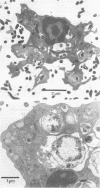
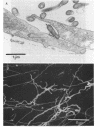
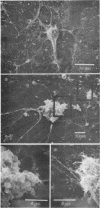
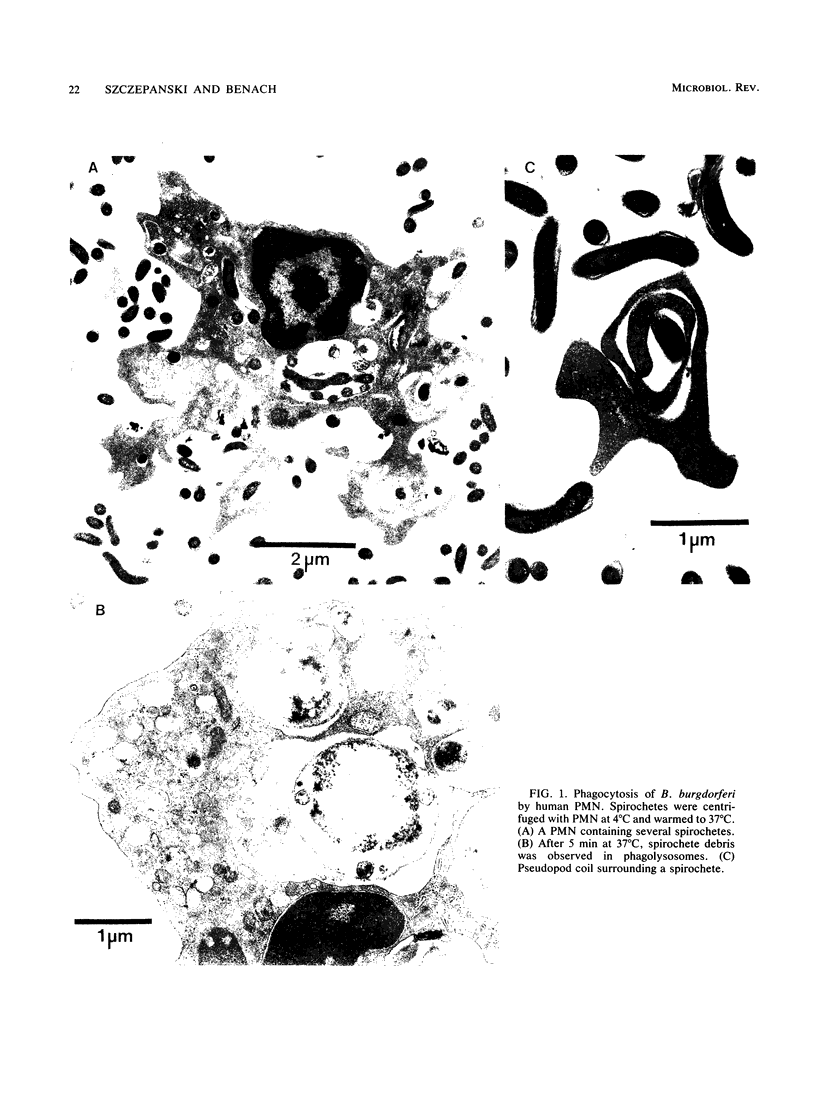
Abstract
The chronic inflammatory condition that develops after infection by B. burgdorferi is a complex process resulting from host responses to a limited number of organisms. Amplification mechanisms driven by potent proinflammatory molecules, i.e., IL-1, may explain the vigorous response to a paucity of organisms. Spirochete dissemination to distant locations involves adherence to and penetration across endothelium and may be facilitated by host responses that increase vessel permeability. The apparent lack of tissue tropism in Lyme disease is reflected in the organism's ability to adhere to different eucaryotic cell types in vitro and the wide distribution of B. burgdorferi in various organs of infected humans and experimentally infected animals. While phagocytosis and complement activation have been observed in vitro, the specific immune response that develops in humans is inefficient in eradicating the organisms, which may possess some mechanism(s) to evade this response. There is significant evidence for host autoreactivity based on antigenic cross-reactivity between the 41-kDa flagellar subunit and stress proteins of the spirochetes and endogenous host cell components. Although the outer surface proteins appear to be suitable candidates as targets for vaccination in animal studies, fundamental differences in the immune response to spirochetal components may preclude their use in humans.
Full text
PDF


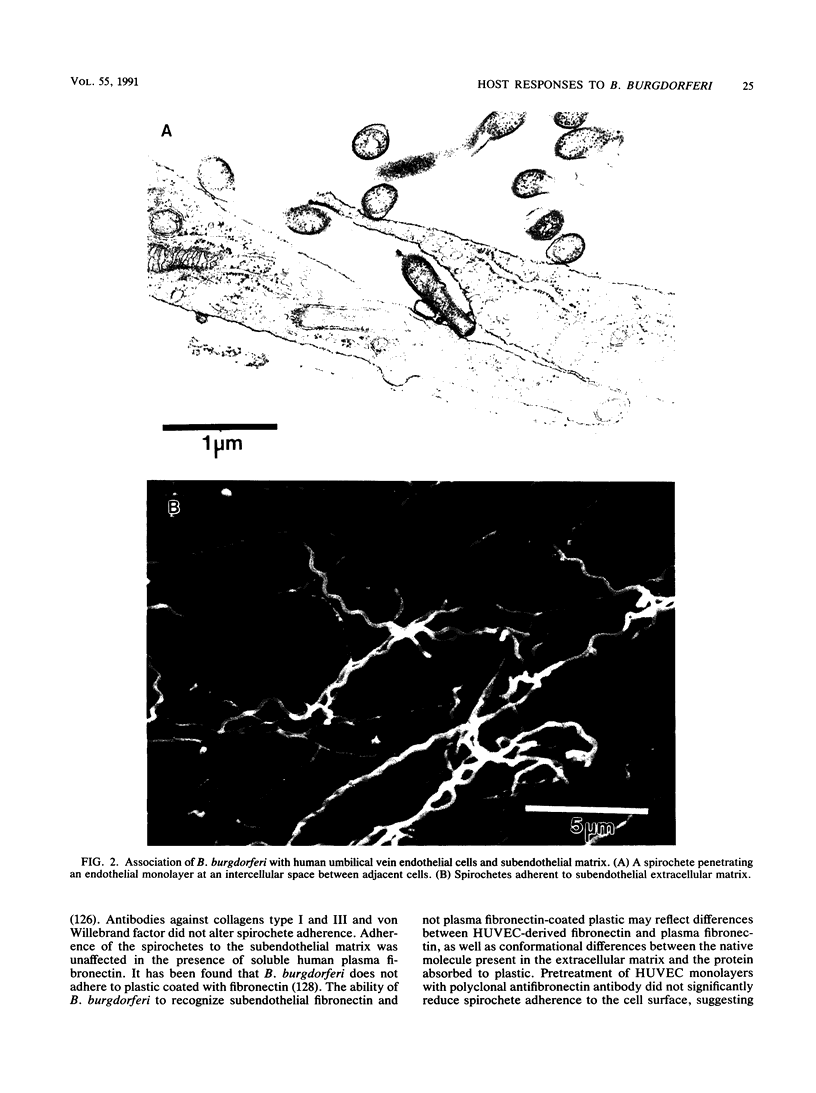








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aberer E., Brunner C., Suchanek G., Klade H., Barbour A., Stanek G., Lassmann H. Molecular mimicry and Lyme borreliosis: a shared antigenic determinant between Borrelia burgdorferi and human tissue. Ann Neurol. 1989 Dec;26(6):732–737. doi: 10.1002/ana.410260608. [DOI] [PubMed] [Google Scholar]
- Asbrink E., Hovmark A., Hederstedt B. The spirochetal etiology of acrodermatitis chronica atrophicans Herxheimer. Acta Derm Venereol. 1984;64(6):506–512. [PubMed] [Google Scholar]
- Asbrink E., Hovmark A. Successful cultivation of spirochetes from skin lesions of patients with erythema chronicum migrans Afzelius and acrodermatitis chronica atrophicans. Acta Pathol Microbiol Immunol Scand B. 1985 Apr;93(2):161–163. doi: 10.1111/j.1699-0463.1985.tb02870.x. [DOI] [PubMed] [Google Scholar]
- Banfi E., Cinco M., Perticarari S., Presani G. Rapid flow cytometric studies of Borrelia burgdorferi phagocytosis by human polymorphonuclear leukocytes. J Appl Bacteriol. 1989 Jul;67(1):37–45. doi: 10.1111/j.1365-2672.1989.tb04952.x. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Burgdorfer W., Grunwaldt E., Steere A. C. Antibodies of patients with Lyme disease to components of the Ixodes dammini spirochete. J Clin Invest. 1983 Aug;72(2):504–515. doi: 10.1172/JCI110998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F., Heiland R. A., Schrumpf M. E., Tessier S. L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986 May;52(2):549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Laboratory aspects of Lyme borreliosis. Clin Microbiol Rev. 1988 Oct;1(4):399–414. doi: 10.1128/cmr.1.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Stoenner H. G. Variable major proteins of Borrellia hermsii. J Exp Med. 1982 Nov 1;156(5):1312–1324. doi: 10.1084/jem.156.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W., Beck D. S., Hansen G. M., Terwilliger G. A., Moody K. D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990 Jul;162(1):133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- Barthold S. W., Moody K. D., Terwilliger G. A., Duray P. H., Jacoby R. O., Steere A. C. Experimental Lyme arthritis in rats infected with Borrelia burgdorferi. J Infect Dis. 1988 Apr;157(4):842–846. doi: 10.1093/infdis/157.4.842. [DOI] [PubMed] [Google Scholar]
- Barthold S. W., Moody K. D., Terwilliger G. A., Jacoby R. O., Steere A. C. An animal model for Lyme arthritis. Ann N Y Acad Sci. 1988;539:264–273. doi: 10.1111/j.1749-6632.1988.tb31860.x. [DOI] [PubMed] [Google Scholar]
- Beck G., Benach J. L., Habicht G. S. Isolation of interleukin 1 from joint fluids of patients with Lyme disease. J Rheumatol. 1989 Jun;16(6):800–806. [PubMed] [Google Scholar]
- Beck G., Habicht G. S., Benach J. L., Coleman J. L. Chemical and biologic characterization of a lipopolysaccharide extracted from the Lyme disease spirochete (Borrelia burgdorferi). J Infect Dis. 1985 Jul;152(1):108–117. doi: 10.1093/infdis/152.1.108. [DOI] [PubMed] [Google Scholar]
- Beck G., Habicht G. S., Benach J. L., Miller F. Interleukin 1: a common endogenous mediator of inflammation and the local Shwartzman reaction. J Immunol. 1986 Apr 15;136(8):3025–3031. [PubMed] [Google Scholar]
- Benach J. L., Bosler E. M., Coleman J. L., Habicht G. S. Experimental transmission of the Lyme disease spirochete to rabbits. J Infect Dis. 1984 Nov;150(5):786–787. doi: 10.1093/infdis/150.5.786-a. [DOI] [PubMed] [Google Scholar]
- Benach J. L., Bosler E. M., Hanrahan J. P., Coleman J. L., Habicht G. S., Bast T. F., Cameron D. J., Ziegler J. L., Barbour A. G., Burgdorfer W. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983 Mar 31;308(13):740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Benach J. L., Coleman J. L., Garcia-Monco J. C., Deponte P. C. Biological activity of Borrelia burgdorferi antigens. Ann N Y Acad Sci. 1988;539:115–125. doi: 10.1111/j.1749-6632.1988.tb31845.x. [DOI] [PubMed] [Google Scholar]
- Benach J. L., Coleman J. L., Golightly M. G. A murine IgM monoclonal antibody binds an antigenic determinant in outer surface protein A, an immunodominant basic protein of the Lyme disease spirochete. J Immunol. 1988 Jan 1;140(1):265–272. [PubMed] [Google Scholar]
- Benach J. L., Coleman J. L., Skinner R. A., Bosler E. M. Adult Ixodes dammini on rabbits: a hypothesis for the development and transmission of Borrelia burgdorferi. J Infect Dis. 1987 Jun;155(6):1300–1306. doi: 10.1093/infdis/155.6.1300. [DOI] [PubMed] [Google Scholar]
- Benach J. L., Fleit H. B., Habicht G. S., Coleman J. L., Bosler E. M., Lane B. P. Interactions of phagocytes with the Lyme disease spirochete: role of the Fc receptor. J Infect Dis. 1984 Oct;150(4):497–507. doi: 10.1093/infdis/150.4.497. [DOI] [PubMed] [Google Scholar]
- Benach J. L., Habicht G. S., Gocinski B. L., Coleman J. L. Phagocytic cell responses to in vivo and in vitro exposure to the Lyme disease spirochete. Yale J Biol Med. 1984 Jul-Aug;57(4):599–605. [PMC free article] [PubMed] [Google Scholar]
- Berger B. W., Kaplan M. H., Rothenberg I. R., Barbour A. G. Isolation and characterization of the Lyme disease spirochete from the skin of patients with erythema chronicum migrans. J Am Acad Dermatol. 1985 Sep;13(3):444–449. doi: 10.1016/s0190-9622(85)70187-9. [DOI] [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Brandt M. E., Riley B. S., Radolf J. D., Norgard M. V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990 Apr;58(4):983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Hayes S. F., Benach J. L. Development of Borrelia burgdorferi in ixodid tick vectors. Ann N Y Acad Sci. 1988;539:172–179. doi: 10.1111/j.1749-6632.1988.tb31851.x. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W. The New Zealand white rabbit: an experimental host for infecting ticks with Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):609–612. [PMC free article] [PubMed] [Google Scholar]
- Carreiro M. M., Laux D. C., Nelson D. R. Characterization of the heat shock response and identification of heat shock protein antigens of Borrelia burgdorferi. Infect Immun. 1990 Jul;58(7):2186–2191. doi: 10.1128/iai.58.7.2186-2191.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluss R. G., Boothby J. T. Thermoregulation of protein synthesis in Borrelia burgdorferi. Infect Immun. 1990 Apr;58(4):1038–1042. doi: 10.1128/iai.58.4.1038-1042.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. L., Benach J. L. Identification and characterization of an endoflagellar antigen of Borrelia burgdorferi. J Clin Invest. 1989 Jul;84(1):322–330. doi: 10.1172/JCI114157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. L., Benach J. L. Isolation of antigenic components from the Lyme disease spirochete: their role in early diagnosis. J Infect Dis. 1987 Apr;155(4):756–765. doi: 10.1093/infdis/155.4.756. [DOI] [PubMed] [Google Scholar]
- Comstock L. E., Thomas D. D. Penetration of endothelial cell monolayers by Borrelia burgdorferi. Infect Immun. 1989 May;57(5):1626–1628. doi: 10.1128/iai.57.5.1626-1628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft J. E., Fischer D. K., Shimamoto G. T., Steere A. C. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest. 1986 Oct;78(4):934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattwyler R. J., Volkman D. J., Halperin J. J., Luft B. J., Thomas J., Golightly M. G. Specific immune responses in Lyme borreliosis. Characterization of T cell and B cell responses to Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:93–102. doi: 10.1111/j.1749-6632.1988.tb31842.x. [DOI] [PubMed] [Google Scholar]
- Dattwyler R. J., Volkman D. J., Luft B. J., Halperin J. J., Thomas J., Golightly M. G. Seronegative Lyme disease. Dissociation of specific T- and B-lymphocyte responses to Borrelia burgdorferi. N Engl J Med. 1988 Dec 1;319(22):1441–1446. doi: 10.1056/NEJM198812013192203. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Doetsch R. N., Sjoblad R. D. Flagellar structure and function in eubacteria. Annu Rev Microbiol. 1980;34:69–108. doi: 10.1146/annurev.mi.34.100180.000441. [DOI] [PubMed] [Google Scholar]
- Donahue J. G., Piesman J., Spielman A. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am J Trop Med Hyg. 1987 Jan;36(1):92–96. doi: 10.4269/ajtmh.1987.36.92. [DOI] [PubMed] [Google Scholar]
- Duray P. H., Johnson R. C. The histopathology of experimentally infected hamsters with the Lyme disease spirochete, Borrelia burgdorferi. Proc Soc Exp Biol Med. 1986 Feb;181(2):263–269. doi: 10.3181/00379727-181-42251. [DOI] [PubMed] [Google Scholar]
- Duray P. H., Steere A. C. Clinical pathologic correlations of Lyme disease by stage. Ann N Y Acad Sci. 1988;539:65–79. doi: 10.1111/j.1749-6632.1988.tb31839.x. [DOI] [PubMed] [Google Scholar]
- Ellis J. Proteins as molecular chaperones. 1987 Jul 30-Aug 5Nature. 328(6129):378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Monco J. C., Benach J. L. The pathogenesis of Lyme disease. Rheum Dis Clin North Am. 1989 Nov;15(4):711–726. [PubMed] [Google Scholar]
- Garcia-Monco J. C., Coleman J. L., Benach J. L. Antibodies to myelin basic protein in Lyme disease. J Infect Dis. 1988 Sep;158(3):667–668. doi: 10.1093/infdis/158.3.667. [DOI] [PubMed] [Google Scholar]
- Garcia-Monco J. C., Fernandez-Villar B., Benach J. L. Adherence of the Lyme disease spirochete to glial cells and cells of glial origin. J Infect Dis. 1989 Sep;160(3):497–506. doi: 10.1093/infdis/160.3.497. [DOI] [PubMed] [Google Scholar]
- Garcia-Monco J. C., Frey H. M., Villar B. F., Golightly M. G., Benach J. L. Lyme disease concurrent with human immunodeficiency virus infection. Am J Med. 1989 Sep;87(3):325–328. doi: 10.1016/s0002-9343(89)80158-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Monco J. C., Villar B. F., Alen J. C., Benach J. L. Borrelia burgdorferi in the central nervous system: experimental and clinical evidence for early invasion. J Infect Dis. 1990 Jun;161(6):1187–1193. doi: 10.1093/infdis/161.6.1187. [DOI] [PubMed] [Google Scholar]
- Gassmann G. S., Kramer M., Göbel U. B., Wallich R. Nucleotide sequence of a gene encoding the Borrelia burgdorferi flagellin. Nucleic Acids Res. 1989 May 11;17(9):3590–3590. doi: 10.1093/nar/17.9.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston J. S., Life P. F., Bailey L. C., Bacon P. A. In vitro responses to a 65-kilodalton mycobacterial protein by synovial T cells from inflammatory arthritis patients. J Immunol. 1989 Oct 15;143(8):2494–2500. [PubMed] [Google Scholar]
- Golightly M., Thomas J., Volkman D., Dattwyler R. Modulation of natural killer cell activity by Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:103–111. doi: 10.1111/j.1749-6632.1988.tb31843.x. [DOI] [PubMed] [Google Scholar]
- Habicht G. S., Beck G., Benach J. L., Coleman J. L., Leichtling K. D. Lyme disease spirochetes induce human and murine interleukin 1 production. J Immunol. 1985 May;134(5):3147–3154. [PubMed] [Google Scholar]
- Habicht G. S., Beck G., Benach J. L. The role of interleukin-1 in the pathogenesis of Lyme disease. Ann N Y Acad Sci. 1988;539:80–86. doi: 10.1111/j.1749-6632.1988.tb31840.x. [DOI] [PubMed] [Google Scholar]
- Hansen K., Bangsborg J. M., Fjordvang H., Pedersen N. S., Hindersson P. Immunochemical characterization of and isolation of the gene for a Borrelia burgdorferi immunodominant 60-kilodalton antigen common to a wide range of bacteria. Infect Immun. 1988 Aug;56(8):2047–2053. doi: 10.1128/iai.56.8.2047-2053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K., Cruz M., Link H. Oligoclonal Borrelia burgdorferi-specific IgG antibodies in cerebrospinal fluid in Lyme neuroborreliosis. J Infect Dis. 1990 Jun;161(6):1194–1202. doi: 10.1093/infdis/161.6.1194. [DOI] [PubMed] [Google Scholar]
- Hardin J. A., Steere A. C., Malawista S. E. Immune complexes and the evolution of Lyme arthritis. Dissemination and localization of abnormal C1q binding activity. N Engl J Med. 1979 Dec 20;301(25):1358–1363. doi: 10.1056/NEJM197912203012502. [DOI] [PubMed] [Google Scholar]
- Hardin J. A., Walker L. C., Steere A. C., Trumble T. C., Tung K. S., Williams R. C., Jr, Ruddy S., Malawista S. E. Circulating immune complexes in Lyme arthritis. Detection by the 125I-C1q binding, C1q solid phase, and Raji cell assays. J Clin Invest. 1979 Mar;63(3):468–477. doi: 10.1172/JCI109324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechemy K. E., Harris H. L., Duerr M. J., Benach J. L., Reimer C. B. Immunoglobulin G subclasses specific to Borrelia burgdorferi in patients with Lyme disease. Ann N Y Acad Sci. 1988;539:162–169. doi: 10.1111/j.1749-6632.1988.tb31849.x. [DOI] [PubMed] [Google Scholar]
- Hechemy K. E., Samsonoff W. A., McKee M., Guttman J. M. Borrelia burgdorferi attachment to mammalian cells. J Infect Dis. 1989 Apr;159(4):805–806. doi: 10.1093/infdis/159.4.805. [DOI] [PubMed] [Google Scholar]
- Hejka A., Schmitz J. L., England D. M., Callister S. M., Schell R. F. Histopathology of Lyme arthritis in LSH hamsters. Am J Pathol. 1989 May;134(5):1113–1123. [PMC free article] [PubMed] [Google Scholar]
- Henriksson A., Link H., Cruz M., Stiernstedt G. Immunoglobulin abnormalities in cerebrospinal fluid and blood over the course of lymphocytic meningoradiculitis (Bannwarth's syndrome). Ann Neurol. 1986 Sep;20(3):337–345. doi: 10.1002/ana.410200311. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Naparstek Y., Ben-Nun A., Cohen I. R. Lines of T lymphocytes induce or vaccinate against autoimmune arthritis. Science. 1983 Jan 7;219(4580):56–58. doi: 10.1126/science.6336851. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Kodner C., Russell M. Active immunization of hamsters against experimental infection with Borrelia burgdorferi. Infect Immun. 1986 Dec;54(3):897–898. doi: 10.1128/iai.54.3.897-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Kodner C., Russell M., Duray P. H. Experimental infection of the hamster with Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:258–263. doi: 10.1111/j.1749-6632.1988.tb31859.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Kodner C., Russell M. Passive immunization of hamsters against experimental infection with the Lyme disease spirochete. Infect Immun. 1986 Sep;53(3):713–714. doi: 10.1128/iai.53.3.713-714.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Marek N., Kodner C. Infection of Syrian hamsters with Lyme disease spirochetes. J Clin Microbiol. 1984 Dec;20(6):1099–1101. doi: 10.1128/jcm.20.6.1099-1101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston Y. E., Duray P. H., Steere A. C., Kashgarian M., Buza J., Malawista S. E., Askenase P. W. Lyme arthritis. Spirochetes found in synovial microangiopathic lesions. Am J Pathol. 1985 Jan;118(1):26–34. [PMC free article] [PubMed] [Google Scholar]
- Joys T. M. The covalent structure of the phase-1 flagellar filament protein of Salmonella typhimurium and its comparison with other flagellins. J Biol Chem. 1985 Dec 15;260(29):15758–15761. [PubMed] [Google Scholar]
- Joys T. M. The flagellar filament protein. Can J Microbiol. 1988 Apr;34(4):452–458. doi: 10.1139/m88-078. [DOI] [PubMed] [Google Scholar]
- Kochi S. K., Johnson R. C. Role of immunoglobulin G in killing of Borrelia burgdorferi by the classical complement pathway. Infect Immun. 1988 Feb;56(2):314–321. doi: 10.1128/iai.56.2.314-321.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Wand-Württenberger A., DeBruyn J., Munk M. E., Schoel B., Kaufmann S. H. T cells against a bacterial heat shock protein recognize stressed macrophages. Science. 1989 Sep 8;245(4922):1112–1115. doi: 10.1126/science.2788923. [DOI] [PubMed] [Google Scholar]
- Kornblatt A. N., Steere A. C., Brownstein D. G. Infection in rabbits with the Lyme disease spirochete. Yale J Biol Med. 1984 Jul-Aug;57(4):613–616. [PMC free article] [PubMed] [Google Scholar]
- Krinsky W. L., Brown S. J., Askenase P. W. Ixodes dammini: induced skin lesions in guinea pigs and rabbits compared to erythema chronicum migrans in patients with lyme arthritis. Exp Parasitol. 1982 Jun;53(3):381–395. doi: 10.1016/0014-4894(82)90081-9. [DOI] [PubMed] [Google Scholar]
- Kujala G. A., Steere A. C., Davis J. S., 4th IgM rheumatoid factor in Lyme disease: correlation with disease activity, total serum IgM, and IgM antibody to Borrelia burgdorferi. J Rheumatol. 1987 Aug;14(4):772–776. [PubMed] [Google Scholar]
- Kurtti T. J., Munderloh U. G., Ahlstrand G. G., Johnson R. C. Borrelia burgdorferi in tick cell culture: growth and cellular adherence. J Med Entomol. 1988 Jul;25(4):256–261. doi: 10.1093/jmedent/25.4.256. [DOI] [PubMed] [Google Scholar]
- Lydyard P. M., van Eden W. Heat shock proteins: immunity and immunopathology. Immunol Today. 1990 Jul;11(7):228–229. doi: 10.1016/0167-5699(90)90091-m. [DOI] [PubMed] [Google Scholar]
- Mackworth-Young C. G., Harris E. N., Steere A. C., Rizvi F., Malawista S. E., Hughes G. R., Gharavi A. E. Anticardiolipin antibodies in Lyme disease. Arthritis Rheum. 1988 Aug;31(8):1052–1056. doi: 10.1002/art.1780310818. [DOI] [PubMed] [Google Scholar]
- Martin R., Ortlauf J., Sticht-Groh V., Bogdahn U., Goldmann S. F., Mertens H. G. Borrelia burgdorferi--specific and autoreactive T-cell lines from cerebrospinal fluid in Lyme radiculomyelitis. Ann Neurol. 1988 Oct;24(4):509–516. doi: 10.1002/ana.410240406. [DOI] [PubMed] [Google Scholar]
- Mensi N., Webb D. R., Turck C. W., Peltz G. A. Characterization of Borrelia burgdorferi proteins reactive with antibodies in synovial fluid of a patient with Lyme arthritis. Infect Immun. 1990 Jul;58(7):2404–2407. doi: 10.1128/iai.58.7.2404-2407.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat C. M., Sigal L. H., Steere A. C., Freeman D. H., Dwyer J. M. Cellular immune findings in Lyme disease. Correlation with serum IgM and disease activity. Am J Med. 1984 Oct;77(4):625–632. doi: 10.1016/0002-9343(84)90352-8. [DOI] [PubMed] [Google Scholar]
- Moody K. D., Barthold S. W., Terwilliger G. A., Beck D. S., Hansen G. M., Jacoby R. O. Experimental chronic Lyme borreliosis in Lewis rats. Am J Trop Med Hyg. 1990 Feb;42(2):165–174. doi: 10.4269/ajtmh.1990.42.165. [DOI] [PubMed] [Google Scholar]
- Murray N., Kristoferitsch W., Stanek G., Steck A. J. Specificity of CSF antibodies against components of Borrelia burgdorferi in patients with meningopolyneuritis Garin-Bujadoux-Bannwarth. J Neurol. 1986 Aug;233(4):224–227. doi: 10.1007/BF00314024. [DOI] [PubMed] [Google Scholar]
- Neumann A., Schlesier M., Schneider H., Vogt A., Peter H. H. Frequencies of Borrelia burgdorferi-reactive T lymphocytes in Lyme arthritis. Rheumatol Int. 1989;9(3-5):237–241. doi: 10.1007/BF00271888. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry as a mechanism for the cause and a probe uncovering etiologic agent(s) of autoimmune disease. Curr Top Microbiol Immunol. 1989;145:127–135. doi: 10.1007/978-3-642-74594-2_11. [DOI] [PubMed] [Google Scholar]
- Pachner A. R., Steere A. C., Sigal L. H., Johnson C. J. Antigen-specific proliferation of CSF lymphocytes in Lyme disease. Neurology. 1985 Nov;35(11):1642–1644. doi: 10.1212/wnl.35.11.1642. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Clawson C. C., Lee D. A., Garlich D. J., Quie P. G., Johnson R. C. Human phagocyte interactions with the Lyme disease spirochete. Infect Immun. 1984 Nov;46(2):608–611. doi: 10.1128/iai.46.2.608-611.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister H. W., Neubert U., Wilske B., Preac-Mursic V., Einhäupl K. M., Borasio G. D. Reinfection with Borrelia burgdorferi. Lancet. 1986 Oct 25;2(8513):984–985. doi: 10.1016/s0140-6736(86)90640-9. [DOI] [PubMed] [Google Scholar]
- Piesman J., Mather T. N., Sinsky R. J., Spielman A. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987 Mar;25(3):557–558. doi: 10.1128/jcm.25.3.557-558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preac Mursic V., Wilske B., Schierz G., Pfister H. W., Einhäupl K. Repeated isolation of spirochetes from the cerebrospinal fluid of a patient with meningoradiculitis Bannwarth. Eur J Clin Microbiol. 1984 Dec;3(6):564–565. doi: 10.1007/BF02013623. [DOI] [PubMed] [Google Scholar]
- Res P. C., Schaar C. G., Breedveld F. C., van Eden W., van Embden J. D., Cohen I. R., de Vries R. R. Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet. 1988 Aug 27;2(8609):478–480. doi: 10.1016/s0140-6736(88)90123-7. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. M., Mather T. N., Piesman J., Spielman A. Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari: Ixodidae). J Med Entomol. 1987 Mar;24(2):201–205. doi: 10.1093/jmedent/24.2.201. [DOI] [PubMed] [Google Scholar]
- Ryberg B., Hindfelt B., Nilsson B., Olsson J. E. Antineural antibodies in Guillain-Barré syndrome and lymphocytic meningoradiculitis (Bannwarth's syndrome). Arch Neurol. 1984 Dec;41(12):1277–1281. doi: 10.1001/archneur.1984.04050230063019. [DOI] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Eichmann K., Modolell M., Museteanu C., Simon M. M. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc Natl Acad Sci U S A. 1990 May;87(10):3768–3772. doi: 10.1073/pnas.87.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Justus C. W., Museteanu C., Simon M. M. Demonstration of antigen-specific T cells and histopathological alterations in mice experimentally inoculated with Borrelia burgdorferi. Infect Immun. 1989 Jan;57(1):41–47. doi: 10.1128/iai.57.1.41-47.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Museteanu C., Zimmer G., Mossmann H., Simon M. M. The severe combined immunodeficiency (scid) mouse. A laboratory model for the analysis of Lyme arthritis and carditis. J Exp Med. 1989 Oct 1;170(4):1427–1432. doi: 10.1084/jem.170.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J. Heat shock proteins. J Biol Chem. 1990 Jul 25;265(21):12111–12114. [PubMed] [Google Scholar]
- Schmidli J., Hunziker T., Moesli P., Schaad U. B. Cultivation of Borrelia burgdorferi from joint fluid three months after treatment of facial palsy due to Lyme borreliosis. J Infect Dis. 1988 Oct;158(4):905–906. doi: 10.1093/infdis/158.4.905. [DOI] [PubMed] [Google Scholar]
- Schmitz J. L., Schell R. F., Hejka A. G., England D. M. Passive immunization prevents induction of Lyme arthritis in LSH hamsters. Infect Immun. 1990 Jan;58(1):144–148. doi: 10.1128/iai.58.1.144-148.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J. L., Schell R. F., Hejka A., England D. M., Konick L. Induction of lyme arthritis in LSH hamsters. Infect Immun. 1988 Sep;56(9):2336–2342. doi: 10.1128/iai.56.9.2336-2342.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutzer S. E., Coyle P. K., Belman A. L., Golightly M. G., Drulle J. Sequestration of antibody to Borrelia burgdorferi in immune complexes in seronegative Lyme disease. Lancet. 1990 Feb 10;335(8685):312–315. doi: 10.1016/0140-6736(90)90606-6. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal L. H., Steere A. C., Dwyer J. M. In vivo and in vitro evidence of B cell hyperactivity during Lyme disease. J Rheumatol. 1988 Apr;15(4):648–654. [PubMed] [Google Scholar]
- Sigal L. H., Steere A. C., Freeman D. H., Dwyer J. M. Proliferative responses of mononuclear cells in Lyme disease. Reactivity to Borrelia burgdorferi antigens is greater in joint fluid than in blood. Arthritis Rheum. 1986 Jun;29(6):761–769. doi: 10.1002/art.1780290609. [DOI] [PubMed] [Google Scholar]
- Sigal L. H., Tatum A. H. Lyme disease patients' serum contains IgM antibodies to Borrelia burgdorferi that cross-react with neuronal antigens. Neurology. 1988 Sep;38(9):1439–1442. doi: 10.1212/wnl.38.9.1439. [DOI] [PubMed] [Google Scholar]
- Srinivasappa J., Saegusa J., Prabhakar B. S., Gentry M. K., Buchmeier M. J., Wiktor T. J., Koprowski H., Oldstone M. B., Notkins A. L. Molecular mimicry: frequency of reactivity of monoclonal antiviral antibodies with normal tissues. J Virol. 1986 Jan;57(1):397–401. doi: 10.1128/jvi.57.1.397-401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere A. C., Berardi V. P., Weeks K. E., Logigian E. L., Ackermann R. Evaluation of the intrathecal antibody response to Borrelia burgdorferi as a diagnostic test for Lyme neuroborreliosis. J Infect Dis. 1990 Jun;161(6):1203–1209. doi: 10.1093/infdis/161.6.1203. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Brinckerhoff C. E., Miller D. J., Drinker H., Harris E. D., Jr, Malawista S. E. Elevated levels of collagenase and prostaglandin E2 from synovium associated with erosion of cartilage and bone in a patient with chronic Lyme arthritis. Arthritis Rheum. 1980 May;23(5):591–599. doi: 10.1002/art.1780230511. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Duray P. H., Butcher E. C. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis. Comparison with rheumatoid synovium and tonsillar lymphoid tissue. Arthritis Rheum. 1988 Apr;31(4):487–495. doi: 10.1002/art.1780310405. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Dwyer E., Winchester R. Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 alleles. N Engl J Med. 1990 Jul 26;323(4):219–223. doi: 10.1056/NEJM199007263230402. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Hardin J. A., Ruddy S., Mummaw J. G., Malawista S. E. Lyme arthritis: correlation of serum and cryoglobulin IgM with activity, and serum IgG with remission. Arthritis Rheum. 1979 May;22(5):471–483. doi: 10.1002/art.1780220506. [DOI] [PubMed] [Google Scholar]
- Steere A. C. Lyme disease. N Engl J Med. 1989 Aug 31;321(9):586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Malawista S. E., Hardin J. A., Ruddy S., Askenase W., Andiman W. A. Erythema chronicum migrans and Lyme arthritis. The enlarging clinical spectrum. Ann Intern Med. 1977 Jun;86(6):685–698. doi: 10.7326/0003-4819-86-6-685. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Malawista S. E., Snydman D. R., Shope R. E., Andiman W. A., Ross M. R., Steele F. M. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three connecticut communities. Arthritis Rheum. 1977 Jan-Feb;20(1):7–17. doi: 10.1002/art.1780200102. [DOI] [PubMed] [Google Scholar]
- Suchanek G., Kristoferitsch W., Stanek G., Bernheimer H. Anti-myelin antibodies in cerebrospinal fluid and serum of patients with meningopolyneuritis Garin-Bujadoux-Bannwarth and other neurological diseases. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):160–168. doi: 10.1016/s0176-6724(86)80119-5. [DOI] [PubMed] [Google Scholar]
- Szczepanski A., Furie M. B., Benach J. L., Lane B. P., Fleit H. B. Interaction between Borrelia burgdorferi and endothelium in vitro. J Clin Invest. 1990 May;85(5):1637–1647. doi: 10.1172/JCI114615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Rothenberg R. J., Barbour A. G. Absence of lipopolysaccharide in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1987 Sep;55(9):2311–2313. doi: 10.1128/iai.55.9.2311-2313.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Comstock L. E. Interaction of Lyme disease spirochetes with cultured eucaryotic cells. Infect Immun. 1989 Apr;57(4):1324–1326. doi: 10.1128/iai.57.4.1324-1326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Higbie L. M. In vitro association of leptospires with host cells. Infect Immun. 1990 Mar;58(3):581–585. doi: 10.1128/iai.58.3.581-585.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Navab M., Haake D. A., Fogelman A. M., Miller J. N., Lovett M. A. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc Natl Acad Sci U S A. 1988 May;85(10):3608–3612. doi: 10.1073/pnas.85.10.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J. J. Immune responses to Borrelia burgdorferi in patients with reactive arthritis. Arthritis Rheum. 1989 Sep;32(9):1057–1064. doi: 10.1002/anr.1780320902. [DOI] [PubMed] [Google Scholar]
- Wheeler C. M., Coleman J. L., Habicht G. S., Benach J. L. Adult Ixodes dammini on rabbits: development of acute inflammation in the skin and immune responses to salivary gland, midgut, and spirochetal components. J Infect Dis. 1989 Feb;159(2):265–273. doi: 10.1093/infdis/159.2.265. [DOI] [PubMed] [Google Scholar]
- Wilske B., Schierz G., Preac-Mursic V., von Busch K., Kühbeck R., Pfister H. W., Einhäupl K. Intrathecal production of specific antibodies against Borrelia burgdorferi in patients with lymphocytic meningoradiculitis (Bannwarth's syndrome). J Infect Dis. 1986 Feb;153(2):304–314. doi: 10.1093/infdis/153.2.304. [DOI] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]
- Young R. A. Stress proteins and immunology. Annu Rev Immunol. 1990;8:401–420. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]
- van Eden W., Holoshitz J., Nevo Z., Frenkel A., Klajman A., Cohen I. R. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5117–5120. doi: 10.1073/pnas.82.15.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]