alcohol abuse or alcoholism (21–23). Our findings suggest that young binge drinkers have developed FMD impairments that are equivalent to those found in individuals who have a lifetime history of daily heavy alcohol consumption (> 6 drinks/day for > 8 years). Since our subjects were tested between 24 and 96 hours following their most recent binge drinking episode, the time course for restoration of FMD that might occur with cessation of binge drinking is unknown. It is important to note that NTG-dilations were also reduced in BDs, suggesting that there could be reduced smooth muscle cell responses to NO and altered post-receptor signal transduction events, such as alterations in the cyclic guanosine monophosphate pathway.
Binge Drinking and Microvascular Function
A unique aspect of our study was the use of subcutaneous gluteal fat pad biopsy technique to study isolated resistance arteries under ex vivo conditions. In contrast to decreased FMD in brachial arteries of the BD group, there were no differences between groups in response to all doses of the endothelium-dependent vasodilator, ACh, and the NO-donor, SNP. Our results suggest that the effects of binge drinking on FMD and ACh and SNP dilations are divergent. In addition, L-NAME had no inhibitory effect on ACh-induced dilations, which implies the possibility that other endothelial or non-endothelium-dependent pathways may be involved in vasodilation in resistance arteries. These differences may relate to the experimental preparation and the absence of circulating vasoconstrictor mediators in the ex vivo preparation, such as norepinephrine or flow stimuli (shear stress vs. ACh). Also, others report that dilations to flow and ACh can be divergent in the context of pathological conditions (i.e., high cholesterol) (24).
In certain CV disease states, NO bioavailability is reduced and other endothelium-derived dilator substances or non-endothelium-derived factors such as hydrogen peroxide (H2O2) may compensate for the lack of NO (14,25). Both acute and chronic ethanol exposure is associated with the excessive generation of reactive oxygen species (ROS) and oxidative stress and is believed to play a central role in alcohol toxicity (26). Yogi et al. found that ethanol (100 mmol) increased superoxide and H2O2 generation in cultured aorta vascular smooth muscle cells (27). Others have found that impairments in NO-dependent vasorelaxation occurred with increased ROS production and overproduction of ET-1 (28). While in our study circulating ET-1 levels were not measured, we found enhanced ET-1-induced constrictions and a lower EC50 in the BD compared to the A group, indicating increased vasoconstrictor responsiveness to ET-1 (Figure 3). ET-1 may reduce NO production and increase NO degradation, and increased plasma levels of ET-1 have been found in heavy alcohol consumers (23,29,30), making it important to elucidate the effects of binge drinking on ET-1 and ET-1 receptors in the setting of binge drinking.
Binge Drinking and Accelerated Atherosclerosis
In other populations, such as healthy postmenopausal women, endothelial dysfunction was found to be significantly associated with the future development of hypertension (31). In the Coronary Artery Risk Development in Young Adults study, Pletcher et al. (2005) found that binge drinking in adults (mean age 25.2 years) was significantly associated with greater coronary calcification, suggesting the presence of coronary artery atherosclerosis (32). Recently, Liu et al. reported that binge drinking rather than daily moderate drinking was associated with accelerated plaque development in a mouse model of accelerated atherosclerosis (33). Also, in a prospective evaluation of adults admitted for first-ever brain infarction, Hillbom et al. found that acute intake of more than 40 grams of ethanol during the 24 hours preceding the brain infarction on weekends and holidays was significantly associated with cerebral infarction in young (16–40 years) and middle-aged (41–60 years) subjects (34). One mechanism suggested for the binge-associated increased stroke risk is hypertension; however, at least in younger individuals, hypertension prevalence is low. These data from both epidemiologic and animal models suggest the need to evaluate the pattern of alcohol consumption in relationship to CV risk and adverse events.
Study limitations
This study had important limitations related to both clinical sample and study design. We had a small sample size, and the study was cross-sectional, warranting future longitudinal assessment. We also used self-report to measure alcohol consumption. Others, though, have shown that self-report methods offer a reliable and valid approach to measuring alcohol consumption (35). Another limitation may relate to the greater baseline brachial artery diameter and lower peak shear rates found in the BD group. The latter may contribute to the lower FMD in the BD group because larger baseline diameters are associated with reduced FMD (36). Our results, though, show that there is a significant effect of binge drinking on FMD and NTG-induced dilation even after correcting for baseline diameter as a covariate.
Conclusions
Young adults without a history of CV disease who engaged in frequent and heavy episodic binge drinking had reduced FMD and NTG-induced dilation. The microvascular vasodilator responses to ACh and SNP were not different between groups; however, microvessels isolated from the BD group had enhanced vasoconstrictor responses to the potent vasoconstrictor ET-1. Our data show alterations in macro- and microvascular function associated with binge drinking that are similar to those seen in association with recognized CV risk factors. This study adds to a growing chain of evidence that suggests that, in contrast to regular and moderate alcohol consumption, binge drinking may be a risk factor for future clinical cardiovascular disease.
Acknowledgments
We are grateful to Dr. Alana Steffen for statistical assistance and Kevin Grandfield for editorial assistance.
Funding Sources This study was supported by National Institutes of Health grants AA015578 (MRP), HL85614 (SAP), HL095701 (SAP), and HL095701-02S1 (SAP). This project was also supported by the University of Illinois at Chicago, Center for Clinical and Translational Science (CCTS), Award Number UL1RR029879 from the National Center for Research Resources (SAP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Abbreviations
- A
abstainers
- ACh
acetylcholine
- BD
binge drinkers
- CV
cardiovascular
- ET-1
endothelin-1
- FMD
flow-mediated dilation
- L-NAME
N-Nitro-L-Arginine Methyl Ester
- NO
nitric oxide
- ROS
reactive oxygen species
- SNP
sodium nitroprusside
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No relationship with industry exists for any author.
References
- 1.Wechsler H, Nelson TF. What we have learned from the Harvard School Of Public Health College Alcohol Study: focusing attention on college student alcohol consumption and the environmental conditions that promote it. J Stud Alcohol Drugs. 2008;69(4):481–90. doi: 10.15288/jsad.2008.69.481. [DOI] [PubMed] [Google Scholar]
- 2.Gunzareth L, Faden V, Zakhari S, Warren K. National Institute on Alcohol Abuse and Alcoholism (NIAAA) Newslettter. Number 3. U.S. Department of Health and Human Services; Rockville, MD: 2004. [Google Scholar]
- 3.Naimi TS, Brewer RD, Mokdad A, Denny C, Serdula MK, Marks JS. Binge drinking among US adults. JAMA. 2003;289:70–5. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention . Vital Signs Binge Drinking 24/7. Jan, 2012. [Google Scholar]
- 5.Sundell L, Salomaa V, Vartiainen E, Poikolainen K, Laatikainen T. Increased stroke risk is related to a binge-drinking habit. Stroke. 2008;39:3179–84. doi: 10.1161/STROKEAHA.108.520817. [DOI] [PubMed] [Google Scholar]
- 6.Mukamal KJ, Maclure M, Muller JE, Mittleman MA. Binge drinking and mortality after acute myocardial infarction. Circulation. 2005;112(5):3839–45. doi: 10.1161/CIRCULATIONAHA.105.574749. [DOI] [PubMed] [Google Scholar]
- 7.Marques-Vidal P, Arveiler D, Evans A, Amouyel P, Ferrieres J, Ducimetiere P. Different alcohol drinking and blood pressure relationships in France and Northern Ireland: The PRIME Study. Hypertension. 2001;38:1361–6. doi: 10.1161/hy1101.095328. [DOI] [PubMed] [Google Scholar]
- 8.Wannamethee G, Shaper AG. Alcohol and sudden cardiac death. Br Heart J. 1992;68:443–8. doi: 10.1136/hrt.68.11.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kauhanen J, Kaplan GA, Goldberg DE, Salonen R, Salonen JT. Pattern of alcohol drinking and progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19(12):3001–6. doi: 10.1161/01.atv.19.12.3001. [DOI] [PubMed] [Google Scholar]
- 10.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 11.Ras RT, Streppel MT, Draijer R, Zock PL. Flow mediated dilation and cardiovascular risk prediction: A systemic review. International Journal of Cardiology. 2012 Oct 4; doi: 10.1016/j.ijcard.2012.09.047. pii: S0167-5273(12)01153-9. doi: 10.1016/j.ijcard.2012.09.047. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.National Institute on Alcohol Abuse and Alcoholism . The Task Force on Recommended Alcohol Questions. Bethesda, MD: 2003. Recommended Alcohol Questions. [Google Scholar]
- 13.Phillips SA, Das E, Wang J, Pritchard K, Gutterman DD. Resistance and aerobic exercise protects against acute endothelial impairment induced by a single exposure to hypertension during exertion. J Appl Physiol. 2011;110(4):1013–20. doi: 10.1152/japplphysiol.00438.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips SA, Hatoum OA, Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am J Physiol Heart Circ Physio. 2007;292:H93–H100. doi: 10.1152/ajpheart.00819.2006. [DOI] [PubMed] [Google Scholar]
- 15.Kizhakekuttu TJ, Gutterman DD, Phillips SA, Jurva JW, Arthur EI, Das E, et al. Measuring FMD in the brachial artery: how important is QRS gating? J Appl Physiol. 2010;109(4):959–65. doi: 10.1152/japplphysiol.00532.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dharmashankar K, Welsh A, Wang J, Kizhakekuttu TJ, Ying R, Gutterman DD, et al. Nitric oxide synthase-dependent vasodilation of human subcutaneous arterioles correlates with noninvasive measurements of endothelial function. Am J Hypertens. 2012;25:528–34. doi: 10.1038/ajh.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smithson MJ. Confidence Intervals. Sage; Belmont, CA: 2003. [Google Scholar]
- 18.Hashimoto M, Kim S, Eto M, Iijima K, Ako J, Yoshizumi M, et al. Effect of acute intake of red wine on flow-mediated vasodilatation of the brachial artery. Am J Cardiol. 2001;88(12):1457–60. doi: 10.1016/s0002-9149(01)02137-3. [DOI] [PubMed] [Google Scholar]
- 19.Bau PF, Bau CH, Naujorks AA, Rosito GA. Early and late effects of alcohol ingestion on blood pressure and endothelial function. Alcohol. 2005;37:53–8. doi: 10.1016/j.alcohol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 20.Hijmering ML, de Lange DW, Lorsheyd A, Kraaijenhagen RJ, van de Wiel A. Binge drinking causes endothelial dysfunction, which is not prevented by wine polyphenols: a small trial in healthy volunteers. Neth J Med. 2007;65:29–5. [PubMed] [Google Scholar]
- 21.Maiorano G, Bartolomucci F, Contursi V, Minenna FS, Di MR, Palasciano A, et al. Noninvasive detection of vascular dysfunction in alcoholic patients. Am J Hypertens. 1999;12:137–44. doi: 10.1016/s0895-7061(98)00173-3. [DOI] [PubMed] [Google Scholar]
- 22.Di Gennaro C, Biggi A, Barilli AL, Fasoli E, Carra N, Novarini A, et al. Endothelial dysfunction and cardiovascular risk profile in long-term withdrawing alcoholics. J Hypertens. 2007;25(2):367–73. doi: 10.1097/HJH.0b013e328010929c. [DOI] [PubMed] [Google Scholar]
- 23.Di Gennaro C, Saccani-Jotti G, Pinelli S, Venturi N, Palombi F, Manfredi G, et al. Endothelial dysfunction and high cardiovascular risk profile in severe alcoholics improve only partially following a medium-term alcohol withdrawal. Alcohol Clin Exp Res. 2012;36(2):242–50. doi: 10.1111/j.1530-0277.2011.01636.x. [DOI] [PubMed] [Google Scholar]
- 24.Mullen MJ, Khaarbanda RK, Cross J, Donald AE, Taylor M, Vallance P, et al. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo. Circ Res. 2001;88:145–51. doi: 10.1161/01.res.88.2.145. [DOI] [PubMed] [Google Scholar]
- 25.Koller A, Huang A, Sun D, Kaley G. Exercise training augments flow-dependent dilation in rat skeletal muscle arterioles. Role of endothelial nitric oxide and prostaglandins. Circ Res. 1995;76:544–50. doi: 10.1161/01.res.76.4.544. [DOI] [PubMed] [Google Scholar]
- 26.Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003;27(4):277–84. [PMC free article] [PubMed] [Google Scholar]
- 27.Yogi A, Callera GE, Hipólito UV, Silva CR, Touyz RM, Tirapelli CR. Ethanol-induced vasoconstriction is mediated via redox-sensitive cyclo-oxygenase-dependent mechanisms. Clin Sci (Lond) 2010;118(11):657–68. doi: 10.1042/CS20090352. [DOI] [PubMed] [Google Scholar]
- 28.Amiri F, Virdis A, Neves MF, Iglarz M, Seidah NG, Touyz RM, Reudelhuber TL, Schiffrin EL. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation. 2004 Oct 12;110(15):2233–2240. doi: 10.1161/01.CIR.0000144462.08345.B9. [DOI] [PubMed] [Google Scholar]
- 29.Soardo G, Donnini D, Varutti R, Moretti M, Milocco C, Basan L, et al. Alcohol-induced endothelial changes are associated with oxidative stress and are rapidly reversed after withdrawal. Alcohol Clin Exp Res. 2005;29(10):1889–98. doi: 10.1097/01.alc.0000183004.28587.23. [DOI] [PubMed] [Google Scholar]
- 30.Iglarz M, Clozel M. Mechanisms of ET-1-induced endothelial dysfunction. J Cardiovasc Pharmacol. 2007;50:621–8. doi: 10.1097/FJC.0b013e31813c6cc3. [DOI] [PubMed] [Google Scholar]
- 31.Rossi R, Chiurlia E, Nuzzo A, Cioni E, Origliani G, Modena MG. Flow-mediated vasodilation and the risk of developing hypertension in healthy postmenopausal women. J Am Coll Cardiol. 2004;44(8):1636–40. doi: 10.1016/j.jacc.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 32.Pletcher MJ, Varosy P, Kiefe CI, Lewis CE, Sidney S, Hulley SB. Alcohol consumption, binge drinking, and early coronary calcification: findings from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 2005;161(5):423–33. doi: 10.1093/aje/kwi062. [DOI] [PubMed] [Google Scholar]
- 33.Liu W, Redmond WM, Morrow D, Cullen JP. Differential effects of daily-moderate versus weekend-binge alcohol consumption on atherosclerotic plaque development. Atherosclerosis. 2011;219:448–454. doi: 10.1016/j.atherosclerosis.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hillbom M, Haapaniemi H, Juvela S, Palomäki H, Numminen H, Kaste M. Recent alcohol consumption, cigarette smoking, and cerebral infarction in young adults. Stroke. 1995;26(1):40–5. doi: 10.1161/01.str.26.1.40. [DOI] [PubMed] [Google Scholar]
- 35.Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;2:1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- 36.Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Heterogeneity in conduit artery function in humans: impact of arterial size. Am J Physiol. 2008;295:H1927–H1934. doi: 10.1152/ajpheart.00405.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]