Abstract
The copy condition of the Rey-Osterrieth Complex Figure (ROCF) is sensitive to Alzheimer’s disease (AD) pathology, but its neural correlates remain unclear. We used FDG-PET to elucidate this association in 77 patients with probable AD. We observed a correlation between ROCF and metabolic rate of bilateral temporal-parietal cortex and occipital lobe, and right frontal lobe. Global and local elements of the ROCF correlated with metabolic rate of these same regions. The copy approach correlated with right lateral temporal cortex. The ROCF appears reflective of posterior temporal-parietal cortex functioning, highlighting the role of visuospatial processing in constructional abilities in AD.
Keywords: Alzheimer’s Disease, Rey-Osterrieth Complex Figure, FDG-PET, visuospatial, construction
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by a progressive decline in neuropsychological functioning across multiple cognitive domains, including the areas of attention/information processing, learning and memory, visuospatial functioning, language, and executive/frontal lobe functioning (Lezak, Howieson, & Loring, 2004). Many tests have been developed to assess visuospatial skills in AD; however, the Rey-Osterrieth Complex Figure Test (ROCF; Osterrieth, 1944; Rey, 1941) represents a benchmark test and is one of the most commonly used in the field of geriatric neuropsychology (Camara, 2000). Use of the ROCF in the field of geriatric neuropsychology is supported by research documenting that this test is effective in discriminating AD, even in the earliest stages (Fujimori et al., 1998).
Despite its widespread use in the field, the underlying neurobiological correlates of the ROCF in patients with AD have yet to be clearly identified. Using a bottom-up region-of-interest approach, Tippet & Black (2008) observed that performance on the ROCF correlated with perfusion of right superior parietal lobule and postcentral gyrus in mild AD patients. Additionally, a top-down connectivity analysis found that regions of bilateral association cortex in frontal, temporal, and parietal lobes were associated with performance on visual tests, although the ROCF was not significant in this analysis. A principal component analysis of metabolism in AD found that performance on the ROCF correlated with two networks: one encompassing bilateral posterior brain regions including retrosplenial/posterior cingulate cortex, precuneus, parietal, and lateral temporal lobes, and a second including those regions plus bilateral prefrontal cortex (Salmon et al., 2009). Finally, in a combined group of patients with Mild Cognitive Impairment (MCI) and Alzheimer’s disease (AD), the ROCF correlated with cortical thinning of the right temporal gyrus, right superior parietal lobule, bilateral posterior cingulate, and right middle frontal gyrus (Ahn et al., 2011). All of these studies identify the right superior parietal lobule as a correlate, but differ as to the role of bilateral temporal and frontal regions in ROCF performance. Likewise, investigations exploring other figure copying tests in AD have differed, documenting associations with only temporal/temporal-parietal (Boxer et al., 2003; Nobili et al., 2005), temporal and frontal (Forster et al., 2009), or temporal-parietal and frontal regions (Teipel et al., 2006). As such, the neurobiology of figure copying in AD remains unclear.
The ROCF is conceptualized as a measure of visuoconstruction, suggesting it may largely localize to the right hemisphere. In a mixed clinical sample, it has been found to correlate with visual neuropsychological tests, including measures of visual perception, judgment of spatial relations, visual organization, and set-shifting (Meyers & Meyers, 1995). In right hemisphere patients, ROCF copy scores appear related to both visuoperceptive and spatial manipulation abilities (Trojano et al., 2004). In AD specifically, figure copying has been shown to correlate with measures of visual exploration and judgment of spatial relations (Guerin, Belleville, & Ska, 2002), as well as design fluency (Mickanin, Grossman, Onishi, Auriacombe, & Clark, 1994). It appears that the ROCF is associated with a host of cognitive abilities thought to reflect right hemisphere functioning.
As summarized above, visual perception has been identified as a ROCF correlate (Meyers & Meyers, 1995; Trojano et al., 2004). Visuoperception may be subdivided into global (gestalt) or local (detailed) processing, reflecting a bias towards right and left hemispheres, respectively (Van Kleeck, 1989). This hemispheric specialization may be particularly present in early visual processing in extrastriate regions (Fink et al., 1996; Peyrin, Baciu, Segebarth, & Marendaz, 2004). Based on the foregoing, it follows that when copying the ROCF, the right hemisphere may be preferentially involved in the figure gestalt, while the left hemisphere may be preferentially involved in copying the figure details.
In addition to visuospatial skills, executive functioning may be important in the ROCF. Research in non-elderly samples has documented an association between performance on the ROCF and executive functioning, specifically planning and organization (Somerville, Tremont, & Stern, 2000; Watanabe et al., 2005). While this has not been extended to AD specifically, work in patients with various types of dementia found that a modified ROCF design correlated significantly with tests of working memory (Freeman et al., 2000). Given this, it is suggested that the frontal cortex may be involved in the organizational approach taken to ROCF copying.
The present study sought to build on the literature assessing ROCF in AD by using a voxel-based approach to examine the correlation between cerebral metabolism as measured using FDG-PET and impaired performance on the ROCF. The advantage of a voxel-based approach is it identifies areas that are not constrained by anatomy, as is the case with region of interest analyses, allowing for identification of small yet functionally meaningful associations. In addition, given the literature implicating global/local differences in visuoperception, and a role for executive functioning and working memory in ROCF performance, follow-up analyses sought to determine if specific aspects of the ROCF are associated with distinct neurobiological substrates. To this end we divided the figure into global (main box) and local (detailed) elements, and characterized the approach to copying these elements of the figure. In light of previous research, it was hypothesized that impaired performance on the ROCF paradigm would show associations with hypometabolism of tempo-parietal regions, with global and detailed elements dissociating between the right and left hemispheres, respectively. Additionally, given the literature implicating aspects of executive processing in ROCF performance in AD, it was hypothesized that impaired ROCF would be associated with hypometabolism in prefrontal cortex, with the organizational approach localizing to this region.
Methods
Participants
Data from eighty-six patients with probable AD was reviewed. Of the eighty-six subjects, seventy-seven completed the ROCF copy condition and were included in subsequent analyses. Of the nine subjects not included in the study, eight were too impaired to complete this measure and one was not administered the measure for other reasons. Subjects were recruited from the VA Greater Los Angeles Healthcare System Geropsychiatry Outpatient Program and from the UCLA Alzheimer’s Disease clinics. Each patient underwent a clinical evaluation that included complete history, cognitive assessment, psychiatric assessment, neurological examination, and structural neuroimaging with magnetic resonance imaging (MRI) or computed tomography (CT). A board certified geriatric psychiatrist (DLS) confirmed final diagnosis using National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorder Association criteria (McKhann et al., 1984), which is consistent with 2011 criteria (McKhann et al., 2011). Patients were excluded from the study if they had a history of psychotic disorder unrelated to dementia, history of head trauma resulting in a loss of consciousness, a psychoactive substance use disorder, or a systemic illness or other neurological condition accounting for cognitive impairment. We included participants on a stable dose of cholinesterase inhibitor (27/77, 25 taking donepezil, 2 taking galantamine) or antidepressant (20/77) medication. Patients taking other psychotropic medications were excluded.
The study was reviewed and approved by the local IRB, and consent to participate was documented according to IRB guidelines.
Neuropsychological testing
Neuropsychological measures were drawn from a larger neuropsychological battery. The Folstein Mini-mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975) and the Dementia Rating Scale (DRS; Mattis, 1988) were administered to assess global cognitive impairment. Complex visuoconstruction was assessed using the ROCF copy condition (Meyers & Meyers, 1995). The ROCF has shown good reliability in patients with memory impairment (Tupler, Welsh, Asare-Aboagye, & Dawson, 1995). Patients were required to copy a complex figure and scoring was done in accordance with the Meyers & Meyers (1995) criteria. Total score ranged from 0-36, with higher scores indicating better performance. In this scoring paradigm, the ROCF is broken into 18 components. One point is awarded for object accuracy (does the element look as it should), and a second point is awarded for location accuracy (is the element in the correct spatial location). We also created sub-scores reflecting the global vs. detailed elements of the Rey (GLOB: main box, main horizontal, vertical, and diagonal lines, and outer right triangle, scores 0-10, 1 point for object accuracy and one point for location accuracy), and all other elements of the score (DET: remaining details of the figure, scores 0-26, 1 point for object accuracy and one point for location accuracy). Finally, we created an ORG score to investigate the approach to copying the figure. This score was calculated using a procedure adapted from Binder (1982). In brief, five lines were rated based on whether they were drawn contiguously (the complete line was drawn as one segment) or not. These ratings were made for the primary horizontal, vertical, and diagonal lines, and the right outer triangle. The total number of contiguous lines was included in subsequent analysis (ORG: scores 0-5, 1 point per line- note each diagonal counted as one line). Ratings were made by visual investigation of the copy; the order in which the patient drew the lines was not available.
FDG-PET image acquisition
Patients were scanned using three different tomographs with similar imaging characteristics over the course of the study: 39 patients were scanned on a Siemens 953/31 tomographic scanner, 23 on a GE Advance PET-CT, and 15 on a Philips Gemini TF PET-CT. Each scanner had an inplane resolution of approximately 5 mm at full-width half maximum and axial slice thickness of 2-4 mm. [18F] fluorodeoxyglucose (FDG) was synthesized at the Veterans Affairs Greater Los Angeles Healthcare System PET Imaging facility in accordance with the technique of Hamacher, Coenen, & Stocklin (1986). Subject received 5-10 mCi of FDG intravenously and rested with their eyes open during a 40-minute uptake phase. Subjects were placed in the scanner with the imaging plane parallel to the canthomeatal plane and metabolic data were acquired for 30-40 minutes.
Analysis of clinical data
Statistical analyses were conducted using SPSS version 20. Data were assessed for normality via visual examination of the histograms, exploring skewness/kurtosis, and the Kolmogorov-Smirnov test (p<.05). To determine if specific cognitive abilities were correlated with the ROCF, we conducted correlations between the DRS subtests and ROCF. The DRS subtests were selected because they are generally free from floor effects in dementia patients.
PET image analysis
PET data were analyzed using SPM2 (Wellcome Trust Centre for Neuroimaging) in Matlab R2012a (MathWorks). Images were normalized to MNI space using trilinear interpolation and resampled to 2×2×2 mm voxels and smoothed using a 6mm FWHM smoothing kernel. We determined the overall metabolic pattern of the sample. To this end we divided each individuals’ pre-processed brain by their global mean. We then averaged these images together to create a group averaged image. Using PMOD software (PMOD Technologies, Ltd) we obtained the average metabolic rate in regions-of-interest across the brain using the AAL atlas (Tzourio-Mazoyer et al., 2002). ROIs included bilateral occipital lobes, temporal lobes, superior and inferior parietal lobules, prefrontal cortex (including the superior, middle, and inferior frontal gyri), and postcentral gyri.
Association between metabolism and the ROCF copy score was assessed using the correlation procedure in SPM. Images were normalized to the global mean using proportional scaling and threshold masking was used to remove signal from structures outside of grey matter (set to .8). Results were thresholded and considered significant at the voxel level at p<.05, corrected for multiple comparisons using the false discover rate (FDR) procedure (height correction; Genovese, Lazar, & Nichols, 2002) and an extent of 50 voxels. Anatomical regions were determined using Human Brain Anatomy in Computerized Images (Damasio, 2005) and approximate Brodmann’s regions were determined using MRIcro (Rorden & Brett, 2000) and Petrides & Pandya (1999).
Follow-up SPM analyses were conducted to investigate the other elements of ROCF performance. The analytic approach was identical to that described above. Correlations were conducted between metabolism and the GLOB, DET, and ORG scores, separately. To further determine if the GLOB and DET elements had unique associations with cerebral metabolism after controlling for the other, we conducted a regression model in SPM, with both GLOB and DET as predictors. In this analysis, to ensure that we were only examining areas that had been identified in the correlation analysis, the results of the unique contribution of the GLOB and DET scores were inclusively masked with the results of their corresponding correlation analysis (threshold at p<.005 uncorrected). For example, the contribution of GLOB when controlling for DET was masked with the findings from the association between GLOB and metabolism when DET was not in the model.
Results
Participants consisted of 77 patients diagnosed with probable AD. This included 62 men and 15 women. Sixty-seven were right handed and 8 were left handed (data missing from 2). Fifty-three identified as white, 15 as African-American, 5 as Asian/Pacific Islander, and 4 as Hispanic. Results of the neuropsychological testing are presented in Table 1. Education level was unavailable from 5 participants and DRS scores were unavailable from 1 participant.
Table 1.
Demographic and cognitive data
| Mean | SD | Range | |
|---|---|---|---|
| Age | 78.4 | 8.0 | 52-97 |
| Education (n=72) | 14.1 | 3.6 | 6-24 |
| Years since dx | 3.0 | 2.4 | 0-10 |
| MMSE | 20.2 | 4.9 | 6-28 |
| Mattis DRS (n=76) | 106.9 | 15.9 | 54-141 |
| Attention | 34.6 | 2.4 | 23-37 |
| Initiation/Perseveration | 25.0 | 7.0 | 7-37 |
| Construction | 5.3 | 1.2 | 1-6 |
| Conceptualization | 29.7 | 5.7 | 14-39 |
| Memory | 12.3 | 4.2 | 4-22 |
| ROCF copy | 18.2 | 9.4 | 1-35 |
| ROCF global (GLOB) | 5.7 | 2.7 | 0-10 |
| ROCF details (DET) | 12.6 | 7.5 | 1-26 |
| ROCF organization (ORG) | 2.2 | 1.5 | 0-5 |
Neuropsychological correlates of the ROCF
Neuropsychological indicators were assessed for normality. The ROCF, GLOB, DET, and ORG scores were all found to be within acceptable limits. If the data were not normally distributed, Spearman’s rho was used to assess associations (DRS attention and DRS construction). There were no correlations between the ROCF and demographic variables, including age, education, or duration of illness (for all, r<0.10, p>.1). Performance on the ROCF copy was directly associated with MMSE (r=0.35, p<.01). ROCF correlated with DRS total (r=0.40, p<.001). Moreover, significant correlations were found between ROCF and all DRS subscales, including DRS attention (Spearman’s rho=0.24, p<.05), DRS initiation/perseveration (r=0.31, p<.01), DRS construction (Spearman’s rho =0.53, p<.001), DRS conceptualization (r=0.28, p<.05), and DRS memory (r=0.30, p<.01). The GLOB and DET sub scores were strongly correlated with one another (r=0.62, p<.001), though showed over 60% unique variance.
Average FDG-PET of sample
Qualitative inspection of the averaged FDG-PET brain of the sample was consistent with the bilateral pattern of hypometabolism characteristic of AD. The metabolic rate was lowest in bilateral posterior cingulate gyri (left: 1.59; right: 1.46), temporal lobes (left: 1.60 ; right 1.64), superior parietal lobules (left: 1.84; right: 1.78), and inferior parietal lobules (left: 1.80; right: 1.83). It was highest in bilateral occipital pole (left: 2.07; right: 2.12) and postcentral gyri (left: 2.06, right: 2.04). The prefrontal cortex was between these values (left: 1.86, right: 1.92).
Imaging correlates of the ROCF
Performance on the ORG score was broken down as follows: 0 (12 patients), 2 (14 patients), 2 (16 patients), 3 (17 patients), 4 (14 patients), and 5 (4 patients). In the normative sample used by Binder et al (1982), healthy volunteers averaged 4.79 configural units, with a standard deviation of .43, suggesting most healthy older adults approach the ROCF in a configural manner. In our sample, 77% of participants scored a 3 or lower (score of 3 is greater than 2 standard deviations below the mean), suggesting overall patients with AD did not approach the ROCF using a configural strategy. The results of the SPM analysis revealed multiple areas of significant positive association between cortical metabolism and ROCF copy score (Table 2, Fig. 1). The largest cluster was located in the posterior part of the brain, and included primarily right hemisphere. Specifically, this cluster included the right inferior, middle, and superior temporal gyri (ITG, MTG, STG) and fusiform gyrus. It also included the supramarginal gyrus (BA40), angular gyrus (BA39), superior parietal lobule (SPL, BA7), and precuneus. It included the occipital pole and lateral aspect of the occipital lobe (including the superior, middle, and inferior occipital gyri, and the lingual gyrus) and anterior cuneus. This same cluster extended into the left hemisphere, including the posterior extent of the SPL, cuneus, lingual gyrus, precuneus, and small regions of superior and middle occipital gyri. There were also significant clusters within the right inferior frontal pole, middle frontal gyrus (dorsolateral prefrontal cortex- DLPFC), posterior middle and superior frontal gyri (MFG/SFG; part of frontal eye field), and precentral gyrus. Finally, small additional clusters were observed in the left hemisphere within the supramarginal gyrus and inferior occipital gyrus.
Table 2.
SPM results of the correlation between ROCF and metabolism.
| Region | BA | Side | x | y | z | T | p-value | k |
|---|---|---|---|---|---|---|---|---|
| Frontal pole | 10 | R | 26 | 66 | -8 | 4.05 | 0.002 | 284 |
| Middle frontal gyrus | 9/46 | R | 42 | 38 | 36 | 4.01 | 0.002 | 477 |
| Middle/superior frontal gyri | 8/9 | R | 28 | 22 | 60 | 3.62 | 0.005 | 693 |
| Precentral gyrus | 6 | R | 62 | 6 | 36 | 2.97 | 0.019 | 52 |
| Supramarginal gyrus | 40 | L | -40 | -44 | 36 | 3.24 | 0.011 | 97 |
| Temporal; Parietal; Occipital |
20/21/22/37; 40/39/7; 17/18/19 |
R/L | 14 | -80 | 50 | 5.72 | 0.001 | 22720 |
| Inferior occipital gyrus | 19 | L | -46 | -64 | -8 | 2.86 | 0.024 | 143 |
| Inferior occipital gyrus | 18 | L | -22 | -90 | -14 | 3.76 | 0.003 | 369 |
Statistics shown for the peak voxel within each cluster.
BA=approximate Brodmann’s region; R=right, L=left; x,y,z=MNI coordinates; T=t-value; p-value after correction using the FDR procedure; k=number of voxels within the cluster.
Figure 1. Association between ROCF and cerebral metabolism.
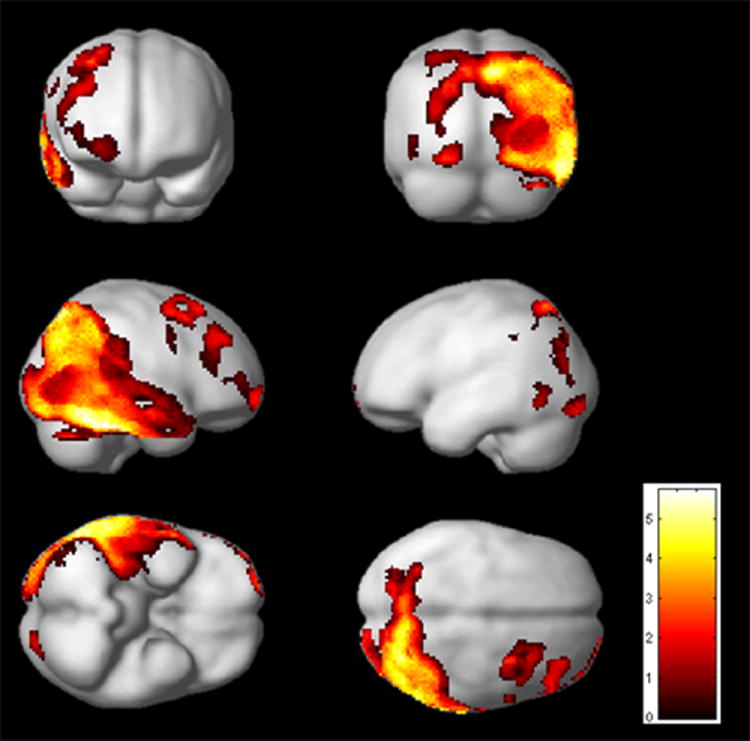
SPM results (p<.05, FDR corrected, extent=50 voxels) displayed on the smoothed average brain in SPM2.
The analysis was repeated, controlling for dementia severity by covarying for MMSE score. The pattern of the results did not change.
Imaging correlates of global and local features
The SPM analysis of the correlation between metabolism and GLOB revealed regions similar to those found in the original SPM analysis (p<.05, FDR corrected, extent=50 voxels) (Table 3, Figure 2a). The largest cluster was again posterior, and comprised the right ITG, MTG, and STG, fusiform, SPL, and inferior parietal lobule (IPL), as well as portions of the precuneus, cuneus, lingual gyrus, and lateral occipital lobe. In the right frontal lobe, we observed separate clusters in the frontal pole, MFG, SFG/MFG (BA6), and inferior frontal gyrus (IFG). In the left hemisphere, clusters were observed in left lingual gyrus, cuneus, and inferior occipital gyrus. From a qualitative perspective, the main differences in the pattern of correlation when compared with the ROCF total score results were that GLOB showed a correlation with right IFG whereas ROCF did not, and that GLOB did not show a correlation with left parietal regions
Table 3.
SPM results of the correlation between GLOB and metabolism.
| BA | Side | x | y | z | T | p-value | K | |
|---|---|---|---|---|---|---|---|---|
| Frontal pole | 10 | R | 30 | 64 | -10 | 3.13 | 0.013 | 176 |
| Middle frontal gyrus | 9/46 | R | 44 | 40 | 34 | 3.93 | 0.003 | 299 |
| Inferior frontal gyrus | 44 | R | 44 | 14 | 26 | 3.37 | 0.008 | 231 |
| Middle/superior frontal gyri | 9/8/6 | R | 40 | 0 | 60 | 4.10 | 0.002 | 723 |
| Temporal; Parietal; Occipital |
20/21/22/37; 40/39/7; 17/18/19 |
R/L | 40 | -32 | -28 | 5.72 | 0.001 | 22178 |
| Inferior occipital gyrus | 19 | L | -44 | -70 | 0 | 2.82 | 0.026 | 71 |
| Lingual gyrus/cuneus | 17/18 | L | -16 | -72 | 26 | 3.34 | 0.009 | 520 |
| Inferior occipital gyrus | 18 | L | -24 | -90 | -14 | 2.85 | 0.025 | 67 |
Statistics shown for the peak voxel within each cluster.
BA=approximate Brodmann’s region; R=right, L=left; x,y,z=MNI coordinates; T=t-value; p-value after correction using the FDR procedure; k=number of voxels within the cluster.
Figure 2. Association between GLOB/DET and cerebral metabolism.
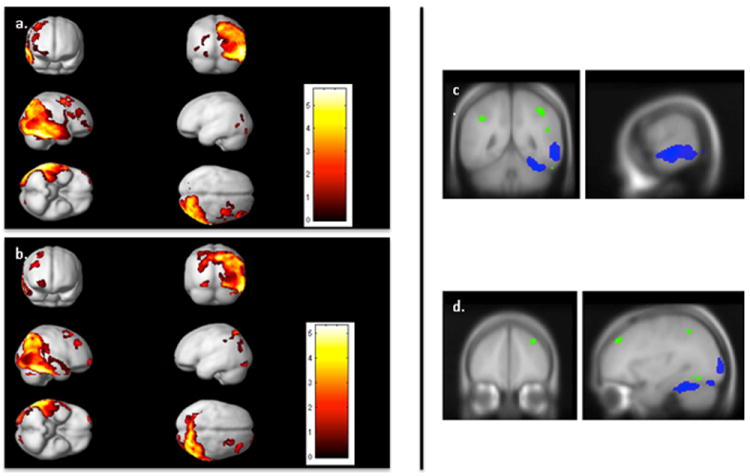
The left side includes SPM results (p<.05 FDR corrected, extent=50 voxels) displayed on the smoothed average brain in SPM2 demonstrating the correlation between (a) GLOB and metabolism and (b) DET and metabolism. The right side illustrates the unique contribution of GLOB after controlling for DET, and DET after controlling for GLOB (p<.005, uncorrected, extent=10 voxels). GLOB in blue and DET in green, through (a) the temporal cortex (x=64, y=-48), and (b) the PFC (x=33, y=44).
The results of the correlation between DET and metabolism (p<.05 FDR corrected, extent=50 voxels) (Table 4, Fig 2b) appeared similar to the main ROCF analysis. The largest cluster included the right ITG, MTG, STG, SPL, IPL, portions of the precuneus, cuneus, lingual gyrus, and lateral occipital lobe, as well as left SPL, IPL, precuneus, cuneus, and occipital regions. There were also clusters located in the right frontal pole, right MFG, right posterior SFG/MFG, and left inferior occipital gyrus. From a qualitative perspective this analysis appeared very similar to the ROCF total score analysis.
Table 4.
SPM results of the correlation between DET and metabolism.
| Region | BA | Side | x | y | z | T | p-value | k |
|---|---|---|---|---|---|---|---|---|
| Frontal pole | 10 | R | 26 | 68 | -6 | 4.08 | .005 | 168 |
| Middle frontal gyrus | 9/46 | R | 36 | 44 | 38 | 4.11 | .005 | 309 |
| Middle/superior frontal gyri | 8/9 | R | 30 | 24 | 58 | 3.48 | .009 | 230 |
| Temporal; Parietal; Occipital |
20/21/22/37; 40/39/7; 17/18/19 |
R/L | 14 | -80 | 50 | 5.33 | .004 | 17938 |
| Inferior occipital gyrus | 18 | L | -20 | -90 | -14 | 3.72 | .007 | 286 |
Statistics shown for the peak voxel within each cluster.
BA=approximate Brodmann’s region; R=right, L=left; x,y,z=MNI coordinates; T=t-value; p-value after correction using the FDR procedure; k=number of voxels within the cluster.
We next created a regression model, entering both GLOB and DET scores as predictors, in order to identify the independent association of each subscore with brain functioning. To examine potential collinearity, we extracted metabolic data from the cluster localized in the right temporoparietal cortex (using the VOI tool) and imported this to SPSS. A linear regression was run entering the VOI as the dependent variable and the GLOB and DET scores as forced-entry predictor variables. Collinearity diagnostics suggested lack of collinearity between the GLOB and DET subscores (VIF<10 and tolerance >.1).
There were no significant regions predicted by either subscore when controlling for the other (p<.05 FDR corrected). We relaxed the statistical threshold (p<.005 uncorrected, extent=10 voxels) and re-examined the data to determine if there were any trends. The GLOB score was associated with hypometabolism of right IFG, premotor cortex, lateral temporal cortex, fusiform, lingual gyrus, precuneus, and lateral occipital cortex (Fig 2c). The DET scores were associated with hypometabolism in right frontal pole, MFG, temporal pole, posterior ITG, fusiform; bilateral IPL, SPL, and precuneus; and left lingual gyrus (Fig 2d). In summary, trends suggested that DET showed a more bilateral representation and included more right prefrontal regions.
Imaging correlates of the organizational approach
Performance on the ORG score was broken down as follows: 0 (12 patients), 2 (14 patients), 2 (16 patients), 3 (17 patients), 4 (14 patients), and 5 (4 patients). In the normative sample used by Binder (1982), healthy volunteers averaged 4.79 configural units, with a standard deviation of .43, suggesting most healthy older adults approach the ROCF in a configural manner. In our sample, 77% of participants scored a 3 or lower (score of 3 is greater than 2 standard deviations below the mean), suggesting overall patients with AD did not approach the ROCF using a configural strategy. We observed a direct correlation between ORG and metabolism in the right lateral temporal cortex. This cluster included the fusiform, ITG, MTG, and STG (Table 5, Fig. 3).
Table 5.
SPM results of the correlation between ORG and metabolism.
| BA | Side | x | y | z | T | p-value | k | |
|---|---|---|---|---|---|---|---|---|
| Lateral temporal lobe | 20/21/22/37 | R | 42 | -50 | -28 | 5.49 | 0.005 | 3693 |
Statistics shown for the peak voxel within each cluster.
BA=approximate Brodmann’s region; R=right, L=left; x,y,z=MNI coordinates; T=t-value; p-value after correction using the FDR procedure; k=number of voxels within the cluster.
Figure 3. Association between ORG and cerebral metabolism.
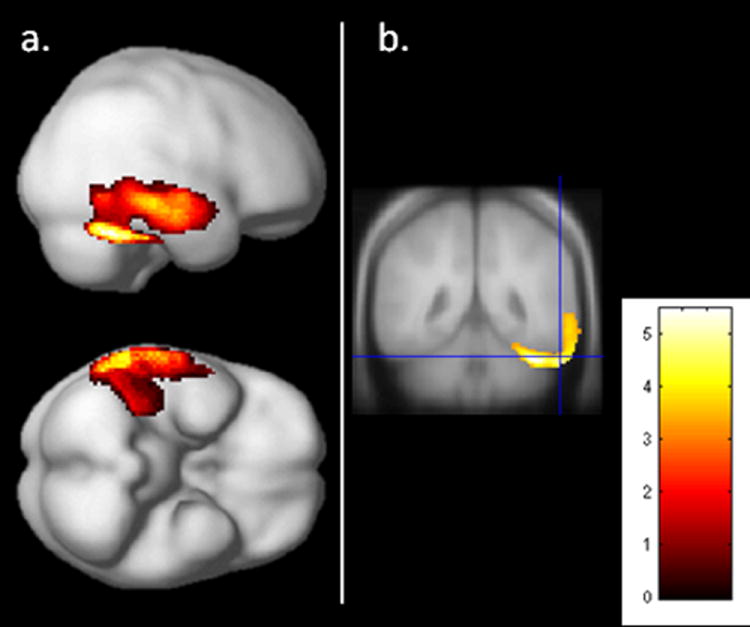
SPM results (p<.05, FDR corrected, extent=50 voxels). Results displayed on (a) the smoothed average brain and on (b) coronal view through the posterior temporal cortex.
Discussion
The current study explored the association between cerebral metabolism as assessed by FDG-PET and performance on the ROCF copy condition in patients with probable AD. The findings documented that performance on the ROCF was associated with cerebral metabolism in many regions of association cortex. The largest cluster was observed in the right posterior cortex, encompassing the superior and inferior parietal lobules, lateral temporal cortex and fusiform, and occipital cortex. Additional correlations were observed in the right prefrontal cortex, right frontal eye fields, right precentral gyrus, and left posterior parietal/occipital cortex. There were no unique associations between metabolism and global versus local features of the ROCF, but trends in the data suggested that details were more associated with dorsolateral prefrontal and bilateral parietal and occipital regions, whereas global elements were more associated with right posterior frontal cortex and right temporal lobe. The organizational approach to drawing the main box of the ROCF was associated with metabolism of the right lateral temporal lobe. These findings suggest that in patients with AD, the ROCF interrogates the functioning of a multi-lobe network of primarily right sided brain regions, but largely reflects functioning of posterior cortex.
Cognitive models of copying an image posit that it involves four processes: preliminary analysis of the target elements and spatial relations; preparation of a drawing plan, which includes storing the plan in short term memory as it is translated into a motor program; execution of the plan (drawing); and monitoring of the accuracy of the drawing (Gross & Trojano, 1999). Neuroimaging studies of drawing have observed activation of the dorsal and ventral visual pathways, as well as premotor/motor areas involved in hand movement (Makuuchi, Kaminaga, & Sugishita, 2003) and prefrontal regions (Harrington, Farias, & Davis, 2009; Miall, Gowen, & Tchalenko, 2009). It has been proposed that visual information about the to-be-copied picture passes through the dorsal visual stream in service of spatial processing. At the same time, information is passed through the ventral pathway, which may activate semantic representations about the target object (Harrington et al., 2009; Makuuchi et al., 2003). Prefrontal regions may subserve planning and self-monitoring (Miall et al., 2009). The present study found an association between ROCF performance and metabolism in many of these areas, including the dorsal and ventral visual streams, premotor cortex, and prefrontal regions. Applying this model to AD, it appears that poor figure drawing represents the breakdown of multiple brain regions that underlie the visuospatial and executive aspects of copying. Given that the largest correlation between metabolism and ROCF was observed in the posterior cortex, it seems that for patients with AD, what/where visuospatial processing is a crucial factor in successful copying.
In addition to assessing overall copy score, further analyses investigated which brain regions were most strongly associated with the global vs. detailed elements of the ROCF. Contrary to predictions, both GLOB and DET subscores showed correlations with metabolism that looked very similar to the results of the total ROCF analysis. When the independent contribution of each subscore was assessed, no significant regions were identified. We observed trends suggesting that the global features were associated with posterior frontal, temporal, and some occipital regions of the right hemisphere, while the details had a more bilateral representation, and included regions of right prefrontal cortex. Some investigators have found support for a global/local dissociation in the ROCF (Kleinman & Gupta, 2008), while others have not (McConley, Martin, Banos, Blanton & Faught, 2006). It may be that the detailed elements as defined did not adequately represent local processing. It is also noteworthy that the overall ROCF score correlated with metabolism of both the right lingual and left inferior occipital regions, which are cortical areas posited to reflect global and local processing, respectively (Fink et al., 1996). Overall, in AD, the brain regions that support the reproduction of the global and detailed elements of the ROCF appear quite similar.
We also examined the approach to the figure, looking to see if patients drew the lines within the main box and right triangle as a contiguous line. This analysis involves elements of both visuospatial processing and executive functioning. It was initially developed as a means of determining if left and right hemisphere stroke patients show different approaches to copying the ROCF, premised on the theory that right hemisphere patients with more global processing deficits were more likely to show fragmented approaches to copying (Binder, 1982). It has also been conceptualized as a measure of organization/planning, demonstrating that an intact organizational approach to copying the global elements mediates delayed recall of the figure in patients with obsessive-compulsive disorder (Savage et al., 1999). In our study, taking an intact approach was associated with higher metabolism in right temporal regions, contrary to the hypothesis that this would correlate with frontal regions involved in planning. The right temporal lobe has been implicated in object recognition (Haxby et al., 1991) and in AD, disturbances in object recognition correlate with right temporal and inferior parietal hypometabolism (Fujimori et al., 2000). Our finding may point to the fact that object recognition is a strong determinant of the approach to copying the figure in AD. Indeed if a patient is unable to visually appreciate the ROCF as a box with additional elements, the approach becomes secondary.
The finding that the copy approach correlated with right temporal metabolism appears consistent with research showing that patients with right hemisphere stroke are less likely to produce configurally organized ROCF copies and may also show poorer copies relative to left hemisphere stroke patients (Binder, 1982; Lange, Waked, Kirshblum, & DeLuca, 2000). The majority of patients in our study showed a fragmented copy approach, which may reflect greater difficulties with global than local processing (Slavin, Mattingley, Bradshaw, & Storey, 2002). Alternatively, previous research has shown that patients with AD who use a piecemeal approach to the ROCF also showed deficits on visuospatial tasks that require not only mental representation of spatial relationships, but also more basic visuoperceptual abilities such as line length and angle judgment (Grossi et al., 2002). As such, the association between ROCF approach and temporal metabolism may reflect that in AD, a fragmented copy approach reflects compromise not to object recognition, but to other visuospatial skills involved in the perception and analysis of spatial associations. A caveat of our analysis is we made ratings via visual inspection of the resulting picture, as the order of the lines of the drawing was not available. Future research that takes advantage of a more comprehensive scoring algorithm such as the Boston Qualitative Scoring System, which addresses planning, fragmentation, neatness, perseveration, and organization (Somerville et al., 2000; Watanabe et al., 2005), may yield greater insight into the role of frontal lobe functioning in figure copying in AD. In addition, the range of ORG scores possible was restricted (0-5), and it is possible that limited variance impeded our ability to identify all brain regions involved in the copy approach.
In integrating the results described here with other studies in AD, it appears that deficits in visuospatial abilities are related to posterior brain dysfunction, and that the role of right or left temporal or parietal lobes vary based on task demands. As highlighted in the introduction, previous investigations of ROCF copying in AD samples have generally reported an association between this ability and functioning/structure of right parietal lobe, but have differed with respect to findings in the right frontal and temporal lobes (Ahn et al., 2011; Salmon et al., 2009; Tippett & Black, 2008). Using methodology designed to examine associations across even small areas of the brain, the results described here suggest that all of these regions are involved in ROCF performance in AD. One of the better researched visuoconstructional tasks in AD has been clock drawing. Results of studies addressing clock drawing and AD have yielded somewhat discrepant results, though associations with right parietal (Lee et al., 2008) and right temporal cortex (Cahn-Weiner et al., 1999) have been documented, consistent with the present findings using the ROCF. Clock drawing studies have also implicated the involvement of left temporal regions (Nagahama, Okina, Suzuki, Nabatame, & Matsuda, 2005; Thomann, Toro, Dos Santos, Essig, & Schroder, 2008; Ueda et al., 2002), which was not observed in the present study. Clock drawing differs from the ROCF in that patients do not copy a figure, but rather generate a clock from internal imagery or in response to a blank circle. Researchers have hypothesized that the association with temporal regions in particular may indicate that successful clock drawing performance requires semantic knowledge about the representation of time. Studies of simple visuoperception in AD have reported an association between perception and right superior parietal lobule integrity (Tippett & Black, 2008) or right temporal-parietal regions (Fujimori et al., 2000). Taken together, the literature suggests that visuospatial dysfunction in AD is largely due to damage of posterior brain regions, with more difficult tasks generally reflecting damage to a more extensive brain network.
Visuospatial abilities remain an important component of AD neuropsychological assessment not only because of the sensitivity in detecting pathology, but because this domain is implicated in the functional impairments observed in AD. The ROCF (Farias, Harrell, Neumann, & Houtz, 2003; Razani et al., 2011) and other measures of visuoconstruction and visuoperception are associated with poorer day to day functioning in AD (Glosser et al., 2002; Jefferson, Barakat, Giovannetti, Paul, & Glosser, 2006; Mahurin, DeBettignies, & Pirozzolo, 1991). Consistent with the findings reported here, poorer functional abilities have been associated with neural changes to right posterior cortex (Melrose et al., 2011; Nadkarni, Levy-Cooperman, & Black, 2010; Salmon et al., 2005; Vidoni, Honea, & Burns, 2010), suggesting that the relation between neurobiological dysfunction and functional impairment in AD may be mediated in part by visuospatial functioning.
It is important to emphasize that the correlational approach here identifies those regions in which brain metabolism is most strongly associated with ROCF performance. AD is a diffuse neurodegenerative process. It is possible that some of the correlations identified here are not necessarily functionally meaningful to ROCF performance, but instead reflect connectivity with other parts of the brain with high burden of AD pathology. In addition, the areas identified in the analysis reflect the brain regions in which the correlation between ROCF and metabolism was most significant. It is also possible that we have failed to identify regions that are functionally important to ROCF performance, yet did not reach significance. Additional limitations include using data from patients scanned on multiple PET tomographs, and including patients both on and off anticholinesterase inhibitors or psychiatric medications.
In summary, findings demonstrate that in Alzheimer’s disease, the ROCF copy condition interrogates the functioning of primarily right parietal, temporal and frontal cortex. This network of regions is consistent with cognitive and neuroimaging models of figure copying, that suggest copying includes temporal and parietal brain regions involved in what/where visuospatial processing, as well as prefrontal regions involved in monitoring the success of the product. In AD, the global vs. more detailed elements of the ROCF do not appear to have strong neural dissociates. The strategic approach to copying the global elements of the ROCF is most strongly associated with right temporal regions, perhaps highlighting the importance of accurate visuospatial processing on organizational approach for patients with AD. When using the ROCF clinically with patients with AD, neuropsychologists may consider this measure as tapping into a multi-lobe network of primarily right sided brain regions, particularly reflecting the integrity of right temporal and parietal regions.
Acknowledgments
Support provided by the Department of Veterans Affairs (Merit Review to D. Sultzer and Career Development Award to R. Melrose); and NIH (R01 MH56031). Dr. David Sultzer has received research grant support from Forest Research Institute.
Footnotes
Potential conflicts to study participants were disclosed.
References
- Ahn HJ, Seo SW, Chin J, Suh MK, Lee BH, Kim ST, et al. The cortical neuroanatomy of neuropsychological deficits in mild cognitive impairment and alzheimer’s disease: A surface-based morphometric analysis. Neuropsychologia. 2011;49(14):3931–3945. doi: 10.1016/j.neuropsychologia.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Binder LM. Constructional strategies on complex figure drawings after unilateral brain damage. Journal of Clinical Neuropsychology. 1982;4(1):51–58. doi: 10.1080/01688638208401116. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Rankin KP, Miller BL, Schuff N, Weiner M, Gorno-Tempini ML, Rosen HJ. Cinguloparietal atrophy distinguishes alzheimer disease from semantic dementia. Archives of Neurology. 2003;60(7):949–956. doi: 10.1001/archneur.60.7.94960/7/949. [DOI] [PubMed] [Google Scholar]
- Cahn-Weiner DA, Sullivan EV, Shear PK, Fama R, Lim KO, Yesavage JA, et al. Brain structural and cognitive correlates of clock drawing performance in alzheimer’s disease. Journal of the International Neuropsychological Society. 1999;5(6):502–509. doi: 10.1017/S1355617799566034. [DOI] [PubMed] [Google Scholar]
- Camara WJ, Nathan JS, Puente AE. Psychological test usage: Implications in professional psychology. Professional Psychology: Research and Practice. 2000;31:141–154. doi: 10.1037//0735-7028.31.2.141. [DOI] [Google Scholar]
- Damasio H. Human brain anatomy in computerized images. New York: Oxford University Press; 2005. [Google Scholar]
- Farias ST, Harrell E, Neumann C, Houtz A. The relationship between neuropsychological performance and daily functioning in individuals with alzheimer’s disease: Ecological validity of neuropsychological tests. Archives of Clinical Neuropsychology. 2003;18(6):655–672. [PubMed] [Google Scholar]
- Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Where in the brain does visual attention select the forest and the trees? Nature. 1996;382(6592):626–628. doi: 10.1038/382626a0. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forster S, Teipel S, Zach C, Rominger A, Cumming P, Fougere C, et al. Fdg-pet mapping the brain substrates of visuo-constructive processing in alzheimer’s disease. Journal of Psychiatric Research. 2009;44(7):462–469. doi: 10.1016/j.jpsychires.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Freeman RQ, Giovannetti T, Lamar M, Cloud BS, Stern RA, Kaplan E, Libon DJ. Visuoconstructional problems in dementia: Contribution of executive systems functions. Neuropsychology. 2000;14(3):415–426. doi: 10.1037//0894-4105.14.3.415. [DOI] [PubMed] [Google Scholar]
- Fujimori M, Imamura T, Hirono N, Ishii K, Sasaki M, Mori E. Disturbances of spatial vision and object vision correlate differently with regional cerebral glucose metabolism in alzheimer’s disease. Neuropsychologia. 2000;38(10):1356–1361. doi: 10.1016/s0028-3932(00)00060-9. [DOI] [PubMed] [Google Scholar]
- Fujimori M, Imamura T, Yamashita H, Hirono N, Ikejiri Y, Shimomura T, Mori E. Age at onset and visuocognitive disturbances in alzheimer disease. Alzheimer Disease and Associated Disorders. 1998;12(3):163–166. doi: 10.1097/00002093-199809000-00007. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037S1053811901910377. [DOI] [PubMed] [Google Scholar]
- Glosser G, Gallo J, Duda N, de Vries JJ, Clark CM, Grossman M. Visual perceptual functions predict instrumental activities of daily living in patients with dementia. Neuropsychiatry Neuropsychology and Behavioral Neurology. 2002;15(3):198–206. [PubMed] [Google Scholar]
- Grossi D, Trojano L. Constructional apraxia. In: Denes G, Pizzamiglio L, editors. Handbook of clinical and experimental neuropsychology. Vol. 2. Hove, UK: Psychology Press; 1999. pp. 441–452. [Google Scholar]
- Grossi D, Fragassi NA, Chiacchio L, Valoroso L, Tuccillo R, Perrotta C, et al. Do visuospatial and constructional disturbances differentiate frontal variant of frontotemporal dementia and alzheimer’s disease? An experimental study of a clinical belief. International Journal of Geriatric Psychiatry. 2002;17(7):641–648. doi: 10.1002/gps.654. [DOI] [PubMed] [Google Scholar]
- Guerin F, Belleville S, Ska B. Characterization of visuoconstructional disabilities in patients with probable dementia of alzheimer’s type. Journal of Clinical and Experimental Neuropsychology. 2002;24(1):1–17. doi: 10.1076/jcen.24.1.1.963. [DOI] [PubMed] [Google Scholar]
- Hamacher K, Coenen HH, Stocklin G. Efficient stereospecific synthesis of no-carrier-added 2-[18f]-fluoro-2-deoxy-d-glucose using aminopolyether supported nucleophilic substitution. Journal of Nuclear Medicine. 1986;27(2):235–238. [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Horwitz B, Ungerleider LG, Mishkin M, Carson RE, et al. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proceedings of the National Academy of Sciences, U S A. 1991;88(5):1621–1625. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington GS, Farias D, Davis CH. The neural basis for simulated drawing and the semantic implications. Cortex. 2009;45(3):386–393. doi: 10.1016/j.cortex.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Barakat LP, Giovannetti T, Paul RH, Glosser G. Object perception impairments predict instrumental activities of daily living dependence in alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology. 2006;28(6):884–897. doi: 10.1080/13803390591001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman JT, Gupta A. The right hand draws the trees, but the left draws the forest? Behavioral Neurology. 2008;20(1-2):55–60. doi: 10.3233/BEN-2008-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange G, Waked W, Kirshblum S, DeLuca J. Organizational strategy influence on visual memory performance after stroke: Cortical/subcortical and left/right hemisphere contrasts. Archives of Physical Medicine and Rehabilitation. 2000;81(1):89–94. doi: 10.1016/s0003-9993(00)90227-2. [DOI] [PubMed] [Google Scholar]
- Lee DY, Seo EH, Choo IH, Kim SG, Lee JS, Lee DS, et al. Neural correlates of the clock drawing test performance in alzheimer’s disease: A fdg-pet study. Dementia and Geriatric Cognitive Disorders. 2008;26(4):306–313. doi: 10.1159/000161055. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4. New York: Oxford; 2004. [Google Scholar]
- Mahurin RK, DeBettignies BH, Pirozzolo FJ. Structured assessment of independent living skills: Preliminary report of a performance measure of functional abilities in dementia. Journal of Gerontology. 1991;46(2):P58–66. doi: 10.1093/geronj/46.2.p58. [DOI] [PubMed] [Google Scholar]
- Makuuchi M, Kaminaga T, Sugishita M. Both parietal lobes are involved in drawing: A functional mri study and implications for constructional apraxia. Brain Research Cognitive Brain Research. 2003;16(3):338–347. doi: 10.1016/s0926-6410(02)00302-6. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia rating scale: Professional manual. Odessa, FL: Psychological Assessment Resource, Inc; 1988. [Google Scholar]
- McConley R, Martin R, Banos J, Blanton P, Faught E. Global/local scoring modifications for the Rey-osterrieth complex figure: Relation to unilateral temporal lobe epilepsy patients. Journal of the International Neuropsychological Society. 2006;12(3):383–390. doi: 10.1017/s1355617706060413. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of alzheimer’s disease: Report of the nincds-adrda work group under the auspices of the department of health and human services task force on alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas, et al. The diagnosis of dementia due to alzheimer’s disease: Recommendations from the national institute on aging-alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimers & Dementia. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose RJ, Ettenhofer ML, Harwood D, Achamallah N, Campa O, Mandelkern M, Sultzer DL. Cerebral metabolism, cognition, and functional abilities in alzheimer disease. Journal of Geriatric Psychiatry and Neurology. 2011;24(3):127–134. doi: 10.1177/089198871140533324/3/127. [DOI] [PubMed] [Google Scholar]
- Meyers J, Meyers K. The meyers scoring system for the rey complex figure and the recognition trial: Professional manual. Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- Miall RC, Gowen E, Tchalenko J. Drawing cartoon faces--a functional imaging study of the cognitive neuroscience of drawing. Cortex. 2009;45(3):394–406. doi: 10.1016/j.cortex.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickanin J, Grossman M, Inishi K, Auriacombe S, Clark C. Verbal and nonverbal fluency in patients with probable alzheimer’s disease. Neuropsychology. 1994;8(3):385–394. [Google Scholar]
- Nadkarni NK, Levy-Cooperman N, Black SE. Functional correlates of instrumental activities of daily living in mild alzheimer’s disease. Neurobiology of Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama Y, Okina T, Suzuki N, Nabatame H, Matsuda M. Neural correlates of impaired performance on the clock drawing test in alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2005;19(5-6):390–396. doi: 10.1159/000084710. [DOI] [PubMed] [Google Scholar]
- Nobili F, Brugnolo A, Calvini P, Copello F, De Leo C, Girtler N, et al. Resting spect-neuropsychology correlation in very mild alzheimer’s disease. Clinical Neurophysiology. 2005;116(2):364–375. doi: 10.1016/j.clinph.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d’une figure complex: Contribution a l’etude de la perception et de la memoire [The test of copying a complex figure: A contribution to the study of perception and memory] Archives de Psychologie. 1944;28:1021–1034. [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: Comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. The European Journal of Neuroscience. 1999;11(3):1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Peyrin C, Baciu M, Segebarth C, Marendaz C. Cerebral regions and hemispheric specialization for processing spatial frequencies during natural scene recognition. An event-related fmri study. Neuroimage. 2004;23(2):698–707. doi: 10.1016/j.neuroimage.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Razani J, Bayan S, Funes C, Mahmoud N, Torrence N, Wong J, et al. Patterns of deficits in daily functioning and cognitive performance of patients with alzheimer disease. Journal of Geriatric Psychiatry and Neurology. 2011;24(1):23–32. doi: 10.1177/0891988710390812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique [Psychological examination of traumatic encephalopathy] Archives de Psychologie. 1941;28:286–340. [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioral Neurology. 2000;12(4):191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Salmon E, Lespagnard S, Marique P, Peeters F, Herholz K, Perani D, et al. Cerebral metabolic correlates of four dementia scales in alzheimer’s disease. Journal of Neurology. 2005;252(3):283–290. doi: 10.1007/s00415-005-0551-3. [DOI] [PubMed] [Google Scholar]
- Salmon E, Kerrouche N, Perani D, Lekeu F, Holthoff V, Beuthien-Baumann B, et al. On the multivariate nature of brain metabolic impairment in alzheimer’s disease. Neurobiology of Aging. 2009;30(2):186–197. doi: 10.1016/j.neurobiolaging.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Savage CR, Baer L, Keuthen NJ, Brown HD, Rauch SL, Jenike MA. Organizational strategies mediate nonverbal memory impairment in obsessive-compulsive disorder. Biological Psychiatry. 1999;45(7):905–916. doi: 10.1016/s0006-3223(98)00278-9. [DOI] [PubMed] [Google Scholar]
- Slavin MJ, Mattingley JB, Bradshaw JL, Storey E. Local-global processing in alzheimer’s disease: An examination of interference, inhibition and priming. Neuropsychologia. 2002;40(8):1173–1186. doi: 10.1016/s0028-3932(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Somerville J, Tremont G, Stern RA. The boston qualitative scoring system as a measure of executive functioning in rey-osterrieth complex figure performance. Journal of Clinical and Experimental Neuropsychology. 2000;22(5):613–621. doi: 10.1076/1380-3395(200010)22:5;1-9;FT613. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Willoch F, Ishii K, Burger K, Drzezga A, Engel R, et al. Resting state glucose utilization and the cerad cognitive battery in patients with alzheimer’s disease. Neurobiology of Aging. 2006;27(5):681–690. doi: 10.1016/j.neurobiolaging.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Thomann PA, Toro P, Dos Santos V, Essig M, Schroder J. Clock drawing performance and brain morphology in mild cognitive impairment and alzheimer’s disease. Brain and Cognition. 2008;67(1):88–93. doi: 10.1016/j.bandc.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Tippett WJ, Black SE. Regional cerebral blood flow correlates of visuospatial tasks in alzheimer’s disease. Journal of the International Neuropsychological Society. 2008;14(6):1034–1045. doi: 10.1017/S1355617708081241. [DOI] [PubMed] [Google Scholar]
- Trojano L, Fragassi NA, Chiacchio L, Izzo O, Izzo G, Di Cesare G, et al. Relationships between constructional and visuospatial abilities in normal subjects and in focal brain-damaged patients. Journal of Clinical and Experimental Neuropsychology. 2004;26(8):1103–1112. doi: 10.1080/13803390490515522. [DOI] [PubMed] [Google Scholar]
- Tupler LA, Welsh KA, Asare-Aboagye Y, Dawson DV. Reliability of the rey-osterrieth complex figure in use with memory-impaired patients. Journal of Clinical and Experimental Neuropsychology. 1995;17(4):566–579. doi: 10.1080/01688639508405146. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in spm using a macroscopic anatomical parcellation of the mni mri single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Ueda H, Kitabayashi Y, Narumoto J, Nakamura K, Kita H, Kishikawa Y, Fukui K. Relationship between clock drawing test performance and regional cerebral blood flow in alzheimer’s disease: A single photon emission computed tomography study. Psychiatry and Clinical Neurosciences. 2002;56(1):25–29. doi: 10.1046/j.1440-1819.2002.00940.x. [DOI] [PubMed] [Google Scholar]
- Van Kleeck MH. Hemispheric differences in global versus local processing of hierarchical visual stimuli by normal subjects: New data and a meta-analysis of previous studies. Neuropsychologia. 1989;27(9):1165–1178. doi: 10.1016/0028-3932(89)90099-7. [DOI] [PubMed] [Google Scholar]
- Vidoni ED, Honea RA, Burns JM. Neural correlates of impaired functional independence in early alzheimer’s disease. Journal of Alzheimers Disease. 2010;19(2):517–527. doi: 10.3233/JAD-2010-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Ogino T, Nakano K, Hattori J, Kado Y, Sanada S, Ohtsuka Y. The rey-osterrieth complex figure as a measure of executive function in childhood. Brain & Development. 2005;27(8):564–569. doi: 10.1016/j.braindev.2005.02.007. [DOI] [PubMed] [Google Scholar]


